Abstract
The development of cytopenia in CLL patients can predict poor prognosis. We evaluated all CLL patients seen in the Division of Hematology at Mayo Clinic Rochester from 1 January 1995 to 31 December 2004 (n = 1,750) for cytopenia, etiology of cytopenia, and clinical outcome. Cytopenia occurred in 423 (24.2%) patients and was attributable to CLL in 303 (17.3%) with 228 (75%) of these having bone marrow (BM) failure and 75 (25%) having autoimmune disease (AID). Survival from onset of cytopenia was significantly better for patients with AID (median 9.1 years) compared to patients with BM failure (median 4.4 years, p < 0.001). Patients with AID diagnosed within 1 year of the diagnosis of CLL (n = 35) had similar survival from diagnosis compared to patients without CLL related cytopenia (median 9.3 vs. 9.7 years, p = 0.881). Although cytopenia caused by BM failure predicts a poorer prognosis in CLL, cytopenia caused by AID is not an adverse prognostic factor. These findings suggest that patients with cytopenia due to AID cannot be meaningfully classified by the current clinical staging systems. Revisions of the NCI-WG 96 criteria should consider the etiology of cytopenia in staging CLL patients.
INTRODUCTION
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) has been defined by the Revised European American Lymphoma (REAL) and World Health Organization (WHO) classifications (Harris, et al 1994, Muller-Hermelink, et al 2001) as a distinct malignancy of mature B-lymphocytes which is clearly different from both other chronic B-cell lymphoproliferative diseases and the leukemic phase of non-Hodgkin lymphomas. Most CLL patients are now diagnosed at an earlier stage of disease with a lower tumor burden. This is because CLL can be easily diagnosed using flow cytometric analyses when incidental absolute lymphocytosis is detected on routine automated white blood cell differential counts (Zent, et al 2001). Recent biological discoveries in CLL have provided more accurate prognostic markers which can affect patient management. Thus CLL patients with unmutated immunoglobulin gene heavy chain variable region (IgVH)(Damle, et al 1999, Hamblin, et al 1999), unfavorable chromosomal abnormalities on interphase FISH analysis (Dewald, et al 2003, Dohner, et al 2000), and cells with elevated expression of ZAP-70 (Crespo, et al 2003) or CD38 (Ibrahim, et al 2001) have a poorer prognosis (Zent, et al 2006). The more precise definition of CLL, increased recognition of early stage disease, the development of relevant biological prognostic factors, and the availability of more efficacious treatments (Kay, et al 2007, Keating, et al 2005), all reinforce the need for redefining the clinical implications of cytopenia in CLL patients.
Anemia and thrombocytopenia have long been considered to be predictive of poor prognosis in CLL (Binet, et al 1981, Rai, et al 1975). Cytopenia in CLL patients can be attributable to CLL or caused by unrelated etiologies. The CLL specific causes of cytopenia are bone marrow (BM) infiltration and autoimmune disease (AID). Extensive BM infiltration by CLL cells (i.e. BM failure) is usually a late event in the course of CLL that causes anemia and subsequently thrombocytopenia (Binet, et al 1981, Rai, et al 1975). In contrast, cytopenia caused by AID in CLL patients can occur at any time in the course of their disease (Kyasa, et al 2003, Mauro, et al 2000) and can present as autoimmune hemolytic anemia (AIHA), immune thrombocytopenia (ITP), pure red blood cell aplasia (PRBCA) and autoimmune agranulocytosis (AIG) (Hamblin 2001). The currently used Rai and Binet clinical classifications do not explicitly distinguish cytopenia caused by BM failure from cytopenia caused by AID (Binet, et al 1981, Rai, et al 1975). However, there are data to suggest that the etiology of cytopenia strongly influences the clinical outcome in CLL. Cytopenia caused by AID is unlikely to have the poor prognosis associated with patients with BM failure (Kyasa, et al 2003, Mauro, et al 2000). In addition, a small but relevant number of patients with CLL can have cytopenia caused by etiologies distinct from their CLL (e.g., another malignancy, iron deficiency, chronic disease), and these patients will require other therapies. A re-evaluation of both the etiology and the prognostic significance of cytopenia in CLL patients could lead to changes in patient care.
We conducted an observational study of all patients seen in the Division of Hematology at Mayo Clinic Rochester (MCR) over a period of 10 years to test the hypothesis that the etiology of cytopenia in CLL patients has prognostic significance. Our data show that cytopenia caused by BM infiltration is associated with a significantly decreased survival compared to that due to AID. Further, we demonstrate that cytopenia due to AID did not result in a worse survival compared to CLL patients without AID. Finally, we observed that the survival of patients with cytopenia attributed to CLL has increased compared to historical studies.
