Abstract
The role of agrin in synaptogenesis has been extensively studied. On the other hand, little is known about the function of this extracellular matrix protein during developmental processes that precede the formation of synapses. Recently, it has been shown that agrin regulates the rate of neurite elongation and the behavior of growth cones in hippocampal and spinal neurons, respectively. However, the molecular mechanisms underlying these effects have not been completely elucidated. In the present study, we analyzed the morphological and molecular changes induced by agrin in growth cones of hippocampal neurons that developed in culture. Morphometric analysis showed a significant enlargement of growth cones of hippocampal neurons cultured in the presence of agrin. These agrin-induced growth cone changes were accompanied by the formation of loops of microtubules highly enriched in acetylated tubulin and an increase in the content of the microtubule-associated protein MAP1B. Together, these data provide further insights into the potential molecular mechanisms underlying the effects of agrin on neurite outgrowth in central neurons.
INTRODUCTION
Agrin is one of the heparan sulfate proteoglycan components of the extracellular matrix. This multi-domain glycoprotein is encoded by a single gene that is alternatively spliced to give rise to several isoforms (Deyst et al., 1998; Glass et al., 1996 & 1997; Godfrey, 1991; Godfrey et al., 1988; Hoch et al., 1994; McMahan, 1990; Ruegg et al., 1992 & 1998; Rupp, et al. 1991 & 1992; Sanes, 1997; Stone and Nikolics, 1995). The neuron-specific isoform was initially detected in motor neurons (Cohen and Godfrey, 1992; Magill-Solc and McMahan, 1988). Later, it was shown that this isoform was also expressed in the brain (Bowe and Fallon, 1995; Hoch et al., 1993; Kroger et al., 1996; Mann and Kroger, 1996; Mantych and Ferreira, 2001; O'Toole et al., 1996; Rupp et al., 1991 & 1992; Stone and Nikolics, 1995). Secreted and transmembrane agrin isoforms are differentially expressed throughout the nervous system. While secreted forms are enriched in motor neurons, transmembrane agrin is mainly expressed in the brain (Burgess et al., 2000; Neumann et al., 2001; Gingras and Ferns, 2002). Numerous studies have focused on the functional role of agrin during synaptogenesis. The results obtained clearly indicated that agrin plays a key role in the formation of neuromuscular junctions by inducing the clustering of the acetylcholine receptors at synaptic sites (reviewed by Bowe and Fallon, 1995; McMahan, 1990; Campanelli et al., 1991; Hall & Sanes, 1993; Haydon & Drapeau, 1995; Magill-Solc and McMahan, 1988; Sanes, 1997). A growing body of evidence suggests that agrin also participates in the formation of interneuronal synapses (Ferreira, 1999; Bose et al., 2000; Mantych and Ferreira, 2001; Gingras et al., 2002; Tournell et al., 2006).
The subcellular localization of agrin at the tip of growing axons, as well as its pattern of expression in the brain, suggested that this extracellular matrix protein might play a role during the initial phases of development in central neurons (Mantych and Ferreira, 2001; Neuhuber and Daniels, 2003). Loss- and gain-of-function experiments seem to further support this view. Thus, agrin-depleted neurons extended longer axons when compared to agrin-expressing ones (Gautman et al., 1996; Mantych and Ferreira, 2001). Conversely, hippocampal neurons cultured in the presence of agrin grew shorter albeit more branched processes (Mantych and Ferreira, 2001). Similar results were obtained when sensory and motor neurons were grown on cells expressing different agrin isoforms (Chang et al., 1997). More recently, it has been shown that agrin also induced repulsive growth cone turning in cultured Xenopus spinal neurons (Xu et al., 2005). In addition to these direct effects, agrin could regulate neurite elongation by modulating the function of neurite-promoting molecules. This seems to be the case in PC12 cells and chick retina neurons treated with fibroblast growth factor (Kim et al., 2003). Little is known, on the other hand, about the mechanisms underlying agrin effects on neurite elongation. Initial studies on the composition of the cytoskeleton in agrin-depleted neurons and in neurons cultured in the presence of agrin showed that changes in the expression of microtubule-associated proteins paralleled the changes observed in the rate of neurite elongation (Mantych and Ferreira, 2001).
In the present study, we analyzed the effects of agrin on the morphology and molecular composition of growth cones of cultured hippocampal neurons. Our results showed a significant enlargement of growth cones in the presence of agrin. Furthermore, they suggested that agrin induced a change in the conformation of microtubules in the central region of these structures. Collectively, our results provide further insights into the potential molecular mechanisms underlying changes in neurite elongation induced by agrin.
RESULTS
Agrin induced the enlargement of axonal growth cones in cultured hippocampal neurons
Experimental evidence obtained recently suggests that agrin modulates neurite elongation in central neurons (Gautman et al., 1996; Chang et al., 1997; Mantych and Ferreira, 2001; Xu et al., 2005). However, little is known about the molecular mechanisms underlying this developmental agrin function. To gain insights into such mechanisms, we analyzed the effects of agrin on growth cones of cultured hippocampal neurons. These cellular compartments play a key role in both neurite elongation and pathfinding in the developing nervous system. For these experiments, we first used hippocampal neurons co-cultured in the presence of a monolayer of untransfected astrocytes or astrocytes transfected with full-length agrin cDNA with inserts in the Y and Z splicing sites. Twenty-four hours after plating, neurons co-cultured as described above were fixed and stained using a tubulin antibody. Light microscopy analysis indicated that the growth cones present at the tip of axons of hippocampal neurons co-cultured with agrin-expressing astrocytes were bigger than the ones present when hippocampal neurons were co-cultured with non-transfected glia (Fig. 1A-F). Morphometric analysis showed that, indeed, the growth cone area in hippocampal neurons cultured in the presence of full-length agrin was significantly larger than that of growth cones of neurons co-cultured with non-transfected glial cells (Fig. 2A).
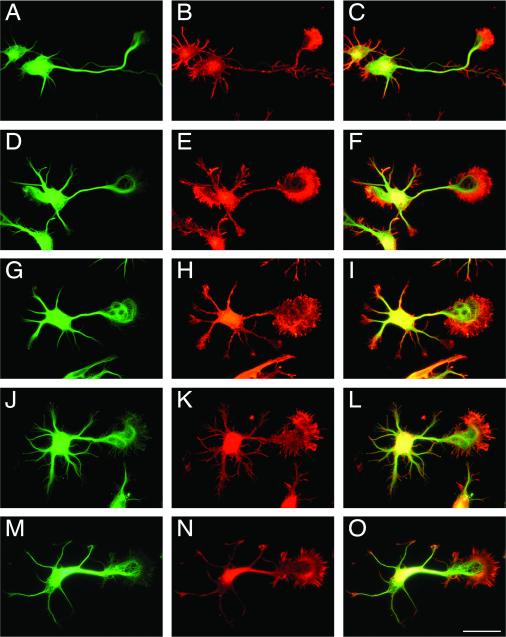
Figure 1. Agrin induced an increase in growth cone size in cultured hippocampal neurons.
