Abstract
The majority of colorectal tumors are aneuploid because of the underlying chromosome instability (CIN) phenotype, in which a defective mitotic checkpoint is implicated. Adenomatous polyposis coli (APC), a tumor suppressor gene that is commonly mutated in colon cancers, has been suggested in causing CIN; however, the molecular mechanism remains unresolved. In this study, we report an interaction of tumor-associated N-terminal APC fragments (N-APC) with Mad2, an essential mitotic checkpoint protein, providing a direct molecular support for linking APC mutations to the generation of CIN. N-APC interacts with Mad2 in Xenopus egg extracts, colon cancer cells, and in vitro with purified components. The interaction between N-APC and Mad2 decreases the soluble pool of Mad2, which is essential for Mad2 cycling and releasing from unattached kinetochores to produce a diffusible |P`wait anaphase|P' signal. Addition of such an N-APC mutant of egg extracts inactivates the mitotic checkpoint. Expressing a tumor-associated N-APC mutant in mammalian cells with an intact mitotic checkpoint produces premature anaphase onset with missegregated chromosomes.
INTRODUCTION
The challenge of mitosis is to deliver one copy of each chromosome to each daughter cell at each cell division. To ensure accurate chromosome segregation, the mitotic checkpoint in vertebrate cells is activated in every cell cycle to delay anaphase onset until all chromosomes have been successfully captured by spindle microtubules. Screening for budding yeast mutants sensitive to the antimicrotubule drug initially identified seven components of the mitotic checkpoint Mad1-3 (Li and Murray, 1991), Bub1-3 (Hoyt et al., 1991), and Mps1 (Weiss and Winey, 1996). There are vertebrate homologues of all of these except Bub2. Initially demonstrated for Mad2 (Chen et al., 1996), all checkpoint proteins have now been shown to be associated with unattached kinetochores, in which they generate a diffusible signal to prevent chromosome segregation. The basic plan of the mitotic checkpoint signaling cascade is established (Cleveland et al., 2003). Before spindle microtubule capture, Mad1-Mad2 complex is targeted to unattached kinetochores, where additional free Mad2 is recruited (De Antoni et al., 2005) and converted into a rapidly released, inhibitory form (Shah et al., 2004; Vink et al., 2006), including possibly assembly of complexes with other checkpoint proteins (Sudakin et al., 2001; Fang, 2002). The activated inhibitor(s), whatever its composition, binds to and inhibits Cdc20/p55Cdc/Fizzy (Fang et al., 1998; Kallio et al., 1998), a specific factor required for an E3 ubiquitin ligase called anaphase-promoting complex/cyclosome (APC/C) to recognize substrates (such as Securins [Zur and Brandeis, 2001] and cyclin B [Raff et al., 2002]) whose destruction are required for anaphase onset (Cohen-Fix et al., 1996; Fang et al., 1998; Zou et al., 1999).
Similar to what has been observed in other cancers, ∼85% of colon cancers have significantly aberrant genomic structure, including abnormal numbers of chromosomes (Weaver and Cleveland, 2006). It has been shown that chromosome instability (CIN) colon cancer cell lines do accumulate in mitosis in response to microtubule-disrupting agents; however, the maximum mitotic index achieved by the CIN lines (40–68%) is diminished relative to microsatellite instability (MIN) cell lines (>80%) (Tighe et al., 2001; Gascoigne and Taylor, 2008). This result indicates that the mitotic checkpoint may be weakened in CIN cells. Expressing colon cancer-associated truncating adenomatous polyposis coli (APC) mutants in human epithelial colon HCT-116 cells (diploid and chromosomally stable) also resulted in premature mitosis exit in the presence of spindle toxins (Tighe et al., 2004). A couple of recent studies have shown that CIN may also arise from an increased incidence of lagging anaphase chromosomes (Gascoigne and Taylor, 2008; Thompson and Compton, 2008). One possibility is that CIN cells may not be able to resolve malorientations, because they are more sensitive to transient Eg5 inhibition (Gascoigne and Taylor, 2008).
APC mutations have been linked to CIN in colon cancer cells (Fodde et al., 2001; Kaplan et al., 2001). Chromosome missegregation has been reported in APC-depleted mammalian cultured cells (Green et al., 2005). Furthermore, APC has been shown to form a complex with mitotic checkpoint proteins Bub1 and Bub3 and is a substrate for Bub1/BubR1 kinases in vitro (Kaplan et al., 2001). However, recent studies have suggested that APC itself is not essential for the mitotic checkpoint (Draviam et al., 2006; Zhang et al., 2007). In APC-defective colorectal cancer cells, one allele acquires a truncating mutation, but the second allele undergoes either loss of heterozygosity or a second truncating mutation (Kinzler and Vogelstein, 1996). Thus, these cells do not completely lack APC proteins and express N-terminal APC fragments (N-APC). Expression of such N-APC fragments in mammalian cultured cells causes chromosome missegregation (Green and Kaplan, 2003; Tighe et al., 2004). However, whether and how the colon cancer-related N-APC mutants dominantly affect the mitotic checkpoint remains untested.
Here, we report an interaction between colon cancer associated N-APC fragments and Mad2, an essential mitotic checkpoint protein. The interaction decreases the soluble pool of Mad2, resulting in a weakened mitotic checkpoint to produce CIN in human cells.