PATIENTS AND METHODS
This observational study was conducted at Mayo Clinic Rochester (MCR) and was approved by the Institutional Review Board. All patients with CLL, seen at least once in the Division of Hematology at MCR from 1 January 1995 to 31 December 2004, were included in the study and were followed through 2006. Patients were identified using the MCR CLL Database and a comprehensive review of MCR clinical records using all the ICD-9 codes appropriate for CLL, SLL, AIHA , PRBCA , ITP, and AIG. The period of study was chosen because patients seen at Mayo Clinic Rochester since 1995 have had a diagnosis of CLL made using current immunophenotypic criteria based on published data from the Mayo Clinic Laboratory (Nowakowski, et al 2005), the World Health Organization (WHO)/REAL classification (Harris, et al 1994, Muller-Hermelink, et al 2001) and the National Cancer Center – Working Group 1996 (NCI-WG 96) criteria (Cheson, et al 1996). CLL was diagnosed in patients with a monoclonal B cell population in the peripheral blood (with lymphocyte count > 5 × 109/L) and a CLL immunophenotype, or a lymph node biopsy with proliferation centers comprising monoclonal B cells as previously described (Nowakowski, et al 2005).
Cytopenia (hemoglobin [Hgb] < 10 g/dL or platelet count < 100 × 109/L) was considered to be due to BM failure in patients with 1) extensive BM involvement by CLL cells (< 20% residual myeloid tissue on BM biopsy) or 2) reticulocytopenia in the absence of other causes of cytopenia persisting for more than 3 months after the last therapy for CLL. Cytopenia was considered to be due to AID in patients with AIHA, ITP, PRCA, or AIG. AIHA was diagnosed in patients with 1) Hgb < 10 g/dL, 2) at least one marker of hemolysis (increased indirect bilirubin without liver disease, increased LDH without alternative etiology, increased absolute reticulocyte count or increased BM erythropoiesis without bleeding), and 3) direct or indirect evidence of an autoimmune mechanism (positive DAT for either IgG or C3d, cold agglutinins, or at least 2 markers of hemolysis without evidence of bleeding or hypersplenism) (Diehl and Ketchum 1998, Gehrs and Friedberg 2002, Kyasa, et al 2003). ITP was diagnosed in patients with 1) platelet counts < 100 × 109/L with no evidence of hypersplenism and 2) no evidence of decreased BM platelet production (normal or increased megakaryocytes on BM examination or normal or increased absolute reticulocyte count if a BM examination was not performed) (Diehl and Ketchum 1998, Gehrs and Friedberg 2002, Hamblin 2001, Kyasa, et al 2003). PRBCA was diagnosed in patients with 1) Hgb < 10 g/dL, 2) reticulocytopenia and 3) an isolated absence of erythrocyte precursors in the BM at the time of the anemia (Diehl and Ketchum 1998, Kyasa, et al 2003). AIG was diagnosed in patients with 1) sustained neutropenia (< 0.5 × 109/L) with no chemotherapy in the preceding 8 weeks and 2) BM showing decreased or absent granulocyte precursors. All available BM from CLL patients with AID were blindly reviewed by a single pathologist (JDH).
Prognostic data collected included clinical stage at diagnosis using the Rai classification (Rai, et al 1975), IgVH mutation status, interphase FISH results, ZAP-70 expression and CD38 expression as previously described (Kay, et al 2007, Shanafelt, et al 2006). Each patient was defined according to their presentation as either a) AID at presentation with simultaneous or a later diagnosis of CLL or b) AID as a complication of previously diagnosed CLL.
Statistical Analysis
Descriptive counts and frequency distributions were computed, and either Wilcoxon rank test or chi-square test was used to compare difference between demographic variables. Survival curves were calculated using the Kaplan-Meier method. Log-rank tests or Cox proportional hazard regression analysis was used for statistical comparisons, with death or censoring (as of December 31, 2006) as our event. When needed, we modeled our binary covariates (such as AID status) as a time-dependent covariate in the Cox regression model. All analyses were done using S-plus (Insightful, Seattle, WA) or SAS (SAS Institute, Inc., Cary, NC). Statistical significance was set at a 5% threshold.
