Abstract
Focal adhesion kinase (FAK) is an essential nonreceptor tyrosine kinase regulating cell migration, adhesive signaling, and mechanosensing. Using FAK-null cells expressing FAK under an inducible promoter, we demonstrate that FAK regulates the time-dependent generation of adhesive forces. During the early stages of adhesion, FAK expression in FAK-null cells enhances integrin activation to promote integrin binding and, hence, the adhesion strengthening rate. Importantly, FAK expression regulated integrin activation, and talin was required for the FAK-dependent effects. A role for FAK in integrin activation was confirmed in human fibroblasts with knocked-down FAK expression. The FAK autophosphorylation Y397 site was required for the enhancements in adhesion strengthening and integrin-binding responses. This work demonstrates a novel role for FAK in integrin activation and the time-dependent generation of cell–ECM forces.
INTRODUCTION
Focal adhesion kinase (FAK) is a widely expressed nonreceptor protein tyrosine kinase that interacts with other focal adhesion components, including Src, Cas, and paxillin, to modulate adhesive interactions (Hankset al., 1992; Schalleret al., 1992; Polte and Hanks, 1995; Schalleret al., 1999). FAK functions as an early mediator of integrin-mediated signaling to regulate cell motility, survival, and proliferation (Ilicet al., 1995a; Zhaoet al., 1998; Owenet al., 1999; Renshawet al., 1999; Sieget al.,1999, 2000). Deletion of the FAK gene results in early embryonic lethality due to defects in cell migration (Furutaet al., 1995). Tissue-specific knockout of FAK also demonstrates a pivotal role for this signaling molecule in cardiac development and innervation (Shenet al., 2005; Brarenet al., 2006; Penget al., 2008; Watanabeet al., 2008). Moreover, elevated FAK levels have been observed in benign, preinvasive, and invasive tumors (Gabarra-Nieckoet al., 2003), and inhibition of FAK reduces experimental metastatic tumor formation (van Nimwegenet al., 2005). Recently, FAK signaling has been implicated in load-induced cardiac hypertrophy as well as bone remodeling in response to mechanical loading, further establishing a role for FAK in mechanotransduction (Clementeet al., 2007; Leuchtet al., 2007). Analyses of the role of FAK in cell migration and mammalian development, however, are complicated by compensatory actions by the FAK-related kinase Pyk2, which is overexpressed or phosphorylated in the absence of FAK (Owenet al., 1999; Limet al., 2008; Weiset al., 2008).
Migration studies and live-cell microscopy analyses have demonstrated that FAK regulates cell migration via modulation of focal adhesion turnover (Owenet al., 1999; Wanget al., 2001). FAK signaling is critical to focal adhesion turnover at the cell front and regulates focal adhesion disassembly, correlating with the disassembly rates for Src, paxillin, extracellular signal–regulated kinase (ERK), and myosin light-chain kinase (MLCK; Webbet al., 2004). In addition, FAK modulates actin cytoskeleton polymerization and lamellipodia protrusion (Serrelset al., 2007). Although FAK has been implicated in mechanosensing (Wanget al., 2001; Pironeet al., 2006; Schoberet al., 2007), the role of FAK in the generation of adhesive forces remains poorly understood as cell migration is a multistep, dynamic process that exhibits complex dependencies on adhesion strength and focal adhesion and cytoskeletal dynamics (Paleceket al., 1997; Gupton and Waterman-Storer, 2006). These outcome measures therefore do not provide direct or sensitive metrics of adhesive force to interpret functional mechanisms. In the present study, we applied an experimental system that integrates a sensitive adhesion strength assay and quantitative biochemical assays (Gallantet al., 2005) to analyze the role of FAK in the adhesion strengthening process. We demonstrate a novel role for FAK in modulating integrin activation to regulate the generation of cell-extracellular matrix (ECM) adhesive forces.
MATERIALS AND METHODS
Cells, Constructs, Antibodies, and Micropatterned Substrates
FAK-null (FAK−) fibroblasts with tetracycline-inducible FAK expression were maintained as described previously (Owenet al., 1999). Two clones for each FAK variant were analyzed. FAK expression was induced 2 d before all experiments to achieve maximal expression levels. Primary human dermal fibroblasts were kindly provided by A. P. Kowalczyk (Emory University). The talin shRNA construct was generated by cloning the short hairpin RNA (shRNA) sequence from the pENTR/D-TOPO plasmid (Tadokoroet al., 2003) into the pMSCV-puro retroviral vector (Paddisonet al., 2004). The FAK small interfering RNA (siRNA) construct has been previously described (Benlimameet al., 2005). Retrovirus packaging and transductions were performed as described previously (Byerset al., 2002).
Antibodies against vinculin (clone V284, Upstate Biotechnology, Lake Placid, NY), talin (clone 8d4, Sigma, St. Louis, MO), integrin subunit α5 (AB1928 polyclonal, Chemicon, Temecula, CA), FAK (06-543 polyclonal, Upstate), and Pyk2 (clone 11, BD Biosciences, San Diego, CA) were used for Western blotting. Monoclonal antibodies against vinculin (clone V4505) and talin (8d4) were purchased from Sigma and used for immunostaining. Monoclonal antibodies against vinculin (clone V284, Upstate) and talin (clone 8d4, Sigma) were used for flow cytometry. Antibodies against mouse (clone 9EG7, PharMingen, San Diego, CA) and human (clones 12G10 and HUTS-4, Chemicon) active β1integrin and mouse (AB1950 polyclonal and clone MAB1997, Chemicon) and human (clone MAB1981, Chemicon) β1integrin were obtained from commercial vendors. The following function-blocking, integrin-specific antibodies were used for blocking studies: AB1950 (Chemicon) and HMα5–1 (PharMingen) against α5; HA2/5 (BD Biosciences) against β1; and H9.2B8 (BD Biosciences) against αv.
Micropatterned substrates to control cell adhesive area and shape were prepared by microcontact printing of alkanethiol self-assembled monolayers on gold as previously described (Gallantet al., 2005). Briefly, arrays of CH3-terminated alkanethiol (HS-(CH2)11-CH3, Sigma) circles were stamped onto gold-coated glass coverslips using a PDMS stamp (Sylgard 184/186 Elastomer kit). The remaining exposed gold was then functionalized with a tri(ethylene glycol)-terminated alkanethiol [HS-(CH2)11-(CH2CH2O)3-OH; ProChimia Surfaces, Sopot, Poland], which resists protein adsorption and cell attachment. The patterned coverslip was coated with human plasma fibronectin (20 μg/ml), blocked with 1% heat-denatured bovine serum albumin to prevent nonspecific cellular binding, and incubated in PBS. This process results in patterned areas of adsorbed fibronectin in an array of circular islands 5 μm in diameter and spaced 75 μm apart to promote single cell attachment to each island.
Adhesion Strength Assay
Adhesion strength was measured using our spinning disk system (Gallantet al., 2005). Micropatterned substrates with adherent cells were spun in PBS + 2 mM dextrose for 5 min at a constant speed. The spinning disk device imparts a shear stress (τ) dependent on radial position (r), such that τ = 0.8r(ρμω3)1/2. In some experiments, the spinning buffer was supplemented with 5% dextran to increase fluid viscosity. After spinning, cells were fixed in 3.7% formaldehyde, permeabilized in 1% Triton X-100, stained with ethidium homodimer (Molecular Probes, Eugene, OR), and counted at specific radial positions using a Nikon TE300 microscope (Melville, NY) equipped with a Ludl motorized stage (Ludl Electronic Products, Hawthorne, NY), Spot RT camera (Diagnostic Instruments, Sterling Heights, MI) and Image-Pro analysis system (Media Cybernetics, Silver Spring, MD). Sixty-one fields (80–100 cells/field before spinning, 10X) were analyzed and cell counts were normalized to the number of cells present at the center of the disk. The fraction of adherent cells (f) was then fit to a sigmoid curve f = 1/(1 + exp[b(τ − τ50)]), where τ50 is the shear stress for 50% detachment and b is the inflection slope. τ50 characterizes the mean adhesion strength for a population of cells because this applied shear stress results in detachment of 50% of the cell population.
