Abstract
Hepatocyte growth factor/scatter factor (HGF/SF) acts through the membrane-anchored Met receptor tyrosine kinase to induce invasive growth. Deregulation of this signaling is associated with tumorigenesis and involves, in most cases, overexpression of the receptor. We demonstrate that Met is processed in epithelial cells by presenilin-dependent regulated intramembrane proteolysis (PS-RIP) independently of ligand stimulation. The proteolytic process involves sequential cleavage by metalloproteases and the γ-secretase complex, leading to generation of labile fragments. In normal epithelial cells, although expression of cleavable Met by PS-RIP is down-regulated, uncleavable Met displayed membrane accumulation and induced ligand-independent motility and morphogenesis. Inversely, in transformed cells, the Met inhibitory antibody DN30 is able to promote Met PS-RIP, resulting in down-regulation of the receptor and inhibition of the Met-dependent invasive growth. This demonstrates the original involvement of a proteolytic process in degradation of the Met receptor implicated in negative regulation of invasive growth.
INTRODUCTION
The Met receptor tyrosine kinase is expressed predominantly in cells of epithelial origin and is activated by its stromal ligand, the hepatocyte growth factor/scatter factor (HGF/SF). Met activation stimulates proliferation, scattering, invasion, morphogenesis, and survival of epithelial cells. The ligand-stimulated Met receptor furthermore acts as an angiogenic factor in endothelial cells and has chemoattractant and neurotrophic activities in various types of neurons (Birchmeieret al.,2003). The biological program triggered by ligand-stimulated Met has been named invasive growth and instructs cells to dissociate, migrate, degrade the surrounding matrix, proliferate, and survive (Comoglioet al.,2008). Targeted disruption of either thehgf or themet gene highlights the essential role of the HGF/SF-Met system during development of the placenta, liver, muscles, and neurons (Bladtet al.,1995; Schmidtet al.,1995; Ueharaet al.,1995; Mainaet al.,1997). HGF/SF and Met also play a role in adults in regulating mammary gland development (Yantet al.,1998) and renal (Kawaidaet al.,1994) or liver regeneration (Borowiaket al.,2004).
Met is synthesized from a single-chain precursor that undergoes posttranslational glycosylation and endoproteolytic cleavage to produce the mature heterodimeric membrane form (Giordanoet al.,1989). Mature Met is a type I transmembrane protein composed of an entirely extracellular 45-kDa α subunit disulfide-linked to a 145-kDa β subunit shared between the extra- and intracellular compartments and containing the catalytic domain (Giordanoet al.,1989). On ligand binding and subsequent dimerization of Met, several tyrosine residues in the intracellular region of the β subunit become phosphorylated. Within the tyrosine kinase domain, two tyrosines are the major autophosphorylation sites, and mutation of these residues abolishes the biological activity (Longatiet al.,1994). Outside the kinase domain, two autophosphorylation sites in the C-terminal region are responsible for recruitment of several proteins involved in initiation of intracellular signaling (Ponzettoet al.,1994).
Aberrant Met and HGF/SF signaling is involved in promoting tumorigenesis and metastasis. In fact, the Met receptor was originally identified as an oncogene resulting from a chromosomal rearrangement and giving rise to a product where the dimerization domain of the translocated promoter region (TPR) is fused with the intracellular region of the Met receptor (Parket al.,1986). A direct link between Met and cancer was later evidenced by characterization of receptor-activating mutations in hereditary papillary renal carcinoma (Schmidtet al., 1997). Most often, activation of Met in cancer occurs through ligand-dependent stimulation, induced by uncontrolled expression of HGF/SF and/or Met, leading to autocrine or paracrine activation (Birchmeieret al.,2003). In addition, aberrant Met activation is induced by overexpression of the receptor without HGF/SF engagement: ligand-independent activation of receptor tyrosine kinases is typically observed in cells expressing high levels of receptor, leading to spontaneous dimerization and subsequent activation (Ponzettoet al.,1991). Overexpression of Met is a consequence of various mechanisms such as gene amplification as in colorectal cancer (Di Renzoet al.,1995), increased transcription induced by oncogenes (Gambarottaet al.,1996; Ivanet al.,1997), or hypoxia (Pennacchiettiet al.,2003). Interference with Met activation appears as a challenging approach to hampering tumorigenic and metastatic processes mediated by Met and/or HGF/SF overexpression (Migliore and Giordano, 2008).
Down-regulation of the HGF/SF-activated receptor is an essential negative regulatory mechanism preventing receptor oversignaling. This down-regulation occurs through recruitment of the ubiquitin ligase c-Cbl, which promotes receptor ubiquitination and subsequent degradation (Petrelliet al.,2002; Peschardet al.,2004). Uncoupling of Met from c-Cbl–mediated ubiquitination, either through loss of the juxtamembrane domain, as in the oncogene TPR-Met, or by mutation of the tyrosine residue serving as a Cbl dock leads to cell transformation (Peschardet al.,2001; Maket al.,2007).
Down-regulation of Met signaling also involves proteolytic cleavages. We have previously demonstrated that Met is cleaved by caspases, which separate the extracellular ligand-binding domain from the intracellular kinase domain. In addition to abolishing the ligand responsiveness of Met, these cleavages generate a cytoplasmic proapoptotic fragment named p40 Met, involved in apoptosis amplification (Tulasneet al.,2004; Foveauet al.,2007; Deheunincket al.,2008; Tulasne and Foveau, 2008). Many other fragments of Met have been described, but their biological functions, and the mechanisms involved in their generation have not been investigated. Examples include an extracellular fragment of Met, released upon ectodomain shedding into the culture supernatant (Pratet al.,1991; Galvaniet al.,1995; Wajihet al.,2002), and a labile 55-kDa intracellular fragment of Met produced in epithelial cells (Jefferset al.,1997).
In the present article we reveal sequential proteolytic cleavages of the full-length Met receptor by the presenilin-dependent regulated intramembrane proteolysis (PS-RIP). We demonstrate that PS-RIP is able to down-regulate Met receptor independently of ligand stimulation and as a consequence down-regulates Met-induced invasive growth.
MATERIALS AND METHODS
Cytokines, Drugs, and Cell Cultures
Human recombinant HGF/SF was purchased from Peprotech (Rocky Hill, NJ). Nerve growth factor (NGF) was purchased from R&D Systems (Minneapolis, MN). The proteasome inhibitors MG132 and ALLN were purchased from Calbiochem (San Diego, CA). The proteasome inhibitor lactacystin and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma (St. Louis, MO). The γ-secretase inhibitor E compound was purchased from Alexis/Coger (Lausen, Switzerland). The γ-secretase inhibitors L-685 458 (inhibitor X), DAPT and the γ-secretase inhibitor VI were purchased from Calbiochem. The matrix metalloproteinase inhibitors GM6001 and TAPI-1 were purchased respectively from Chemicon (Temecula, CA) and Calbiochem. The inhibitor of protein biosynthesis cycloheximide (CHX) was purchased from ICN Biomedicals (Costa Mesa, CA). The Met kinase inhibitor SU11274 was purchased from Calbiochem.
Madin-Darby canine kidney (MDCK) epithelial cells, epithelial cells from cervix carcinoma Henrietta Lacks (HeLa), human mammary adenocarcinoma cells MDA-MB231, and human gastric carcinoma cell line GTL16 cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS, Invitrogen) and antibiotics. MCF-10A, human mammary epithelial cells, spontaneously immortalized, were cultured in DMEM and HAM’s F12 (Invitrogen; vol/vol) supplemented with 5% horse serum (HS, Invitrogen), 500 ng/ml hydrocortisone (Calbiochem), 20 ng/ml epidermal growth factor (Peprotech), 10 μg/ml insulin (Sigma), and 100 ng/ml cholera toxin (Calbiochem). Murine embryonic fibroblast cells (MEFs), WT or deficient for presenilin 1 and/or presenilin 2, (PS1 and or PS2) were kindly provided by Paul Saftig (Christian-Albrechts-Universität, Germany) and Bart deStrooper (VIB, the Flanders Institute for Biotechnology, Belgium) and were cultured in DMEM (Invitrogen) supplemented with 10% FCS and antibiotics. Cells were cultured at 37°C in a water-saturated 5% CO2 atmosphere.