Hippocampal neurons maintained for 24 hrs in the presence of untransfected astrocytes (A-C), astrocytes transfected with a full-length agrin construct (D-F), a Z(+) agrin construct (G-I), a Z(-) agrin construct (J-L), or in the presence of recombinant C-terminal agrin (10 ng/ml) (M-O) were fixed and stained with an anti-α-tubulin antibody (A, D, G, J & M) and rhodamine-phalloidin (B, E, H, K & N). Growth cone measurements were made using merged pictures (C, F, I, L & O). Note the increased growth cone size in neurons cultured in the presence of either agrin-transfected astrocytes or purified recombinant C-terminal agrin when compared to untreated controls. Scale bar: 20 μm.
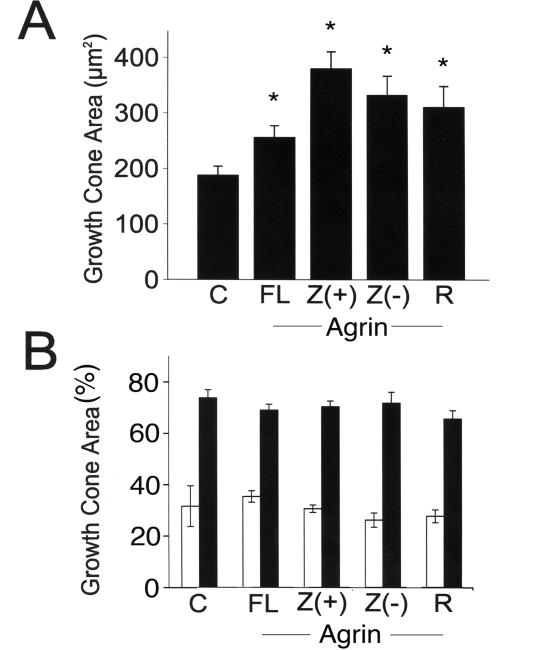
Figure 2. Morphometric analysis of growth cones in hippocampal neurons cultured in the presence of different agrin isoforms.
A) Growth cone area was determined in control cultures (C), and in neurons maintained in the presence of astrocytes transfected with full-length agrin (FL), Z (+) agrin (Z (+)), Z (-) agrin (Z (-)) or recombinant C-terminal agrin (R). The numbers represent the mean ± s.e.m. obtained from 100 growth cones for each experimental condition. *Differs from untreated controls, P<0.01. B) Quantitative analysis of the area enriched in microtubules (open bars) and actin filaments (black bars) in growth cones of hippocampal neurons under the same experimental conditions described in A. The numbers represent the mean ± s.e.m. obtained from 100 growth cones for each experimental condition.
We then performed a series of experiments to determine whether this agrin effect depended on the presence of an insert in the Z splicing site (neuron-specific isoform). For these experiments, we co-cultured hippocampal neurons with astrocyte monolayers transfected with agrin C-terminal constructs containing the insert in the Z splicing site (Z (+) agrin) or lacking this insert (Z (-) agrin). Morphological analysis of hippocampal neurons co-cultured in the presence of agrin-transfected astrocytes showed a significant increase in growth area when compared to the ones cultured in the presence of non-transfected glia monolayers, independent of the cDNA used to transfect the astrocytes (Fig. 1G-L & 2A). Finally, we repeated these experiments using recombinant C-terminal Z (+) agrin. This recombinant agrin was added directly to the culture medium at a final concentration of 10 ng/ml. We have previously shown that when added at this dose, recombinant C-terminal agrin significantly slowed the rate of axonal elongation (Mantych and Ferreira, 2001). Twenty-four hrs after the addition of recombinant agrin, the neurons were fixed, stained, and the growth cones analyzed. Growth cone areas were also significantly larger in hippocampal neurons cultured in the presence of recombinant C-terminal Z (+) agrin when compared with those cultured in its absence (Fig. 1M-O & 2A).
Agrin induced structural changes in growth cones of cultured hippocampal neurons
The central and the peripheral regions of growth cones are rich in microtubules and actin filaments, respectively. Therefore, we took advantage of markers of these cytoskeletal components to determine first whether the agrin-induced increase in growth cone size described above was due to an increase in the microtubule-rich and/or the actin-rich areas. For these experiments, hippocampal neurons were cultured for 24 hrs in the presence of either untransfected astrocytes, agrin-transfected astrocytes, or recombinant agrin as described above (see also Experimental Procedures). Neurons were then immunostained using a tubulin antibody and counterstained with rhodamine-phalloidin to label the central and peripheral regions of these structures, respectively. Morphometric analysis of the total, central, and peripheral growth cone areas showed that ~ 30 % of the total growth cone area was occupied by microtubules and ~70 % was occupied by actin filaments in control hippocampal neurons (Fig. 2). This analysis also showed that although the total growth cone area was bigger in agrin-treated neurons, the microtubule-/actin-rich area ratio was conserved in growth cones of agrin-treated hippocampal neurons independently of the type of agrin isoform present in the culture system (Fig. 2).
We next analyzed the composition and content of microtubules in the central area of growth cones in hippocampal neurons grown in the presence or absence of agrin. These quantitative analyses were performed using recombinant C-terminal agrin to avoid potential differences in the levels of expression of transfected constructs that could either mask or accentuate agrin effects. Thus, untreated controls and hippocampal neurons cultured for 24 hrs in the presence of recombinant C-terminal agrin (10 ng/ml) were fixed directly or extracted using a microtubule-stabilizing buffer prior to their fixation as described in the method section. The cells were then stained using a tubulin antibody (all isoforms) and antibodies that recognize unstable (tyrosinated tubulin), and stable (acetylated tubulin) microtubules, as well as MAP1B, MAP2, and tau. Immunostaining using any of the tubulin antibodies showed that the morphological appearance of microtubules was significantly different in growth cones of hippocampal neurons cultured in the presence of agrin when compared to untreated ones (Fig. 1D, G, J, & M and Fig. 3). In untreated cultures, tubulin immunoreactivity decorated microtubules that penetrated straight into the central domain of growth cones (Fig. 1 & 3). In contrast, microtubular loops were readily detectable in the palm area of growth cones in agrin-treated neurons (Fig. 1D, G, J, & M, see also Fig. 3). Differences in the content of stable microtubules, as assessed by their content in acetylated tubulin, were also detected in growth cones of hippocampal neurons grown in the presence of agrin when compared to untreated controls. While acetylated tubulin immunoreactivity was barely detected in the growth cones of untreated controls, intense immunoreactivity for this post-translationally modified tubulin was detected in agrin-treated neurons (Fig. 3, Table 1). A similar pattern of staining was detected when growth cones were stained using an antibody against MAP1B, a microtubule-associated protein enriched at the tip of neurites. Thus, a stronger MAP1B immunoreactivity was detected throughout the central area of agrin-treated growth cones when compared to untreated ones (Fig. 4, Table 1). On the other hand, no changes were detected in the content of MAP2 and tau when agrin-treated growth cones were compared to those present in untreated cultures (data not shown).