Integrin Binding and Focal Adhesion Assembly
The number of bound integrins was quantified using a cross-linking, extraction, and reversal technique (Gallantet al., 2005). Adherent cells were incubated in 1.0 mM 3,3′-Dithiobis[sulfosuccinimidylpropionate] (Pierce Biotechnology, Rockford, IL) + 2 mM dextrose for 30 min to chemically cross-link integrins to the underlying ECM. After quenching in 50 mM Tris (pH 7.6), bulk cellular components were removed via SDS extraction (0.1% SDS, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). After stringent washing, cross-linkers were cleaved in 50 mM DTT in 0.1% SDS for 30 min, thus releasing bound integrins that were collected, concentrated, and quantified by Western blotting.
For visualization of focal adhesions, cells were permeabilized in cytoskeleton-stabilizing buffer (0.5% Triton X-100 + 50 mM NaCl + 150 mM sucrose + 3 mM MgCl2 + 20 μg/ml aprotinin + 1 μg/ml leupeptin + 1 mM PMSF + 50 mM Tris, pH 6) for 10 min, fixed in 3.7% formaldehyde for 5 min, blocked in 5% fetal bovine serum, and incubated with primary antibodies against focal adhesion components followed by AlexaFluor-labeled secondary antibodies (Molecular Probes). Mouse anti-vinculin (V4505) and anti-talin (8d4) antibodies were used. Images were captured using a Nikon 100× objective (1.3 NA) and Spot RT camera and software. Focal adhesion area fractions and intensities were quantified using calibrated image analysis software (ImagePro 4.5, Media Cybernetics).
Integrin Activation and Blocking
Integrin activation was assessed via flow cytometry using antibodies that recognize activation-associated epitopes and soluble fibronectin binding. For experiments with antibodies that recognize activation-associated epitopes (9EG7 for murine cells, 12G10 and HUTS-4 for human cells), integrin surface expression levels were determined by flow cytometry using antibodies that recognize both active and total (active + inactive) receptors (AB1950 or MAB 1997 for murine cells, MAB 1981 for human cells). Cells were plated on fibronectin-coated substrates for 15 min and harvested by trypsinization. After washing, cells were incubated in primary antibodies for 1 h, washed three times in DPBS, incubated in fluorochrome-labeled secondary antibodies. Isotype controls (rat IgG2aK, goat AB758, Chemicon) and secondary-only antibody controls were also included. The mean geometric fluorescence intensity (mGFI) value for 9EG7 (corrected for background by subtracting the mean mGFI for the antibody control) was divided by the mean mGFI for the total integrin antibody (1997 or 1950, corrected for background). This normalization procedure allowed for statistical analyses of 9EG7 binding across independent experimental runs.
For soluble fibronectin binding (Tadokoroet al., 2003; Bouaouinaet al., 2008), we used a monobiotinylated recombinant fragment spanning the seventh to tenth type III repeat of human fibronectin (FN7-10; Petrieet al., 2006). For talin knockdown studies, FAK-expressing (FAK+) and FAK− cells (1 × 106) were cotransfected with the talin shRNA pENTR/D-TOPO plasmid and the Amaxa pmaxGFP plasmid (Gaithersburg, MD) or only pmaxGFP with an Amaxa Nucleofector II (program T-20, solution MEF 2) according to manufacturer’s instructions. Seventy-two hours after transfection, cells were plated on fibronectin-coated dishes for 15 min, detached, and resuspended in buffer. Cells were incubated in biotinylated FN7-10 for 20 min, washed, incubated in allophycocyanin (APC)-conjugated streptavidin, washed, and analyzed by flow cytometry for transfection (green channel) and FN7-10 binding (red channel). For statistical analysis, cells were gated for FN7-10 binding and green fluorescent protein (GFP) expression using untransfected cells and binding in the presence of 10 mM EDTA to inactivate the integrins. The amount of FN7-10 binding was computed as follows: (mGFIi − mGFIo)/mGFIo, where mGFIi is the mean geometry fluorescence intensity for the experimental condition and mGFIo is the mean geometry fluorescence intensity for FN7-10 binding in the presence of 10 mM EDTA to inactivate the integrin (background).
Blocking of integrin activation adhesion strength measurements were performed using function-blocking antibodies (AB1950 and HMα5–1 against α5; HA2/5 against β1, H9.2B8 against αv). Cells were counted, incubated for 15 min in either experimental antibody or isotype control and plated as described above.
Flow Cytometry Assessment of Vinculin and Talin Expression
Expression levels for talin and vinculin were quantified by flow cytometry (Tadokoroet al., 2003). Cells were trypsinized and resuspended in serum-containing media. After rinses in serum-free media, cells were suspended in 875 μl of cold fixing solution (0.025% paraformaldehyde final concentration in PBS, pH 7.2) and incubated for 30 min under gentle agitation. Fixed cells were then permeabilized in 0.2% Tween-20 in 1% bovine serum albumin in PBS for 15 min under gentle agitation. After blocking in 0.02% Tween-20 + 2% fetal bovine serum for 30 min, cells were incubated in primary and secondary antibodies (in 0.02% Tween-20 + 1% bovine serum albumin for 30 min) for 30 min, washed, and analyzed by flow cytometry.
Statistical Analyses
Nonlinear regression analysis was performed using SigmaPlot 2001 software (SPSS; Systat, San Jose, CA). Analysis of variance (ANOVA) statistical analyses were performed using SYSTAT 11 software. Because each data point of adhesion strength is a separate experimental measurement, hypothesis testing comparing nonlinear regression parameters of adhesion strength was performed as a t test of parameters with known variance. Comparisons of integrin binding parameters were completed by collecting profiles of binding from each Western blot membrane and analyzing variance using ANOVA.
RESULTS
FAK Regulates Adhesive Force Generation
We used FAK-null cells engineered for tetracycline-regulated expression of wild-type FAK (Owenet al., 1999). In this cellular system, FAK is expressed to normal levels in the absence of tetracycline, whereas in the presence of tetracycline in the culture media, FAK expression is repressed. A significant advantage of this system is that direct comparisons between FAK expression states can be made within the same clone cell population. We analyzed two independent clones with equivalent results. We use the nomenclature FAK+ and FAK− for cells expressing FAK and cells with repressed FAK expression (FAK-null), respectively, throughout this work.
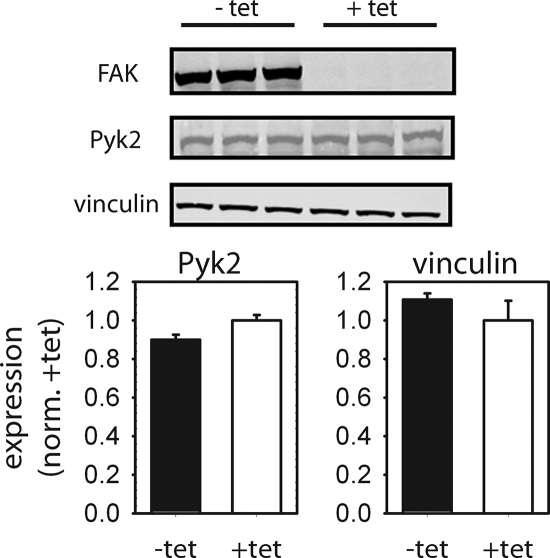
Western blot analyses demonstrated that FAK is expressed at high levels in the absence of tetracycline, whereas FAK expression is effectively repressed in the presence of tetracycline (Figure 1). No differences in vinculin expression were observed between these two culture conditions. We also examined Pyk2 expression levels for potential compensatory effects in the absence of FAK. Schlaepfer and coworkers recently demonstrated that loss of FAK expression results in the up-regulation of Pyk2 (Limet al., 2008). However, we were unable to show an effect of inducible FAK expression on Pyk2 expression for these cells (Figure 1). We note that Pyk2 levels are elevated in our inducible FAK cells compared with wild-type FAK+/+ cells (Owenet al., 1999), suggesting that cells expressing high levels of Pyk2 may have been selected during the initial establishment of the FAK-null cells.
Figure 1.
Tetracycline-regulated expression of FAK in FAK-null cells. In the absence of tetracycline (−tet), FAK expression is activated. Addition of tetracycline to the culture media (+tet) represses FAK expression. No differences in expression levels for Pyk2 or vinculin were observed between tet conditions. Representative Western blots (left) and quantification of protein levels (normalized to FAK-null condition [+tet], right) are shown.