Antibodies
Mouse mAb directed against mouse Met (B-2) and rabbit polyclonal antibody directed against C-terminal region of human Met (C-12) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal antibodies 25H2 and 3D4 directed against the kinase domain of Met were purchased respectively from Cell Signaling Technology (Danvers, MA) and Zymed Laboratories (South San Francisco, CA). Antibodies directed against extracellular region of Met (clones DL-21 and DO-24) were purchased from Upstate Biotechnology (Lake Placid, NY). mAb against extracellular region of Met, used in flow cytometry, was purchased from R&D Systems. Antibody directed against phosphorylated tyrosine of the Met kinase domain was purchased from BioSource (Camarillo, CA). Rabbit polyclonal antibody against ADAM-17 was purchased from Millipore (Billerica, MA). Antibodies directed against ERK2 and β-actin were purchased from Santa Cruz Biotechnology. Antibody directed against phosphorylated extracellular signal–regulated kinase (ERK) was purchased from Cell Signaling Technology. Mouse mAb directed against extracellular domain of Met was previously described (Petrelliet al.,2006). Peroxydase, fluorescein, and rhodamine-conjugated antibodies directed against rabbit and mouse IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Plasmid Constructions
The TRK-Met chimera expressing vector was constructed as follows. The original TRK-Met chimera cloned in pBAT vector, which allows expression of extracellular TRKA receptor fused to the transmembrane and cytoplasmic region of mouse Met (Weidneret al.,1995), was cloned in pcDNA 3.1 plasmid (Invitrogen). The TRK sequence, up to the BamHI restriction site, was cut out by digestion using HindIII and BamHI restriction enzymes and cloned into pcDNA 3.1 plasmid. The remaining TRK-Met sequence, from the BamHI restriction site to the 3′ end, was amplified by PCR using pBAT TRK-Met as a template and the following primers: 5′ GCAGGCCGGCTGGATCCTCACAGAGCTGG 3′ containing the BamHI restriction site and 5′ GGTGGGCCTCTCGAGCATCATGTGTTCC 3′ containing the EcoRI restriction site. The PCR product was subcloned into a pGEM plasmid (pGEM Easy kit; Stratagene, La Jolla, CA). Finally, the remaining TRK-Met sequence was inserted in pcDNA3 TRK using BamHI and XhoI restriction sites. The TRK-Met-juxta, in which 50 amino acids of the extracellular juxtamembrane domain of mouse Met were added, was constructed as follows. The mouse Met sequence was amplified by PCR using pMB11 mouse Met as template (kindly provided by Dr. G. Vande Woude, Van Andel Research Institute, Grand Rapids, MI) and the following primers: 5′ AAAATGTACTGGAATTCAAGGGAAATGATA 3′ containing the EcoRI restriction site and 5′ GCCTCTCGAGCATCATGTGTTCCCCTC 3′ containing the XhoI restriction site. The PCR product was inserted in the pcDNA 3.1 containing the TRKA sequence using EcoRI and XhoI restriction sites.
The full-length human Met expressing vector was constructed as follows. The human Met sequence WT and K1108A (Kinase dead), previously described (Foveauet al.,2007), were obtained by cutting out by digestion using EcoRI restriction enzyme from PRS2 hu Met (kindly provided by Dr G. Vande Woude) and cloned into pCAGGS vector (kindly provided by Dr P. Mehlen, Centre Léon Bérard, Lyon, France).
Western Blotting, Immunofluorescence, and Immunoprecipitation
Western blotting and immunofluorescence were performed as previously described (Foveauet al.,2007). Results are representative of at least three experiments. For F-actin staining, cells were incubated with phalloidin-FITC (Molecular Probes, Eugene, OR). Cell nuclei were counterstained using Hoechst 33258. Cover slips were mounted with Glycergel mounting medium (Dako, Carpenteria, CA), and fluorescence was examined using a Zeiss Axio Imager Z1 (Thornwood, NY). For quantification of protein expression, luminescence was captured with a digital imaging using a cooled charge coupled device (CCD) camera (LAS 3000, Fuji, Tokyo, Japan), and quantification was performed using Multigauge V3.0 software. The background adjusted volume was normalized to empty well. For immunoprecipitation, cell supernatants were clarified by centrifugation (10 min, 20,800 ×g) and precleared for 30 min at 4°C with protein A Sepharose 4B (GE Healthcare, Waukesha, WI). After centrifugation to remove beads (10 min, 20,800 ×g, 4°C), the supernatant was incubated with the indicated antibody at 4°C overnight. The immune complex was collected with protein A Sepharose 4B at 4°C for 1 h. The beads were washed four times with lysis buffer and boiled in Laemmli sample buffer.
Transfections
Transient transfections of MDCK cells were performed as previously described using the lipofection method (Tulasneet al.,1999). For stable transfections of MDCK cells, 2 d after transfection, cells were split into four 100-mm dishes containing DMEM-10% FCS, and the next day the medium was supplemented with 800 μg/ml G418 (Invitrogen). Resistant clones were isolated after 10 d, and clones expressing the transfected constructs were selected by Western blot.
Small Interfering RNA
MCF10A cells were cultured (2 × 105 cells/well in six-well plates) in DMEM-10% FCS. The next day, cells were incubated for 6 h in serum-free OptiMEM, 5 μl transfectant (Lipofectamine 2000; Invitrogen), and 50 nM small interfering RNA (siRNA; Invitrogen) targeting the ADAM-17 (sequence 1: 5′-CCAGGGAGGGAAAUAUGUCAUGUAU-3′ and sequence 2: 5′-GAGGAAAGGAAAGCCCUGUACAGUA-3′) or a random sequence. The cells were rinsed in DMEM-10% FCS before further treatment.
Scattering Assay
Scattering from cell islets was performed as follows. MDCK cells (6 × 104 cells/well) were seeded onto six-well plates and cultured for 24 h in DMEM and 10% FCS, with or without 10 ng/ml HGF/SF or 100 ng/ml NGF. At the end of the experiments, cells were fixed and stained with Carazzi’s hematoxylin and eosin, and their morphologies were examined by light microscopy.
Morphogenesis Assay
MDCK cells (4 × 104 cells/well) were plated on a layer of 300 μl of Matrigel (Becton Dickinson, Franklin Lakes, NJ) in 24-well plates in DMEM-10% FCS. The next day, cultures were treated or not with HGF/SF for 24 h. At the end of the experiments, cells were stained 10 min at 37°C with neutral red (0.5% wt/vol) and fixed with 4% paraformaldehyde, and their morphologies were examined by light microscopy.
Migration Assay
MDCK cells (1 × 105 cells/wells), treated or not with SU11274, were seeded in triplicates in invasion chambers (Transwell, BD Biosciences, San Jose, CA). The next day, HGF/SF or NGF were added in the lower compartment of the chamber. After 48 h, the filters were removed, and the cells on the lower surface of the filter were stained with Hoechst. The total number of cells from five microscopic fields at 10× magnification was counted from each filter.
Flow Cytometric Analysis
Cells were collected and washed in ice-cold PBS, 1% FCS. Cells were then incubated for 1 h with 25 ng/ml phycoerythrin-coupled antibody directed against the TRKA (R&D Systems) or 2.5 μg/ml antibody directed against extracellular domain of Met followed by incubation with 7.5 μg/ml fluorescein-conjugated anti-mouse IgG. Cells were analyzed by flow cytometry using an EPICS XL-MCL Coulter (Beckman, Fullerton, CA). Data were recorded with Expo II software and analyzed with Win-MDI 2.9 software (Windows Multiple Document Interface for Flow Cytometry, The Scripps Research Institute).
