Abstract
Many lupus patients develop neuropsychiatric manifestations, including cognitive dysfunction, depression, and anxiety. However, it is not clear if neuropsychiatric lupus is a primary disease manifestation, or is secondary to non-CNS disease. We found that MRL/lpr lupus-prone mice exhibited significant depression-like behavior already at 8 weeks of age, despite normal visual working memory, locomotor coordination and social preference. Moreover, depression was significantly correlated with titers of autoantibodies against DNA, NMDA receptors and cardiolipin. Our results indicate that lupus mice develop depression and CNS dysfunction very early in the course of disease, in the absence of substantial pathology involving other target organs.
Keywords: Systemic Lupus Erythematosus, Neuropsychiatric lupus, Anti-DNA antibodies, Depression
1. Introduction
Systemic Lupus Erythematosus (SLE) is an autoimmune disease affecting multiple organ systems, including the heart, skin, joints, and kidney. In addition, approximately 70% of lupus patients suffer central nervous system (CNS) manifestations including cognitive dysfunction and mood disorders (Bluestein, 1992), with major depression being one of the most common psychiatric presentations (as high as 40%). However, it is unclear if the neuropsychiatric signs and symptoms are secondary manifestations of widespread organ dysfunction, or if the CNS itself is a primary target of autoimmune dysfunction in lupus. Some studies suggest that the neurological manifestations in lupus may be a secondary consequence of lupus nephritis, due to uremia or inflammatory changes and increased permeability of the brain-blood-barrier (BBB). Alternatively, although lupus neuropathology can include neuronal death (Kowal et al., 2004) and loss of brain volume, behavioral abnormalities may also be evident when there is no gross CNS pathology (Hermosillo-Romo and Brey, 2002), suggesting that chemokines, autoantibodies and other inflammatory mediators may be instrumental in the pathogenesis of the neuropsychiatric manifestations of lupus (Fragoso-Loyo et al., 2007). Furthermore, it has been reported that the mood and cognitive deficits prevalent in lupus patients may not reliably correlate with measures of active disease and/or disease flares involving other organ systems (Hermosillo-Romo and Brey, 2002), suggesting primary CNS involvement in this disease. Thus, behavioral outcomes, such as depression, may represent a sensitive measure of underlying CNS disease mechanisms that have yet to be elucidated.
The diagnostic criteria for lupus include high titers of anti-nuclear autoantibodies (ANA), especially anti-double stranded (ds) DNA antibodies, which are particularly important in the pathogenesis of lupus nephritis (Deocharan et al., 2002; Putterman, 2004). In addition, autoantibodies to neuronal and/or other CNS antigens are also typically present, including N-methyl-D-aspartate receptors (NMDAR). SLE autoantibodies can damage neurons and cause cognitive deficits when the blood brain barrier is breached (Kowal et al., 2004), as can occur as a consequence of lupus related nephritis. Moreover, high immunoglobulin G (IgG) and albumin levels are present in cerebrospinal fluids in murine models of lupus, and these are cytotoxic to neurons in vitro (Sidor et al., 2005). However, it remains unclear whether the CNS lupus syndromes are directly caused by neurocytoxicity induced by high titers of autoantibodies, since immunoglobulins can not cross the intact blood brain barrier in normal individuals.
There are several lupus-prone mouse strains in current use, including MRL/lpr, NZB/W F1, and BxSB (Sakic et al., 1997b). Among the listed strains, MRL/lpr mice have been most extensively used in lupus related neuropsychiatric studies. In MRL/lpr mice, an insertion of a retrotransposon in the gene for Fas (CD95) results in defective apoptosis of lymphocytes and massive lymphoproliferation, resulting in impaired regulation of autoreactive B cells and high autoantibody titers (Chu et al., 1993). Previous studies in MRL/lpr mice have indicated numerous characteristics validating the model, such as a higher prevalence in females, increased ANA titers, and severe kidney pathology (Theofilopoulos, 1992). Moreover, emotional and cognitive deficits present in human lupus such as depression and impaired memory have been demonstrated in older male MRL/lpr mice (12–16 weeks of age) (Sakic et al., 1994), although female mice have not been systematically studied.