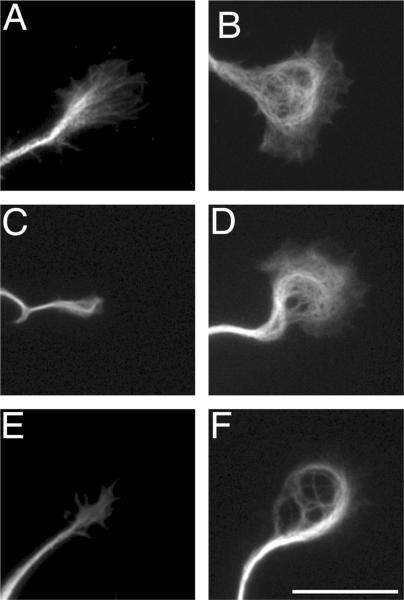
Figure 3. Agrin induced changes in the organization of microtubules in growth cones of cultured hippocampal neurons.
Hippocampal neurons were maintained for 24 hours in the absence (A, C & E) or in the presence of agrin (B, D, F), fixed and stained using a total tubulin (A & B), tyrosinated tubulin (C & D), or acetylated tubulin (E & F) antibodies. Note the presence of loops of microtubules (B) and intense acetylated tubulin immunoreactivity (F) in growth cones of agrin-treated hippocampal neurons. Scale bar: 20 μ-m.
Table 1.
Quantification of tubulin, acetylated tubulin, F-actin, and MAP 1B fluorescence intensity in growth cones of hippocampal neurons cultured in the presence or absence of recombinant agrin.
| Fluorescence | Treatment |
|
|---|---|---|
| Control | Agrin1 | |
| α-tubulin (clone DM1 A) | 48.4 ± 3.7 | 56.6 ± 4.1 |
| F-actin (Rhodamine-phalloidin) | 39.1 ± 2.4 | 46.8 ± 2.4 |
| Acetylated tubulin (clone 6-1 1B-1) | 23.5 ± 1.6 | 45.2 ± 9.0* |
| MAP1B (clone AA6) | 10.7 ± 0.9 | 27.5 ± 2.0** |
Values represent the mean ± S.E.M of fluorescence intensity expressed in pixels within a 5 μm2 area located in the central (tubulin, acetylated tubulin and MAP 1B) or peripheral (phalloidin) growth cone regions.
Differs from control cultures, P< 0.05.
Differs from control cultures, P< 0.01.
The dose of agrin used for these experiments was 10 ng/ml.
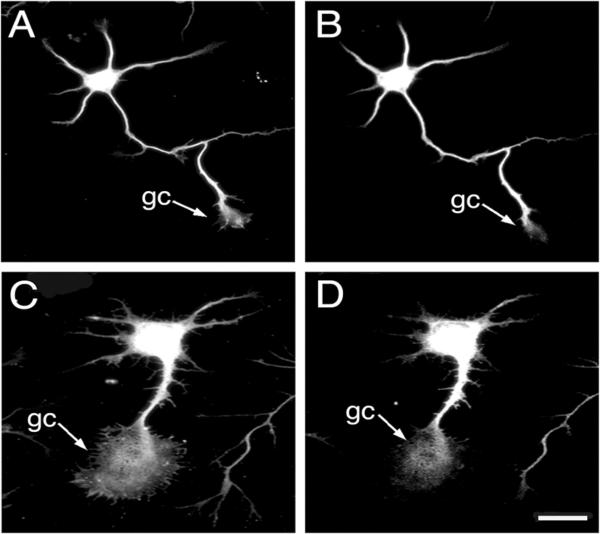
Figure 4. MAP1B immunoreactivity was detected throughout the growth cones of hippocampal neurons cultured in the presence of agrin.
Hippocampal neurons grown in the absence (A & B) or presence of recombinant C-terminal agrin (C & D) for 24 hrs were fixed and double stained using tubulin (A & C) and MAP1B (B & D) antibodies. Note the presence of MAP1B immunoreactivity in the central area of a growth cone (gc) of agrin-treated hippocampal neurons (D). Scale bar: 20 μ-m.
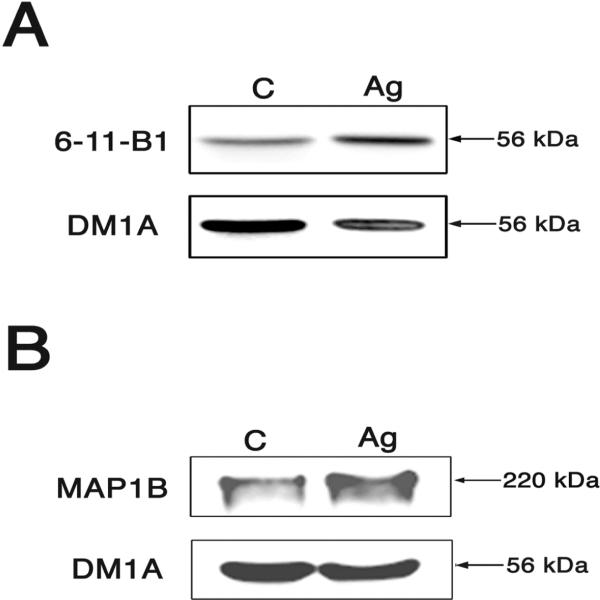
We determined next whether these changes in the content of microtubular proteins could be detected biochemically. Unfortunately, the limited amount of material that could be obtained from our culture system precluded us from obtaining pure growth cone fractions. Therefore, we determined the levels of these proteins in whole cells extracts by means of Western blot analysis. When normalized using tubulin levels as internal controls, quantitative analysis of immunoreactive bands showed a significant increase in both acetylated tubulin (124 % ± 0.9 vs. 101 % ± 2) and MAP1B (151 % ± 17 vs. 105 % ± 10, n= 6 independent culture preparations) levels in agrin-treated neurons when compared to untreated controls (Fig. 5). Membranes were then stripped and re-blotted using an antibody that recognizes phosphorylated MAP1B (at serine 1260). Immunoreactive bands were quantified and the ratio of phosphorylated MAP1B to total MAP1B was determined. No significant differences in this ratio were detected when samples obtained from agrin-treated neurons were compared to untreated controls (116 % ± 27 vs. 100 % ± 2, n= 6 independent culture preparations).