RESULTS
Proteasome Inhibitors Stabilize Met Fragments
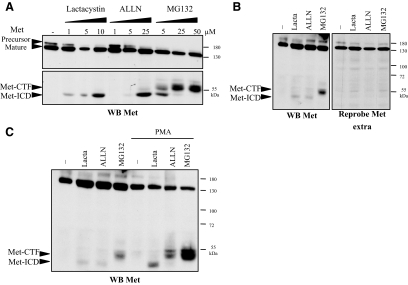
To observe potential labile degradation fragments of Met, MDCK epithelial cells were treated with various proteasome or lysosome inhibitors, including lactacystin, ALLN, MG132, and concanamycin A, and Western blot analyses were performed with an anti-Met antibody directed against the intracellular kinase domain (Figure 1A). After lactacystin treatment, we detected a 50-kDa fragment in addition to the full-length receptor. Similarly, ALLN treatment allowed detection of this 50-kDa fragment, and at higher concentration an additional 55-kDa fragment was observed as a doublet. After MG132 treatment, we detected the 50- and 55-kDa fragments, the 55-kDa band gaining in intensity at the expense of the 50-kDa at high doses. No Met fragments were detected after treatment with the lysosome inhibitor concanamycin A (data not shown). Results consistent with these findings were obtained with HeLa epithelial cells, where the 50- and 55-kDa fragments of Met were detected with antibody directed against the kinase domain. No fragments were detected with antibody directed against the extracellular domain of Met (Figure 1B), which shows that the observed fragments are intracellular C-terminal fragments. When MDCK cells were cotreated with PMA plus the proteasome inhibitor lactacystin, ALLN, or MG132, the levels of both 50- and 55-kDa fragments increased, demonstrating that their generation can be stimulated by PMA, a common inducer of membrane receptor cleavages (Figure 1C). Thus, proteasome inhibitors stabilize fragments of Met detected at ∼55- and 50-kDa, which we named Met-CTF (C-terminal fragment) and Met-ICD (intracellular domain), respectively.
Figure 1.
Stabilization of Met fragments by proteasome inhibitors. (A) MDCK epithelial cells were treated for 5 h with increasing concentrations of lactacystin or for 10 h with increasing concentrations of ALLN or MG132. (B) HeLa cells were treated for 5 h with lactacystin or for 10 h with ALLN or MG132. (C) HeLa cells were treated for 5 h with lactacystin or for 10 h with ALLN or MG132 and for 45 min with 100 ng/ml PMA. (A–C) For each condition, the same amount of protein was resolved by 10% SDS-PAGE and analyzed by Western blotting with antibodies against the kinase domain of Met (WB Met) or against the extracellular domain of Met (WB Met extra). The positions of prestained molecular weight markers are indicated. Arrows indicate positions of precursor and mature full-length Met, Met-CTF, and Met-ICD.
γ-Secretase Converts Met-CTF to Met-ICD
On the basis of the sizes of these fragments, we estimated that the relevant cleavages of full-length Met were likely to occur near the extracellular juxtamembrane domain for generation of Met-CTF and near the transmembrane domain for Met-ICD. These putative cleavage sites are consistent with shedding and γ-secretase cleavages observed for other type I membrane receptors. Extracellular shedding within the extracellular juxtamembrane region of several type I transmembrane proteins has been shown to be the initial step of a more complex proteolytic process named PS-RIP (Landman and Kim, 2004). In most cases, shedding involves metalloproteases of the ADAM (a disintegrin and metalloprotease) family, generating an N-terminal fragment (NTF) released into the extracellular space and a membrane-anchored C-terminal fragment (CTF). This CTF is further cleaved by the γ-secretase complex (of which presenilin is the catalytic subunit), releasing a fragment containing the ICD (De Strooper, 2003).
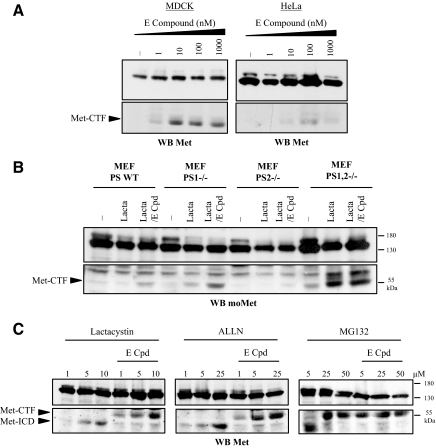
To address these hypotheses, we treated MDCK and HeLa epithelial cells with increasing concentrations of E compound, a γ-secretase inhibitor (Figure 2A). We found inhibition of γ-secretase to stabilize the Met-CTF fragment (55 kDa) in a dose-dependent manner. These results suggest that Met-CTF is an intermediate fragment that is further cleaved by γ-secretase. Cellular γ-secretase activity requires expression of PS1 and/or PS2 (Herremanet al.,2000). To assess processing of Met-CTF by γ-secretase, we compared MEFs from wild-type (WT) and PS1-, PS2-, and PS1,2-knockout mice. In the absence of any treatment, cells from both wild-type and single-knockout animals failed to yield any detectable Met-CTF fragment. As expected, treatment by E compound in combination with proteasome inhibitors allowed stabilization of this fragment (Figure 2B). It is worth noticing that lactacystin did not stabilize Met-ICD in MEF, suggesting alternate mechanism of degradation of this fragment in these cells. In contrast, cells from PS1,2-knockout mice showed a detectable level of Met-CTF even in the absence of any treatment. Lactacystin stabilized further this Met-CTF but E compound had no effect. These results demonstrate that both PS1 and PS2 of the γ-secretase complex are involved in cleavage of Met-CTF. In addition, the generated Met-CTF is also labile through a proteosomal degradation.
Figure 2.
γ-Secretase inhibition prevents the Met-CTF-to-Met-ICD transition. (A) MDCK and HeLa cells were treated or not overnight with increasing concentrations of E compound. (B) Wild-type mouse embryonic fibroblasts (MEF PS WT), presenilin 1–deficient MEF (PS1−/−), presenilin 2–deficient MEF (PS2 −/−), and presenilin 1– and 2–deficient MEF (PS1,2 −/−) were treated or not overnight with 1 μM E compound (E-Cpd). The following day, the cells were treated or not for 5 h with 10 μM lactacystin. (C) MDCK epithelial cells were treated or not overnight with 1 μM E compound. The following day the cells were treated for 5 h with increasing concentrations of lactacystin or for 10 h with increasing concentrations of ALLN or MG132. (A–C) For each condition, the same amount of protein was resolved by 10% SDS-PAGE and analyzed by Western blotting with an antibody directed against the kinase domain of Met (WB Met) or the C-terminal domain of mouse Met for the Western blot produced with MEF extracts (WB moMet). Arrows indicate positions of Met-CTF and Met-ICD.
We next examined the effect of cotreating MDCK cells with a proteasome inhibitor (lactacystin, ALLN, or MG132) and E compound. Met-ICD (50 kDa) was visualized in cells treated with increasing doses of proteasome inhibitor (Figure 2C). Interestingly, cotreatment with E compound abolished the release of Met-ICD, causing concomitant accumulation of Met-CTF. This suggests that Met-CTF is converted to Met-ICD upon γ-secretase cleavage. The MG132 at high concentration induced an effect similar to that caused by cotreatment of MDCK cells with lactacystin and E compound. This may be attributable to inhibition of γ-secretase activity by MG132, as previously reported (Zhanget al.,1999). Similar results were obtained when MDCK cells were cotreated with the proteasome inhibitor lactacystin and two other γ-secretase inhibitors L-685 458 and DAPT (Supplementary Data, Supplementary Figure S1). Taken together, these results suggest that Met-CTF is cleaved by γ-secretase to generate Met-ICD, both Met fragments being sensitive to proteasome degradation.
Metalloprotease-mediated Shedding of Met Is a Prerequisite for γ-Secretase Cleavage
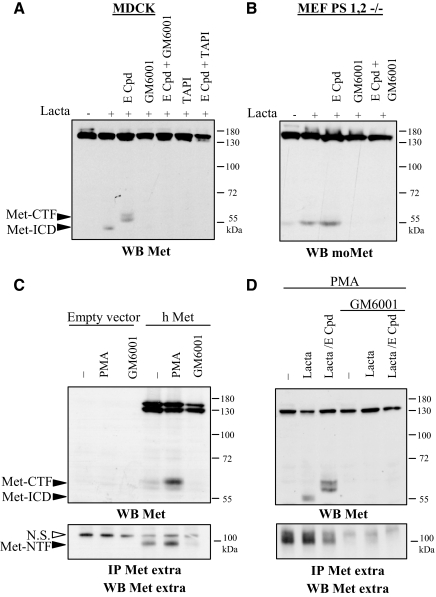
In most cases, presenilin cleavages are preceded by ectodomain shedding, notably involving proteases of the ADAM family. We checked the effect of metalloprotease inhibitors on generation of Met fragments. Inhibition of zinc metalloprotease activity by the broad-spectrum inhibitor GM6001 (Galardyet al.,1994) or the ADAM inhibitor TAPI-1 (Slacket al.,2001) prevented stabilization of both Met-ICD and Met-CTF induced in MDCK cells by E compound and lactacystin (Figure 3A). Similar inhibition of Met-CTF generation by TAPI-1 was observed in human mammary epithelial cells MCF10A (Supplementary Figure S2). Inhibition of metalloprotease activity likewise prevented Met-CTF generation in PS1,2-deficient MEF, which normally accumulate this fragment, particularly upon lactacystin treatment (Figure 3B).