Lupus becomes manifest earlier, and is notably more severe, in females as compared to males in human patients as well as in most murine models of the disease, including MRL/lpr mice. Therefore, we hypothesized that neuropsychiatric manifestations would be present even earlier in female than in male mice. Thus, we decided to investigate the age of onset and severity of cognitive and affective outcomes in female MRL/lpr mice, and explore a possible relationship of neuropsychiatric manifestations to several categories of autoantibodies. To this end, we conducted a behavioral battery including tests of depression (forced swim), anxiety (elevated plus maze), sickness behavior (social preference), locomotor activity (open field), motor coordination (balance beam), and cognition (novel object recognition test). Finally, we investigated neuropathology in this lupus-prone strain by magnetic resonance imaging (MRI) and magnetic resonance spectroscopic imaging (MRSI).
2. Materials and methods
2.1. Mice
Ten 3–4 week old MRL/MpJ-Faslpr (MRL/lpr; stock #006825) female mice and 10 age and background matched MRL/MpJ (MRL/+; stock #000486) female mice were purchased from The Jackson Laboratory (Bar Harbor, Maine), and housed five mice per cage in the animal facility of the Albert Einstein College of Medicine (Bronx, NY). The housing conditions were controlled, with the temperature at 21–23°C and a 12:12 hours light:dark cycle. All animal studies were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine. All the behavior tests were done in the light phase (7AM–7PM).
2.2. Assessment of lupus
Mice were monitored bi-weekly for the development of proteinuria and autoantibody titers. Urinary protein excretion was measured by dipstick analysis (Uristix; Bayer), where +1 is 30 mg/dl, +2 is 100 mg/dl, +3 is 300 mg/dl, and +4 is ≥2000 mg/dl. Mouse IgG anti-double stranded (ds) DNA antibody titers were determined by ELISA, as previously described in detail (Deocharan et al., 2007). Similar ELISA protocols were also used for anti-chromatin, anti-cardiolipin and anti-N-methyl-D-aspartate receptor antibody ELISAs (Putterman and Diamond, 1998). Briefly, antigens were coated on 96-well plates overnight (chromatin 5 μg/ml; cardiolipin at 75 μg/ml; multimeric DWEYSVWLSN (containing a linear peptide sequence present in NMDA receptors) at 20 μg/ml) and blocked with 3% fetal calf serum in PBS for 1 hour. Sera samples were diluted 1:250 in PBS before being added to the plates for a 2 hour incubation. Alkaline phosphotase-conjugated goat anti-mouse IgG was used as the detection antibody.
2.3. Behavioral testing
For all the behavioral tests, mice were transferred to the test room and equilibrated for 30–40 minutes prior to the tests which were performed under low incandescent light. Except for the balance beam walking test, all tests were digitally recorded by Viewer tracking software (Biobserve, Bonn, Germany).
2.3.1. Open field test
General locomotor activity was assessed in an open field (39 cm × 39 cm) for 15 minutes. Central and peripheral zone entries, track lengths and durations were recorded in 5 minute bins using Viewer Software, with the middle of the body of the animal defined as the criteria point for tracking a zone entry. The central zone was defined as a 15 cm × 15 cm area in the center of the box (Sakic et al., 1994; Ziporen et al., 1997).
2.3.2. Balance beam walking test
The beam used for the test is 100 cm long with a diameter of 1.5 cm. The start end was brightly illuminated whereas the goal box was dimly lit and contained a palatable food (cocoa puffs) to foster reliable beam crossing. The animals were pre-exposed to the palatable food for 3–5 days prior to testing to reduce neophobia. Motor coordination was assessed as the latency to cross the beam (time in seconds) and the number of slips (both recorded manually). A slip was defined as one of the paws passing below the midline of the beam (Sakic et al., 1994; Shoenfeld et al., 2003).
2.3.3. Social preference test
The typical preference for a conspecific over an inanimate object was assessed in a Y maze (each arm 5 cm × 30 cm). Within a 5 minute period, social preference was assessed as time spent exploring a novel, ovariectomized stimulus mouse or a novel object, placed at the end of each of two arms with a transparent plastic barrier (with holes) separating them from the rest of the maze. Social preference was defined as the percent time exploring the ovariectomized mouse/total exploration time. Normal mice generally spend >50% of the time exploring the conspecific living mouse, whereas animals with high levels of anxiety, depression, or sickness behavior demonstrate reduced social exploration (Dantzer et al., 1987).