RESULTS
During the 10 year time span, 1,750 CLL patients were seen in the Division of Hematology (Table I). Of these patients, 423 (24.2%) had cytopenia at some point during their disease. In 303 patients, the cytopenia was attributable to CLL with 228 (75.2%) of these patients having BM failure and 75 (24.8%) patients having AID. The remaining 120 patients had cytopenia due to either other CLL related factors (splenomegaly, n = 11; suggestive of AID but not meeting the diagnostic criteria, n = 18; long term complications of treatment of CLL, n = 16) or due to non CLL causes (another malignancy, n = 12; iron deficiency, n = 11; anemia of chronic disease, n = 10; renal failure, n = 6; other drugs, n = 4; surgery, n = 4). The cause of cytopenia could not be definitively determined in 28 (6.6%) patients.
Table I.
Demographic and Clinical characteristics of participants
| Characteristic | All CLL patients (N=1,750) N (%) |
Bone Marrow Failure (N = 228) N (%) |
AID Patients (N=75) N (%) |
|---|---|---|---|
| Age at CLL diagnosis (years) median (range) |
63.7 (17.3–94.7) | 63.3 (32.4–94.5) | 66.7 (30.0–85.0) |
| Gender | |||
| Male | 1198 (68%) | 160 (70%) | 62 (83%) |
| Female | 552 (32%) | 68 (30%) | 13 (17%) |
| Clinical stage at diagnosis of CLL (Rai)* |
|||
| 0 | 1010 (59%) | 74 (33%) | 26 (35%) |
| I | 484 (28%) | 35 (15%) | 24 (32%) |
| II | 119 (7%) | 22 (10%) | 11 (15%) |
| III | 46 (3%) | 40 (18%) | 6 (8%) |
| IV | 65 (4%) | 57 (25%) | 8 (11%) |
| Unknown | 26 | ||
| FISH | |||
| Normal, 13q, trisomy 12 | 379 (66%) | 42 (48%) | 15 (56%) |
| 17p-, 11q- | 198 (34%) | 45 (52%) | 12 (44%) |
| Missing | 1173 | 141 | 48 |
| Unmutated IgVH | |||
| No | 261 (58%) | 23 (33%) | 7 (35%) |
| Yes | 189 (42%) | 46 (67%) | 13 (65%) |
| Missing | 1300 | 159 | 55 |
| ZAP 70 | |||
| Negative | 187 (58%) | 15 (37%) | 1 (20%) |
| Positive (≥20%) | 135 (42%) | 26 (63%) | 4 (80%) |
| Missing | 1428 | 187 | 70 |
| CD38 | |||
| Negative | 393 (69%) | 47 (58%) | 22 (56%) |
| Positive (≥30%) | 176 (31%) | 34 (42%) | 17 (44%) |
| Missing | 1181 | 147 | 36 |
| Local Patient+ | |||
| No | 1437 (82%) | 196 (86%) | 43 (57%) |
| Yes | 313 (18%) | 32 (14%) | 32 (43%) |
Patients were staged using standard criteria which did not distinguish between cytopenia caused by bone marrow failure vs. AID
Patients were considered to be non-referred (local) if they lived within 120 miles of Mayo Clinic Rochester excluding the Minneapolis – St. Paul metropolitan area.
Of the 228 patients having BM failure, 97 (42.5%) had cytopenia at the time of diagnosis. More than 50% of these subjects had at least one poor prognostic marker (ZAP-70, CD38, FISH, or mutation status) (Table I). However, these results should be interpreted with caution because these prognostic markers only became available after 2000 and are not available for more than 60% of the study subjects.
We found that 54 (72%) of the 75 AID patients had their AID diagnosed after their CLL diagnosis, 14 (19%) patients had AID and CLL diagnosis within one month of each other, and 7 (9%) patients had AID diagnosed at least one month before their CLL (median difference in time between diagnosis of CLL and AID was 1.7 years). The most common AID observed was AIHA (n = 41), followed by ITP (n = 35), PRCA (n = 8), and AIG (n = 3) with 9 patients having more than one manifestation of AID.