Figure 5. Increased acetylated tubulin and MAP1B levels in agrin-treated hippocampal neurons.
Western blot analysis of whole cell extracts prepared from agrin-treated and untreated hippocampal neurons were reacted with antibodies that recognized acetylated tubulin (clone 6-11-B1) and alpha-tubulin (clone DM1A) (A) or MAP1B and alpha-tubulin (clone DM1A)(B). Note the increase in the ratio of acetylated tubulin/tubulin (A) and MAP1B/alpha-tubulin (B) in agrin-treated hippocampal neurons as compared to untreated controls.
Since a growing body of evidence suggests that actin filament organization and dynamics plays an essential role in determining the morphology and motility of the growth cone, we next analyzed the content of F-actin in growth cones of hippocampal neurons that developed in the presence or absence of agrin (Mitchison and Kirschner, 1988; Tanaka and Sabry, 1995; Bradke and Dotti, 1999; Kunda et al., 2001). For these experiments, hippocampal neurons were cultured for 24 hrs in the presence or absence of recombinant C-terminal agrin, fixed, and stained using rhodamine-phalloidin. Quantitative immunofluorescence analysis showed no significant differences in F-actin fluorescence per area (pixels/5 μm2 area) in growth cones of hippocampal neurons cultured in the presence of agrin when compared to untreated ones (Table 1). Nevertheless, there was an increase in total F-actin that was proportional to the increased area of the outer portion of the growth cone in agrin-treated neurons when compared to control ones. However, we did not detect these differences in the content of actin filaments in lysates prepared from hippocampal neurons cultured in the presence or absence of recombinant C-terminal agrin for 24 hrs and extracted with a F-actin stabilizing buffer as determined by quantitative Western blot (97 % ± 9 vs. 99 % ± 1, respectively; n= 6 independent culture preparations).
Finally, we analyzed whether agrin induced changes in signal transduction pathways known to participate in the organization of actin filaments. We focused on the small GTPase Rac 1 and cofilin since recent reports indicated that activation and/or phosphorylation of these proteins underlie changes in the morphology of growth cones (Meberg et al., 1998; Meberg and Bamburg, 2000; Endo et al., 2003; Rosso et al., 2004). To assess Rac 1 activity, we harvested untreated hippocampal neurons and neurons incubated in the presence of recombinant C-terminal agrin for 20 minutes. This incubation time was chosen because we have previously shown that the activation of signal transduction pathways in the presence of agrin peaked between 15 and 20 minutes after the addition of this recombinant protein (Karasewski and Ferreira, 2003). GTPase activity was determined by means of a pulldown assay followed by Western blot analysis (see experimental procedures). Quantitative analysis of immunoreactive bands showed no differences in Rac 1 activity when agrin-treated neurons were compared to untreated controls (89 % ± 7 vs. 100 % ± 10; n= 3 independent culture preparations).
In the last set of experiments, we assessed the activity of cofilin, an actin binding protein that severs actin filaments inducing their depolymerization, in the presence of agrin. Cofilin activity is regulated by phosphorylation at Ser3 (Agnew et al., 1995; Bamburg, 1999). Therefore, we determined the levels of total cofilin and its phosphorylated form in whole cell extracts prepared from untreated hippocampal neurons and agrin-treated ones by means of Western Blot analysis. Quantification of immunoreactive bands showed no significant changes in the total levels of cofilin (105 % ± 13 vs. 100 % ± 0.3, n= 6 independent culture preparations) or phosphorylated cofilin (113 % ± 9 vs. 100 % ± 0.5, n= 6 independent culture preparations) when whole cell extracts prepared from agrin-treated neurons were compared to untreated controls.
DISCUSSION
The results presented herein indicate that agrin induces both the enlargement of growth cones and the reorganization of the microtubules in these cellular domains in developing hippocampal neurons. Together, they provide further insights into the molecular mechanisms underlying agrin effects on neurite elongation in central neurons. A potential role for this extracellular matrix protein during neurite outgrowth was first suggested based on its predominant expression during the early stages of development and its subcellular localization in growth cones (Cohen and Godfrey, 1992; Mann and Kroger, 1996; Ferreira, 1999; Serpinskaya et al., 1999; Neuhuber and Daniels, 2003). Functional studies provided additional evidence for a regulatory role of agrin on the rate of axonal and dendritic elongation in central neurons (Chang et al., 1997; Gautman et al., 1996; Ferreira, 1999; Mantych and Ferreira, 2001). This agrin effect was accompanied by clear changes in neuronal morphology including an enhanced branching pattern and the formation of filopodia-like processes protruding from axonal and dendritic shafts (Ferreira, 1999; Mantych and Ferreira, 2001; Annies et al, 2006; McCroskery et al., 2006). In this study, we showed that agrin also induced morphological changes in the growth cones of hippocampal neurons. One of these changes is the significant enlargement of the growth cones of agrin-treated hippocampal neurons when compared to untreated controls. In addition, our data indicate that full-length agrin as well as C-terminal isoforms, including or excluding inserts in the Y and Z splicing sites, were all capable of inducing the increase in growth cone areas. These results suggest that the C-terminal half of the molecule is sufficient for this activity. While we have previously shown that, when transfected into glial cells, all of these isoforms could be detected in the culture medium, the levels of expression might vary (Tournell et al., 2006). Hence, we cannot rule out differences in activity among agrin isoforms. Regardless, the changes in growth cone morphology observed in agrin-treated cultures could reflect specific growth conditions. It has been reported that during active phases of axonal elongation, growth cones became streamlined. On the other hand, growth cone enlargement has been associated with pauses in neurite elongation (Mason and Wang, 1997; Dent et al., 1999). Therefore, the enlargement of growth cones detected under the experimental conditions used in this study could be consistent with an inhibitory effect of agrin on neurite outgrowth. These results are in agreement with previous observations that indicated that agrin slows axonal elongation. Those observations included the inhibition of neurite elongation when embryonic motor and sensory neurons were grown on agrin expressing CHO cells, the failure of motor neuron axons to stop at their normal synaptic sites in agrin knockout mice, and the presence of shorter axons when hippocampal neurons were cultured with agrin (Campagna et al., 1995; Gautman et al., 1996; Chang et al., 1997; Ferreira, 1999; Mantych and Ferreira, 2001).
In addition to the enlargement of growth cones, our results indicated that agrin induced the formation of loops of microtubules in the central area of these structures. The formation of this type of loop has been described before (Tsui et al., 1984; Lankford and Klein, 1990; Sabry et al., 1991; Tanaka and Kirschner, 1991; Dent et al., 1999). The picture emerging from those studies indicates that microtubule loops are formed under experimental conditions that induce the pause or slow of the rate of elongation of developing axons. Together, the increase in growth cone size and the reorganization of microtubules into loops further supported a role of agrin as a stop signal in developing central neurons. Acting as a stop signal, agrin could enhance the interactions of developing axons and potential targets leading to the formation of synapses.