2.3.4. Object recognition (visual memory) test
The novel object recognition test of memory is based on the robust tendency of rodents to preferentially explore novel objects, and was performed essentially as described (Ennaceur et al., 1997) in an opaque Perspex arena (39 × 39 cm). In Trial 1, mice were given 3 minutes to freely explore 2 identical, non-toxic objects (such as glass, plastic and ceramic items). Mice were then removed to the home cage for a 45 minute retention interval, and returned to the arena for 3 minutes after one object had been replaced with a novel object (Trial 2). Data from Trial 2 were expressed as a preference score (time exploring novel object/total exploration) × 100. A preference score of 50% indicates chance performance. Exploration of the objects was defined as whisking, sniffing, rearing on or touching the object, and approach and obvious orientation to the object within 3–5 cm. Novelty-induced exploration in Trial 1 was also assessed and expressed as the total time spent exploring both the objects. This ensures that all groups had equal tendency to explore novel objects. One of the 8 week old MRL/+ mice was used for setting up the system and was excluded from the analysis.
2.3.5. Forced swim test
Individual mice were placed into a glass beaker (25 cm height, 15 cm diameter) containing 3000 ml (17 cm height) of water maintained at approximately 27°C. Each 10 minute test session was digitally recorded with the Viewer software and manually scored. Mice placed in this situation with no way to escape begin struggling and swimming but eventually exhibit behavioral despair, assessed as immobility (Porsolt et al., 1977a; Katzav et al., 2007). Depression-like behavior was defined as immobility (floating) and is illustrated as the % time spent immobile (Porsolt et al., 1977a). The latency to begin floating was also assessed and was defined as the first bout of immobility lasting at least 8 seconds. The scorers were blinded to the experimental groups. Two of the 8 week MRL/+ mice were used for setting up the system, and their total floating time were not recorded for the analysis.
2.3.6. Elevated-plus-maze test
Mice were tested on a plus maze, elevated 50 cm above the floor, in a room with low, indirect incandescent lighting and low noise levels. The plus maze consists of 2 enclosed arms (10 cm × 43 cm × 20 cm) and 2 open arms (10 cm × 43 cm × 20 cm), and has been validated in detail elsewhere (Pellow et al., 1985). At the time of testing, each animal was evaluated for 15 minutes after exiting to the center platform of the plus maze. To be considered as an entry into any arm, the mouse must pass the line of the open platform with all four paws. The duration (in seconds) of time spent in the open arm from the time of entry was recorded, in addition to the number of entries into both arms. Decreased % of open arm entries and decreased % time spent in the open arm generally indicates higher levels of anxiety-like behavior (Pellow et al., 1985).
2.3.7. Estrous cycle measurement
Estrous cycle stage was determined by microscopic examination of the vaginal lavage, as described previously (Montes and Luque, 1988) daily (5 pm) for 2 weeks.
2.4. Proton magnetic resonance imaging and magnetic resonance spectroscopic imaging of mouse brain
Proton magnetic resonance spectroscopic imaging (1H MRSI) of mice brains was performed on a 9.4T 21 cm horizontal bore imaging system (Varian INOVA, Palo Alto, CA), equipped with home-made coils. The coils included an actively detunable volume transmitter coil and a 12 mm × 8 mm elliptical surface receiver coil. Animals were anesthetized and maintained on 2% isoflurane administered through a specially designed nose cone. The animals were maintained at a constant temperature throughout the experiment using heated circulating water.
An inversion recovery gradient echo imaging sequence (128 × 128 acquisition matrix, 17 slices, 0.5 mm slice thickness, 24 mm × 24 mm field of view, 900 ms inversion time) was employed to acquire scout images. After performing non-iterative shimming as previously described (Miyasaka et al., 2006), water suppressed MRSI was obtained using an adiabatic refocusing method (LASER) (Garwood and DelaBarre, 2001) with 1 mm slice thickness in combination with a broad-band semiselective excitation sequence and a selective 90 degree water suppression pulse. Two dimensions of 24 × 24 phase encodes were acquired over a FOV of 24 mm × 24 mm. The data was acquired with a repetition time of 2000 ms, and an echo time of 50 ms using two averages resulting in an acquisition time of approximately 38 minutes. Spatial dimensions of the data were filtered with a Hanning filter. After the acquisition of MRSI data, images with a higher resolution (256 × 256 acquisition matrix) were acquired using the same inversion recovery gradient echo imaging sequence with two averages in order to quantify the hippocampal volume of the mouse brain.