Cells were plated in 10% serum on fibronectin-coated micropatterned substrates (5-μm diameter circles spaced 75 μm apart) to ensure constant adhesive area and equal cell shape among experimental conditions. This well-defined adhesive condition eliminates differences in cell adhesive area and shape between FAK+ and FAK− cells that may confound the analysis and allows for direct comparison among experimental groups. Importantly, we previously demonstrated that these analyses for adhesion strength, integrin binding, and vinculin localization on micropatterned substrates provide an appropriate model to study adhesive interactions applicable to unpatterned cells (Gallantet al., 2005).
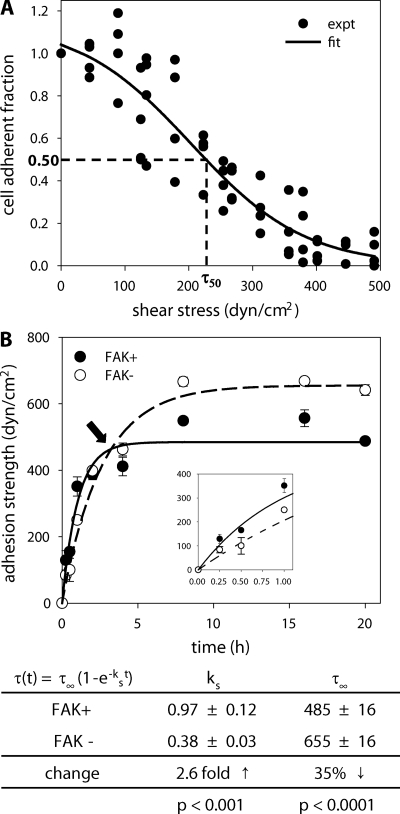
We analyzed adhesive force over time for FAK+ and FAK− cells using a hydrodynamic adhesion assay that provides direct and sensitive population-based measurements of adhesion strength to examine the contribution of FAK to the kinetics of adhesion strengthening. In this assay, substrates containing adherent cells are placed on the rotating disk and spun in buffer at prescribed speeds. The fluid flow associated with the disk rotation applies a well-characterized hydrodynamic force to adherent cells. The hydrodynamic force increases linearly with radial position along the surface of the sample, such that cells at the center of the sample experience negligible forces, whereas cell numbers decrease toward the outside of the disk as the applied cell detachment force increases. Thus, in a single sample, a linear range of forces is applied to a large cell population (>6000 cells analyzed/sample). After spinning, adherent cells are fixed and stained, and cell numbers at different radial positions are quantified using a motorized microscope stage and image analysis system. The fraction of adherent cells (f) is calculated by dividing the number of cells in each field by the number of cells at the center of the array, where negligible forces are applied. The detachment profile (cell adherent fraction vs. shear stress; τ [stress = force/area]) is then fit to a sigmoid curve to obtain the shear stress for 50% detachment, which represents the mean adhesive force. Figure 2A shows a typical detachment profile (each experimental point represents cell densities at a specific radial position) and sigmoid fit (adhesion strength = 232 dyn/cm2, R2 = 0.88) for a single sample.
Figure 2.
FAK modulates adhesion strengthening. (A) Cell detachment profile showing the fraction of adherent cells (f) as a function of wall shear stress τ (stress = force/area) for a single experiment. Experimental cell counts (●) are fit to a sigmoid curve (line) to obtain the shear stress for 50% detachment (τ50), which represents the mean adhesive strength. For the experiment shown (FAK− cells, 1-h adhesion), the adhesion strength equals 232 dyn/cm2 (R2 = 0.88). (B) Top, adhesion strengthening profile over time for FAK-expressing (FAK+, ●) and FAK-null (FAK−, ○) cells. Bottom, strengthening profile was curve fit to a first-order kinetic model to extract strengthening parameters (mean ± SE). A minimum of 27 data points was used for the curve fits, and mean ± SE for each time point is shown. FAK expression had divergent effects on the kinetic parameters strengthening rate (ks) and steady-state adhesion strength (τ∞) as demonstrated by “cross-over” point (arrow). Inset shows adhesion strengthening behavior at short adhesion times showing significant differences between FAK+ and FAK− cells.
Adhesion strength was measured at discrete time points (Figure 2B), and the results were fit to the solution of a first-order kinetic equation that describes the strengthening process: τ(t) = τ∞(1 − e−kst; Gallantet al., 2005; Michael and Garcia, 2007). This analysis yielded two parameters describing the strengthening response: 1) ks represents the strengthening rate, i.e., the time required to reach 67% of the steady-state strength, and 2) τ∞ is equal to the steady-state adhesion strength. We note that the numerical algorithm used weights all data points equally for the nonlinear regression and there is no bias introduced for any time points. Importantly, regression analyses demonstrated that this model captures the experimental results with a high degree of confidence (p < 0.0001, R2 > 0.83). In summary, this analysis provides sensitive and direct measurements of the adhesive strengthening process described by a kinetic strengthening rate constant ks and a steady-state adhesion strength value τ∞.
Expression of FAK in FAK-null cells resulted in significant changes in both the strengthening rate and steady-state adhesion strength (Figure 2B). Surprisingly, FAK expression had divergent effects in the adhesion strengthening parameters as illustrated by the “cross-over” point in the strengthening profile (arrow in Figure 2B). FAK expression in FAK-null cells increased the strengthening rate 2.6-fold over matched nonexpressing control cells (p < 0.001). In contrast, the steady-state adhesion strength for FAK+ cells decreased by 35% compared with FAK− cells (p < 0.0001). These adhesive responses were observed in two separate inducible FAK clones. These results show that FAK increases the initial strength of cell–ECM adhesion; however, in long-term, steady-state cell–ECM interactions, the presence of FAK decreases adhesive force. Differences in both strengthening parameters suggest that FAK modulates adhesion strength via different mechanisms.
FAK Modulates Adhesion Strengthening Rate via Integrin Activation and Binding
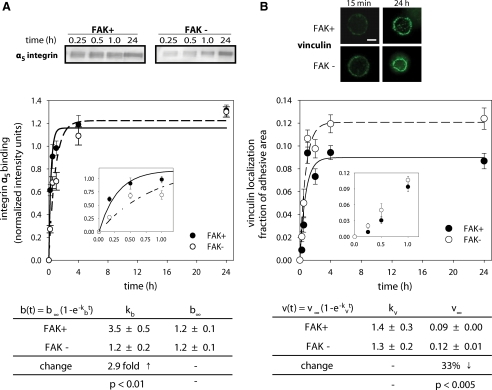
The enhancement in the adhesion strengthening rate in the presence of FAK indicates an increase in the rate of mechanical coupling of the cell to the ECM. Modulation of the strengthening rate could arise from 1) differences in the number of integrin–ECM bonds, 2) enhanced recruitment of strengthening molecules to the site of interaction, or 3) optimization of bond positions for more efficient force distribution (Michael and Garcia, 2007). Bound integrin levels were quantified using a cross-linking/extraction/reversal technique that provides for direct quantitative comparisons among experimental conditions (Garcíaet al., 1999; Gallantet al., 2005). Initial adhesion blocking experiments demonstrated that integrin α5β1 provided the dominant adhesion mechanism, so the integrin binding analysis was performed on the α5 integrin subunit. We have previously demonstrated good correspondence in this assay between α5 and β1 binding to fibronectin (Garcíaet al., 1999). Bound integrins were measured at different adhesion times (Figure 3A), and the results were curve-fit to a simple exponential rise function to obtain a bound integrin rate constant kb and a steady-state bound level b∞ to characterize integrin binding kinetics (Michael and Garcia, 2007). The results presented comprise a minimum of 31 data points from five to six independent experiments. Analysis of the kinetics of bound α5β1 integrins revealed that the bound integrin rate was 2.7-fold higher in FAK+ cells than in FAK− cells (p < 0.01; Figure 3A). The differences in bound integrin levels at early time points can be clearly seen in the inset of Figure 3A. This increase in bound integrin rate correlates well with the 2.6-fold strengthening rate increase (Figure 2B). No differences in the steady-state levels of bound α5β1 integrins were detected between FAK+ and FAK− cells.