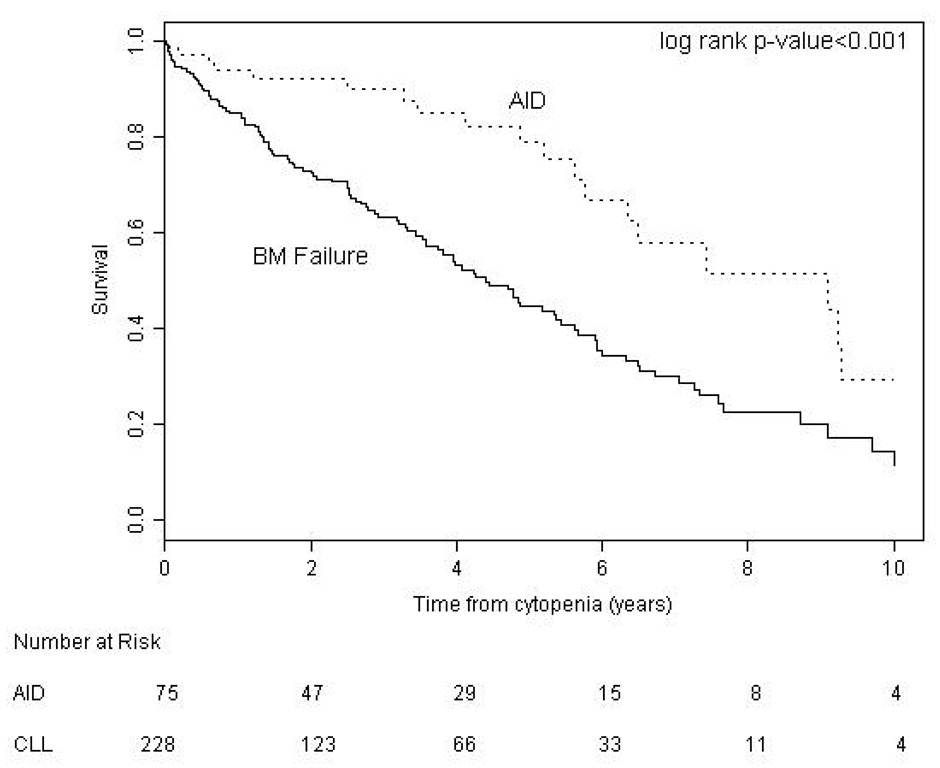
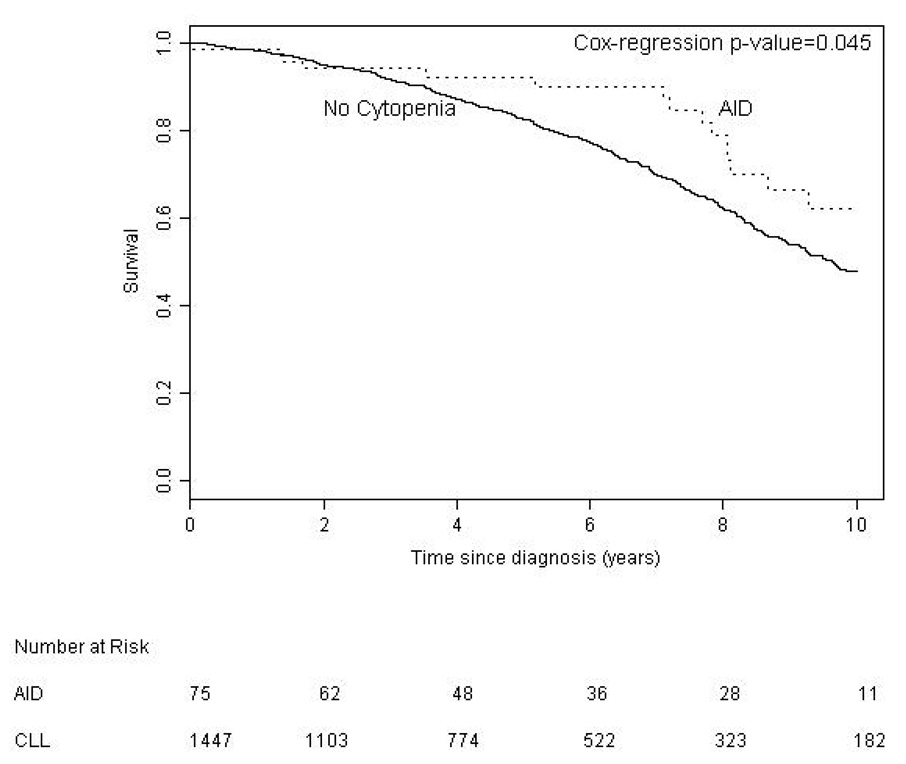
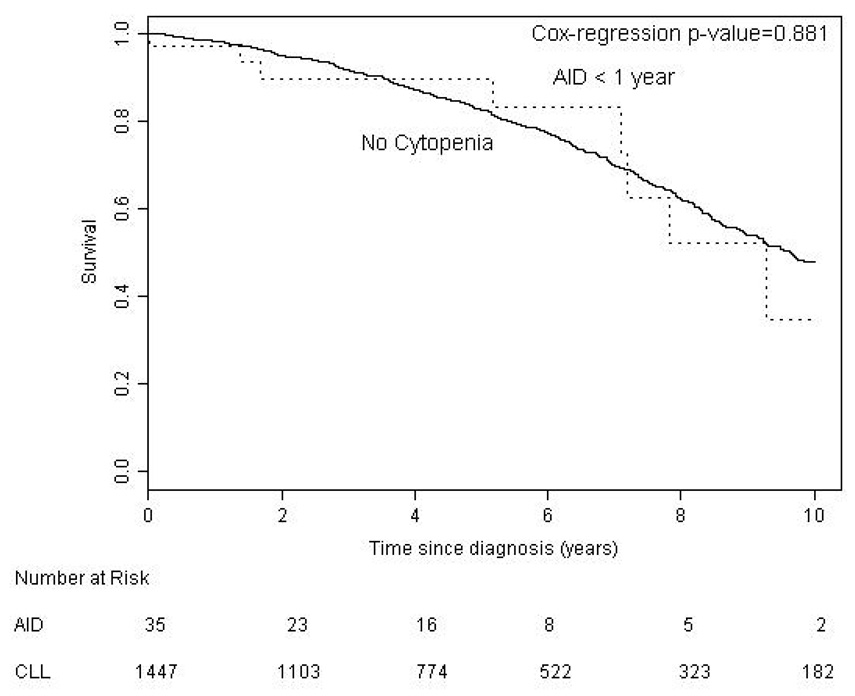
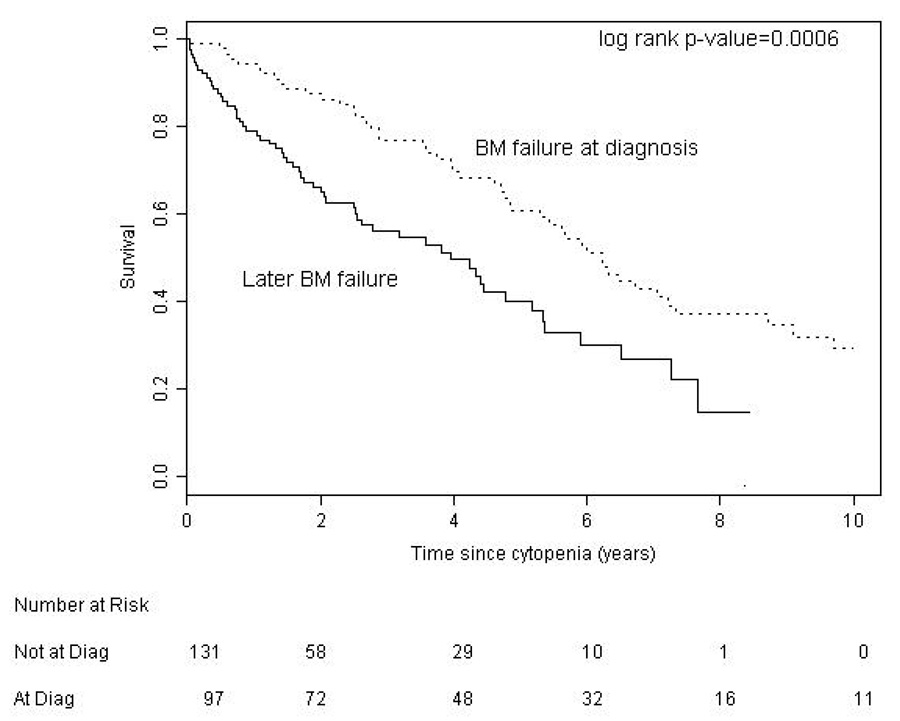
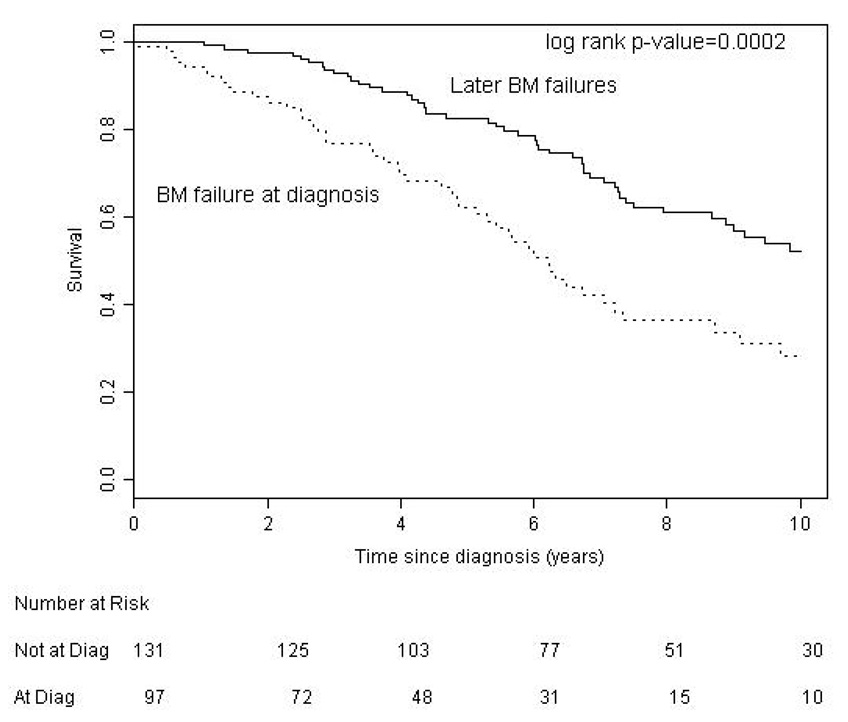
We found no significant difference between BM failure and AID patients with regard to age (p = 0.094) but our results suggest that AID is more common in males (p = 0.05). We compared survival in CLL patients according to the etiology of their cytopenia. Survival from time of onset of cytopenia was significantly better for patients with AID (median 9.1 years) than patients with bone marrow failure (median 4.4 years, p < 0.001) (Figure 1). Patients with AID as the cause of their cytopenia had a significantly better overall survival from the time of diagnosis of CLL (median 12.4 years) compared to both the entire CLL population (median 9.5 years, p = 0.020), and CLL patients without CLL related cytopenia (n = 1447, median 9.7 years, p = 0.045) (Figure 2a). To examine if this result reflected a survival bias (i.e. living long enough to develop AID), we determined the survival of patients with AID diagnosed within 1 year of the diagnosis of CLL (n = 35). Patients in this group had a median survival of 9.3 years which was not significantly different from that of the CLL patients who never developed CLL related cytopenia (Figure 2b), suggesting that patients with AID had equivalent survival compared to CLL patients without CLL related cytopenia. Survival from the onset of cytopenia caused by BM failure was longer for patients with BM failure at diagnosis compared to those patients developing