To compare differences in brain metabolites between control and MRL/lpr mice, N-acetyl aspartate/creatine (NAA/Cr), NAA/choline (NAA/Ch) and creatine/choline (Cr/Ch) ratios were measured from the spectra obtained from the hippocampus, thalamus and cortex in each animal. The voxels across each brain region, e.g., hippocampus, thalamus and cortex, were selected using an image-guided single voxel reconstruction algorithm via voxel-shifting methods (Miyasaka et al., 2006; Pan et al., 2005; Twieg et al., 1989). The values over the reconstructed voxels were averaged to give a mean value in each region. All data were processed using MATLAB® (The MathWorks, Natick, MA).
2.5. Statistics
Graphpad/Prism4 software was used for the statistical analysis. An unpaired t test (two tailed) or non-parametric Mann-Whitney test was used to compare the differences between two groups. Two way repeated measures ANOVA (age by genotype) with Bonferroni post-hoc tests were used to analyze the data from repeated measurements. Correlation and linear regression analysis were also performed. For all the statistics, significance was defined as p<0.05.
3. Results
3.1. Lupus-prone MRL/lpr mice develop higher autoantibody titers by 8 weeks of age
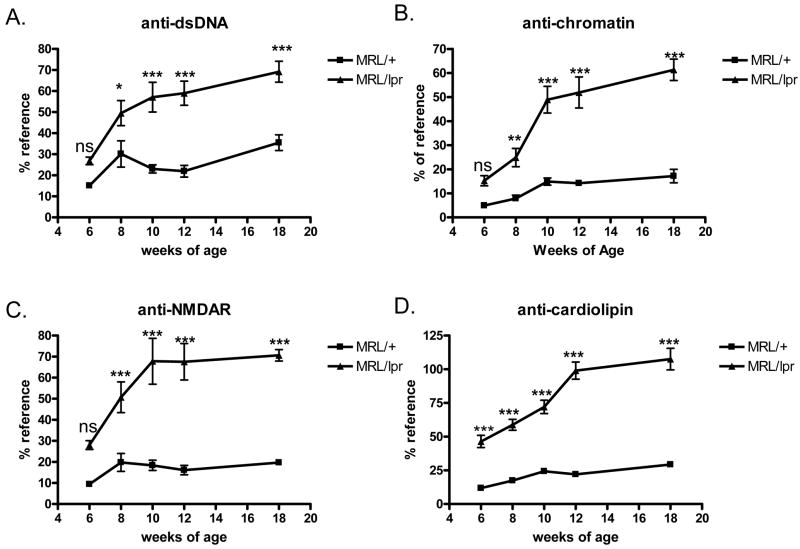
The onset and severity of murine lupus is usually measured by autoantibody levels, especially titers of anti-dsDNA and other autoantibodies, and the amount of urinary protein excretion (proteinuria), which reflects kidney dysfunction. We monitored these parameters in lupus-prone MRL/lpr female mice and the congenic wild type control MRL/+ mice over the time-course of the study, starting from 6 weeks to the mid-disease point at 18 weeks of age.
We found that typical serologic indicators of lupus were evident by 8 weeks in MRL/lpr female mice, and continued to increase over time. At 6 weeks of age, anti-dsDNA Ab titers were similar in MRL/lpr and the congenic MRL/+ mice. Starting at week 8 and throughout week 18, MRL/lpr mice displayed significantly higher anti-dsDNA autoantibody titers than MRL/+ controls (Fig. 1A). A two-way repeated measures (RM) ANOVA analyzing anti-dsDNA Ab titers revealed a main effect of genotype (F(1,18)=32.80; p<0.0001) and a main effect of age (F(4,18) =26.80; p<0.0001). The Bonferroni post-hoc tests indicated that the anti-dsDNA Ab titers were significantly different between strains at week 8 (p<0.05) and weeks 10–18 (p<0.001), but not week 6 (p>0.05). This result is consistent with previous reports that no disease is yet present in MRL/lpr mice at 6 weeks of age, and that the disease-onset time is around 8 weeks of age (Sakic et al., 1992).
