The preliminary X-ray data of β-microseminoprotein isolated from human seminal plasma at 2.4 Å resolution are reported.
Keywords: β-microseminoprotein
Abstract
β-Microseminoprotein (β-MSP) is a small cysteine-rich protein with a molecular mass of 10 kDa. It was first isolated from human seminal plasma and has subsequently been identified from several species. Comparison of the amino-acid sequences of β-MSP proteins suggests that the protein is a rapidly evolving protein. The function of β-MSP is poorly understood. Furthermore, no crystal structure has been reported of any β-MSP; therefore, determination of the crystal structure of β-MSP is the foremost task in order to understand the function of this protein completely. Here, the purification, crystallization and preliminary X-ray diffraction analysis of β-MSP from human seminal plasma are described. The protein was purified using anion-exchange and size-exclusion chromatography and the purified protein was crystallized using 0.1 M ammonium sulfate, 0.1 M HEPES buffer pH 7.0 and 20%(w/v) PEG 3350. The crystals belonged to the tetragonal space group P4322 and contained three β-MSP molecules in the asymmetric unit. X-ray intensity data were collected to 2.4 Å resolution.
1. Introduction
Human β-microseminoprotein (β-MSP) is one of three predominant prostate secretory proteins; the other two are prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP) (Lilja & Abrahamsson, 1988 ▶). β-MSP was first purified from human seminal plasma and constitutes approximately 20% of the total seminal plasma protein (Thakur et al., 1981 ▶). It is a hydrophilic nonglycosylated cysteine-rich protein with a molecular mass of 10.7 kDa (Akiyama et al., 1985 ▶; Dubé et al., 1987 ▶; Seidah et al., 1984 ▶). It is synthesized as a precursor molecule of 114 amino acids with a 20-amino-acid signal sequence, which is cleaved to yield a 94-amino-acid mature protein (Mbikay et al., 1987 ▶). Its synonyms include PSP 94 (prostatic secretory protein of 94 amino acids; Mbikay et al., 1987 ▶; Dubé et al., 1987 ▶), immunoglobulin-binding factor (IGBF; Kamada et al., 1998 ▶), prostatic inhibin-like peptide (β-inhibin; Seidah et al., 1984 ▶) and sperm motility inhibitor (SPMI; Chao et al., 1996 ▶). In addition to being found in human seminal plasma, it is also present in tracheobronchial fluid, gastric juice, secretions from the uterine cervix, tears, saliva, pancreatic juice, bile and mucus from the colon (Weiber et al., 1990 ▶; Ohkubo et al., 1995 ▶; Fernlund et al., 1996 ▶; Kwong et al., 2000 ▶, 2003 ▶).
Since its isolation, similar homologous proteins have been identified from man (Seidah et al., 1984 ▶), chicken (Kokan-Moore et al., 1991 ▶), pig (Fernlund et al., 1994 ▶), rat (Fernlund et al., 1996 ▶) and ostrich (Lazure et al., 2001 ▶). The amino-acid sequences of the various β-MSP proteins revealed extensive sequence divergence between species and marks them as members of a unique protein family known as β-microseminoproteins.
Previous investigations have suggested β-MSP to be an inhibitor of sperm motility (Chao et al., 1996 ▶; Anahi Franchi et al., 2008 ▶), an immunoglobulin-binding factor (Mori et al., 1998 ▶) and a regulator of tumour growth in prostate cancer (Garde et al., 1999 ▶; Shukeir et al., 2003 ▶; Annabi et al., 2005 ▶). Recently, it has been shown that a peptide corresponding to the region 31–45 of β-MSP reduces tumour growth without any noticeable side effects and thus can act as a therapeutic treatment for prostate cancer, reducing morbidity and mortality (Shukeir et al., 2005 ▶; Annabi et al., 2005 ▶). These findings demonstrate the importance of β-MSP in clinical diagnosis of prostate pathophysiology. However, the exact biological function of this protein has remained poorly understood since its first isolation in 1984. The most promising observation that could lead to an understanding of the biology and function of this protein is the recent detailed analysis of the NMR structures of human and porcine β-MSP (Wang et al., 2005 ▶; Ghasriani et al., 2006 ▶). These two structures are very similar, even though their sequence identity is only 51%. They both show a two-domain structure with β-sheets as the only predominant secondary-structure elements and an identical disulfide pattern involving ten cysteines.