The formation of these microtubule loops in growth cones of hippocampal neurons grown in the presence of agrin were associated with an increase in MAP1B and acetylated tubulin. These results confirmed and extended our previous observation using hippocampal neurons grown in the presence of agrin for 4 days. Under those experimental conditions, agrin induced an increase in MAP1B, at least in part, by regulating its transcription (Mantych and Ferreira, 2001). The induction of this microtubule-associated protein (MAP) has been correlated with tubulin polymerization underlying neurite extension in PC12 cells (Greene et al., 1983; Avila et al., 1994; Black et al., 1994). Conversely, the suppression of MAP1B reduced NGF-induced neurite outgrowth in PC12 cells (Brugg et al., 1993). Biochemical studies also showed that MAP1B promotes microtubule polymerization by suppressing dynamic instability (Vandecandelaere et al., 1996). Based on these data, it is tempting to speculate that the presence of this MAP could induce the polymerization of microtubules in growth cones of neurons in which the rate of growth has been slowed down by the presence of agrin. Hence, these microtubules, unable to be polymerized in the preferred directional growth, might form loops. These loops could become a source of readily available microtubules once neurons resume an active growth phase. The presence of this MAP in growth cones could also induce the stabilization of microtubules as detected by acetylated tubulin. The effect of MAP1B on this post-translational modification of tubulin has previously been reported. Thus, an increase in acetylated tubulin was detected in non-neuronal cells transfected with MAP1B (Takemura et al., 1992). Conversely, a reduced level of acetylated tubulin has been detected in the growth cone of dorsal root ganglion axons obtained from MAP1B knockout mice (Bouquet et al., 2004). The levels of stable microtubules were also decreased when sympathetic neurons were immunodepleted of MAP1B (Tint et al., 2005).
Our results suggest that the reorganization of the cytoskeleton in growth cones of agrin-treated hippocampal neurons primarily affects the microtubular system. On the other hand, no changes in the content of actin filaments were detected in agrin-treated neurons. Nor did we detect changes in the activity of cofilin and Rac 1, two proteins known to regulate actin polymerization leading to changes in the morphology of growth cones. Reports on the agrin regulation of Rac 1 activity are controversial. It has been shown that agrin induced a strong activation of Rac 1 in differentiated myotubules (Weston et al., 2000). Furthermore, this activation seems to be essential for the clustering of acetylcholine receptors in the presence of agrin (Weston et al., 2000). In contrast, agrin decreased Rac 1 activation in cultured hippocampal neurons (Xu et al., 2005). The lack of activation of Rac 1 under our experimental conditions, together with those previous observations, suggested that the ability of agrin to activate this signaling pathway might be cell-type specific.
Collectively, our results suggest that agrin induced changes in the morphology and composition of growth cones compatible with its function as a stop signal in cultured hippocampal neurons. In addition, they suggest that these effects might be the result of the reorganization of the microtubular system rather than the local rearrangement of actin filaments. Further studies will be required to completely elucidate the mechanisms underlying agrin-dependent regulation of neurite elongation.
EXPERIMENTAL PROCEDURES
Preparation of hippocampal cultures
Neuronal cultures were prepared from the hippocampi of embryonic day 18 (E18) rat embryos as previously described (Goslin and Banker, 1991). In brief, embryos were removed and their hippocampi dissected and freed of meninges. The cells were dissociated by trypsinization (0.25% for 15 min at 37°C) followed by trituration with a fire-polished Pasteur pipette and plated onto poly-L-lysine-coated coverslips in Minimum Essential Medium (MEM) with 10% horse serum. Coverslips were then transferred to dishes containing an astroglial monolayer. For biochemical experiments, hippocampal neurons were plated at high density (500,000 cells/60-mm dish) in MEM with 10% horse serum. After 4 hrs, the medium was replaced with glia-conditioned MEM with 10% horse serum.
Preparation of astrocyte cultures
Astrocyte cultures were prepared from the cerebral cortex of E18 rat embryos as previously described (Ferreira and Loomis, 1998). Briefly, embryos were removed and their cerebral cortex dissected and freed of meninges. The cells were dissociated by trypsinization (0.25% for 35 min at 37°C) and then centrifuged in MEM with 10% horse serum at 1000 rpm for 10 min. The cells were resuspended in fresh MEM with 10% horse serum, triturated with a fire-polished pipette, and plated at high density (1,600,000 cells/60-mm dish) on non-coated culture dishes. All the experiments included in this study were performed using astrocytes kept in culture for 14 days.
Astrocyte transfection
Three different agrin constructs, a full-length construct with inserts in the Y and Z splicing sites, and C-terminal constructs with and without an insert in the Z splice site, were used in this study (Campanelli et al., 1991; Ferns et al., 1993). These constructs were transfected into astrocytes with the Nucleofector™ apparatus (Amaxa, Gaithersburg, MD) as previously described (Paganoni and Ferreira, 2005; Tournell et al., 2006). Briefly, astrocytes were re-suspended in rat nucleofector solution, transferred to an electroporation cuvette and “nucleofected” according to the manufacturer's protocol (program T-20). For each reaction, 4 million astrocytes and 5 μg of cDNA were used. Transfection efficiency was determined by means of Western blot and immunocytochemistry using antibody against agrin and c-myc as described below. The experiments included in this study were performed using glia monolayers in which ~30% of the cells expressed the transfected constructs.
Recombinant agrin
In some experiments, recombinant rat C-terminal agrin (R&D Systems, Minneapolis, MN) containing inserts in the Y and Z splice sites (Agrin3,4,8) was added to the glia-conditioned MEM with 10% horse serum at a final concentration of 10 ng/ml as previously described (Mantych and Ferreira, 2001).