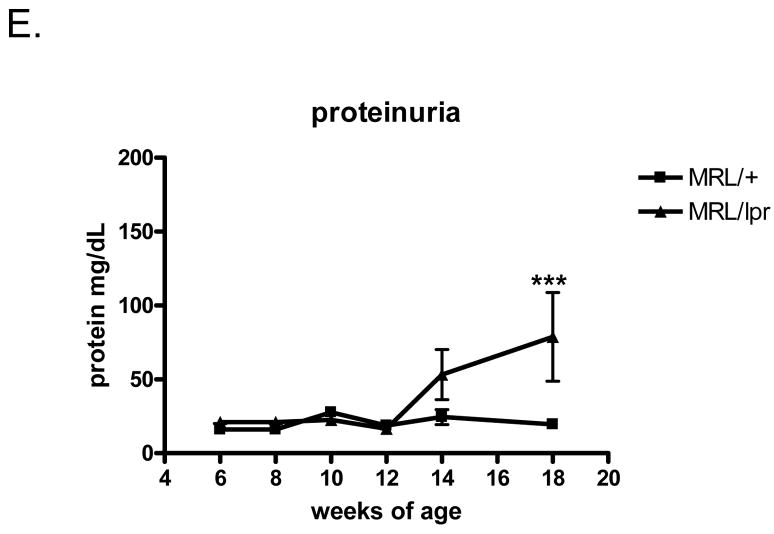
Fig. 1.
Proteinuria and autoantibody levels in MRL/lpr and MRL/+ mice. A–D, Mouse serum autoantibody titers examined by ELISA, from age 6w to 18w. A. IgG anti-dsDNA titers. B. IgG anti-chromatin titers. C. IgG anti-NMDAR titers. D. IgG anti-cardiolipin titers. E. Urine protein concentrations in MRL/+ and MRL/lpr mice at 6–18 weeks. N=10 in each of the mice groups in this Figure. *, p<0.05; **, p<0.01; ***, p<0.001
We also measured titers of anti-chromatin antibodies, another anti-nuclear autoantibody characteristic of SLE. At 6 weeks of age, anti-chromatin antibody titers were similar in MRL/lpr and MRL/+ mice. Starting at week 8, MRL/lpr mice developed significantly higher anti-chromatin autoantibody titers than the congenic MRL/+ controls, which continued to increase throughout week 18 (Fig. 1B). A two-way RM ANOVA analyzing anti-chromatin Ab titers revealed a main effect of genotype (F(1,18)=117.30; p<0.0001) and a main effect of age (F(4,18) =27.03; p<0.0001). The Bonferroni post-hoc tests indicated that the anti-chromatin antibody titers were significantly different between strains from week 8 to week 18 (p<0.01 or 0.001 at each time point), but not at week 6 (p>0.05).
It has been reported that the anti-dsDNA antibodies from SLE patients can cross-react with NMDA receptors and mediate neuronal cell death or damage (DeGiorgio et al., 2001). The resulting damage to relevant brain regions, such as the hippocampus, cortex (DeGiorgio et al., 2001), and amygdala (Emmer et al., 2006) may manifest in several possible behavioral outcomes, including cognitive and emotional dysfunction. Thus, we also examined anti-NMDAR autoantibody titers over time. At 6 weeks of age, anti-NMDAR autoantibody titers were similar between MRL/lpr and MRL/+ controls. From week 6 to week 10, a rapid increase in anti-NMDAR Ab titers was found in the MRL/lpr group but not in the MRL/+ group (Fig. 1C). After week 10, the anti-NMDAR Ab titers reached a plateau in MRL/lpr mice but still remained significantly higher than in the MRL/+ group. A two-way RM ANOVA (age by genotype) analyzing anti-NMDAR Ab titers revealed a main effect of genotype (F(1,18) =132.3; p<0.0001) and a main effect of age (F(4,18) =8.129; p<0.0001). Bonferroni post tests showed significantly higher levels of anti-NMDAR autoantibodies in MRL/lpr mice than the control group from week 8 to week 18 (p<0.001 at each time point). No significant difference between the two groups was present at the week 6 time point (p>0.05).