Figure 3.
Metalloprotease inhibitors prevent generation of both Met-CTF and Met-ICD. (A) MDCK cells were left untreated (−) or were treated overnight with 1 μM E compound and/or 25 μM GM6001 and 50 μM TAPI-1. The following day the cells were treated for 5 h with 10 μM lactacystin. (B) MEF PS1,2 −/− were left untreated or treated overnight with 1 μM E compound and/or 25 μM GM6001. The following day the cells were treated for 5 h with 10 μM lactacystin. (C) MDCK cells transiently transfected with either the empty vector or a vector expressing human Met (h Met) were treated or not overnight with 25 μM GM6001. The following day, the cells were treated for 45 min with 100 ng/ml PMA. The culture medium was collected and immunoprecipitation was performed with an antibody directed against the extracellular domain of human Met. (D) HeLa cells were treated or not overnight with 1 μM E compound and/or 25 μM GM6001. The following day, the cells were treated for 5 h with 10 μM lactacystin and for 45 min with 100 ng/ml PMA. The culture medium was collected, and immunoprecipitation was performed with an antibody directed against the extracellular domain of human Met. (A–D) For each condition, the same amount of protein was resolved by 10% SDS-PAGE or 7% SDS-PAGE for IP and analyzed by Western blotting with different anti-Met antibodies as indicated. Arrows indicate positions of Met-CTF, Met-ICD, and Met-NTF.
Ectodomain shedding could generate, in addition to Met-CTF, a soluble 90-kDa Met-NTF. To test for the generation of extracellular fragments, we transiently transfected MDCK cells with a construct encoding human Met and then used an antibody against the extracellular part of human Met to immunoprecipitate Met-NTF from the culture supernatant, before detection by Western blotting. Detection of Met-NTF was found to increase with PMA treatment and to disappear with GM6001 treatment (Figure 3C). Similarly, in the culture medium of HeLa cells treated with PMA to promote PS-RIP, we detected soluble Met-NTF. In addition, Met-CTF and Met-ICD were detected in the cell lysate upon treatment with γ-secretase and proteasome inhibitor, respectively (Figure 3D). Inhibition of metalloprotease activity by GM6001 prevented generation of both intracellular fragments Met-ICD and -CTF and of the extracellular fragment Met-NTF. This suggests the involvement of metalloprotease in an initial step of shedding, necessary for subsequent γ-secretase cleavage.
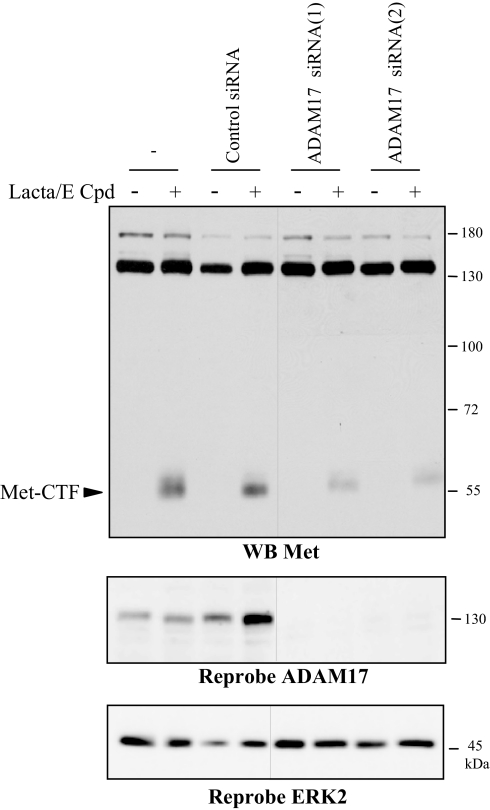
In most known cases of metalloprotease-dependent shedding followed by γ-secretase proteolysis, ADAM-17 and ADAM-10 are involved (Huovilaet al.,2005). To check the involvement of these proteases in Met shedding, we used siRNA to knock down their expression in human epithelial cells MCF10A. Silencing of ADAM-17 partially inhibited generation of Met-CTF, indicating that ADAM-17 participates to Met processing (Figure 4). In contrast, silencing of ADAM-10 did not inhibit Met cleavages (data not shown).
Figure 4.
ADAM-17 is involved in Met shedding. MCF10A cells were transfected or not with siRNA control or targeting the ADAM-17. Cells were then treated or not overnight with 1 μM E compound and the following day 5 h with 10 μM lactacystin. Proteins were resolved by 10% SDS-PAGE and analyzed by Western blotting with antibodies directed against the kinase domain of Met (WB Met). The filter was stripped and reprobed with an antibody directed against the ADAM-17 and against ERK2 to assess the loading. The two parts of the panels were on the same gel. Arrows indicate the position of Met-CTF.
Taken together, our results demonstrate that metalloprotease-mediated shedding from Met generates both a N-terminal fragment Met-NTF and a C-terminal fragment Met-CTF, which is in turn cleaved by γ-secretase to generate the unstable Met-ICD. Furthermore, Met-NTF is released into the extracellular medium, suggesting that the ectodomain shedding of Met targets membrane-anchored receptor.
Sequential Proteolysis of Met Is Independent of Kinase Activation
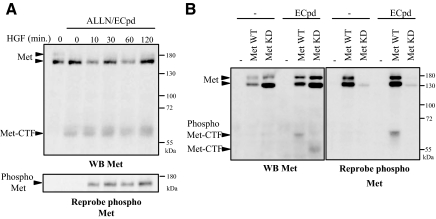
PS-RIP of several type I membrane receptors such as Notch and ErbB4 is regulated by ligand stimulation (Brouet al.,2000; Mummet al.,2000; Niet al.,2001). Therefore, we cotreated MDCK cells with a proteasome and γ-secretase inhibitors to stabilize Met-CTF and stimulated the cells with HGF/SF (Figure 5A). HGF/SF stimulation induced tyrosine phosphorylation of Met. However, as we did not observe any modulation of Met-CTF generation in response to HGF/SF over the test period, it appears that ligand activation of Met is not involved in this process. Furthermore, in MDCK cells transiently transfected with a construct encoding either wild-type or kinase-dead full-length Met (Met WT and Met kinase dead, respectively), γ-secretase inhibition was found to allow stabilization of Met-CTF generated from either full-length proteins (Figure 5B). As expected, antibody against phosphorylated tyrosine residues of Met detected efficiently the Met WT, whose overexpression induced constitutive phosphorylation, in contrast to the Met kinase-dead variant. It is noteworthy that the Met-CTF generated from Met WT migrated more slowly than that generated from Met kinase dead, probably as a result of Met WT phosphorylation. PS-RIP of Met, which seems independent from ligand stimulation and kinase activity, thus contrasts with the main down-regulation mechanism described for Met, e.g., ubiquitin-dependent degradation, which requires HGF/SF stimulation and a proper kinase activity (Peschardet al.,2004).
Figure 5.
HGF/SF does not influence PS-RIP of Met. (A) MDCK cells were treated or not overnight with 1 μM E compound and the following day 10 h with ALLN. Cells were then stimulated with HGF/SF (30 ng/ml) at the indicated time. (B) MDCK cells transiently transfected with either the empty vector or the vector expressing full-length human wild-type Met (Met WT) or kinase-dead Met (Met KD) were treated or not overnight with 1 μM E compound. (A and B) Proteins were resolved by 10% SDS-PAGE and analyzed by Western blotting with antibodies directed against the kinase domain of Met (WB Met). The filter was stripped and reprobed with an antibody directed against the phosphorylated tyrosine residues of the kinase domain of Met. Arrows indicate the positions of Met, Met-CTF and phosphorylated Met and Met-CTF.