Immunocytochemistry
Cultures were fixed for 15 min with 4% paraformaldehyde in phosphate buffered saline (PBS) containing 0.12 M sucrose. The cultures were then permeabilized in 0.3% Triton X-100 in PBS for 4 min and rinsed three times in PBS. The coverslips were pre-incubated in 10% bovine serum albumin (BSA) in PBS for 1 hr at room temperature and exposed to the primary antibodies (diluted in 1% BSA in PBS) overnight at 4°C. Finally, the cultures were rinsed in PBS and incubated with secondary antibodies for 1 hr at 37°C. The following primary antibodies were used: anti-α-tubulin (clone DM1A, 1:600; Sigma, St Louis, MO), anti-acetylated tubulin (clone 6-11-B1, 1:10,000; Sigma), anti-tyrosinated-tubulin (1:500, Sigma), anti-tau (clone tau 5, 1:1,000; Biosource, Camarillo, CA); anti-MAP2 (clone AP20, 1:1,000; Sigma), anti-MAP1B (clone AA6, 1:50; Sigma), anti-phosphorylated MAP1B (clone SMI31, 1:250; Covance Research Product, Inc. Berkely, CA), anti-agrin 530 (1:100; Stressgen, Victoria, BC, Canada), and anti-c-myc (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). The following secondary antibodies were used: Alexa Fluor® 488 goat anti-mouse IgG and Alexa Fluor® 568 goat anti-rabbit (both diluted 1:200, Molecular Probes, Eugene, OR). For some experiments, hippocampal neurons were stained for 1 hr at 37°C with rhodamine-phalloidin to detect actin filaments (1:1,000; Sigma). Fluorescent images of equal exposure from hippocampal neurons cultured under different experimental conditions were acquired with a Photometric Cool Snap FX color digital camera on a Nikon microscope and analyzed using Metamorph Image Analysis software (Fryer Company, Huntley, IL). For some experiments the relative intensities of tubulin, acetylated tubulin, MAP1B immunofluorescence as well as of phalloidin staining were evaluated using quantitative fluorescence techniques as described previously (Paglini et al., 1998). Briefly, fluorescence intensity measurements were perfomed pixel by pixel within the central and peripheral regions of growth cones using the Metamorph Image Analysis software.
Morphometric analysis
To determine growth cone area, hippocampal neurons grown in the presence of untransfected astrocytes, astrocytes transfected with different agrin cDNA constructs, or recombinant C-terminal agrin were fixed and stained using a tubulin antibody (clone DM1A) and rhodamine phalloidin (as described above). Growth cones from randomly selected cells were traced from the screen by using the morphometric menu of the Metamorph Image Analysis Software (Fryer Company) and their area quantified using the same software. The proximal limit of the growth cone was defined as the distal part of the neurite where the diameter is twice as big as the neurite itself (Bradke and Dotti, 1997). All results of the morphometric analysis were expressed as means ± S.E.M. Comparisons of the means between groups were made using one-way analysis of variance (ANOVA) followed by Fisher test for multiple comparison of means.
Protein electrophoresis and immunoblotting
To prepare whole cell extracts, cultures were rinsed twice in warmed PBS, scraped into Laemmli buffer, and homogenized in a boiling water bath for 10 min. For some experiments, cytoskeletal- and F-actin-enriched fractions were prepared. Cytoskeletal fractions were prepared as described previously (Ferreira et al., 1989). Briefly, cultures were rinsed in a microtubule-stabilizing buffer (MTSB: HEPES 130 mM, MgCl2 4 mM, EGTA 10 mM, pH 6.9) for 30 seconds and then extracted in MTSB plus 0.2% Triton X-100 (Sigma) for 2 min. F-actin fractions were prepared using the G-actin/F-actin In Vivo assay kit (# BK037; Cytoskeleton, Denver, CO) as described below. Sodium dodecyl sulfate (SDS)-polyacrylamide gels were run according to Laemmli (1970). Transfer of protein to Immobilon membranes (Millipore, Bedford, MA) and immunodetection were performed as described by Towbin et al. (1979) and modified by Ferreira et al. (1989). The following antibodies were used: anti-α-tubulin (clone DM1A, 1:10,000; Sigma), anti-acetylated tubulin (clone 6-11-B1, 1:25,000; Sigma), anti-actin (clone AC40, 1:100; Sigma), anti-MAP1B (clone AA6, 1:100; Sigma), anti-cofilin (1:1,000, Cell Signaling Technology, Danvers, MA), and anti-phospho-cofilin (1:1,000; Cell Signaling Technology). Secondary antibodies conjugated to HRP (1:1,000, Promega, Madison, WI) followed by enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Chicago, IL) were used for the detection of proteins. In some experiments, blots were stripped in 60 mM Tris, 2% SDS and 0.8% β-mercaptoethanol at 55°C for 35 min and then re-probed with a different antibody. Membranes were imaged on a ChemiDoc gel documentation system (BioRad, Hercules, CA). The appropriate bands were analyzed using Quantity One Analysis Software (BioRad). Densitometric values were normalized using α-tubulin as an internal control. Scanning of the Western blots demonstrated the curve to be linear in the range used for each antibody. All results were expressed as mean ± S.E.M. obtained from at least 3 independent experiments.
Preparation of cytoskeletal fractions
To prepare cytoskeletal fractions, hippocampal neurons plated on coverslips were rinsed in the MTSB for 30 seconds and then extracted in MTSB plus 0.2% Triton X-100 for 15 seconds. Hippocampal neurons were then fixed and processed for immunocytochemistry as previously described.
Determination of F-actin content
F-actin/G-actin In Vivo assay kit (#BK037; Cytoskeleton, Denver, CO) was used to detect the content of F-actin in hippocampal neurons cultured in the presence or absence of agrin. Neurons were incubated in the lysis and F-actin stabilizing buffers for 45 seconds and processed according to the manufacturer's instructions. Neurons were then scraped in Laemmli buffer and used for Western blot analysis as described above.
Rac 1 activation assay
Rac 1 activity was measured using a Rac 1 activation kit (# BK035, Cytoskeleton). Briefly, untreated hippocampal neurons and neurons cultured in the presence of recombinant C-terminal agrin for 30 min were washed in ice-cooled PBS, lysed and immunoprecipitated according to the manufacturer's instructions. Positive and negative controls were conducted in parallel as indicated in manufacturer's protocol. The amount of bound (active) Rac 1 was determined by means of quantitative Western blot analysis as described above.
Statistical analysis
All experiments were done at least in triplicate using hippocampal neurons obtained from independent culture preparations. Data were presented as means ± S.E.M. and statistically analyzed using the Student's T test or one-way ANOVA followed by Fisher's LSD post-hoc test.