Anti-cardiolipin (aCL) antibodies, which react against phospholipid antigens, are associated with manifestations of neuropsychiatric SLE (NPSLE) including stroke and transient ischemic attack (Brey et al., 2001; Gorelick, 2002; Petri, 2000). At 6 weeks of age, higher aCL Ab titers were already detected in MRL/lpr mice, compared to MRL/+ controls. From week 6 to week 18, aCL Ab titers progressively increased in the MRL/lpr group, but rose much more slowly in MRL/+ mice (Fig 1D). From week 6 to week 18, the aCL Ab titers were consistently higher in MRL/lpr mice than the MRL/+ group. A two way RM ANOVA (age by genotype) analyzing aCL Ab titers revealed a main effect of genotype (F(1,18) =282.4; p<0.0001) and a main effect of age (F(4,18) =32.51; p<0.0001). Bonferroni post tests showed significantly higher levels of aCL autoantibodies in MRL/lpr mice than in the control group at each time point tested between week 6 and week 18 (p<0.001 at each time point).
In contrast to the early onset of autoantibody formation, proteinuria increased much more gradually. Proteinuria began to increase in MRL/lpr mice at week 14, and was significantly different in MRL/lpr as compared to MRL/+ mice at 18 weeks of age (Fig. 1E, p<0.001). A two way RM ANOVA (age by genotype) analyzing proteinuria revealed a main effect of genotype (F(1,18) =4.28; p<0.05) and a main effect of age (F(4,18) =3.57; p<0.01). Bonferroni post tests indicated that the proteinuria levels were significantly higher in 18w old MRL/lpr mice than in control group (p<0.001). No significant difference were found from week 6 to week 14 (p>0.05). Severe proteinuria (>300 mg/dL urine protein) was not observed in the period of study (≤18 weeks of age). These results indicate that despite displaying increased autoantibody titers, kidney function was not yet severely affected by 18 weeks of age in the studied mice.
3.2. MRL/lpr mice had normal general activity, motor coordination, cognition activity and social preference
To examine the time course of potential behavioral outcomes of lupus, we tested the mice at 8 weeks (approximate time of lupus onset) and at 18 weeks of age. A battery of behavioral tests was selected to investigate general activity, motor coordination, cognition, anxiety-like behavior, and depression at these time points.
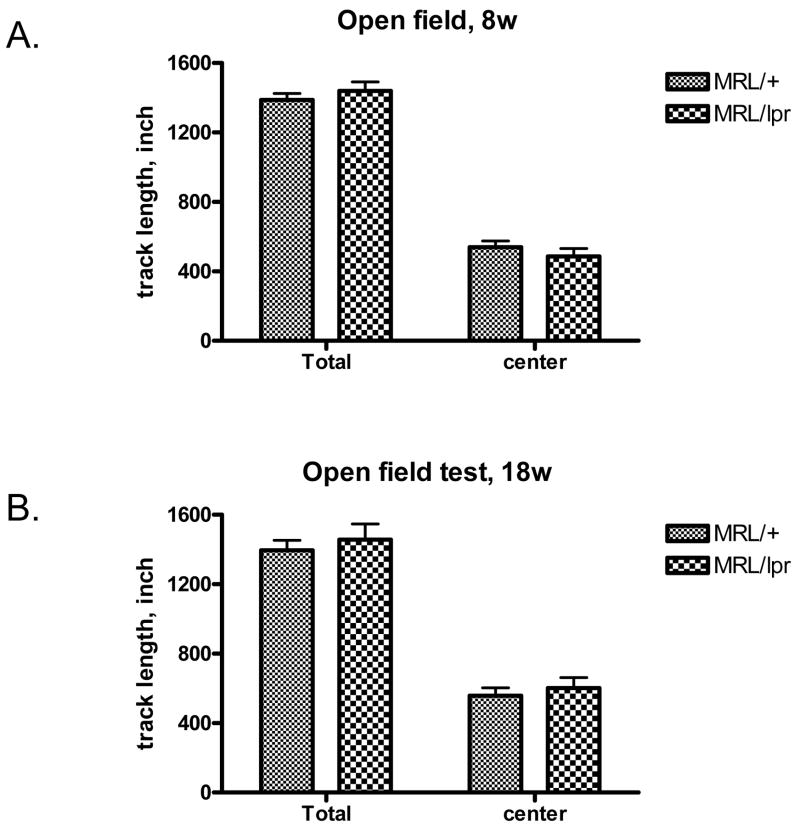
To test the general activity of the mice, open field tests were performed. We found that MRL/lpr mice had similar total track lengths and center track lengths as MRL/+ controls at both age 8w (Fig. 2A) and 18w (Fig. 2B), suggesting that normal general locomotor activity was present in MRL/lpr mice. Two way RM ANOVA (age by genotype) analyzing total track lengths and center track lengths revealed no main effect of genotype and age in the performance of mice in this open field test.