No crystal structure of an MSP has been reported to date. Here, we describe the successful purification, crystallization and crystallographic characterization of human β-MSP. The crystal structure of human β-MSP is expected to provide an alternative structural basis and ultimately a functional illustration of β-MSP.
2. Materials and methods
2.1. Protein purification and identification
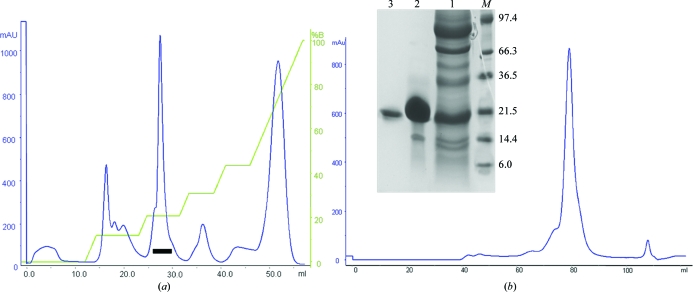
β-MSP was isolated and purified from human seminal plasma fluid, which was obtained from five normal healthy volunteers from the Department of Laboratory Medicine, All India Institute of Medical Sciences, New Delhi. A cocktail of protease inhibitors (Sigma, USA) was added to the samples immediately after ejaculation. All other chemicals and reagents were of analytical grade and were purchased from local suppliers. Seminal plasma was further separated from spermatozoa by centrifugation at 1300g for 15 min at 277 K and was stored at 253 K until use. Human seminal plasma (30 ml) was dialyzed overnight against 20 mM Tris–HCl buffer pH 8.0 and applied onto a DEAE-Sephacel anion-exchange chromatography column (1.6 × 25 cm) equilibrated with 20 mM Tris–HCl buffer pH 8.0 at a flow rate of 18 ml h−1. The bound proteins were eluted with a linear gradient of NaCl (0–500 mM) in equilibrating buffer. All subsequent purification steps were monitored using 15% SDS–PAGE. Fractions containing β-MSP were collected and concentrated by ultrafiltration with a 3 kDa membrane. Final purification was achieved by size-exclusion chromatography on a Superdex 75 column (16 × 60 cm) pre-equilibrated in 50 mM Tris–HCl pH 8.0 containing 150 mM NaCl. Fractions containing β-MSP were pooled and concentrated to 15 mg ml−1 using centrifugal devices and were stored at 193 K until the crystallization experiments. The protein purity was checked by 15% SDS–PAGE as described by Laemmli (1970 ▶) (Fig. 1 ▶).
Figure 1.
Purification profile of β-MSP from human seminal plasma. (a) Purification of β-MSP on an anion-exchange column (DEAE-Sephacel). The graph was plotted of elution volume in millilitres (x axis) against absorbance (y axis) at 280 nm. Fraction 2 (indicated by a bar) was collected and concentrated. (b) The fraction from the previous step was loaded onto a HiLoad Superdex 75 (16/60) column. The fraction containing β-MSP was pooled and concentrated for crystallization. Inset: SDS–PAGE of purified β-MSP from human seminal plasma under reducing conditions. Lane M, molecular-weight markers (kDa); lane 1, fraction 2 (indicated by a bar) from the DEAE column; lane 2, purified β-MSP from the Superdex 75 column; lane 3, β-MSP band from a crystal dissolved in acetonitrile and washed with crystallization buffer.
2.2. Crystallization
Prior to crystallization trials, dynamic light scattering (Laser-Spectroscatter 201, RiNA GmbH, Berlin) was used to confirm the monodispersity of the protein sample. For crystallization, the protein was concentrated to 10 mg ml−1 (in 25 mM Tris–HCl and 150 mM NaCl pH 8.0) and crystallized by the hanging-drop vapour-diffusion method in a robotic setup (Heinemann et al., 2003 ▶) using a 96-well Greiner plate (Crystal Quick low profile) at 277 K. Initial crystallization conditions were screened using the commercially available JCSG, Classics I and Classics II suites (Qiagen, Hilden, Germany) with drops formed by mixing equal volumes (0.4 µl) of protein and precipitant solution; the drops were equilibrated against 100 µl reservoir solution. The crystals obtained were transferred into crystallization buffer with the addition of 25%(v/v) glycerol for cryoprotection and flash-cooled in liquid nitrogen.