Membrane Accumulation of an Uncleavable TRK-Met Chimera
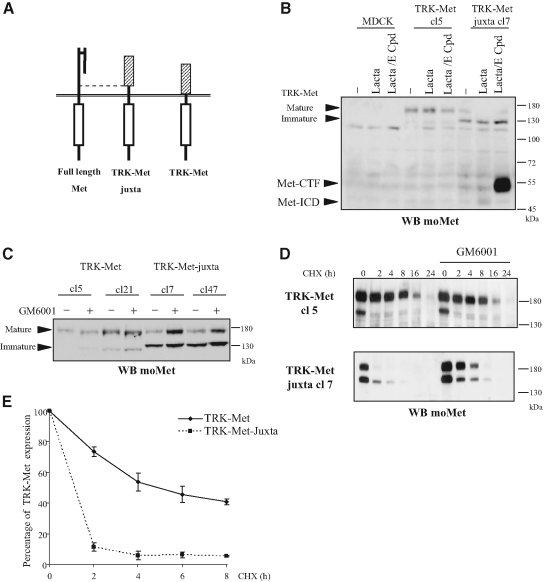
Chimeric receptors, possessing the intracellular region of a studied receptor fused to the extracellular region of another receptor not expressed by the recipient cells, are widespread tools for elucidating the intracellular signaling of RTKs. The advantage is that it is possible to stimulate the chimera without activating the endogenous receptor. Chimeric TRK-Met receptors mediate Met-specific signals in epithelial cells in response to NGF (Tulasneet al.,1999). To investigate further the prerequisites to extracellular cleavage of Met by metalloproteases, we produced constructs encoding the extracellular TRKA domain fused either directly to the transmembrane domain of the Met receptor, so as to replace the entire the extracellular domain (TRK-Met), or to the 50-amino acid extracellular juxtamembrane domain of Met (TRK-Met-juxta; Figure 6A). MDCK epithelial cells were stably transfected with TRK-Met and TRK-Met-juxta, and two clones expressing each construct were analyzed (TRK-Met clones 5 and 21; TRK-Met-juxta clones 7and 47).
Figure 6.
PS-RIP regulates the stability of TRK-Met-juxta. (A) Schematic representation of full-length Met, TRK-Met (consisting of the extracellular portion of TRKA fused to the transmembrane and intracellular domains of Met), and TRK-Met-juxta, possessing 50 additional amino acids of the extracellular juxtamembrane domain of Met. (B) MDCK cells transfected with either the empty vector or the vector expressing TRK-Met (clones 5) or TRK-Met-juxta (clones 7) were treated or not overnight with 1 μM E compound. The following day the cells were treated for 5 h with lactacystin (10 μM) before lysis. (C) MDCK cells stably expressing TRK-Met or TRK-Met-juxta were treated or not overnight with 25 μM GM6001. The following day the cells were lysed. (D) MDCK cells stably expressing TRK-Met or TRK-Met-juxta were treated or not overnight with 25 μM GM6001. The following day the cells were treated with 25 μg/ml CHX for the indicated time. (B–D) Proteins were resolved by 10% SDS-PAGE and analyzed by Western blotting with antibodies directed against the C-terminal domain of mouse Met (WB moMet). Arrows indicate the positions of the mature and immature forms of TRK-Met and TRK-Met-juxta, Met-CTF, and Met-ICD. (E) MDCK cells expressing TRK-Met or TRK-Met-juxta were treated or not with 25 μg/ml CHX for the indicated time. Luminescence from the Western blot was captured with a CCD camera and the expression levels of mature TRK-Met were quantified. The percentage of expression was calculated using the untreated control as the reference (n = 3, ± SD).
In cells expressing TRK-Met-juxta, Met-ICD was weakly detected in lactacystin-treated cells, and cotreatment with lactacystin and E compound generates important amount of Met-CTF, suggesting that γ-secretase cleavage of TRK-Met-juxta is very efficient. In contrast, no fragment was detected in cells expressing TRK-Met (Figure 6B and Supplementary Figure S3). This suggests that the extracellular region of TRK does not allow proper metalloprotease cleavage leading to further γ-secretase cleavage, whereas addition of the juxtamembrane domain of Met rescues the entire proteolytic process.
Another interesting observation was made on the TRK-Met and TRK-Met-juxta profiles (Figure 6B). TRK-Met chimeras are detected as a 180-kDa glycosylated mature form and a 140-kDa nonglycosylated immature form (Tulasneet al.,1999). The mature form was more abundant than the immature form in TRK-Met–expressing cells but much less abundant in TRK-Met-juxta–expressing cells. To assess the effect of PS-RIP on the expression profile, we treated cells stably expressing one or the other chimera with a metalloprotease inhibitor (Figure 6C). On treatment, we observed an increase of the mature form in cells expressing TRK-Met-juxta, whereas expression of the immature form was not significantly modified. In cells expressing TRK-Met, metalloprotease inhibition did not modify the expression profile. Next we checked whether metalloprotease inhibition might affect the receptor half-life. Protein synthesis was inhibited with CHX in cells expressing TRK-Met and TRK-Met-juxta, and receptor expression was measured over time (Figures 6, D and E). Under these conditions, the half-life of TRK-Met was ∼6 h and that of TRK-Met-juxta was <2 h. The matrix metalloprotease inhibitor GM6001 partially restored the stability of the TRK-Met-juxta chimera (Figure 6D). These results demonstrate that PS-RIP is able to down-regulate the expression of the mature receptor.
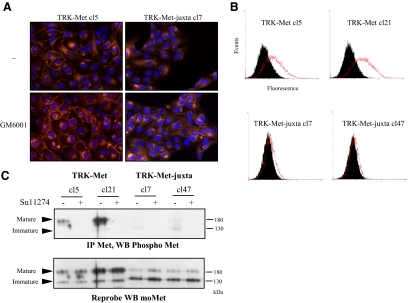
To evaluate subcellular localization of TRK-Met and TRK-Met-juxta, immunofluorescence staining with an antibody recognizing the intracellular domain of mouse Met was performed on MDCK cells expressing one or the other chimera (Figure 7A). When untreated, cells expressing TRK-Met displayed both membrane-localized and perinuclear staining, whereas cells expressing TRK-Met-juxta displayed only perinuclear staining. Perinuclear staining likely corresponds to intracellular maturation steps. Flow cytometry analysis applied to nonpermeabilized cells exposed to an antibody recognizing the extracellular domain of TRKA confirmed the membrane localization of TRK-Met but not TRK-Met-juxta (Figure 7B). Treatment with a metalloprotease inhibitor partially restored expression of TRK-Met-juxta at the plasma membrane, without affecting the localization of TRK-Met (Figure 7A). These observations are consistent with the rescue of the expression of the mature TRK-Met-juxta upon metalloprotease inhibitor treatment. This demonstrates that different expressions between cleavable and uncleavable chimeras are the consequence of their different post transcriptional processing by PS-RIP.
Figure 7.
PS-RIP occurs on the mature TRK-Met chimera at the membrane. (A) MDCK cells stably expressing TRK-Met-juxta or TRK-Met were treated or not overnight with 25 μM GM6001. Nuclei were detected by Hoechst staining (Hoechst, blue staining), and immunofluorescence staining was done with an anti-mouse Met antibody (Anti-mouse Met, red staining). Magnification, ×100. (B) TRK-Met– and TRK-Met-juxta–expressing cells were stained (red, open histogram) or not (black, filled histogram) with a phycoerythrin-coupled antibody directed against the extracellular domain of TRKA, and fluorescence was analyzed by flow cytometry. (C) TRK-Met– and TRK-Met-juxta–expressing cells were treated or not overnight with 2.5 μM SU11274. Immunoprecipitation was performed with an anti-mouse Met antibody. Proteins were resolved by 10% SDS-PAGE and analyzed by Western blotting with an antibody against phosphorylated tyrosine residues of the kinase domain of Met. The filter was stripped and reprobed with an antibody directed against mouse Met. Arrows indicate the positions of the mature and immature forms of TRK-Met and TRK-Met-juxta.
Finally, we evaluated the phosphorylation state of the cleavable and uncleavable TRK-Met versions by immunoprecipitating the chimeras (to avoid detecting the endogenous Met receptor) and evaluating their phosphorylation with antibodies directed against phosphorylated Met tyrosine residues (Figure 7C). The mature TRK-Met was detected as a phosphorylated receptor, despite the absence of ligand stimulation. In contrast, no phosphorylation of mature TRK-Met-juxta receptor was detected. As expected, treatment of cells with a specific ATP competitor of the Met kinase abolished TRK-Met phosphorylation. Taken together, these results demonstrate that impairment of PS-RIP induces membrane accumulation of activated receptors in Met-transfected cells.