MATERIALS AND METHODS
Xenopus Egg Extracts
Cytostatic factor (CSF)-arrested extracts were prepared from Xenopus egg as described previously (Murray, 1991). For the activation of the mitotic checkpoint, egg extracts were incubated with ∼9000 demembranated sperm nuclei/μl and 10 μg/ml nocodazole for 30 min. The addition of 0.4 mM CaCl2 to the egg extracts is used to inactivate CSF.
Isolation of chromosomal proteins was performed as described previously (Chen, 2002). Briefly, 40-μl aliquots of CSF-arrested egg extracts were incubated with sperm and nocodazole for 30 min. The samples were then diluted 10-fold with CSF-XB containing 0.1% Triton X-100 and layered onto a 30% sucrose-containing CSF-XB cushion and centrifuged at 10,000 rpm for 15 min at 4°C. The pellets were washed in the same buffer, and the centrifugation was repeated. After removing the supernatant, the pellets were dissolved in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer.
In Vitro Binding Assay
Glutathione transferase (GST) pull-down assays were performed using standard procedures. Xenopus GST-tagged Mad2 was expressed in Escherichia coli and purified over glutathione-Sepharose beads. His-tagged Xenopus N-APC was expressed in insect cells infected with a baculovirus encoding amino acids 1-1450 of APC and purified over immobilized nickel-nitrilotriacetic acid agarose. The binding buffer used contained 20 mM HEPES, pH 7.5, 300 mM NaCl, 1 mM dithiothreitol, and 0.5% NP-40.
Cell Culture, Transfection, and Treatments
HeLa and SW480 cells were cultured in DMEM with 10% fetal bovine serum and 1% penicillin-streptavidin at 37°C in 5% CO2. Transfection of Myc tagged N-APC was accomplished using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Transfected cell populations were isolated using magnetic activated cell sorting (Miltenyi Biotec, Auburn, CA) by cotransfected with pCMV-CD20 in a 1:10 ratio, and isolation was performed according to the manufacturer's instruction. In live-cell imaging, transfected cells were also identified with cotransfection with a Ds-Red plasmid in 1:10 ratio. Nocodazole and MG132 (Sigma-Aldrich, St. Louis, MO) were added to a final concentration of 100 ng/ml and 10 μM, respectively.
Immunoprecipitation
Affinity-purified antibodies were couple to Dynal beads (Invitrogen), and the beads were washed twice with lysis buffer. The beads were then incubated with egg extracts or cell lysates at 4°C for 1 h. After incubation, precipitates were washed twice with lysis buffer and twice with phosphate-buffered saline. The following antibodies were used: mouse APC (Calbiochem, San Diego, CA), rabbit APC (Santa Cruz Biotechnology, Santa Cruz, CA), human Cdc 20 and BubR1 (Abcam, Cambridge, MA), rabbit human Mad2 (Covance Research Products, Princeton, NJ), Xenopus cyclin B1 (a kind gift from S. Yoshitome, Tottori University, Yonago, Japan), and Myc (Clontech, Mountain View, CA).
Immunofluorescence and Live-Cell Imaging
Immunofluorescence with Xenopus egg extracts was performed as described previously (Zhang et al., 2007). All images in each experiment were collected on the same day by using identical exposure times. Image acquisition and data analysis were performed at the room temperature by using an inverted microscope (IX81; Olympus, Tokyo, Japan) with a 60× numerical aperture (NA) 1.42 plan Apo oil immersion objective lens (Olympus), a monochrome charge-coupled device camera (Sensicam QE; Cooke, Romulus, MI), and Slidebook software package (Olympus).
For live-cell imaging, HeLa cells stably expressing H2B-EYFP (a kind gift from D. Foltz, Ludwig Institute for Cancer Research, San Diego, CA) were plated onto 35-mm glass-bottomed dishes. Images were acquired using a 40× NA dry objective lens (Olympus) every 3 min on the microscope with a 37°C chamber (Olympus). The data were analyzed with the Slidebook software.
RESULTS
Addition of N-APC to Xenopus Egg Extracts Inactivates the Mitotic Checkpoint
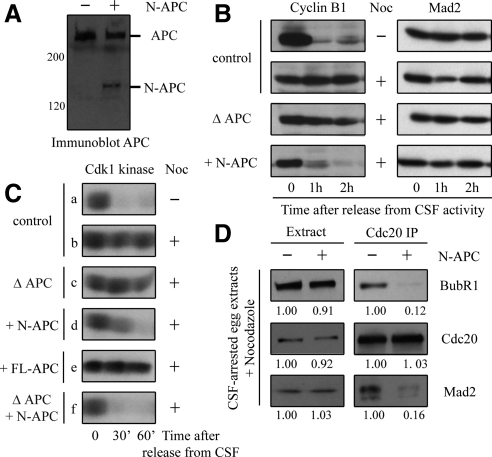
To determine whether N-APC affects the mitotic checkpoint, we added purified N-APC recombinant proteins to CSF-arrested Xenopus egg extracts to a level comparable with that of endogenous APC (Figure 1A). We then followed cyclin B1 degradation in egg extracts supplemented with sperm nuclei. CSF release upon addition of Ca++ caused a steady degradation of cyclin B1 (Figure 1B), whereas when the mitotic checkpoint was provoked by incubation with nocodazole, cyclin B1 remained stable (Figure 1B). APC-depleted egg extracts exhibited a chronically activated checkpoint response as judged by the persistence of intact Cyclin B1 (Figure 1B), suggesting that APC does not play a role in the mitotic checkpoint. In contrast, addition of N-APC induced pronounced cyclin B1 degradation (Figure 1B). Similar results were obtained when the mitotic checkpoint was judged by a continuous high level of Cdk1 kinase activity after the calcium-mediated inactivation of CSF in egg extracts. High numbers of sperm nuclei were added to CSF-arrested egg extracts, followed by addition of nocodazole (for 30 min) to disrupt spindle microtubule assembly and produce unattached kinetochores. The presence of an activated mitotic checkpoint is assessed by Cdc2/cyclin B (Cdk1) kinase activity (assayed on phosphorylation of histone H1, Figure 1C) 0, 30, and 60 min after addition of calcium to release the CSF arrest. Addition of N-APC (Figure 1C, rows d and f), but not full-length APC (Figure 1C, row e), inactivates the mitotic checkpoint.