BM failure later in the course of their disease (median 6.2 vs. 3.9 years, respectively, p = 0.0006) (Figure 3a). Additional analysis suggested that this finding could reflect a survival bias or a possible change in the biology of the CLL. Survival from the time of diagnosis of CLL in patients who present with BM failure was significantly shorter than patients who develop BM failure during the course of their disease (median 6.2 vs. 10.4 years respectively, p = 0.0002) (Figure 3b). In addition, survival from the time of diagnosis of CLL was similar for patients who developed BM failure during the course of their CLL compared to patients who never had BM failure (median 10.4 vs. 9.7 years, respectively, log rank p = 0.889).
Figure 1. Survival from Diagnosis of Cytopenia.
Patients with cytopenia caused by autoimmune complications of CLL (AID) had a significantly better survival (median 9.1 years) than patients with cytopenia caused by bone marrow failure (BM failure) (median 4.4 years)
Figure 2. Survival from Diagnosis of CLL.
a. Patients with autoimmune cytopenia (AID) had better survival from diagnosis of CLL (median 12.4 years) than patients without cytopenia (median 9.7 years).
b. Patients with AID diagnosed within 1 year of their CLL (AID < 1 year) had equivalent survival (median 9.3 years) compared to patients without cytopenia.
Figure 3. Survival in CLL Patients with Bone Marrow Failure.
a. Patient survival from cytopenia was better for presentation with cytopenia (median 6.2 years) compared to later BM failure (median 3.9 years).