Fig. 2.
Lupus-prone MRL/lpr mice behave normally in the open field test. The total track lengths and center track lengths in the open field of 8w (A) and 18w (B) MRL/+ and MRL/lpr mice (n=10) were measured. Total time in the field: 15 minutes.
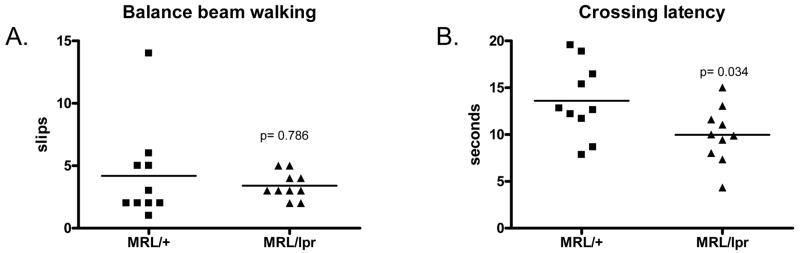
There were no evident deficits in motor coordination in MRL/lpr mice, as assessed in the balance beam test (number of slips and latency to cross a round beam). We found that MRL/lpr mice made a similar number of slips as controls while crossing the beam (Fig. 3A, 3.4±0.3 vs. 4.2±1.2, p>0.05). Interestingly, MRL/lpr mice actually crossed the beam more quickly than MRL mice (Fig. 3B, 9.98±0.96 vs. 13.60±1.25 seconds, p=0.034). The open field and balance beam experiments also indicate that impaired locomotor function (caused by inflammatory joint disease, general disability, or other causes) is not likely to be a confounding factor in the subsequent behavioral tests.
Fig. 3.
Lupus-prone MRL/lpr mice display normal behavior in the balance beam walking test. 8w old MRL/+ and MRL/lpr mice were tested in beam walking for locomotor coordination. A. Number of slips recorded during beam crossing. B. Total time each group spent crossing the beam.
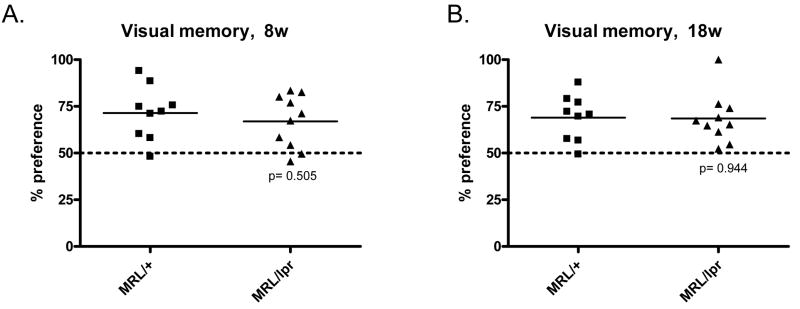
An object recognition (visual memory) test was performed to evaluate cognition and memory performance. In this behavioral test, both 8w old and 18w old MRL/lpr mice showed similar visual memory preference as MRL/+ controls (Fig. 4A & B, p>0.05), indicating normal cognition in these lupus-prone mice. Two-way RM ANOVA (age by genotype) analyzing visual memory preference revealed no main effect of genotype or age in the performance of mice in this test.
Fig. 4.
Cognition is preserved in 8w and 18w MRL/lpr mice. Object recognition (visual memory) tests on 8w (A) and 18w (B) mice were performed. A 45 minute delay time was used between the first and second object exposures. Mice having normal visual memory are expected to have >50% preference for a novel object (above the dotted line).