Figure 1.
N-APC inactivates the mitotic checkpoint in Xenopus egg extracts. (A) APC and N-APC levels were determined by immunoblot in egg extracts. (B) Addition of N-APC triggers cyclin B1 degradation. CSF-arrested egg extracts were mock depleted, APC depleted, or supplemented with N-APC as indicated. High number of sperms and nocodazole (Noc) were then added for 30 min to initiate the mitotic checkpoint. Aliquots were blotted with anti-cyclin B1 and anti-Mad2 antibodies at the times indicated upon release from the CSF arrest. (C) Addition of N-APC inactivates the mitotic checkpoint. CSF extracts were APC depleted or supplemented with N-APC or full-length APC (FL-APC) and then incubated with sperm nuclei and nocodazole for 30 min. Subsequently, at the times indicated after release from CSF, aliquots were assayed for Cdk1 kinase activity on histone H1. (D) N-APC reduces the interaction between Cdc20 and the mitotic checkpoint proteins. The mitotic checkpoint was activated in CSF-arrested egg extracts supplemented with or without N-APC upon addition of sperm nuclei and nocodazole for 30 min. Cdc20 was then immunoprecipitated from CSF-arrested mitotic extracts and proteins in the extracts or associated with Cdc20 immunoprecipitates were blotted for Cdc20, BubR1, and Mad2. Immunoreactivities in the presence of N-APC were relative to the values of that in the absence of N-APC.
The target of the mitotic checkpoint is Cdc20, an activator of the E3 ubiquitin ligase APC/C. We immunoprecipitated Cdc20 from CSF-arrested and checkpoint-activated (with sperm and nocodazole) egg extracts supplemented with or without N-APC recombinant proteins, and then we blotted two known Cdc20-binding proteins: BubR1 and Mad2. Consistent with the finding that the mitotic checkpoint was inactivated in the presence of N-APC, the amount of BubR1 and Mad2 associated with Cdc20 was substantially decreased in egg extracts supplemented with N-APC compared with that in control extracts (Figure 1D).
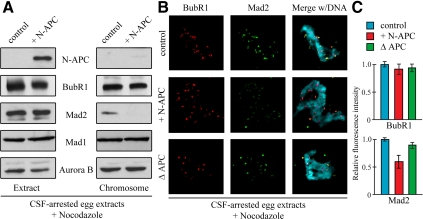
To understand the molecular basis by which N-APC dominantly affects the mitotic checkpoint, we isolated mitotic chromosomes from CSF-arrested egg extracts supplemented with nocodazole and immunoblotted diverse kinetochore components with specific antibodies. Immunoblotting analysis revealed a significant portion of checkpoint proteins, including BubR1 and Mad2, in the purified chromosome fraction from control egg extracts (Figure 2A). However, addition of N-APC to the egg extracts substantially decreased the level of Mad2 associated with chromosomes (Figure 2B, right) without effect on total Mad2 or other checkpoint proteins levels (Figure 2B, left). Immunofluorescence analysis also showed that addition of N-APC to egg extracts caused a marked reduction (∼40%) in the level of Mad2 but not BubR1 (Figure 2, B and C).
Figure 2.
Addition of N-APC to egg extracts decreases Mad2 recruitment onto unattached kinetochores. (A) N-APC decreases Mad2 association with chromosomes. Mitotic chromosomes were isolated from checkpoint activated CSF-arrested egg extracts supplemented with or without N-APC. The egg extracts and chromosomal fractions were blotted for the checkpoint proteins as indicated. (B) Addition of N-APC reduces Mad2 from unattached kinetochores. CSF-arrested egg extracts were mock depleted, APC depleted, or supplemented with N-APC. Sperm nuclei and nocodazole were added and after 30 min, BubR1 (red) and Mad2 (green) kinetochore localization were visualized by immunofluorescence. DAPI was used to visualize the chromatin (blue). (C) Quantitation of the normalized integrated intensities of the kinetochore signals in (B). At least 50 kinetochores from more than three mitotic figures were quantified for each bar. p < 0.05. Error bars represent SEs.