Acknowledgements
We wish to thank Drs. Martin Smith (University of California, Irvine, California) and Robert Burgess (The Jackson Laboratory, Bar Harbor, Maine) for the generous gift of the agrin constructs used in this study. This work was supported by NIH grant NS046834 to AF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Avila J, Dominguez J, Diaz-Nido J. Regulation of microtubule dynamics by microtubule associated protein expression and phosphorylation during neuronal development. Int J Dev Biol. 1994;38:13–25. [PubMed] [Google Scholar]
- Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem. 1995;270:17582–17587. doi: 10.1074/jbc.270.29.17582. [DOI] [PubMed] [Google Scholar]
- Annies M, Bittcher G, Ramseger R, Loschinger J, Woll S, Porten E, Abraham C, Ruegg M, Kroger S. Clustering transmembrane-agrin induces filopodia-like processes on axons and dendrites. Mol Cell Neurosci. 2006;31:515–524. doi: 10.1016/j.mcn.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bamburg JR. Proteins of the ADF/cofilin family:essential regulators of actin dynamics. Ann Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Black MM, Slaughter T, Fischer I. Microtubule-associated protein 1b (MAP-1b) is concentrated in the distal regions of growing axons. J Neurosci. 1994;14:857–870. doi: 10.1523/JNEUROSCI.14-02-00857.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose CM, Qui D, Bergamaski A, Gravante B, Bossi M, Villa A, Rupp F, Malgaroli A. Agrin controls synaptic differentiation in hippocampal neurons. J Neurosci. 2000;20:9086–9095. doi: 10.1523/JNEUROSCI.20-24-09086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquet C, Soares S, von Boxberg Y, Ravaille-Veron M, Propst F, Nothias F. Microtubule-associated protein 1B controls directionality and growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons. J Neurosci. 2004;24:7204–7213. doi: 10.1523/JNEUROSCI.2254-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe MA, Fallon JR. The role of agrin in synapse formation. Ann Rev Neurosci. 1995;18:443–462. doi: 10.1146/annurev.ne.18.030195.002303. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Brugg B, Reddy D, Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligodeoxinucleotides inhibits initiation of neurite outgrowth. Neurosci. 1993;52:489–496. doi: 10.1016/0306-4522(93)90401-z. [DOI] [PubMed] [Google Scholar]
- Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini. Differential expression, localization, and function. J Cell Biol. 2000;151:41–52. doi: 10.1083/jcb.151.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna JA, Ruegg MA, Bixby JL. Agrin is a differentiation-inducing “stop signal” for motoneurons in vitro. Neuron. 1995;15:1365–1374. doi: 10.1016/0896-6273(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Hoch W, Rupp F, Kreiner T, Scheller RH. Agrin mediates cell contact-induced acetylcholine receptor clustering. Cell. 1991;67:909–916. doi: 10.1016/0092-8674(91)90364-5. [DOI] [PubMed] [Google Scholar]
- Chang D, Woo JS, Campanelli J, Scheller RH, Ignatius MJ. Agrin inhibits neurite outgrowth but promotes attachment in embryonic motor and sensory neurons. Dev Biol. 1997;181:21–35. doi: 10.1006/dbio.1996.8435. [DOI] [PubMed] [Google Scholar]
- Cohen MW, Godfrey EW. Early appearance of and neuronal contribution to agrin-like molecules at embryonic frog nerve-muscle synapses formed in culture. J Neurosci. 1992;12:2982–2992. doi: 10.1523/JNEUROSCI.12-08-02982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyst KA, McKechnie BA, Fallon JR. The role of alternative splicing in regulating agrin binding to muscle cells. Dev Brain Res. 1998;110:185–191. doi: 10.1016/s0165-3806(98)00105-9. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Sasaki Y, Goshima Y, Niwa R, Uemura T, Mizuno K. Control of growth cone motility and morphology by LIM kinase and slingshot via phosphorylation and dephosphorylation of cofilin. J Neurosci. 2003;23:2527–2537. doi: 10.1523/JNEUROSCI.23-07-02527.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Ferreira A. Abnormal synapse formation in agrin depleted hippocampal neurons. J Cell Sci. 1999;112:4729–4738. doi: 10.1242/jcs.112.24.4729. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Loomis PA. Isolation and culture of primary neural cells. In: Spector D, Goldman R, Leinwand L, editors. Cells: A laboratory manual. Cold Spring Harbor Laboratory Press; Woodbury, NY: 1998. pp. 9.1–9. [Google Scholar]
- Ferreira A, Busciglio J, Caceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: Evidence for the involvement of the microtubule-associated proteins MAP-1a, HMW-MAP-2 and Tau. Dev Brain Res. 1989;49:215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Gingras J, Ferns M. Expression and localization of agrin during sympathetic synapse formation in vitro. J Neurobiol. 2002;48:228–242. doi: 10.1002/neu.1053. [DOI] [PubMed] [Google Scholar]
- Gingras J, Rassadi S, Cooper E, Ferns M. Agrin plays an organizing role in the formation of sympathetic synapses. J Cell Biol. 2002;158:1109–1118. doi: 10.1083/jcb.200203012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ, Yancopoulos GD. Sequential roles of agrin, MuSK and rapsyn during neuromuscular junction formation. Curr Opin in Neurobiol. 1997;7:379–384. doi: 10.1016/s0959-4388(97)80066-9. [DOI] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Sitti TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Budern SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Godfrey EW. Comparison of agrin-like proteins from the extracellular matrix of chicken kidney and muscle with neural agrin, a synapse organizing protein. Exp Cell Res. 1991;195:99–109. doi: 10.1016/0014-4827(91)90504-n. [DOI] [PubMed] [Google Scholar]
- Godfrey EW, Dietz ME, Morstad AL, Wallskog PA, Yorde DE. Acetylcholine receptor-aggregating proteins are associated with the extracellular matrix of many tissues in Torpedo. J Cell Biol. 1988;106:1263–1272. doi: 10.1083/jcb.106.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. MIT; Cambridge, MA: 1991. pp. 251–283. [Google Scholar]
- Greene LA, Liem RK, Shelanski M. Regulation of the high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J Cell Biol. 1983;96:76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell 72/Neuron. 1993;10(Supplement):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Drapeau P. From contact to connection: early events during synaptogenesis. Trends in Neurosci. 1995;18:196–201. doi: 10.1016/0166-2236(95)93901-9. [DOI] [PubMed] [Google Scholar]
- Hoch W, Ferns M, Campanelli JT, Hall ZW, Scheller RH. Developmental regulation of highly active alternatively spliced forms of agrin. Neuron. 1993;11:479–490. doi: 10.1016/0896-6273(93)90152-h. [DOI] [PubMed] [Google Scholar]
- Hoch W, Campanelli JT, Harrison S, Scheller RH. Structural domains of agrin required for clustering of nicotinic acetylcholine receptors. EMBO J. 1994;13:2814–2821. doi: 10.1002/j.1460-2075.1994.tb06575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasewski L, Ferreira A. The MAPK signal transduction pathway mediates agrin effects on neurite elongation in cultured hippocampal neurons. J Neurobiol. 2003;55:14–24. doi: 10.1002/neu.10197. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Cotman SL, Halfter W, Cole GJ. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. J Neurobiol. 2003;55:261–277. doi: 10.1002/neu.10213. [DOI] [PubMed] [Google Scholar]