Figure 3.
Kinetics of bound integrin and vinculin localization to focal adhesions for FAK-expressing (FAK+, ●) and FAK-null (FAK−, ○) cells. (A) Bound integrins were quantified using a cross-linking/extraction/reversal biochemical assay and Western blotting. Binding kinetics were fit to a simple kinetic model revealing differences in bulk bound integrin rate (kb) due to FAK expression, but no differences were found in the number of steady-state bound integrins (b∞). A minimum of 31 data points (five to six independent experiments) was used for the curve fits, and mean ± SE for each time point is shown. Inset shows bound integrin levels at short adhesion times showing significant differences between FAK+ and FAK− cells. (B) Immunostaining for vinculin recruitment to micropatterned adhesive area at early (15 min) and long-term (24 h) adhesion times (scale bar, 2 μm). Quantification of vinculin localization to adhesive area demonstrated no differences in vinculin recruitment rate (kv). At steady-state, localized vinculin levels (v∞) were significantly lower in the presence of FAK. A minimum of 129 data points was used for the curve fits, and mean ± SE for each time point is shown. Inset shows no differences in vinculin localization levels between FAK+ and FAK− at short adhesion times.
We next quantified vinculin localization to the adhesive area using immunostaining and image analysis (Figure 3B). The use of micropatterned substrates with well-controlled adhesive areas provides a particularly robust platform to obtain quantitative measurements of the recruitment of cytoskeletal elements to a well-defined adhesive interface. In a manner analogous to the adhesive force and integrin-binding kinetic analyses, we measured vinculin recruitment to focal adhesions as a function of time and extracted values for the vinculin recruitment rate constant kv and steady-state vinculin levels v∞ (Figure 3B). This analysis demonstrated no differences in vinculin recruitment rate between FAK+ and FAK− cells. Although we observed increases in vinculin staining intensity as a function of time, there were no differences in vinculin intensity between FAK+ and FAK− at early time points (<1 h). Importantly, no differences in talin recruitment were detected between FAK+ and FAK− cells at any time point (data not shown). These results suggest that the functional differences in adhesive strengthening rate between FAK-expressing and FAK-null cells arise from differences in the rates of integrin binding and not recruitment of vinculin or talin to the adhesive interface. It is important to note that our measurements of integrin binding and focal adhesion recruitment rates represent “bulk” rates relating to the total numbers of these molecules at the adhesive interface at a given time point and do not constitute biomolecular “on”/“off” reaction rates typically measured by FRAP (fluorescence recovery after photobleaching) in live cells.
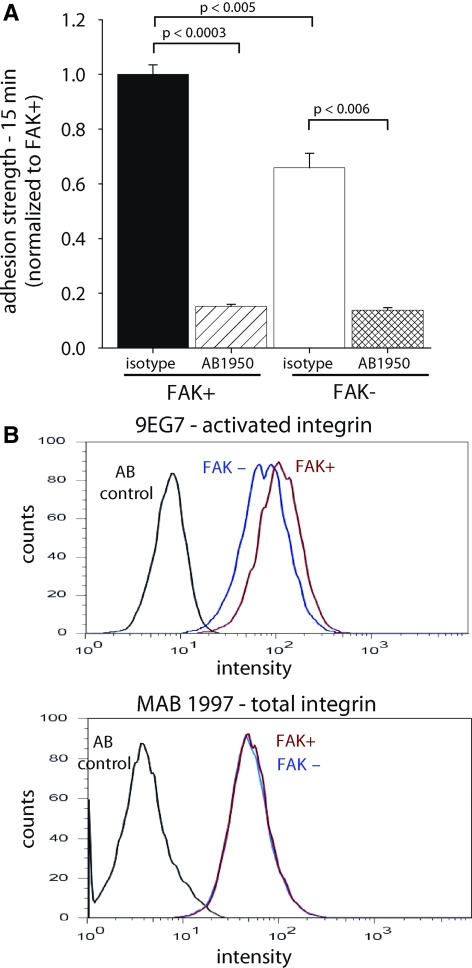
We analyzed the contributions of integrin binding to adhesion strengthening at early time points using blocking antibodies against integrins. Cells were incubated with blocking antibodies for 15 min before seeding on the fibronectin-coated substrates, and adhesive force was measured at 15 min of attachment. Function-blocking antibodies against integrin α5 (AB1950) significantly decreased adhesion strength at 15 min in both FAK+ (>85%) and FAK− (>80%) cells (Figure 4A), indicating that binding of integrin α5β1 to fibronectin provides the primary adhesion mechanism in this system. Importantly, treatment with the blocking antibody eliminated the differences in adhesion strength between FAK+ and FAK− cells (Figure 4A). Similar results were obtained with the anti-β1 integrin, function-blocking HMα5–1 mAb (88 and 85% inhibition for FAK+ and FAK− cells, respectively). No differences in adhesion strength were observed among untreated, isotype control IgG, and anti-αV (H9.2B8) antibodies (data not shown). This result indicates that the differences in adhesive force between FAK+ and FAK− cells arise from differences in α5β1 integrin binding.
Figure 4.
FAK modulates short-term adhesive force via integrin activation. (A) Blocking α5β1 integrin binding to fibronectin (AB1950) reduced adhesion strength at 15 min for both FAK-expressing (FAK+) and FAK-null (FAK−) cells and eliminated strength differences between FAK+ and FAK− cells. (B) Flow cytometry analyses for integrin expression (MAB1997) and activation (9EG7) at 15 min adhesion to fibronectin. Integrin α5β1 surface expression (MAB1997, AB1950) was equal for both FAK-expressing and FAK-null cells. In contrast, FAK-expressing cells had elevated expression of the 9EG7-dependent activated integrin epitope. Results were confirmed in six independent experiments.
We next examined the mechanism by which FAK modulates integrin binding and adhesion strengthening during early adhesion times. Surface expression of integrin α5β1 was equal for FAK+ and FAK− cells (Figure 4B), indicating that FAK modulates binding of integrins already expressed on the cell membrane. Short-term (15 min) differences in adhesion strength were also observed under serum-free conditions (Supplementary Figure S1), indicating that these differences do not arise from secondary, serum-dependent mechanisms such as growth factor signaling. A simple explanation for the differences in integrin binding in the absence and presence of FAK is that FAK modulates integrin activation. To assess integrin activation during these early adhesion times, we analyzed expression of activated integrins using a conformation-specific antibody (9EG7). For these experiments, FAK+ and FAK− cells were plated on fibronectin for 15 min. Cells were then detached with trypsin and assessed for integrin total expression and activation via flow cytometry using MAB1997 and 9EG7, respectively. Flow cytometry experiments with conformation-sensitive 9EG7 antibody revealed that β1 integrins had a higher frequency of being in an active conformation in FAK+ cells than in FAK− cells (Figure 4B). We also observed differences in the 9EG7 staining pattern of adherent cells (15 min) for FAK+ cells, which exhibited more and a wider spatial distribution of 9EG7-positive structures than that of FAK− cells (Supplementary Figure S2). We note that the staining patterns vary considerably across these cells, significantly limiting quantitative analysis of these images. This variability is most likely due to the kinetic nature of the spreading process. Importantly, differences in 9EG7-binding were only observed at early adhesion times (15 min) and not for long-term adhesion (24 h, Supplementary Figure S3). Adhesion to fibronectin was absolutely required for the differences in 9EG7 binding: when cells were maintained in suspension for 45 min before 9EG7 binding analysis, there were no differences in 9EG7 binding between FAK+ and FAK− cells. These integrin activation results are in excellent agreement with the integrin-binding experiments that demonstrated differences in initial integrin binding but no differences in bound integrins at steady state (Figure 3A). Consistent with our observations for steady-state adhesion, Schoberet al. (2007) reported no differences in integrin activation between FAK-null and normal keratinocytes at long adhesion times.