Uncleavable TRK-Met Receptor Displays Ligand-independent Invasive Growth
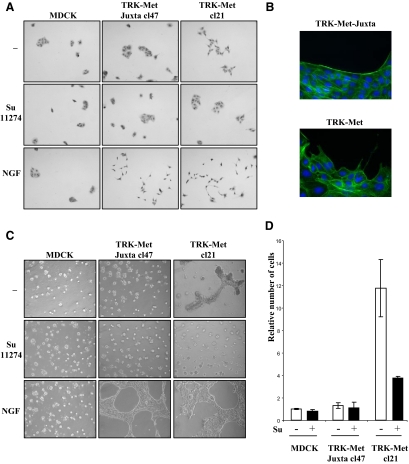
To evaluate the impact of the defect of PS-RIP on the biological responses triggered by Met in epithelial cells, we compared the phenotypes of MDCK cells expressing TRK-Met and TRK-Met-juxta, cultured under different conditions and ligand-stimulated or not. The cells were seeded on plastic at low density. Under such conditions, they form small islets in the absence of stimulation and scatter in response to ligand stimulation (Figure 8A). The cells were also cultured on Matrigel, a basement membrane preparation. On this, ligand stimulation promotes the generation of extensions from small aggregates, resulting in formation of a spider web-like network (Figure 8C).
Figure 8.
PS-RIP of TRK-Met chimera prevents its basal activation and the induction of invasive growth. (A) Scattering from cell islets. MDCK stably expressing TRK-Met and TRK-Met-juxta were seeded at low density. The next day the cells were cultured in the presence or absence of 2.5 μM SU11274 and/or 100 ng/ml NGF. Magnification, ×40 (B) MDCK stably expressing TRK-Met and TRK-Met-juxta were seeded at low density. The next day, the cells were fixed and stained for F-actin with phalloidin. Magnification, ×100. (C) Morphogenesis on Matrigel gels. MDCK stably expressing TRK-Met and TRK-Met-juxta were cultured on Matrigel gels. The following day, cells were incubated with or without SU11274 and/or NGF. Magnification, ×60. (D) MDCK stably expressing TRK-Met and TRK-Met-juxta, cultured in invasion chambers, were treated or not with 5 μM SU11274, a Met kinase inhibitor. The relative number of infiltrated cells was determined after staining of nuclei with Hoechst (n = 3, ± SD).
In response to NGF, MDCK cells expressing TRK-Met and TRK-Met-juxta scattered similarly and formed comparable networks on Matrigel, demonstrating that PS-RIP processing does not affect ligand-dependent responses (Figures 8, A and C). As expected, untransfected MDCK cells did not respond to NGF. In the absence of NGF stimulation, cells expressing TRK-Met and TRK-Met-juxta displayed different phenotypes. TRK-Met-juxta cells were phenotypically indistinguishable from untransfected MDCK cells, both forming condensed islets on plastic or round cysts on Matrigel. TRK-Met cells, in contrast, displayed basal scattering. When the islets were stained with phalloidin to reveal actin fibers, those formed by TRK-Met-juxta–expressing cells showed intense staining at their borders, whereas islets of TRK-Met–expressing cells displayed more weakly stained borders, with disrupting pseudopods (Figure 8B). On Matrigel, accordingly, TRK-Met–expressing cells displayed a basal connective structure between cysts (Figure 8C). Treatment with a specific ATP competitor of the Met kinase (SU11274) abolished the distinctive scattering and morphogenetic patterns of TRK-Met cells, demonstrating that these phenotypes depend on Met activity. As expected, this Met inhibitor also inhibited scattering and morphogenesis induced in response to HGF/SF and NGF (Supplementary Figures S4A and S5, A and B). We obtained similar results with the two independent clones of each type (expressing either TRK-Met or TRK-Met-juxta; Supplementary Figures S4B and S5B). We next tested TRK-Met– and TRK-Met-juxta–expressing MDCK cells in an in vitro migration assay (Figure 8D). In this assay, TRK-Met–expressing cells proved to be about 10 times more invasive than the TRK-Met-juxta–expressing cells, but their migration was markedly reduced in the presence of a Met inhibitor. Similar strong basal migration was observed with two additional clones of TRK-Met–expressing MDCK cells (Supplementary Figure S6). Taken together, these results show that failure of the Met receptor tyrosine kinase to undergo PS-RIP results in its accumulation, leading to ligand-independent activation of epithelial invasive growth.
Met Inhibitory Antibody Promotes PS-RIP of the Receptor
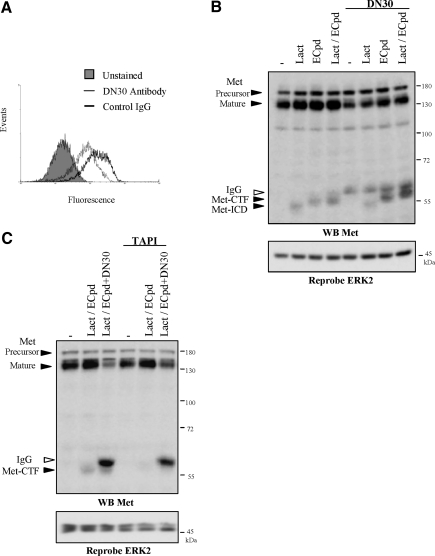
It has been recently shown that the mAb DN30, directed against extracellular region of human Met, is able to induce Met degradation (Petrelliet al.,2006). In consequence, this inhibitory antibody hampers ligand-independent biological activity triggered by the overexpressed Met, including transformed phenotypes of cancer cells and reduction of tumor growth.
Because this forced degradation involved proteolytic cleavages by molecular mechanisms which were not identified, we hypothesized the involvement of the PS-RIP. Treatment with DN30 induced decrease of membrane-anchored Met from the mammary metastatic cancer cells line MDA-MB231 overexpressing Met, detected by flow cytometry (Figure 9A). Consistently to down-regulation of Met, DN30 treatment reduced migration of the MDA-MB231 cells subjected to an in vitro invasion assay (data not shown). Next, we showed that Met-CTF generation induced by γ-secretase inhibitor is increased in presence of DN30 antibody, concomitantly to down-regulation of the mature Met, suggesting that the inhibitory antibody favors PS-RIP of the receptor (Figure 9B). Similar results were obtained in other transformed cell lines, including the gastric and the prostatic carcinoma cell lines GTL16 (Supplementary Figure S7) and PC-3 (data not shown). Finally treatment with the metalloprotease inhibitor TAPI-1, which interferes with the initial step of the PS-RIP, inhibited generation of Met-CTF and partially rescued the degradation of the mature full-length Met (Figure 9C). This demonstrates that the DN30 inhibitory antibody promotes Met PS-RIP leading to receptor degradation.
Figure 9.
Induction of the Met PS-RIP by the Met inhibitory antibody DN30. (A) MDA-MB231 cells were treated 48 h with 40 μg/ml DN30 antibody (open histogram, thin line) or nonrelevant IgG (open histogram, thick line). The cells were then incubated or not (filled histogram) with an antibody directed against the extracellular domain of Met and fluorescein-conjugated antibody. The fluorescence was analyzed by flow cytometry. (B) MDA-MB231 cells were treated or not with 40 μg/ml DN30 antibody. The following day the cells were incubated or not overnight with 1 μM E compound and then treated for 5 h with 10 μM lactacystin. (C) MDA-MB231 cells were treated or not with 40 μg/ml DN30 antibody. The following day the cells were treated or not overnight with 1 μM E compound and 50 μM TAPI-1. The cells were then treated for 5 h with 10 μM lactacystin. (B and C) Proteins were resolved by 10% SDS-PAGE and analyzed by Western blotting with antibodies directed against the kinase domain of Met (WB Met). The filter was stripped and reprobed with an antibody directed against ERK2. Arrows indicate the positions of precursor and mature full-length Met, Met-CTF, Met ICD, and IgG from DN30 antibody.