b. Patient survival from CLL diagnosis was better with later BM failure (median 10.4 years) compared to presentation with BM failure (median 6.2 years).
Discussion
This study shows that the etiology of cytopenia in CLL patients determines its prognostic importance. Anemia and thrombocytopenia caused by CLL were recognized as important markers of poor prognosis by Rai et al and Binet et al and are crucial components in their clinical staging systems (Binet, et al 1981, Rai, et al 1975). However, these staging systems do not distinguish between cytopenia caused by BM failure and cytopenia caused by AID. Our data confirm that in CLL, anemia and thrombocytopenia caused by BM failure are associated with a significantly worse prognosis. In contrast, cytopenia caused by AID is not a poor prognostic factor in CLL patients. These data demonstrate the importance of accurate determination of the etiology of cytopenia in all CLL patients. Patients with cytopenia caused by AID should not automatically be considered to have advanced stage disease because of their cytopenia, and will often require different management compared to patients with cytopenia caused by BM failure. Because cytopenia caused by AID can be difficult to distinguish from BM failure in some patients, we recommend that a bone marrow study should be done in all patients with CLL before initiation of treatment for cytopenia. Patients with cytopenia due to AID cannot be meaningfully classified by the current clinical staging systems and an additional clinical staging based on assessment of disease burden and molecular markers of disease aggression may need to be developed.