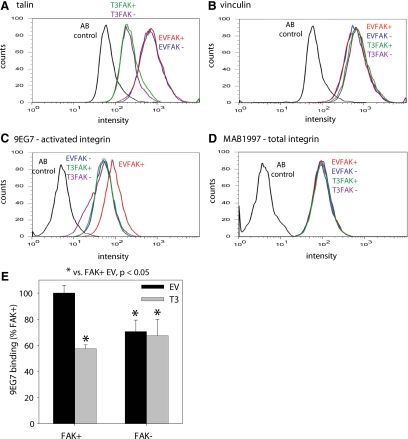
To gain further insights into FAK-mediated integrin activation, we analyzed the requirement for talin in FAK-dependent effects on 9EG7 binding. Specific binding of talin to integrin β tails is a final common step in integrin activation (Tadokoroet al., 2003). Talin expression was knocked down to equivalent levels (60–70%) in FAK+ and FAK− cells using a talin shRNA retrovirus (Figure 5A). Expression levels of vinculin remained unchanged in these cells (Figure 5B), demonstrating that the knockdown was specific to talin. We examined 9EG7 binding for cells in the early stages of adhesion via flow cytometry after adhesion for 15 min. Again, we observed higher 9EG7 binding on FAK+ cells than in FAK− cells (Figure 5C). Talin knockdown reduced 9EG7 epitope expression in FAK+ cells but not in FAK− cells. Moreover, there were no differences in 9EG7 binding between FAK− cells and cells lacking talin, independent of FAK expression. No differences in total integrin expression levels were evident among these experimental groups (Figure 5D). To compare across independent runs, 9EG7 binding was normalized to binding of an antibody that recognizes total integrin and was averaged across all flow cytometry experiments (four to six runs; Figure 5E). ANOVA demonstrated significantly higher levels of 9EG7 binding between FAK+ cells and FAK− cells as well as FAK+ and FAK− cells with talin knocked down. These results indicate that talin is required for FAK-dependent differences in 9EG7 binding.
Figure 5.
Figure 5. Talin is required for FAK-dependent effects on integrin activation. (A) Flow cytometry histograms for talin expression (using 8d4 antibody) for FAK-expressing (FAK+) and FAK-null (FAK−) cells after transduction with talin shRNA (T3) or empty vector (EV) retrovirus showing efficient talin knockdown. (B) Flow cytometry histograms for vinculin expression (using V284 antibody) for FAK+ and FAK− cells showing equivalent levels among treatments. (C) Levels of 9EG7 binding for activated integrin expression for FAK+ and FAK− cells transduced with talin shRNA or control retrovirus. Talin knockdown reduced 9EG7 epitope expression in FAK+ cells but not in FAK− cells. (D) Total β1 integrin surface expression levels for FAK+ and FAK− cells are not altered by talin knockdown. (E) 9EG7 binding normalized to binding of an antibody that recognizes total integrin (AB1950 or MAB1997, equivalent results obtained for both antibodies) and averaged across all flow cytometry experiments (four to six runs).
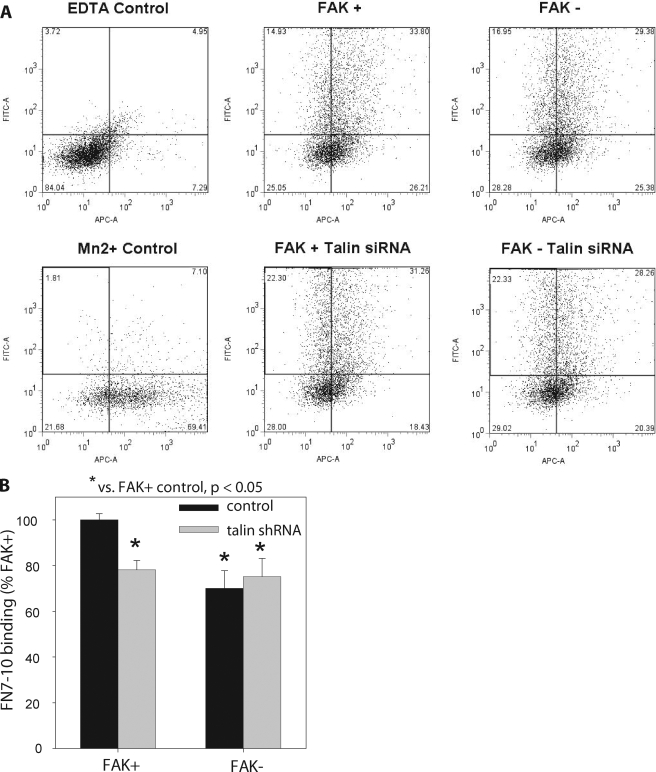
To further strengthen our interpretation that FAK regulates integrin activation, we conducted additional experiments with soluble fibronectin binding. We measured the binding of a monobiotinylated fibronectin fragment (FN7-10) by flow cytometry using similar methods to those established by Calderwood and Ginsberg (Tadokoroet al., 2003; Bouaouinaet al., 2008). FAK+ and FAK− cells, cotransfected 72 h before with a talin shRNA plasmid and a GFP-expressing construct or only the GFP control construct, were plated on fibronectin-coated dishes for 15 min, detached, and resuspended in buffer. Flow cytometry analysis indicated >40% talin knockdown, whereas vinculin levels remained unchanged (data not shown). Cells were incubated in biotinylated FN7-10 for 20 min, washed, incubated in APC-conjugated streptavidin, washed, and analyzed by flow cytometry for transfection of talin shRNA (FITC channel) and FN7-10 binding (APC channel). The cell population was gated for GFP expression and FN7-10 background using untransfected cells under nonactivating conditions (10 mM EDTA). Figure 6A shows dot plots for the experimental conditions analyzed. FAK+ cells displayed a higher percentage of cells in the top, right quadrant, and the FAK+ population was shifted to the right, indicating higher FN7-10 binding than in FAK- and talin-depleted FAK+ and FAK− cells. Figure 6A also shows a dot plot for fibronectin binding in the presence of Mn+2 (2 mM) which promotes maximal integrin activation and fibronectin binding. The mean geometric fluorescence intensity for the FAK+ condition is ∼25% of the maximal fibronectin binding condition, consistent with Calderwood’s observations for control and Mn2+-activated cells (Bouaouinaet al., 2008). For quantitative analysis, we compared the levels of FN7-10 binding for GFP+ cells using the mean geometric fluorescence intensity for the experimental condition normalized to background (10 mM EDTA to inactivate integrins; see Materials and Methods). In contrast to the percentage of cells within each quadrant of the dot blot, the mean geometric fluorescence intensity provides a robust measure of fibronectin binding because it weighs the fluorescence intensity for each cell within the population. Figure 6B presents results for FN7-10 binding as the percentage of binding for FAK+ cells. In excellent agreement with our 9EG7 binding data, FAK+ cells exhibited 30% higher levels of fibronectin binding than in FAK− cells. Talin knockdown significantly reduced fibronectin binding for FAK+ but not FAK− cells. There were no differences in fibronectin binding between talin-depleted FAK+ and FAK− cells. In addition, there were no significant differences in fibronectin binding between FAK− cells and talin-depleted FAK+ and FAK− cells. These results indicate that talin is required for FAK-dependent differences in fibronectin binding. Finally, we point out that no differences in fibronectin binding were detected in cells that were plated overnight, consistent with the 9EG7 results. Taken together, the results for 9EG7 and fibronectin binding demonstrate that FAK regulates integrin binding and that talin is required for these FAK-dependent effects on integrin activation. This requirement of talin in FAK-dependent integrin activation suggests that FAK acts upstream of talin in regulating integrin function.
Figure 6.
FAK expression regulates integrin binding to a soluble fibronectin fragment. FAK+ and FAK− cells, cotransfected 72 h before with the talin shRNA construct and the Amaxa pmaxGFP control plasmid that encodes for GFP or only pmaxGFP plasmid, were plated on fibronectin-coated dishes for 15 min, detached, and resuspended in buffer. Cells were incubated in biotinylated FN7-10 for 20 min, washed, incubated in APC-conjugated streptavidin, washed, and analyzed by flow cytometry for transfection efficiency. (A) Dot blots for GFP expression (FITC channel, y-axis) and FN7-10 binding (APC channel, x-axis). (B) Normalized FN7-10 binding as the percentage of binding for FAK+ cells averaged across three independent experiments.