N-APC Associates with Mad2 in Xenopus Egg Extracts, In Vitro, and in Colon Cancer Cells
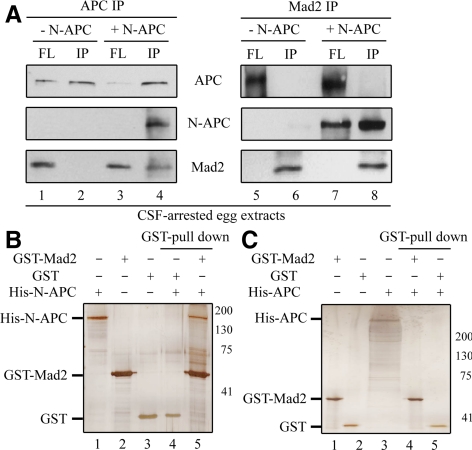
Because addition of N-APC to egg extracts affects recruitment of Mad2 onto unattached kinetochores, we next tested whether N-APC directly associates with Mad2. We performed coimmunoprecipitation assays with a specific anti-APC antibody in CSF-arrested egg extracts supplemented with or without the purified N-APC fragment. This APC antibody recognizes the N-terminal region of APC and is able to immunoprecipitate both endogenous APC and N-APC recombinant proteins from egg extracts (Figure 3A, lanes 2 and 4). A substantial portion of Mad2 was copelleted from egg extracts supplemented with N-APC, but none was precipitated without N-APC (Figure 3A, compare lanes 2 and 4). Reciprocal immunoprecipitation with an anti-Mad2 antibody verified the interaction between Mad2 and N-APC (Figure 3A, lane 8). To determine whether N-APC-Mad2 association is direct, purified recombinant His-N-APC proteins were incubated with purified GST or GST-Mad2 proteins in the in vitro binding assays. As shown in Figure 3B, a considerable amount of His-N-APC was precipitated with GST-Mad2 but not GST alone (compare lane 4 with lane 5). In contrast, purified full-length APC protein did not precipitate with GST-Mad2 by using a similar approach (Figure 3C, lane 5).
Figure 3.
N-APC interacts with Mad2 in Xenopus egg extracts and in vitro with purified components. (A) N-APC associates with Mad2 in egg extracts. Immunoprecipitates from CSF-arrested egg extracts supplemented with or without N-APC with affinity purified anti-APC (left) or Mad2 (right) antibody and then probed with APC and Mad2. (B and C) N-APC, but not FL-APC, binds to Mad2 in vitro. Purified recombinant proteins were incubated with glutathione-Sepharose 4B as indicated. Proteins pull-down with the beads was assayed by SDS-PAGE and Coomassie Blue staining.
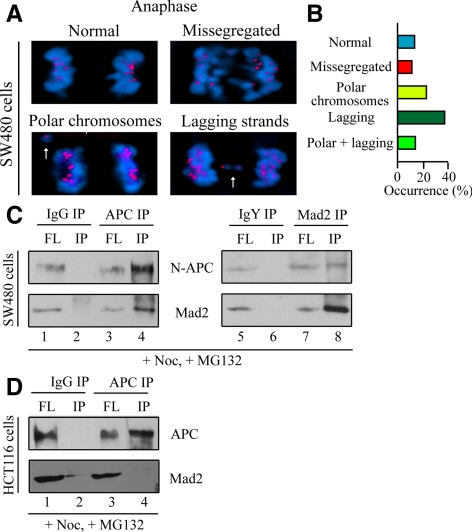
A similar situation was found for cultured colon cancer cells. The SW480 cell line was established from a primary adenocarcinoma of the colon and is known to express truncated APC lacking the carboxy half of the protein (APC1-1338) (Nishisho et al., 1991). SW480 cells have been shown to have abnormal response to nocodazole, with no significant increase in mitotic index (Cahill et al., 1998; Kasai et al., 2002), indicating a defective mitotic checkpoint. Indeed, SW480 cells exhibited severe defects in chromosome segregation during anaphase, including missegregated chromosomes and chromosomal bridge (Figure 4A, arrows; and B). To test N-APC and Mad2 interaction in SW480 cells, N-APC was immunoprecipitated from accumulated mitotic cells upon treatment with nocodazole and MG132 (to prevent premature anaphase onset) for 16 h. Immunoprecipitates obtained with APC antibody were found to contain the truncated form of APC and a substantial amount of Mad2 (Figure 4C, lane 4). Reciprocally, immunoprecipitates with a Mad2 antibody also contained N-APC proteins (Figure 4C, lane 8). In contrast, in another colorectal cell line HCT116, which does not have any APC mutations (Rowan et al., 2000), Mad2 did not coimmunoprecipitate with full-length APC (Figure 4D, lane 4). Thus, Mad2 also interacts with N-APC, but not full-length APC, in colon cancerous cell lines.
Figure 4.
N-APC associates with Mad2 in cultured CIN colon cancer cell lines. (A) Representative examples of anaphase onset in SW480 cells (ACA in red and chromatin in blue). Note missegregated chromosomes and chromosome bridges in anaphase (arrows). (B) Quantitative analysis of anaphase spindle structures that occurred in SW480 cells was shown on the right. Fifty cells were quantified. (C and D) N-APC interacts with Mad2 in SW480 cells but not in HCT116 cells. Cell lysates were prepared from nocodazole-arrested SW480 or HCT116 cells in the presence of MG132 and then subjected to APC or Mad2 immunoprecipitation as indicated. FL, extracts after immunoprecipitation; IP, immunoprecipitates. A three-fold higher proportion of the bead bound fractions relative to the depleted extracts was analyzed.