2.3. X-ray intensity data collection and processing
Prior to data collection, the crystal was flash-cooled in a liquid-nitrogen stream at 100 K. Diffraction experiments were performed on the Protein Structure Factory beamline BL14.2 (Heinemann et al., 2003 ▶) of Freie Universität Berlin at BESSY, Berlin, Germany. Diffraction data were collected on a MAR345 imaging-plate detector using synchrotron radiation with a wavelength of 0.9814 Å. A data set was collected using 100 frames with a 1.0° oscillation angle and an exposure time of 0.35 s per frame. All diffraction images were processed to 2.4 Å resolution with HKL-2000 (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
Human β-MSP (residues 21–114) was successfully purified using anion-exchange chromatography followed by size-exclusion chromatography. The method described in this study is a rapid, convenient and efficient two-step procedure for the purification of β-MSP when compared with previously described procedures (Ohkubo et al., 1995 ▶; Dubé et al., 1987 ▶; Baijal-Gupta et al., 1996 ▶; Garde et al., 2007 ▶). As the Superdex 75 (16/60 column) had been calibrated with proteins covering the range 67–14 kDa, the elution volume of the protein in the gel filtration of 86 ml was estimated to correspond to a molecular mass of 21 kDa. Under SDS denaturing and reducing conditions, SDS–PAGE showed a single band corresponding to a molecular mass of 21 kDa (Fig. 1 ▶), suggesting that β-MSP either forms a homodimer or has residual three-dimensional structure under the experimental conditions. Although β-MSP has a calculated molecular mass of 10.7 kDa, several reports have indicated that it runs at a higher apparent molecular mass on SDS gels (Lilja & Abrahamsson, 1988 ▶; Liang et al., 1991 ▶; Dubé et al., 1987 ▶; Baijal-Gupta et al., 1996 ▶).
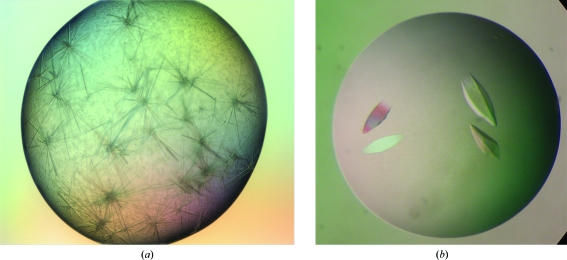
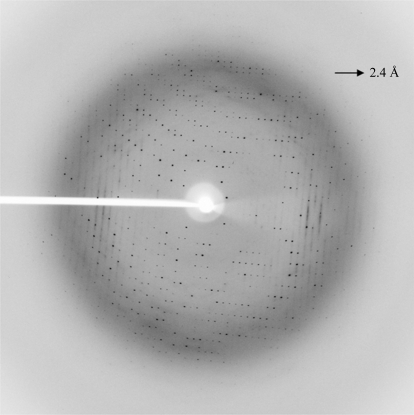
Crystallization trials were performed with drops formed by mixing equal volumes (0.4 µl) of protein and precipitant solution; the drops were equilibrated against 100 µl reservoir solution. Immediate heavy precipitation was observed in nearly 75% of all conditions. After one week, small needle-shaped crystals appeared in one condition together with precipitation [Classic II suite condition No. 68; 0.2 M ammonium sulfate, 0.1 M HEPES pH 7.5 and 25%(w/v) PEG 3350]. The final conditions after optimization produced rhombohedrally shaped crystals of up to 1 mm in length in 3 d at 277 K using the hanging-drop method (Figs. 2 ▶ a and 2 ▶ b). Drops of 1 µl protein solution at 14 mg ml−1 (in 25 mM Tris–HCl pH 8.0 containing 50 mM NaCl) and 1 µl reservoir solution were equilibrated against 1 ml reservoir solution containing 0.1 M ammonium sulfate, 0.1 M HEPES buffer pH 7.0 and 20%(w/v) PEG 3350. The crystal showed good diffraction to 2.4 Å resolution. A diffraction pattern is shown in Fig. 3 ▶. The parameters for X-ray diffraction data collection are shown in Table 1 ▶. Analysis of the diffraction pattern indicated that these crystals exhibit tetragonal symmetry and systematic absences indicated the space group to be P4322, with unit-cell parameters a = b = 76.3, c = 103.4 Å. Assuming the presence of three β-MSP molecules per asymmetric unit, the Matthews coefficient (V M) is calculated to be 2.3 Å3 Da−1, corresponding to a solvent content of 46%, which is well within the normal range for globular protein crystals (1.68–3.5 Å3 Da−1; Matthews, 1968 ▶). Solution structures have been reported for porcine and human β-MSP. The two proteins (51% sequence identity) have a very similar three-dimensional structure with well conserved secondary-structure elements and with most of the amino-acid substitutions resulting in a different charge distribution which is localized on one side of the molecule. In solution, MSP is a β-sheet-rich protein with two distinct domains. The N-terminal domain is composed of a four-stranded β-sheet with a Greek-key motif arrangement. The C-terminal domain contains two two-stranded β-sheets with unique structural motifs. The two domains are connected to each other by the peptide backbone, one disulfide bond and interaction between the N- and C-termini, resulting in an extended structure (Ghasriani et al., 2006 ▶).