Patients with cytopenia caused by BM failure which developed during the course of their CLL had a poorer survival from the onset of cytopenia than patients with BM failure at the time of diagnosis of their CLL. However, patients developing BM failure after their diagnosis of CLL had the same overall survival from the time of diagnosis of CLL compared to CLL patients who never developed cytopenia. This finding could simply reflect differences in the time of presentation and diagnosis of CLL or could alternatively be caused by a fundamental change in the biology of the CLL. One possible mechanism for such a change would be clonal evolution which has been shown to occur in a sizable fraction of patients with CLL over the course of their disease (Shanafelt, et al 2006). Identification of these putative changes could contribute to our understanding of the biology of CLL.
The results of this study reinforce the need to consider alternative etiologies for cytopenia in CLL patients. In 11.1% of the patients, the etiology of cytopenia was not directly related to their CLL. This finding could reflect the known association between CLL and other malignancies (Kyasa, et al 2004) and the increased risk of comorbidities in an older population, and further emphasizes the need for careful evaluation of the cause of cytopenia in all patients with CLL prior to initiation of therapy for presumed BM failure.
The findings of this study are likely to be generally applicable to most patients with CLL because the age at diagnosis and sex distribution of the population studied is characteristic of most CLL populations. The study included all patients seen over a 10 year period during which time CLL was accurately diagnosed and patients with CLL were comprehensively investigated to determine the etiology of cytopenia. In addition, this cohort was monitored through the end of 2006. However, the study does have several limitations. MCR serves both a local and referred population of patients with CLL. The inclusion of referred patients could have introduced bias because of the indications for referral and possible loss of data on patients who were not regularly followed at MCR. We examined the influence of this bias by using each patient’s home address to determining if they were likely to be local or referred. Patients living within 2 hours drive of MCR (120 mile radius excluding the Minneapolis - St. Paul metropolitan area) were considered local and all other patients as referred (Table 1). The percentage of patients with cytopenia due to bone marrow failure who were local (14%) was comparable to the entire CLL population (18%). However, a larger percentage of patients with AID were local (43%). This difference could reflect the availability of more follow up data on local patients or more intensive investigation of the cause of cytopenia in these patients. Conversely, the lower number of referred patients could reflect lack of data in patients who were not closely followed at MCR and could have been exacerbated by the incomplete nature of the retrospective collection of most data prior to 2002. Finally, the patient cohort studied included patients seen since 1995 which was prior to the discovery of many molecular prognostic factors. We thus had insufficient data to compare the prognostic significance of cytopenia due to BM failure to that of other molecular prognostic markers, which only became available in the latter half of our study. These data are being collected in an ongoing prospective study.
We conclude that cytopenia in CLL is a marker of poor prognosis only in those patients with BM failure. Cytopenia in CLL thus requires careful investigation of its etiology before its implications can be determined. Patients with cytopenia caused by CLL induced BM failure need treatment for their CLL which will likely improve survival. In contrast, patients with cytopenia due to AID do not always have an adverse prognosis and initial decisions about treatment of their AID may often be made independently of management of their CLL. We propose that the distinction between AID and BM failure in should be applied to clinical management and research of CLL patients.
Acknowledgements
This study was supported by the Mayo Clinic Hematological Malignancies Fund, University of Iowa/Mayo Clinic NIH SPORE Grant CA97274, Bayer Health Care Pharmaceuticals, and National Cancer Institute grant K07 CA94919.