Expression of N-APC in HeLa Cells Weakens the Mitotic Checkpoint
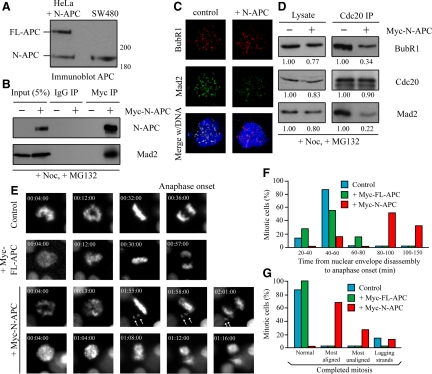
We next investigated whether expression of N-APC alone in mammalian cultured cells affects the mitotic checkpoint. We transfected HeLa cells with a Myc-tagged N-APC mutant expression vector at a level similar to endogenous APC and mutant APC level in SW480 cells (Figure 5A). Consistent with the results from Xenopus egg extracts and SW480 cells, Mad2 was coimmunoprecipitated with N-APC with a specific anti-Myc antibody from the mitotic population of Myc-N-APC\Ntransfected cells upon treatment of nocodazole and MG132 (Figure 5B). Expression of N-APC in HeLa cells also substantially reduced kinetochore recruitment of Mad2, but not BubR1 (Figure 5C), and the amount of BubR1 and Mad2 in Cdc20 immunoprecipitates in response to unattached kinetochores (Figure 5D).
Figure 5.
Expression of N-APC in mammalian cultured cells results in premature anaphase onset with unaligned chromosomes. (A) HeLa cells transfected with the N-APC\Nexpressing vector, the whole-cell lysates of the transfected population and SW480 cell lysates were blotted for APC. (B) Ectopic expressed N-APC associates with Mad2 in HeLa cells. HeLa cells were transfected with Myc:N-APC and arrested in mitosis upon nocodazole treatment 48 h posttransfection in the presence of MG132. Cells were then lysed and subjected to Myc immunoprecipitation. Immunoprecipitates, along with 5% lysates, were blotted with APC and Mad2 antibodies. (C) Transient expression of myc-N-APC in cultured HeLa cells. BubR1 (red) and Mad2 (green) were observed by indirect immunofluorescence. Chromatin in merged panels was visualized with 4,6-diamidino-2-phenylindole (DAPI) (blue). (D) Expression of N-APC reduces the interaction between Cdc20 and the mitotic checkpoint proteins in HeLa cells. Cell lysates were prepared from nocodazole and MG132-arrested cells expressing myc:N-APC or not and immunoprecipitated with a Cdc20 antibody. The lysates and immunoprecipitates were blotted with Cdc20, BubR1, and Mad2. Immunoreactivities in the presence of N-APC were relative to the values of that in the absence of N-APC. (E) Expression of N-APC weakens the mitotic checkpoint in HeLa cells. Still frames of live-cell microscopy of H2B-EYFP HeLa cells transfected with or without Myc-N-APC or Myc-FL-APC. Arrows indicate unaligned chromosomes in metaphase and missegregated during anaphase. (F) The time elapsed between nuclear envelope disassembly and the anaphase onset in cells expressing N-APC or FL-APC and control cells. (G) Living cells assayed by time-lapse microscopy for anaphase defects (n ≥ 10 for each bar). Most aligned, cells have an apparent metaphase plate with some polar chromosomes; most unaligned, cells do not have an apparent metaphase plate.
Next, we used live-cell microscopy to follow mitotic progression and chromosome segregation in individual cells. Filming of mitoses in HeLa cells with H2B-EYFP revealed mitotic errors in cells expressing N-APC, but not full-length APC (Figure 5E). The most prominent errors include a short delay in prometaphase (Figure 5, E and F) and the presence of missegregated chromosomes during anaphase (Figure 5E). Whereas anaphase timing (from nuclear envelope disassembly) in cells expressing full-length APC (t = 45.8 ± 12.7 min) was indistinguishable from that of control cells (t = 46.1 ± 6.0 min), cells expressing N-APC exhibited transient anaphase delay with many unaligned chromosomes (t = 101.5 ± 30.3 min), suggesting that N-APC affects kinetochore microtubule attachment and metaphase chromosome alignment. Conversely, it has been shown that one chromatid pair trapped behind one centrosome is able to generate a sustained block to anaphase in HeLa cells (Bomont et al., 2005). Such cases were rare in cells expressing N-APC. Majority of the N-APC expressing cells initiated anaphase with polar chromosomes within 2 h (Figure 5, E and G). Thus, N-APC weakens the mitotic checkpoint in cultured cells, in which one or a few unattached kinetochores are insufficient to prevent premature entry into anaphase, whereas high numbers of unaligned chromosomes in early prometaphase can still produce sufficient overall checkpoint signal to prevent anaphase onset.