- Kroger S, Horton SE, Honig LS. The developing avian retina expresses agrin isoforms during synaptogenesis. J Neurobiol. 1996;29:165–182. doi: 10.1002/(SICI)1097-4695(199602)29:2<165::AID-NEU4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kunda P, Paglini G, Kosik KS, Quiroga S, Caceres A. Evidence for the involvement of Tiam1 in axon formation. J Neurosci. 2001;21:2361–2372. doi: 10.1523/JNEUROSCI.21-07-02361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lankford KL, Klein WL. Ultrastructure of individual neurons isolated from avian retina: occurrence of microtubule loops in dendrites. Brain Res Dev Res. 1990;51:217–224. doi: 10.1016/0165-3806(90)90278-7. [DOI] [PubMed] [Google Scholar]
- Magill-Solc C, McMahan UJ. Motor neurons contain agrin-like molecules. J Cell Biol. 1988;107:1825–1833. doi: 10.1083/jcb.107.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S, Kroger S. Agrin is synthesized by retinal cells and colocalizes with gephyrin. Mol Cell Neurosci. 1996;8:1–13. doi: 10.1006/mcne.1996.0039. [DOI] [PubMed] [Google Scholar]
- Mantych K, Ferreira A. Agrin differentially regulates the rates of axonal and dendritic elongation in cultured hippocampal neurons. J Neurosci. 2001;21:6802–6809. doi: 10.1523/JNEUROSCI.21-17-06802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CA, Wang LC. Growth cone form is behavior-specific and, consequently, position-specific along the retinal axon pathway. J Neurosci. 1997;17:1086–1100. doi: 10.1523/JNEUROSCI.17-03-01086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan UJ. The agrin hypothesis. Cold Spring Harbor Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Chaudhry A, Lin L, Daniels MP. Transmenbrane agrin regulates filopodia in rat hippocampal neurons in culture. Mol cell neurosci. 2006;33:15–28. doi: 10.1016/j.mcn.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Meberg P, Bamburg JR. Increase in neurite outgrowth mediated by overexpression of actin depolymerizing factor. J Neurosci. 2002;20:2459–2469. doi: 10.1523/JNEUROSCI.20-07-02459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg P, Ono S, Minamide L, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Mot Cytoskeleton. 1998;39:172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Daniels MP. Targeting of recombinant agrin to axonal growth cones. Mol Cell Neurosci. 2003;24:1180–1196. doi: 10.1016/j.mcn.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neumann FR, Bittcher G, Annies M, Schumacher B, Kroger S, Ruegg M. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol Cell Neurosci. 2001;17:208–225. doi: 10.1006/mcne.2000.0932. [DOI] [PubMed] [Google Scholar]
- Paganoni S, Ferreira A. Neurite extension in central neurons: a novel role for the receptor tyrosine kinases Ror1 and Ror2. J Cell Sci. 2005;118:433–446. doi: 10.1242/jcs.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglini G, Kunda P, Quiroga S, Kosik KS, Caceres A. Suppression of radixina and moesin alters groth cone morphology, motility, and process formation in primary cultured neurons. J Cell Biol. 1988;143:443–455. doi: 10.1083/jcb.143.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole JJ, Deyst KA, Bowe MA, Nastuk MA, McKechnie BA, Fallon JR. Alternative splicing of agrin regulates its binding to heparin alpha-dystroglycan, and the cell surface. Proc Natl Acad Sci USA. 1996;93:7369–9374. doi: 10.1073/pnas.93.14.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S, Peretti D, Bollati F, Sumi T, Nakamura T, Quiroga S, Ferreira A, Caceres A. Limk 1 regulates Golgi dynamics, traffic of synaptophysin vesicles, and process extension in primary cultured neurons. Mol Biol Cell. 2004;15:3433–3449. doi: 10.1091/mbc.E03-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg MA, Tsim KW, Horton SE, Kroger S, Escher G, Gensch EM, McMahan UJ. The agrin gene codes for a family of basal lamina protein that differ in function and distribution. Neuron. 1992;8:691–699. doi: 10.1016/0896-6273(92)90090-z. [DOI] [PubMed] [Google Scholar]
- Ruegg MA, Bixby JL. Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 1998;21:22–27. doi: 10.1016/s0166-2236(97)01154-5. [DOI] [PubMed] [Google Scholar]
- Rupp F, Payan DG, Magill-Solc C, Cowan DM, Scheller RH. Structure and expression of rat agrin. Neuron. 1991;6:811–823. doi: 10.1016/0896-6273(91)90177-2. [DOI] [PubMed] [Google Scholar]
- Rupp F, Ozcelik TH, Linial M, Peterson K, Francke U, Scheller RH. Structure and chromosomal localization of the mammalian agrin gene. J Neurosci. 1992;12:3535–3544. doi: 10.1523/JNEUROSCI.12-09-03535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry J, O'Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR. Genetic analysis of postsynaptic differentiation at the vertebrate neuromuscular junction. Curr Opin in Neurobiol. 1997;7:93–100. doi: 10.1016/s0959-4388(97)80126-2. [DOI] [PubMed] [Google Scholar]
- Serpinskaya AS, Feng G, Sanes JR, Craig AM. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev Biol. 1999;205:65–78. doi: 10.1006/dbio.1998.9112. [DOI] [PubMed] [Google Scholar]
- Stone D, Nikolics K. Tissue- and age-specific expression patterns of alternatively spliced agrin mRNA transcripts in embryonic rat suggest novel developmental roles. J Neurosci. 1995;15:6767–6778. doi: 10.1523/JNEUROSCI.15-10-06767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axonal elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Sabry J. Making connections: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha tubulin Acetylation in cells transfected with microtubule-associated protein MAP1B, MAP2, or tau. J Cell Sci. 1992;103:953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- Tint I, Fischer I, Black M. Acute inactivation of MAP1b in growing sympathetic neurons destabilizes axonal microtubules. Cell Motility Cytoskeleton. 2005;60:48–65. doi: 10.1002/cm.20045. [DOI] [PubMed] [Google Scholar]
- Tournell CE, Bergstrom RA, Ferreira A. Progesterone-induced agrin expression in astrocytes modulates glia-neuron interactions leading to synapse formation. Neuroscience. 2006;141:1327–1338. doi: 10.1016/j.neuroscience.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelein T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4354–4356. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui HT, Lankford KL, Ris H, Klein WL. Novel organization of microtubules in cultured central nervous system neurons: formation of hairpin loops at ends of maturing neurites. J Neurosci. 1984;4:3002–3013. doi: 10.1523/JNEUROSCI.04-12-03002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecandelaere A, Pedrotto B, Ulton MM, Calvert RA, Bayley PM. Differences in the regulation of microtubule dynamics by microtubule-associated proteins MAP1B and MAP2. Cell Mot Cytoskeleton. 1996;35:134–146. doi: 10.1002/(SICI)1097-0169(1996)35:2<134::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Weston C, Yee B, Hod E, Prives J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J Cell Biol. 2000;150:205–212. doi: 10.1083/jcb.150.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Fu AKY, Ip FCF, Wu CP, Duan S, Poo MM, Yuan XB, Ip NY. Agrin regulates growth cone turning of Xenopus spinal motoneurons. Development. 2005;132:4309–4316. doi: 10.1242/dev.02016. [DOI] [PubMed] [Google Scholar]