References
- Apelgren P, Hasselblom S, Werlenius O, Nilsson-Ehle H, Andersson P-O. Evaluation of clinical staging in chronic lymphocytic leukemia - a population based study. Leukemia and Lymphoma. 2006;47:2505–2516. doi: 10.1080/10428190600881322. [DOI] [PubMed] [Google Scholar]
- Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, Vaugier G, Potron G, Colona P, Oberling F, Thomas M, Tchernia G, Jacquillat C, Boivin P, Lesty C, Duault MT, Monconduit M, Belabbes S, Gremy F. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–205. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE, Shepherd L, Hines JD, Schiffer CA, Larson RA. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, Marce S, Lopez-Guillermo S, Campo E, Montserrat E. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. NewEngland Journal of Medicine. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J, Lichtman SM, Schulman P, Vinciguerra VP, Rai KR, Ferrarini M, Chiorazzi N. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- Dewald GW, Brockman SR, Paternoster SF, Bone ND, O'Fallon JR, Allmer C, James CD, Jelinek DF, Tschumper RC, Hanson CA, Pruthi RK, Witzig TE, Call TG, Kay NE. Chromosome anomalies detected by interphase fluorescence in situ hybridization: correlation with significant biological features of B-cell chronic lymphocytic leukaemia. British Journal of Haematology. 2003;121:287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- Diehl L, Ketchum L. Autoimmune disease and chronic lymphocytic leukemia: Autoimmune hemolytic anemia, pure red cell aplasia, and autoimmune thrombocytopenia. Seminars in Oncology. 1998;25:80–97. [PubMed] [Google Scholar]
- Dohner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, Döhner K, Bentz M, Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. New England Journal of Medicine. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- Gehrs B, Friedberg R. Autoimmune hemolytic anemia. American Journal of Hematoogyl. 2002;69:258–271. doi: 10.1002/ajh.10062. [DOI] [PubMed] [Google Scholar]
- Hamblin T. Autoimmune disease and its management in chronic lymphocytic leukemia. In: Cheson B, editor. Chronic lymphocytic leukemias. New York: Marcel Dekker; 2001. pp. 435–458. [Google Scholar]
- Hamblin T, Davis Z, Gardiner A, Oscier D, Stevenson F. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- Harris N, Jaffe E, Stein H, Banks P, Chan J, Cleary M, Delsol G, De Wolf-Peeters C, Falini B, Gatter K. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- Ibrahim S, Keating M, Do KA, O'Brien S, Huh YO, Jilani I, Lerner S, Kantarjian HM, M A. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood. 2001;98:181–186. doi: 10.1182/blood.v98.1.181. [DOI] [PubMed] [Google Scholar]
- Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF, Tschumper R, Bone ND, Dewald GW, Lin TS, Heerema NA, Smith L, Grever MR, Byrd JC. Combination chemoimmunotherapy with pentostatin, cyclophosphamide and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B-chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating MJ, O'brien S, Albitar M, Lerner S, Plunkett W, Giles F, Andreeff M, Cortes J, Faderl S, Thomas D, Koller C, Wierda W, Detry MA, Lynn A, Kantarjian H. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. Journal of Clinical Oncology. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leukemia and Lymphoma. 2004;45:507–513. doi: 10.1080/10428190310001612939. [DOI] [PubMed] [Google Scholar]
- Kyasa MJ, Parrish RS, Schichman SA, Zent CS. Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic leukemia/small lymphocytic lymphoma. American Journal of Hematology. 2003;74:1–8. doi: 10.1002/ajh.10369. [DOI] [PubMed] [Google Scholar]
- Mauro F, Foa R, Cerretti R, Giannarelli D, Coluzzi S, Mandelli F, Girelli G. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood. 2000;95:2786–2792. [PubMed] [Google Scholar]
- Muller-Hermelink HK, Catovsky D, Montserrat E, Harris NL. Chronic lymphocytic leukemia/small lymphocytic lymphoma. In: Jaffe E, Harris N, Stein H, Vardiman J, editors. Tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 127–130. [Google Scholar]
- Nowakowski GS, Dewald GW, Hoyer JD, Paternoster SF, Stockero KJ, Fink SR, Smoley SA, Remstein ED, Phyliky RL, Call TG, Shanafelt TD, Kay NE, Zent CS. Interphase fluorescence in situ hybridization with an IGH probe is important in the evaluation of patients with a clinical diagnosis of chronic lymphocytic leukaemia. British Journal of Haematology. 2005;130:36–42. doi: 10.1111/j.1365-2141.2005.05548.x. [DOI] [PubMed] [Google Scholar]
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Witzig TE, Fink SR, Jenkins RB, Paternoster SF, Smoley SA, Stockero KJ, Nast DM, Flynn HC, Tschumper RC, Geyer S, Zent CS, Call TG, Jelinek DF, Kay NE, Dewald GW. Prospective evaluation of clonal evolution during long-term followup of patients with untreated early-stage chronic lymphocytic leukemia. Journal of Clinical Oncology. 2006;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- Wierda WG, O'Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, Cortes J, Thomas D, Garcia-Manero G, Koller C, Beran M, Giles F, Ravandi F, Lerner S, Kantarjian H, Keating M. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- Zent CS, Call TG, Hogan WJ, Shanafelt TD, Kay NE. Update on risk-stratified management for chronic lymphocytic leukemia. Leukemia and Lymphoma. 2006;47:1738–1746. doi: 10.1080/10428190600634036. [DOI] [PubMed] [Google Scholar]
- Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–1330. doi: 10.1002/1097-0142(20010901)92:5<1325::aid-cncr1454>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]