DISCUSSION
We report here that the Met receptor tyrosine kinase is processed by PS-RIP. Mechanistically, PS-RIP of Met involves two cleavage steps. The first step is metalloprotease-mediated shedding of the extracellular domain, generating a soluble N-terminal fragment (Met-NTF) and a membrane-anchored fragment (Met-CTF). This step is a prerequisite to an additional cleavage by γ-secretase, yielding a labile Met-ICD fragment. Met ectodomain shedding has been described previously on the basis of initial observations of a soluble extracellular form of Met (Pratet al.,1991; Crepaldiet al.,1994). This process has been observed in various cell types and has been induced with different agents such as PMA, suramine, epidermal growth factor (EGF), lysophosphatidic acid (LPA), and HGF/SF (Pratet al.,1991; Galvaniet al.,1995; Nathet al.,2001; Wajihet al.,2002). In addition, other fragments have been observed, such as phosphorylated membrane-anchored fragments degraded via the proteosomal pathway or labile nuclear fragments (Jefferset al.,1997; Pozner-Mouliset al.,2006). Our work suggests that these previously observed fragments of Met, initially described independently, could be produced through a common proteolytic process, PS-RIP.
The metalloprotease most commonly implicated in shedding of transmembrane protein is the ADAM-17. ADAM-17 notably contributes to the regulation of signaling pathways by mediating ectodomain shedding from cytokine receptors, including the tyrosine kinase receptors ErbB4 and TRKA (Huovilaet al.,2005). We demonstrated that ADAM-17 participates also to the initial step of the Met PS-RIP. However, although silencing of ADAM-17 was efficient in the MCF10A epithelial cells, the inhibition of the Met cleavages was partial, suggesting that other metalloproteases could process Met. Interestingly, it has been recently shown in NIH3T3 cells that shedding of Met involves the ADAM-10 (Kopitzet al.,2007). Therefore, the shedding of Met could implicate several metalloproteases, which could be differently engaged according to the cell type.
Met ectodomain shedding is immediately followed by γ-secretase cleavage, because Met-CTF is observed only upon γ-secretase inhibition. Furthermore, a 50-amino acid stretch of the Met extracellular juxtamembrane domain, added to the TRK-Met chimera, is necessary and sufficient to initiate ectodomain shedding and further γ-secretase cleavage. This is consistent with the observation that γ-secretase cleavage depends on prior ectodomain shedding (Struhl and Adachi, 2000). Presenilin is the catalytic core of the γ-secretase complex (De Strooper, 2003). Accordingly, we show here that both PS1 and PS2 are involved in Met cleavage. This is consistent with results obtained with APP and Notch, whose γ-secretase–mediated cleavage is abolished only in embryonic stem cells devoid of both PS1 and PS2 (Herremanet al.,2000).
We then explored the functional consequences of PS-RIP using cleavable and uncleavable version of chimeric TRK-Met receptor. We found that the TRK-Met Juxta is efficiently processed by the PS-RIP; consequently the mature form of the cleavable TRK-Met-juxta chimera has a shorter half-life than that of the uncleavable TRK-Met and does not accumulate at the plasma membrane. Furthermore, we showed that the anti-Met antibody DN30, known to induce down-regulation of the membrane-anchored Met promotes Met PS-RIP. Therefore, inhibition of Met PS-RIP through expression of an uncleavable receptor induces its membrane accumulation, whereas inversely forced Met PS-RIP by antagonist antibody induces its membrane depletion. Taken together, our results demonstrate that PS-RIP of Met leading to generation of intracellular instable fragments is able to regulate membrane expression of the receptor. It is worth noticing that in MDCK cells for instance, inhibition of basal PS-RIP by metalloprotease inhibitor does not significantly increase expression of Met. This suggests that although induced PS-RIP destabilizes Met, the basal PS-RIP could be insufficient to reduce significantly the expression of Met.
Although we demonstrated that Met PS-RIP can be enhanced by specific antibody, the physiological stimuli able to promote the process are unknown. Indeed, stimulation of Met by HGF/SF does not induce its PS-RIP in normal epithelial cells. This contrast with induction of the Met shedding in response to HGF/SF observed in normal human aortic smooth muscle cells (Wajih et al., 2002), suggesting that Met proteolysis can be constitutive or activated by its ligand according to the cell type. Our data also highlight how PS-RIP of Met differs from Met degradation involving the E3 ubiquitin ligase c-Cbl. In this latter process, ligand stimulation induces recruitment of c-Cbl to phosphorylated tyrosines of the receptor. This promotes ubiquitination of Met, its endocytosis, and ultimately its lysosomal degradation (Hammondet al.,2003), thereby attenuating the signaling induced by the activated receptor. In contrast, PS-RIP of Met in epithelial cells is ligand-independent and does not require the kinase activity of the receptor.
A well-described cause of deregulated invasive growth induced by Met is its overexpression, which causes autocrine or paracrine stimulation by HGF/SF or induces self-activation of the receptor independently of ligand stimulation (Birchmeieret al.,2003). Overexpression has been attributed to gene amplification or to increased transcription induced by oncogenes. Yet aberrant activation of Met can be the consequence of a defective down-regulation mechanism, as it is well documented in the case of enhanced cell transformation resulting from uncoupling of Met from c-Cbl–mediated down-regulation (Peschardet al.,2004). Aberrant Met activation can also result from defective proteolytic cleavage. In a colon carcinoma cell line, noncleavage of the Met precursor was found to lead to expression of a constitutively active receptor (Mondinoet al.,1991). In this same line, we demonstrate here that impairment of TRK-Met chimera cleavage by PS-RIP results in accumulation of the receptor at the plasma membrane, accompanied with ligand-independent scattering, morphogenesis, and invasion of normal epithelial cells. Inversely, the Met inhibitory antibody DN30, inhibiting transformed phenotype of cancer cells (Petrelliet al.,2006), take advantage of the PS-RIP mechanism to induce Met down-regulation in cells dependent of Met for their transformation. Thus, regulation of the Met PS-RIP could play an important role against cellular transformation by preventing overexpression of Met at the plasma membrane.
Although development of inhibitory antibodies directed against membrane oncogenes is a promising therapeutic approach, the molecular mechanisms underlying their activity are not fully understood. Our data demonstrate that the Met inhibitory antibody acts through an original mechanism involving forced induction of an intrinsic mechanism of degradation, leading to depletion of the receptor. Thus, forced induction of Met PS-RIP might be used therapeutically to prevent uncontrolled activation of the receptor.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Centre National de la Recherche Scientifique, the Institut Pasteur de Lille, and INSERM, and by grants from the Ligue contre le Cancer, comité Nord, the Association pour la Recherche sur le Cancer, the Fondation Recherche Médicale, Comité Nord-Pas de Calais, and Agence Nationale de la Recherche, Young Investigator Program. B. F. was supported by a Institut Pasteur/Région Nord-Pas de Calais fellowship and F. A. by a grant from the French Research and Technology Minister. Financial support was given to K. R. by the Deutsche Forschungsgemeinschaft Grant SFB 617 and the Center of Excellence program Inflammation at Interfaces.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-09-0969) on March 18, 2009.