FAK Knockdown in Human Fibroblasts Modulates Integrin Activation
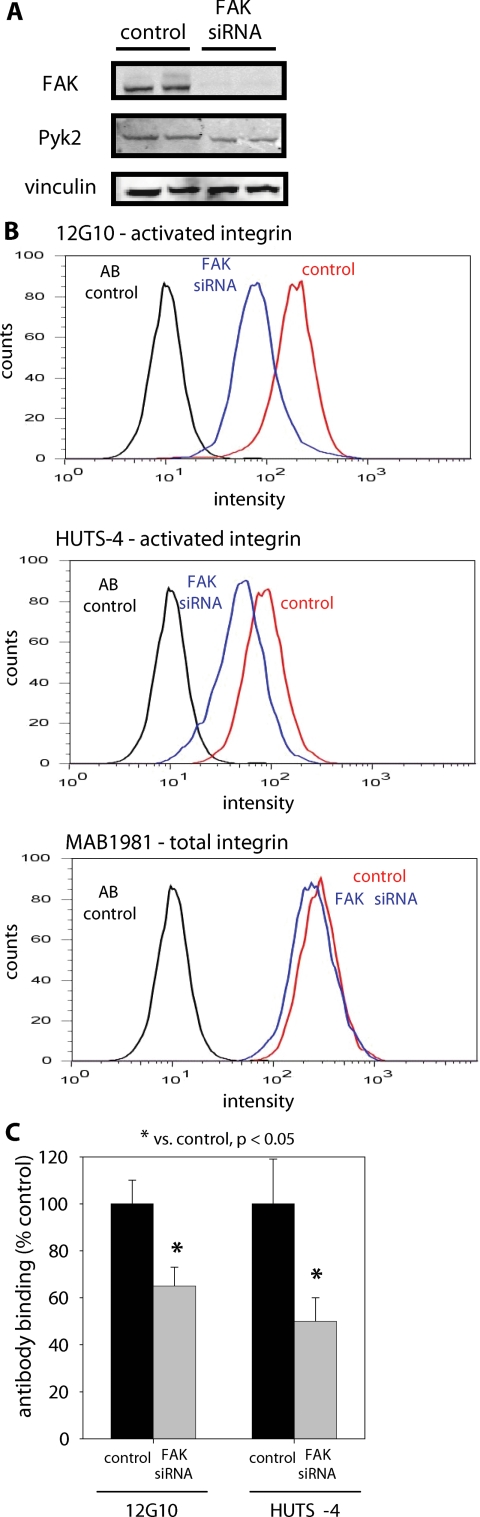
We next examined whether FAK expression regulated integrin activation in primary fibroblasts to rule out any artifacts associated with the FAK-inducible cells. Human dermal fibroblasts were transduced with FAK siRNA-puromycin or control puromycin retrovirus, and puromycin-resistant cells were selected. No differences were observed in functional assays between fibroblasts transduced with control puromycin retrovirus and unmodified cells. Cells treated with the FAK siRNA retrovirus exhibited a 95% reduction in FAK expression, whereas vinculin levels remained unchanged (Figure 7A). No significant differences in Pyk2 expression were observed between FAK-deficient and control human fibroblasts (Figure 7A). Integrin activation experiments were performed as described in the previous section using the conformation-specific human β1 antibodies 12G10 and HUTS-4. Antibody-binding experiments demonstrated that FAK knockdown reduces integrin activation, but not total integrin levels, in these human cells (Figure 7B). 12G10 and HUTS-4 antibody binding was normalized to binding of an antibody that recognizes total integrin and averaged across all flow cytometry experiments (three to four runs; Figure 7C). Knockdown of FAK via siRNA significantly reduced expression of the active integrin receptor as determined by the expression of two conformation-sensitive epitopes. These findings confirm our observations with the FAK-inducible cell system that FAK modulates integrin activation.
Figure 7.
FAK modulates integrin activation in human dermal fibroblasts. Human dermal fibroblasts were transduced with FAK siRNA-puro or control retrovirus, and puromycin-resistant cells were selected. (A) FAK siRNA-puro retrovirus efficiently reduced FAK levels compared with control. No significant differences in Pyk2 or vinculin expression levels were detected. (B) Antibody-binding profiles for 12G10 (top), HUTS-4 (middle), and total integrin (MAB1981, bottom) demonstrating reduced activated integrin expression in FAK siRNA-treated cells. No differences were observed in total β1integrin surface expression levels between FAK siRNA-treated and control cells. (C) 12G10 and HUTS-4 binding normalized to binding of an antibody that recognizes total integrin (MAB1981) and averaged across all flow cytometry experiments (three to four runs).
Role of FAK Autophosphorylation Site on Adhesive Force Responses
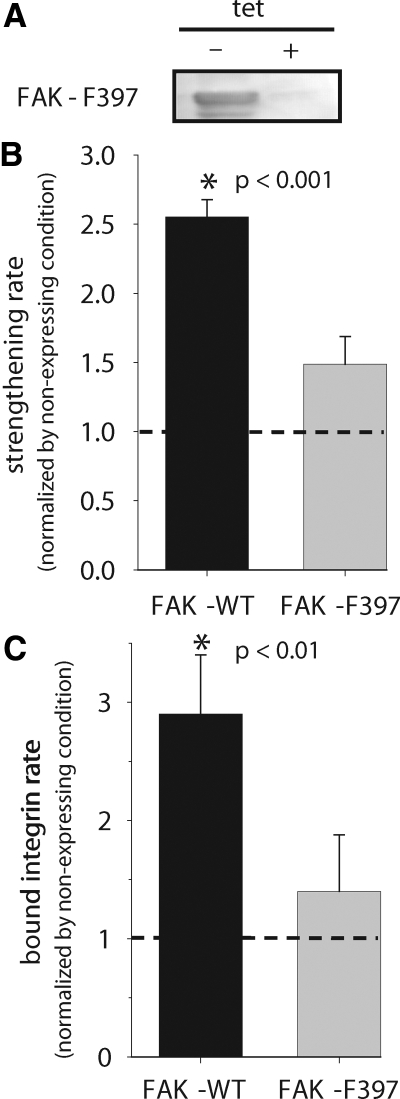
We next examined the role of the Y397 autophosphorylation site in FAK in adhesive force generation using FAK-null cells expressing FAK mutants under the tetracycline-inducible system. FAK Y397 is an autophosphorylation site and a high-affinity binding site for Src homology 2 (SH2) domains of Src family kinases, including c-Src and Fyn, and PI 3-kinase (Schalleret al., 1994; Xinget al., 1994; Polte and Hanks, 1995; Chenet al., 1996). FAK-null cells expressing the mutant FAK-F397 have been previously described (Owenet al., 1999). In the absence of tetracycline in the culture media, these cells express mutant FAK to high levels (Figure 7A).
Cells expressing FAK-F397 exhibited adhesion strengthening kinetics accurately described by a simple exponential function (Supplementary Figure S4), similar to the functional dependence observed for FAK-null cells expressing wild-type FAK. Results for expressed FAK proteins were normalized to the values for the FAK-null matched controls. Analysis of the adhesion strengthening profiles revealed significant differences in the adhesion strengthening rate among wild-type and mutant FAK-F397 (Figure 8A). Compared with the 2.6-fold increase in strengthening rate for wild-type FAK, expression of FAK-F397 resulted in a modest but not significant increase in the strengthening rate over its FAK-null matched control (Figure 8B). This result indicates that phosphorylation of Y397 is required for the adhesive strengthening response.
Figure 8.
FAK Y397 autophosphorylation site is required for FAK-mediated adhesion strengthening response. (A) Western blots demonstrating inducible expression of FAK mutant. (B) Strengthening rate parameter showing differences between wild-type and mutant FAK. Results for expressed FAK proteins were normalized to values for the FAK-null matched controls. (C) Bound integrin rate for wild-type and mutant FAKs demonstrating reduction of integrin binding upon mutation of Y397. Results for expressed FAK proteins were normalized to values for the FAK-null matched controls. The strengthening and binding rates were determined from regression analyses of the experimental data as described for wild-type FAK.