Addition of Recombinant Mad2 in N-APC Egg Extracts Restores the Mitotic Checkpoint
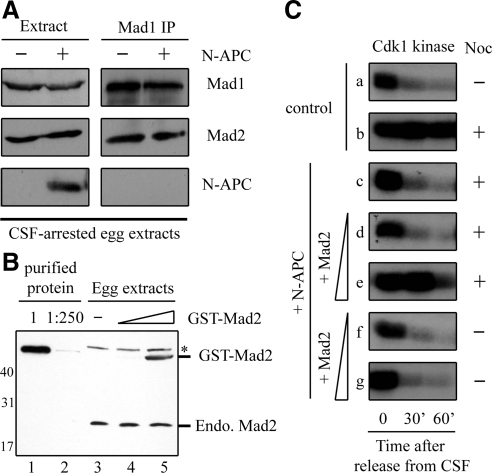
Current studies have suggested that Mad2 cycles through a Mad1-Mad2 template on unattached kinetochores for activation to become a “wait anaphase” signal, possibly in a complex form (Luo et al., 2004; De Antoni et al., 2005). Thus, the mitotic checkpoint requires both Mad1-bound and Mad1-free Mad2 (Chung and Chen, 2002). To understand the molecular basis for how N-APC binding blocks Mad2 function in the mitotic checkpoint, we first examined whether N-APC directly competes for Mad1-binding with Mad2. Immunoprecipitation of egg extracts, in the presence or absence of N-APC, with anti-Mad1 antibodies showed that Mad2 coimmunoprecipitated with Mad1 in a similar amount (Figure 6A), indicating N-APC does not interfere Mad1-Mad2 complex formation. Furthermore, N-APC was not found in the Mad1 immunoprecipitates (Figure 6A).
Figure 6.
Addition of recombinant Mad2 restores the mitotic checkpoint in egg extracts supplemented with N-APC. (A) N-APC does not affect Mad1-Mad2 interaction. The mitotic checkpoint was activated in egg extracts supplemented with or without N-APC upon addition of sperm nuclei and nocodazole. Thirty minutes later, Mad1 was immunoprecipitated from CSF-arrested egg extracts, and proteins in the extracts or associated with Mad1 immunoprecipitates were blotted for Mad1, Mad2, and N-APC. (B) Purified recombinant Mad2 protein level and Mad2 levels in control egg extracts or egg extracts supplemented with 1:20 and 1:100 purified Mad2 were determined by immunoblot. (C) Addition of recombinant Mad2 restores the mitotic checkpoint in egg extracts supplemented with N-APC. Cdk1 kinase activities assayed on histone H1 at 0, 30, and 60 min after CSF release in control, supplemented with N-APC alone, and supplemented with both N-APC and either 1:100 or 1:20 Mad2. Nocodazole was added in egg extracts as indicated.
When the free Mad2 level was reduced to 23% of normal concentration by addition of extra Mad1 to ∼2.7-fold over the endogenous level, the egg extracts failed to support the mitotic checkpoint (Chung and Chen, 2002). If interaction between N-APC and Mad2 results in a decrease of free Mad2 level, then the addition of purified recombinant Mad2 along with N-APC should restore the fraction of Mad1-free Mad2 and thus rescue the mitotic checkpoint defect. Indeed, addition of Mad2 at about one-fold over the endogenous level (Figure 6B) restored the checkpoint function in the N-APC egg extracts (Figure 6C, row e). Although addition of excess recombinant Mad2 (10-fold), which spontaneously binds and inhibits Cdc20, has been shown to overcome the necessity for kinetochore-dependent checkpoint signaling, the amount of Mad2 we added cannot inhibit Cdc20 activity by itself in the absence of unattached kinetochores (Figure 6C, row g). These results strongly suggest that N-APC binds to free Mad2 and interferes with its function, rather than other molecules.
DISCUSSION
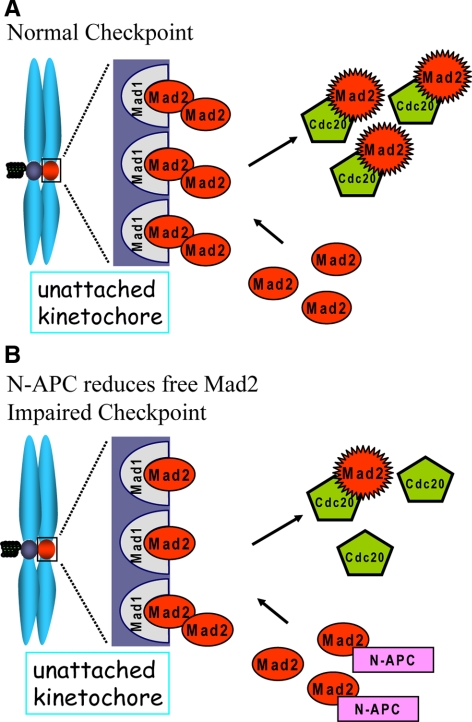
In colorectal neoplastic development, genetic instability is one of the first characteristics of malignant development. There are two different categories of genetic instability: CIN and MIN. It has been shown that MIN is because of second mutations in mismatch repair genes (Kinzler and Vogelstein, 1996), whereas the molecular mechanism remains unknown for the CIN phenotype. Almost all CIN human colorectal cancer cell lines (SW480, COLO205, HT29, KM12, SW620, CACO2, and LOVO) have N-terminal truncating APC mutations, whereas most of the MIN lines (LS180, SW48, LS174T, and HCT116) have mutations in β-catenin but not APC (Rowan et al., 2000). We now show that these colon cancer-associated N-APC mutants weakened the mitotic checkpoint through a direct association with an essential checkpoint protein Mad2 in human cells. Under normal conditions Mad1-Mad2 complexes are recruited onto unattached kinetochores. Free Mad2 proteins dynamically bind to Mad1-Mad2 complexes to become an active form, including a possible complex formation with Cdc20 and BubR1 (Sudakin et al., 2001), and then they are released into cytosol as a wait anaphase signal. The pool of Mad1-free Mad2 molecules is essential to ensure Mad1-Mad2 complexes at unattached kinetochores quickly bind new Mad2 molecules after activated Mad2 have dissociated. Titrating out free Mad2 with an excess of Mad1 results in an abolishment of the mitotic checkpoint in Xenopus egg extracts (Chung and Chen, 2002). In this study, we show that the interaction between N-APC and free Mad2 decreases the pool of free Mad2 proteins, resulting in less recruitment of Mad2 onto unattached kinetochores and less production of the wait anaphase inhibitors demonstrated by less complex formation among Cdc20, Mad2, and BubR1 (Figure 7). Evidence for this model includes the following. 1) N-APC itself does not present on chromosomes. 2) Recruitment of other mitotic checkpoint proteins onto unattached kinetochores is not affected in the presence of N-APC. 3) N-APC does not affect Mad1-Mad2 complex formation. Finally, addition of extra Mad2 to Xenopus egg extracts supplemented with N-APC restores the mitotic checkpoint.