REFERENCES
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G. F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Borowiak M., Garratt A. N., Wustefeld T., Strehle M., Trautwein C., Birchmeier C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J. R., Cumano A., Roux P., Black R. A., Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Comoglio P. M., Giordano S., Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- Crepaldi T., Prat M., Giordano S., Medico E., Comoglio P. M. Generation of a truncated hepatocyte growth factor receptor in the endoplasmic reticulum. J. Biol. Chem. 1994;269:1750–1755. [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- Deheuninck J., Foveau B., Goormachtigh G., Leroy C., Ji Z., Tulasne D., Fafeur V. Caspase cleavage of the MET receptor generates an HGF interfering fragment. Biochem Biophys. Res. Commun. 2008;367:573–577. doi: 10.1016/j.bbrc.2007.12.177. [DOI] [PubMed] [Google Scholar]
- Di Renzo M. F. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin. Cancer Res. 1995;1:147–154. [PubMed] [Google Scholar]
- Foveau B., Leroy C., Ancot F., Deheuninck J., Ji Z., Fafeur V., Tulasne D. Amplification of apoptosis through sequential caspase cleavage of the MET tyrosine kinase receptor. Cell Death Differ. 2007;14:752–764. doi: 10.1038/sj.cdd.4402080. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Grobelny D., Foellmer H. G., Fernandez L. A. Inhibition of angiogenesis by the matrix metalloprotease inhibitor N-[2R-2-(hydroxamidocarbonymethyl)-4-methylpentanoyl)]-L-tryptophan methylamide. Cancer Res. 1994;54:4715–4718. [PubMed] [Google Scholar]
- Galvani A. P., Cristiani C., Carpinelli P., Landonio A., Bertolero F. Suramin modulates cellular levels of hepatocyte growth factor receptor by inducing shedding of a soluble form. Biochem. Pharmacol. 1995;50:959–966. doi: 10.1016/0006-2952(95)00219-p. [DOI] [PubMed] [Google Scholar]
- Gambarotta G., Boccaccio C., Giordano S., Ando M., Stella M. C., Comoglio P. M. Ets up-regulates MET transcription. Oncogene. 1996;13:1911–1917. [PubMed] [Google Scholar]
- Giordano S., Di Renzo M. F., Narsimhan R. P., Cooper C. S., Rosa C., Comoglio P. M. Biosynthesis of the protein encoded by the c-met proto-oncogene. Oncogene. 1989;4:1383–1388. [PubMed] [Google Scholar]
- Hammond D. E., Carter S., McCullough J., Urbe S., Vande Woude G., Clague M. J. Endosomal dynamics of Met determine signaling output. Mol. Biol. Cell. 2003;14:1346–1354. doi: 10.1091/mbc.E02-09-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A., Serneels L., Annaert W., Collen D., Schoonjans L., De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- Huovila A. P., Turner A. J., Pelto-Huikko M., Karkkainen I., Ortiz R. M. Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ivan M., Bond J. A., Prat M., Comoglio P. M., Wynford-Thomas D. Activated ras and ret oncogenes induce over-expression of c-met (hepatocyte growth factor receptor) in human thyroid epithelial cells. Oncogene. 1997;14:2417–2423. doi: 10.1038/sj.onc.1201083. [DOI] [PubMed] [Google Scholar]
- Jeffers M., Taylor G. A., Weidner K. M., Omura S., Vande-Woude G. F. Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaida K., Matsumoto K., Shimazu H., Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc. Natl. Acad. Sci. USA. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitz C. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67:8615–8623. doi: 10.1158/0008-5472.CAN-07-0232. [DOI] [PubMed] [Google Scholar]
- Landman N., Kim T. W. Got RIP? Presenilin-dependent intramembrane proteolysis in growth factor receptor signaling. Cytokine Growth Factor Rev. 2004;15:337–351. doi: 10.1016/j.cytogfr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Longati P., Bardelli A., Ponzetto C., Naldini L., Comoglio P. M. Tyrosines1234–1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor) Oncogene. 1994;9:49–57. [PubMed] [Google Scholar]
- Maina F., Hilton M. C., Ponzetto C., Davies A. M., Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997;11:3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak H. H., Peschard P., Lin T., Naujokas M. A., Zuo D., Park M. Oncogenic activation of the Met receptor tyrosine kinase fusion protein, Tpr-Met, involves exclusion from the endocytic degradative pathway. Oncogene. 2007;26:7213–7221. doi: 10.1038/sj.onc.1210522. [DOI] [PubMed] [Google Scholar]
- Migliore C., Giordano S. Molecular cancer therapy: can our expectation be MET? Eur. J. Cancer. 2008;44:641–651. doi: 10.1016/j.ejca.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Mondino A., Giordano S., Comoglio P. M. Defective posttranslational processing activates the tyrosine kinase encoded by the MET proto-oncogene (hepatocyte growth factor receptor) Mol. Cell. Biol. 1991;11:6084–6092. doi: 10.1128/mcb.11.12.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm J. S., Schroeter E. H., Saxena M. T., Griesemer A., Tian X., Pan D. J., Ray W. J., Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol. Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Nath D., Williamson N. J., Jarvis R., Murphy G. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J. Cell Sci. 2001;114:1213–1220. doi: 10.1242/jcs.114.6.1213. [DOI] [PubMed] [Google Scholar]
- Ni C. Y., Murphy M. P., Golde T. E., Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C. S., Schmidt M., O’Brien S. J., Blair D. G., Vande Woude G. F. Mechanism of met oncogene activation. Cell. 1986;45:895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Pennacchietti S., Michieli P., Galluzzo M., Mazzone M., Giordano S., Comoglio P. M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Peschard P., Fournier T. M., Lamorte L., Naujokas M. A., Band H., Langdon W. Y., Park M. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell. 2001;8:995–1004. doi: 10.1016/s1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- Peschard P., Ishiyama N., Lin T., Lipkowitz S., Park M. A conserved DpYR motif in the juxtamembrane domain of the Met receptor family forms an atypical c-Cbl/Cbl-b tyrosine kinase binding domain binding site required for suppression of oncogenic activation. J. Biol. Chem. 2004;279:29565–29571. doi: 10.1074/jbc.M403954200. [DOI] [PubMed] [Google Scholar]
- Petrelli A., Circosta P., Granziero L., Mazzone M., Pisacane A., Fenoglio S., Comoglio P. M., Giordano S. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc. Natl. Acad. Sci. USA. 2006;103:5090–5095. doi: 10.1073/pnas.0508156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli A., Gilestro G. F., Lanzardo S., Comoglio P. M., Migone N., Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., Graaziani A., Panayotou G., Comoglio P. M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Giordano S., Peverali F., Della Valle G., Abate M. L., Vaula G., Comoglio P. M. c-met is amplified but not mutated in a cell line with an activated met tyrosine kinase. Oncogene. 1991;6:553–559. [PubMed] [Google Scholar]
- Pozner-Moulis S., Pappas D. J., Rimm D. L. Met, the hepatocyte growth factor receptor, localizes to the nucleus in cells at low density. Cancer Res. 2006;66:7976–7982. doi: 10.1158/0008-5472.CAN-05-4335. [DOI] [PubMed] [Google Scholar]
- Prat M., Crepaldi T., Gandino L., Giordano S., Longati P., Comoglio P. C-terminal truncated forms of Met, the hepatocyte growth factor receptor. Mol. Cell. Biol. 1991;11:5954–5962. doi: 10.1128/mcb.11.12.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., Gherardi E., Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Schmidt L. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- Slack B. E., Ma L. K., Seah C. C. Constitutive shedding of the amyloid precursor protein ectodomain is up-regulated by tumour necrosis factor-alpha converting enzyme. Biochem. J. 2001;357:787–794. doi: 10.1042/0264-6021:3570787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol. Cell. 2000;6:625–636. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- Tulasne D., Deheuninck J., Lourenco F. C., Lamballe F., Ji Z., Leroy C., Puchois E., Moumen A., Maina F., Mehlen P., Fafeur V. Proapoptotic function of the MET tyrosine kinase receptor through caspase cleavage. Mol. Cell. Biol. 2004;24:10328–10339. doi: 10.1128/MCB.24.23.10328-10339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulasne D., Foveau B. The shadow of death on the MET tyrosine kinase receptor. Cell Death Differ. 2008;15:427–434. doi: 10.1038/sj.cdd.4402229. [DOI] [PubMed] [Google Scholar]
- Tulasne D., Paumelle R., Weidner K. M., Vandenbunder B., Fafeur V. The multisubstrate docking site of the MET receptor is dispensable for MET-mediated RAS signaling and cell scattering. Mol. Biol. Cell. 1999;10:551–565. doi: 10.1091/mbc.10.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Minowa O., Mori C., Shlota K., Kuno J., Noda T., Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Wajih N., Walter J., Sane D. C. Vascular origin of a soluble truncated form of the hepatocyte growth factor receptor (c-met) Circ. Res. 2002;90:46–52. doi: 10.1161/hh0102.102756. [DOI] [PubMed] [Google Scholar]
- Weidner K. M., Sachs M., Birchmeier W. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the met receptor in epithelial cells. Proc. Natl. Acad. Sci. USA. 1995;92:2597–2601. doi: 10.1073/pnas.92.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant J., Buluwela L., Niranjan B., Gusterson B., Kamalati T. In vivo effects of hepatocyte growth factor/scatter factor on mouse mammary gland development. Exp. Cell Res. 1998;241:476–481. doi: 10.1006/excr.1998.4028. [DOI] [PubMed] [Google Scholar]
- Zhang L., Song L., Parker E. M. Calpain inhibitor I increases beta-amyloid peptide production by inhibiting the degradation of the substrate of gamma-secretase. Evidence that substrate availability limits beta-amyloid peptide production. J. Biol. Chem. 1999;274:8966–8972. doi: 10.1074/jbc.274.13.8966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.