Our analyses of wild-type FAK indicated that FAK increases the adhesion strengthening rate by increasing the rate of integrin binding to fibronectin. Expression of the FAK-F397 mutant altered the integrin binding rate in a manner consistent with the observed effects in adhesion strengthening (Figure 8C). Expression of the FAK-F397 autophosphorylation site mutant resulted in a modest but not significant increase in integrin binding compared with the FAK-null matched control. This change represents a significant decrease compared with the 3.1-fold increase associated with wild-type FAK. These results indicate that Y397 is necessary for the functional effects of FAK on integrin binding rate and adhesion strengthening.
DISCUSSION
We demonstrate that FAK regulates cell adhesion strengthening via integrin activation and binding. This work establishes a new role for FAK in the kinetics of the generation of adhesive forces. Because of the central importance of force generation in cell spreading, migration, muscle contraction, and neurite extension, a thorough understanding of adhesive forces is important to numerous physiological processes and pathological conditions. During the early stages of adhesion, FAK up-regulates integrin activation to enhance integrin binding and, hence, the adhesion strengthening rate. As the adhesive process reaches equilibrium, FAK expression reduces steady-state adhesive force. In addition, there are no differences in steady-state integrin binding between FAK+ and FAK− cells. The differences in steady-state adhesive force between FAK+ and FAK− cells correlate with the levels of vinculin recruitment to focal adhesions in the adhesive interface, consistent with previous observations of focal adhesion turnover and vinculin contributions to adhesive force (Ilicet al., 1995a; Webbet al., 2004; Gallantet al., 2005). These results indicate that FAK plays a multifaceted, time-dependent role in adhesive force generation. These findings provide new insights into the regulation of adhesive interactions. Previous analyses did not reveal a significant role for FAK in the development of traction forces. Using deformable substrates, Wang and colleagues showed no differences in integrated traction forces between FAK-null cells and cells reexpressing wild-type FAK (Wanget al., 2001). Similarly, Chen and colleagues reported no differences in traction force between FAK-null and FAK-reexpressing cells (Pironeet al., 2006). In contrast, FAK expression has been associated with a transient inhibition of Rho activity (Renet al., 2000; Pironeet al., 2006), suggesting a down-regulation in cytoskeletal tension. This transient inhibition in Rho activity may be related to the time-dependent differences in integrin binding and adhesive force observed in the present analysis. These distinctions between traction force and adhesion strength results illustrate important differences between these measurement modalities and underscore the importance of obtaining direct functional measurements for adhesive force.
The importance of FAK in regulating adhesion strengthening kinetics provides a new perspective on the role of FAK in migratory and mechanosensing responses. FAK-regulated, time-dependent generation of strong adhesive forces (i.e., strengthening rate) would significantly alter lamellipodium dynamics and traction forces at the leading edge of migratory and spreading cells. Impairment of adhesive forces at the cell front provides an explanation for the defects in migration speed and mechanosensing in FAK-null cells (Owenet al., 1999; Sieget al., 2000; Wanget al., 2001; Webbet al., 2004). This explanation is consistent with recent observations that FAK is involved in actin polymerization via interactions with Arp2/3 (Serrelset al., 2007). The present work contributes a new aspect to the current model of cell migration in which FAK regulates focal adhesion disassembly to allow protrusion as well as cell body translocation (Ilicet al., 1995b; Webbet al., 2004).
Analysis of FAK phosphorylation mutants demonstrated an important role for the Y397 autophosphorylation site in adhesion strengthening and integrin binding rate. Mutation of the Y397 autophosphorylation site blocked FAK-mediated adhesive responses. The dependence of adhesion strengthening on FAK Y397 phosphorylation is consistent with the role of this site in other adhesive processes. Migratory and spreading activities exhibit a strong dependence on Y397 (Owenet al., 1999; Sieget al., 2000; Wanget al., 2001; Webbet al., 2004), and mutation of this residue often results in complete blocking of these responses.
A major finding of our studies is the identification of FAK as a modulator of integrin activation. To our knowledge, this is the first report demonstrating a role for FAK in integrin activation. FAK-mediated changes in integrin activation support previous reports demonstrating a role for FAK in controlling cell adhesion activation (Glodeket al., 2007; Thamilselvanet al., 2007). Our conclusion that FAK modulates integrin activation is based on the results for 1) integrin binding to immobilized fibronectin, 2) binding of multiple conformation-sensitive antibodies, and 3) integrin binding to a soluble fragment of fibronectin presenting the integrin binding domain. Integrin binding analyses using a biochemical assay that specifically isolates bound integrins demonstrated significantly increased levels of integrin binding to adsorbed fibronectin in FAK+ cells compared with FAK− cells at the early time points. Because there were no differences in total integrin expression between these two cells, the increased levels of bound integrins arise from higher numbers of activated integrins in the FAK+ cells. The experiments with conformation-sensitive antibodies provide an independent confirmation of this result. Conformation-sensitive antibody binding represents the generally accepted method to assess integrin activation (Calderwood, 2004; Humphries, 2004), and recent studies have used the same β1-specific antibody (9EG7) to establish integrin activation in adherent cells (Galbraithet al., 2007; Humphrieset al., 2007). Furthermore, the observation of integrin activation in the FAK-inducible expression system was confirmed in an independent experiment with human dermal fibroblasts treated with a FAK RNAi construct and using two different conformation-sensitive antibodies (12G10, HUTS-4). Finally, because interpretation of studies with conformation-sensitive antibodies is complicated by the fact that these antibodies are not pure reporters of integrin activity but can modulate the conformational state of the receptor (Humphries, 2004), we also performed functional binding experiments with a soluble fibronectin fragment. Binding experiments with a soluble fibronectin fragment presenting the central integrin binding domain confirmed that FAK expression regulates integrin activation.
Recent work has demonstrated that the FAK-related kinase Pyk2 is overexpressed in the absence of FAK and modulates focal adhesion assembly and cell migration via changes in p190RhoGEF expression (Limet al., 2008). We do not observe significant differences in Pyk2 expression between FAK-expressing and FAK-null inducible FAK cells. However, we previously showed that these FAK inducible cells express elevated levels of Pyk2 (possibly as a compensatory mechanism) and that FAK expression reduces the level of Pyk2 tyrosine phosphorylation (Owenet al., 1999; Royet al., 2002). To rule out effects related to this specific cell model, we also examined the role of FAK expression in integrin activation in primary human fibroblasts. RNAi-mediated knockdown of FAK in these primary cells also reduced integrin activation and no differences in Pyk2 expression were observed.
Experiments with talin knockdown showed that talin is required for the FAK-dependent effects on integrin activation. This result is consistent with the current model of talin representing a final step in integrin activation (Calderwood and Ginsberg, 2003; Tadokoroet al., 2003) and suggests that FAK acts upstream of talin in regulating integrin activation. Although the molecular mechanisms involved in FAK-mediated integrin activation remain unknown, a model in which FAK interacts with talin to regulate talin binding to the integrin tails is particularly attractive. The C-terminal focal adhesion targeting (FAT) domain of FAK is known to bind to talin (Chenet al., 1995; Hayashiet al., 2002). Additional analyses beyond the scope of the present work are required to establish the mechanistic links among FAK, talin, and integrin in the adhesive-strengthening response. On the basis of the present findings, we propose a model for FAK-dependent modulation of adhesion strengthening. Integrin receptor binding, clustering, and focal adhesion assembly provide mechanical coupling to the ECM. The major contributor of cell–ECM adhesive force is the integrin–ECM bond (Gallantet al., 2005; Gallant and Garcia, 2007). Clustering of integrin/ECM bonds increases adhesive force by increasing the number of force bearing members within a small area. Focal adhesion assembly further enhances adhesive force by efficiently distributing mechanical loads among bound integrin clusters. During the early stages of adhesion, FAK activates integrins to increase the number of bound integrins over time, resulting in adhesion strengthening. These findings demonstrate an important role for FAK in the time-dependent generation of cell–ECM forces.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank A. P. Kowalczyk (Emory University) for human dermal fibroblasts, D. A. Calderwood (Yale University), and S. W. Craig (Johns Hopkins University), D. Boettiger (University of Pennsylvania), and C. Zhu (Georgia Institute of Technology) for helpful discussions. The talin shRNA plasmid and FAK siRNA-puro constructs were kindly provided by M. H. Ginsberg (Scripps Research Institute) and M. A. Alaoui-Jamali (McGill University), respectively. The pMSCV-puro plasmid was kindly provided by G. J. Hannon (Cold Spring Harbor Laboratory). This work was supported by National Institutes of Health Grants R01-GM065918 and R01-GM049882 and an National Science Foundation Graduate Fellowship (K.E.M.).