Figure 7.
Schematic of impairing the mitotic checkpoint by N-APC. (A) Under normal conditions, Mad1-Mad2 complex is recruited onto unattached kinetochores. A pool of free Mad2 ensures the quick dynamic association and dissociation between Mad1-Mad2 complex and another Mad2 molecule to produce active form of Mad2, the wait anaphase signal. (B) The interaction between N-APC and Mad2 reduces the pool of free Mad2, so that less Mad2 is available to associate with Mad1-Mad2 complex at kinetochores, resulting in a decrease of mitotic checkpoint strength on each unattached kinetochores.
APC depletion in HeLa cells induces a delayed anaphase onset, which is abolished by codepleting of Mad2 (Draviam et al., 2006). This result suggests that APC depletion does not affect a functional mitotic checkpoint. Kinetochore recruitment of Mad2 and BubR1 is also not affected in APC-depleted cells (Draviam et al., 2006) and Xenopus egg extracts (Zhang et al., 2007). Indeed, the mitotic checkpoint is normally activated in APC-depleted egg extracts (Figure 1C, row c). Although it seems APC depletion strengthens the effect of N-APC addition on the mitotic checkpoint activation (Figure 1C, compare rows d and f), it may be simply because depletion of APC frees more N-APC, which originally bind to APC through the N-terminal oligomerization domain (Joslyn et al., 1993; Su et al., 1993), to bind to Mad2.
It has been shown that expressing N-terminal APC fragments in cells causes a mitotic checkpoint defect (Tighe et al., 2004). We now show N-APC, but not full-length APC, interacts with Mad2 in Xenopus egg extracts, cultured CIN colon cancer cells (but not MIN cells), and in vitro with purified recombinant proteins, providing a molecular mechanism for how N-APC can dominantly affect the mitotic checkpoint. The possible reason why only N-APC is capable of interacting with Mad2 may reside in the structure of APC. It has been shown that N-APC interacts with the extreme C-terminal 300 amino acids (Li et al., 2008), which may prevent full-length APC from binding to Mad2.
Although addition of N-APC in Xenopus egg extracts seems to abolish the mitotic checkpoint, it only weakens the checkpoint in human cells, resulting in premature anaphase onset with one or two unattached chromosomes. This may just be an underlying difference in mitotic checkpoint strength between the egg extracts and mammalian cultured cells. It is known that Xenopus egg extracts produce a weak mitotic checkpoint that >9000 sperm head nuclei/μl are needed to activate the checkpoint (Murray, 1991). Indeed, immunodepletion of the kinetochore-associated microtubule motor CENP-E or addition of anti-CENP-E antibody to Xenopus egg extracts completely inhibited the mitotic checkpoint (Abrieu et al., 2000), whereas without CENP-E, the mammalian cultured cells (Weaver et al., 2003) or mice (Putkey et al., 2002) entered anaphase with one or a few unattached kinetochores, resulting in aneuploidy.
Our live-cell imaging analysis confirmed that N-APC dominantly affects kinetochore microtubule attachments (Green and Kaplan, 2003; Tighe et al., 2004). Cells with expression of N-APC exhibited transient delay in prometaphase with a high degree of continued chromosome misalignment. Thus, a large number of unattached and misattached chromosomes are able to generate a high overall wait anaphase inhibitor, despite that each kinetochore generates a reduced checkpoint signal. However, when there is only one or a few unattached chromosomes existing, the N-APC\Nexpressing cells with a weakened mitotic checkpoint can no longer be arrested in mitosis, resulting in a premature anaphase onset with missegregated chromosomes, which may induce CIN phenotype. Thus, our findings revealed a direct molecular support for linking the tumor-associated APC mutations to the generation of CIN in human colon cancer cells.
ACKNOWLEDGMENTS
We thank all members of the Mao laboratory for stimulating discussion. This work has been supported by a start-up fund from the Department of Pathology and Cell Biology, Columbia University College of Physicians and Surgeons, and an American Cancer Society Research Scholar grant (RSG-09-027-01-CCG) (to Y. M.). Y. M. is a Basil O'Connor Starter Scholar supported by the March of Dimes Birth Defects Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-12-1206) on March 4, 2009.