Figure 2.
(a) Native crystals of β-MSP initially grown with 0.2 M ammonium sulfate, 0.1 M HEPES pH 7.5 and 25%(w/v) PEG 3350 by the hanging-drop vapour-diffusion method using a robotic setup. (b) Crystals of β-MSP obtained by optimization of the initial screen conditions in a manual setup. The crystals were grown using 0.1 M ammonium sulfate, 0.1 M HEPES buffer pH 7.0 and 20%(w/v) PEG 3350 in the reservoir. The approximate dimensions of the crystal were 0.3 × 0.2 × 1.0 mm.
Figure 3.
A typical diffraction pattern of the β-MSP crystal at 2.4 Å resolution.
Table 1. Crystal diffraction statistics of β-MSP.
Values in parentheses are for the highest resolution shell.
| Space group | P4322 |
| Unit-cell parameters (Å) | a = b = 76.3, c = 103.4 |
| No. of molecules in unit cell | 8 |
| No. of molecules in ASU | 3 |
| VM (Å3 Da−1) | 2.3 |
| Solvent content (%) | 46 |
| Resolution range (Å) | 52.0–2.4 (2.5–2.4) |
| Total No. of measured reflections | 82016 |
| No. of unique reflections | 11878 |
| Completeness of data (%) | 98.5 (86.7) |
| Rmerge† (%) | 5.8 (35.6) |
| 〈I/σ(I)〉 | 16.7 (2.1) |
R
merge = 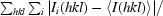
 , where I
i(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
, where I
i(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
MSP proteins have been isolated and characterized from several species: man, pig, rat, chicken and ostrich. Comparison of the known MSP sequences with other protein sequences in public databases suggests that the MSPs constitute a unique protein family and also have a unique three-dimensional structure. To date, no crystal structure of an MSP has been reported. This is the first crystallization report of human β-MSP. Crystal structure analysis by molecular replacement is in progress.
Acknowledgments
We thank Dr Sarman Singh, Professor, Department of Laboratory Medicine, AIIMS, New Delhi for arranging the provision of human seminal samples. This work was supported by financial grants from the Department of Biotechnology (DBT), Government of India. VK is grateful to the DAAD, Germany and the Council of Scientific and Industrial Research (CSIR), New Delhi for the fellowship granted to him.
References
- Akiyama, K., Yoshioka, Y., Schmid, K., Offner, G. D., Troxler, R. F., Tsuda, R. & Hara, M. (1985). Biochim. Biophys. Acta, 829, 288–294. [DOI] [PubMed]
- Anahi Franchi, N., Avendano, C., Molina, R. I., Tissera, A. D., Maldonado, C. A., Oehninger, S. & Coronel, C. E. (2008). Reproduction, 136, 157–166. [DOI] [PubMed]
- Annabi, B., Bouzeghrane, M., Currie, J. C., Hawkins, R., Dulude, H., Daigneault, L., Ruiz, M., Wisniewski, J., Garde, S., Rabbani, S. A., Panchal, C., Wu, J. J. & Beliveau, R. (2005). Clin. Exp. Metastasis, 22, 429–439. [DOI] [PubMed]
- Baijal-Gupta, M., Fraser, J. E., Clarke, M. W., Xuan, J. W. & Finkelman, M. A. (1996). Protein Expr. Purif.8, 483–488. [DOI] [PubMed]
- Chao, C. F., Chiou, S. T., Jeng, H. & Chang, W. C. (1996). Biochem. Biophys. Res. Commun.218, 623–628. [DOI] [PubMed]
- Dubé, J. Y., Frenette, G., Paquin, R., Chapdelaine, P., Tremblay, J., Tremblay, R. R., Lazure, C., Seidah, N. & Chrétien, M. (1987). J. Androl.8, 182–189. [DOI] [PubMed]