Abbreviations used:
- FAK
focal adhesion kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0076) on March 18, 2009.
REFERENCES
- Benlimame N., He Q., Jie S., Xiao D., Xu Y. J., Loignon M., Schlaepfer D. D., Alaoui-Jamali M. A. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J. Cell Biol. 2005;171:505–516. doi: 10.1083/jcb.200504124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaouina M., Lad Y., Calderwood D. A. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate beta1 and beta3 integrins. J. Biol. Chem. 2008;283:6118–6125. doi: 10.1074/jbc.M709527200. [DOI] [PubMed] [Google Scholar]
- Braren R., Hu H., Kim Y. H., Beggs H. E., Reichardt L. F., Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B. A., Pavlath G. K., Murphy T. J., Karsenty G., García A. J. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J. Bone Miner. Res. 2002;17:1931–1944. doi: 10.1359/jbmr.2002.17.11.1931. [DOI] [PubMed] [Google Scholar]
- Calderwood D. A. Integrin activation. J. Cell Sci. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- Calderwood D. A., Ginsberg M. H. Talin forges the links between integrins and actin. Nat. Cell Biol. 2003;5:694–697. doi: 10.1038/ncb0803-694. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Appeddu P. A., Isoda H., Guan J. L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Appeddu P. A., Parsons J. T., Hildebrand J. D., Schaller M. D., Guan J. L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Clemente C. F., Tornatore T. F., Theizen T. H., Deckmann A. C., Pereira T. C., Lopes-Cendes I., Souza J. R., Franchini K. G. Targeting focal adhesion kinase with small interfering RNA prevents and reverses load-induced cardiac hypertrophy in mice. Circ. Res. 2007;101:1339–1348. doi: 10.1161/CIRCRESAHA.107.160978. [DOI] [PubMed] [Google Scholar]
- Furuta Y., Ilic D., Kanazawa S., Takeda N., Yamamoto T., Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Gabarra-Niecko V., Schaller M. D., Dunty J. M. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- Galbraith C. G., Yamada K. M., Galbraith J. A. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science. 2007;315:992–995. doi: 10.1126/science.1137904. [DOI] [PubMed] [Google Scholar]
- Gallant N. D., Garcia A. J. Model of integrin-mediated cell adhesion strengthening. J. Biomech. 2007;40:1301–1309. doi: 10.1016/j.jbiomech.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Gallant N. D., Michael K. E., Garcia A. J. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell. 2005;16:4329–4340. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A. J., Vega M. D., Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol. Biol. Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodek A. M., Le Y., Dykxhoorn D. M., Park S. Y., Mostoslavsky G., Mulligan R., Lieberman J., Beggs H. E., Honczarenko M., Silberstein L. E. Focal adhesion kinase is required for CXCL12-induced chemotactic and pro-adhesive responses in hematopoietic precursor cells. Leukemia. 2007;21:1723–1732. doi: 10.1038/sj.leu.2404769. [DOI] [PubMed] [Google Scholar]
- Gupton S. L., Waterman-Storer C. M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I., Vuori K., Liddington R. C. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat. Struct. Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- Humphries J. D., Wang P., Streuli C., Geiger B., Humphries M. J., Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J. Monoclonal antibodies as probes of integrin priming and activation. Biochem. Soc. Trans. 2004;32:407–411. doi: 10.1042/BST0320407. [DOI] [PubMed] [Google Scholar]
- Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995a;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ilic D., Furuta Y., Suda T., Atsumi T., Fujimoto J., Ikawa Y., Yamamoto T., Aizawa S. Focal adhesion kinase is not essential for in vitro and in vivo differentiation of ES cells. Biochem. Biophys. Res. Comm. 1995b;209:300–309. doi: 10.1006/bbrc.1995.1503. [DOI] [PubMed] [Google Scholar]
- Leucht P., Kim J. B., Currey J. A., Brunski J., Helms J. A. FAK-mediated mechanotransduction in skeletal regeneration. PLoS ONE 2. 2007:e390. doi: 10.1371/journal.pone.0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael K. E., Garcia A. J. Cell adhesion strengthening: measurement and analysis. Methods Cell Biol. 2007;83:329–346. doi: 10.1016/S0091-679X(07)83014-7. [DOI] [PubMed] [Google Scholar]
- Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison P. J., Caudy A. A., Sachidanandam R., Hannon G. J. Short hairpin activated gene silencing in mammalian cells. Methods Mol. Biol. 2004;265:85–100. doi: 10.1385/1-59259-775-0:085. [DOI] [PubMed] [Google Scholar]
- Palecek S. P., Loftus J. C., Ginsberg M. H., Lauffenburger D. A., Horwitz A. F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Peng X., Wu X., Druso J. E., Wei H., Park A. Y., Kraus M. S., Alcaraz A., Chen J., Chien S., Cerione R. A., Guan J. L. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc. Natl. Acad. Sci. USA. 2008;105:6638–6643. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie T. A., Capadona J. R., Reyes C. D., Garcia A. J. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459–5470. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Pirone D. M., Liu W. F., Ruiz S. A., Gao L., Raghavan S., Lemmon C. A., Romer L. H., Chen C. S. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 2006;174:277–288. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte T. R., Hanks S. K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B., Sieg D. J., Otey C. A., Schlaepfer D. D., Schwartz M. A. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Renshaw M. W., Price L. S., Schwartz M. A. Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J. Cell Biol. 1999;147:611–618. doi: 10.1083/jcb.147.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Ruest P. J., Hanks S. K. FAK regulates tyrosine phosphorylation of CAS, paxillin, and PYK2 in cells expressing v-Src, but is not a critical determinant of v-Src transformation. J. Cell. Biochem. 2002;84:377–388. doi: 10.1002/jcb.10025. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Hildebrand J. D., Parsons J. T. Complex formation with focal adhesion kinase: A mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell. 1999;10:3489–3505. doi: 10.1091/mbc.10.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M., Raghavan S., Nikolova M., Polak L., Pasolli H. A., Beggs H. E., Reichardt L. F., Fuchs E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 2007;176:667–680. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrels B., Serrels A., Brunton V. G., Holt M., McLean G. W., Gray C. H., Jones G. E., Frame M. C. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 2007;9:1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- Shen T. L., Park A. Y., Alcaraz A., Peng X., Jang I., Koni P., Flavell R. A., Gu H., Guan J. L. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Sieg D. J., Hauck C. R., Schlaepfer D. D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Thamilselvan V., Craig D. H., Basson M. D. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007;21:1730–1741. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- van Nimwegen M. J., Verkoeijen S., van Buren L., Burg D., van de W. B. Requirement for focal adhesion kinase in the early phase of mammary adenocarcinoma lung metastasis formation. Cancer Res. 2005;65:4698–4706. doi: 10.1158/0008-5472.CAN-04-4126. [DOI] [PubMed] [Google Scholar]
- Wang H. B., Dembo M., Hanks S. K., Wang Y. Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe F., Miyazaki T., Takeuchi T., Fukaya M., Nomura T., Noguchi S., Mori H., Sakimura K., Watanabe M., Mishina M. Effects of FAK ablation on cerebellar foliation, Bergmann glia positioning and climbing fiber territory on Purkinje cells. Eur. J. Neurosci. 2008;27:836–854. doi: 10.1111/j.1460-9568.2008.06069.x. [DOI] [PubMed] [Google Scholar]
- Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Weis S. M., Lim S. T., Lutu-Fuga K. M., Barnes L. A., Chen X. L., Gothert J. R., Shen T. L., Guan J. L., Schlaepfer D. D., Cheresh D. A. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J. Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Chen H. C., Nowlen J. K., Taylor S. J., Shalloway D., Guan J. L. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol. Biol. Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. H., Reiske H., Guan J. L. Regulation of the cell cycle by focal adhesion kinase. J. Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.