REFERENCES
- Abrieu A., Kahana J. A., Wood K. W., Cleveland D. W. CENP-E as an essential component of the mitotic checkpoint in vitro. Cell. 2000;102:817–826. doi: 10.1016/s0092-8674(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Bomont P., Maddox P., Shah J. V., Desai A. B., Cleveland D. W. Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 2005;24:3927–3939. doi: 10.1038/sj.emboj.7600848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill D. P., Lengauer C., Yu J., Riggins G. J., Willson J. K., Markowitz S. D., Kinzler K. W., Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;92:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Chen R.-H. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 2002;158:487–496. doi: 10.1083/jcb.200204048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Waters J. C., Salmon E. D., Murray A. W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Chung E., Chen R. H. Spindle checkpoint requires Mad1-bound and Mad1-free Mad2. Mol. Biol. Cell. 2002;13:1501–1511. doi: 10.1091/mbc.02-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Mao Y., Sullivan K. F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O., Peters J. M., Kirschner M. W., Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- De Antoni A., et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 2005;15:214–225. doi: 10.1016/j.cub.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Draviam V. M., Shapiro I., Aldridge B., Sorger P. K. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 2006;25:2814–2827. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G., Yu H., Kirschner M. W. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- Fodde R., et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Gascoigne K. E., Taylor S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Green R. A., Kaplan K. B. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 2003;163:949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. A., Wollman R., Kaplan K. B. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol. Biol. Cell. 2005;16:4609–4622. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Totis L., Roberts B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Joslyn G., Richardson D. S., White R., Alber T. Dimer formation by an N-terminal coiled coil in the APC protein. Proc. Natl. Acad. Sci. USA. 1993;90:11109–11113. doi: 10.1073/pnas.90.23.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M., Weinstein J., Daum J. R., Burke D. J., Gorbsky G. J. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J. Cell Biol. 1998;141:1393–1406. doi: 10.1083/jcb.141.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K. B., Burds A. A., Swedlow J. R., Bekir S. S., Sorger P. K., Nathke I. S. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat. Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Kasai T., Iwanaga Y., Iha H., Jeang K. T. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J. Biol. Chem. 2002;277:5187–5193. doi: 10.1074/jbc.M110295200. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Li R., Murray A. W. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Li Z., Kroboth K., Newton I. P., Nathke I. S. Novel self-association of the APC molecule affects APC clusters and cell migration. J. Cell Sci. 2008;121:1916–1925. doi: 10.1242/jcs.029470. [DOI] [PubMed] [Google Scholar]
- Luo X., Tang Z., Xia G., Wassmann K., Matsumoto T., Rizo J., Yu H. The Mad2 spindle checkpoint protein has two distinct natively folded states. Nat. Struct. Mol. Biol. 2004;1:338–345. doi: 10.1038/nsmb748. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Putkey F. R., Cramer T., Morphew M. K., Silk A. D., Johnson R. S., McIntosh J. R., Cleveland D. W. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev. Cell. 2002;3:351–365. doi: 10.1016/s1534-5807(02)00255-1. [DOI] [PubMed] [Google Scholar]
- Raff J. W., Jeffers K., Huang J. Y. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J. Cell Biol. 2002;157:1139–1149. doi: 10.1083/jcb.200203035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. J., Lamlum H., Ilyas M., Wheeler J., Straub J., Papadopoulou A., Bicknell D., Bodmer W. F., Tomlinson I. P. APC mutations in sporadic colorectal tumors: a mutational “hotspot” and interdependence of the “two hits”. Proc. Natl. Acad. Sci. USA. 2000;97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. V., Botvinick E., Bonday Z., Furnari F., Berns M., Cleveland D. W. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 2004;14:942–952. doi: 10.1016/j.cub.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Su L. K., Johnson K. A., Smith K. J., Hill D. E., Vogelstein B., Kinzler K. W. Association between wild type and mutant APC gene products. Cancer Res. 1993;53:2728–2731. [PubMed] [Google Scholar]
- Sudakin V., Chan G. K., Yen T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. L., Compton D. A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe A., Johnson V. L., Albertella M., Taylor S. S. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2001;2:609–614. doi: 10.1093/embo-reports/kve127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe A., Johnson V. L., Taylor S. S. Truncating APC mutations have dominant effects on proliferationspindle checkpoint control, survival and chromosome stability. J. Cell Sci. 2004;117:6339–6353. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- Vink M., et al. In vitro FRAP identifies the minimal requirements for Mad2 kinetochore dynamics. Curr. Biol. 2006;16:755–766. doi: 10.1016/j.cub.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Weaver B. A., Bonday Z. Q., Putkey F. R., Kops G. J., Silk A. D., Cleveland D. W. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J. Cell Biol. 2003;162:551–563. doi: 10.1083/jcb.200303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver B. A., Cleveland D. W. Does aneuploidy cause cancer? Curr. Opin. Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Weiss E., Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ahmad S., Mao Y. BubR1 and APC/EB1 cooperate to maintain metaphase chromosome alignment. J. Cell Biol. 2007;178:773–784. doi: 10.1083/jcb.200702138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., McGarry T. J., Bernal T., Kirschner M. W. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- Zur A., Brandeis M. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 2001;20:792–801. doi: 10.1093/emboj/20.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]