- Fernlund, P., Granberg, L. B. & Larsson, I. (1996). Arch. Biochem. Biophys.334, 73–82. [DOI] [PubMed]
- Fernlund, P., Granberg, L. B. & Roepstorff, P. (1994). Arch. Biochem. Biophys.309, 70–76. [DOI] [PubMed]
- Garde, S. V., Basrur, V. S., Li, L., Finkelman, M. A., Krishan, A., Wellham, L., Ben-Josef, E., Haddad, M., Taylor, J. D., Porter, A. T. & Tang, D. G. (1999). Prostate, 38, 118–125. [DOI] [PubMed]
- Garde, S., Fraser, J. E., Nematpoor, N., Pollex, R., Morin, C., Forte, A., Rabbani, S., Panchal, C. & Gupta, M. B. (2007). Protein Expr. Purif.54, 193–203. [DOI] [PubMed]
- Ghasriani, H., Teilum, K., Johnsson, Y., Fernlund, P. & Drakenberg, T. (2006). J. Mol. Biol.362, 502–515. [DOI] [PubMed]
- Heinemann, U., Büssow, K., Mueller, U. & Umbach, P. (2003). Acc. Chem. Res.36, 157–163. [DOI] [PubMed]
- Kamada, M., Mori, H., Maeda, N., Yamamoto, S., Kunimi, K., Takikawa, M., Maegawa, M., Aono, T., Futaki, S. & Koide, S. S. (1998). Biochim. Biophys. Acta, 1388, 101–110. [DOI] [PubMed]
- Kokan-Moore, N. P., Bolender, D. L. & Lough, J. (1991). Dev. Biol.146, 242–245. [DOI] [PubMed]
- Kwong, J., Lui, K., Chan, P. S., Ho, S. M., Wong, Y. C., Xuan, J. W. & Chan, F. L. (2003). Prostate, 56, 81–97. [DOI] [PubMed]
- Kwong, J., Xuan, J. W., Choi, H. L., Chan, P. S. & Chan, F. L. (2000). Prostate, 42, 219–229. [DOI] [PubMed]
- Laemmli, U. K. (1970). Nature (London), 227, 680–685. [DOI] [PubMed]
- Lazure, C., Villemure, M., Gauthier, D., Naude, R. J. & Mbikay, M. (2001). Protein Sci.10, 2207–2218. [DOI] [PMC free article] [PubMed]
- Liang, Z. G., Kamada, M. & Koide, S. S. (1991). Biochem. Biophys. Res. Commun.180, 356–359. [DOI] [PubMed]
- Lilja, H. & Abrahamsson, P. A. (1988). Prostate, 12, 29–38. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Mbikay, M., Nolet, S., Fournier, S., Benjannet, S., Chapdelaine, P., Paradis, G., Dubé, J. Y., Tremblay, R., Lazure, C., Seidah, N. G. & Chrétien, M. (1987). DNA, 6, 23–29. [DOI] [PubMed]
- Mori, H., Kamada, M., Maegawa, M., Yamamoto, S., Aono, T., Futaki, S., Yano, M., Kido, H. & Koide, S. S. (1998). Biochem. Biophys. Res. Commun.246, 409–413. [DOI] [PubMed]
- Ohkubo, I., Tada, T., Ochiai, Y., Ueyama, H., Eimoto, T. & Sasaki, M. (1995). Int. J. Biochem. Cell Biol.27, 603–611. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Seidah, N. G., Arbatti, N. J., Rochemont, J., Sheth, A. R. & Chrétien, M. (1984). FEBS Lett.175, 349–355. [DOI] [PubMed]
- Shukeir, N., Arakelian, A., Kadhim, S., Garde, S. & Rabbani, S. A. (2003). Cancer Res.63, 2072–2078. [PubMed]
- Shukeir, N., Garde, S., Wu, J. J., Panchal, C. & Rabbani, S. A. (2005). Anticancer Drugs, 16, 1045–1051. [DOI] [PubMed]
- Thakur, A. N., Vaze, A. Y., Dattatreyamurthy, B. & Sheth, A. R. (1981). Indian J. Exp. Biol.19, 307–313. [PubMed]
- Wang, I., Lou, Y. C., Wu, K. P., Wu, S. H., Chang, W. C. & Chen, C. (2005). J. Mol. Biol.346, 1071–1082. [DOI] [PubMed]
- Weiber, H., Andersson, C., Murne, A., Rannevik, G., Lindstrom, C., Lilja, H. & Fernlund, P. (1990). Am. J. Pathol.137, 593–603. [PMC free article] [PubMed]