3.3. Lupus mice did not exhibit typical anxiety-like behavior in the elevated plus maze test
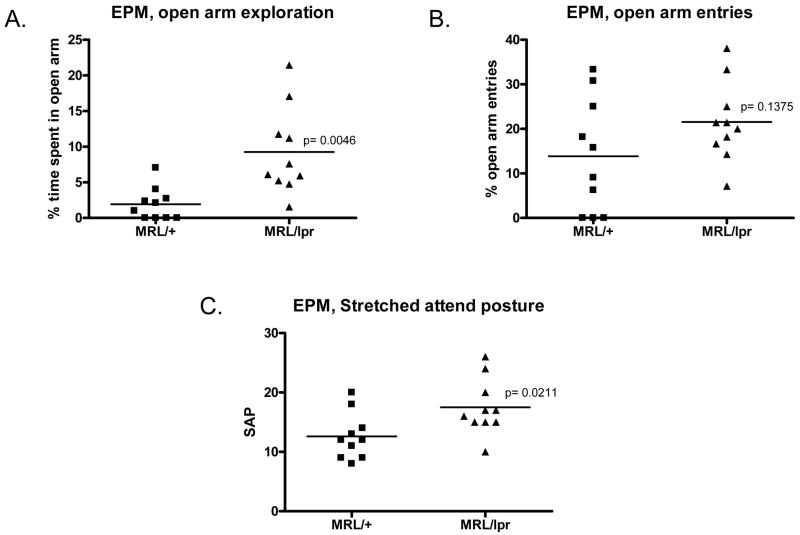
Anxiety is a mood disorder often found in NPSLE patients (Hermosillo-Romo and Brey, 2002). To investigate anxiety levels in lupus-prone mice and the disease-free controls, we utilized the elevated plus maze (EPM). Anxiety-like behavior in this test was defined as decreased exploration of the open arms during the first exposure to the EPM test (Pellow et al., 1985). Since responses in subsequent exposures to the EPM are regulated by a complicated interplay between learning, anxiety, habituation and sensitization (Bessa et al., 2005), we only assessed 18 week old mice. Within 5 minutes in the EPM, MRL/lpr mice exhibited significantly lower levels of anxiety-like behavior than MRL/+ mice, as evident in the higher percentage of open arm exploration (9.27±1.96% vs. 1.93±0.73% of the total time, respectively, Fig. 5A; p=0.005) and a similar number of open arm entries as MRL/+ mice (Fig. 5B; p>0.05). Interestingly, MRL/lpr mice showed significantly more stretched attend posture (Fig. 5C, p=0.021) than MRL/+ mice. Together, these data indicate a moderate but significantly lower level of anxiety-like behavior in female MRL/lpr mice.
Fig. 5.
Elevated plus maze test detects less anxiety in lupus mice than control mice. Anxiety levels were tested in an EPM test. A. Time spent in the open arms was recorded and converted to a percentage of the total time in the EPM (5 minutes). The percent time spent on exploration is shown in the graph. B. Open arm entries were recorded. C. stretched attend postures counted during EPM tests.
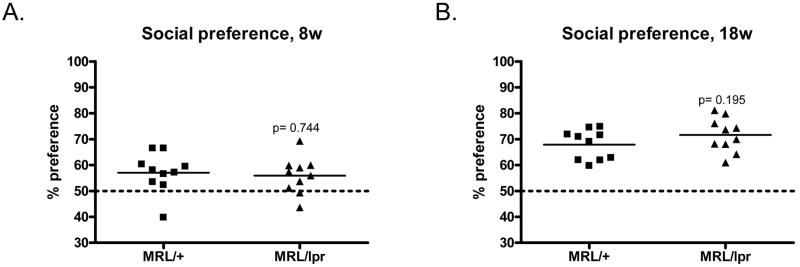
One hallmark of sickness behavior and anxiety-like behavior is social withdrawal (Dantzer et al., 1987), as assessed in a social preference test. We found that both 8w old and 18w old MRL/lpr mice had normal preference for a conspecific living mouse compared to an inanimate object (Fig. 6A & B, p>0.05). Thus, the normal social preference test, together with results in the EPM, did not indicate any increase in anxiety-like behavior or general debility.
Fig. 6.
MRL/lpr mice had similar social preference as MRL/+ controls. 8w (A) and 18w (B) MRL/lpr mice were tested for social activity in a Y maze. Preference was calculated based on the social target contacting time over total exploring time. Mice having normal social activities are expected to have >50% preference to a social target (above the dotted line).
3.4. Lupus mice develop depression very early in the forced swim test
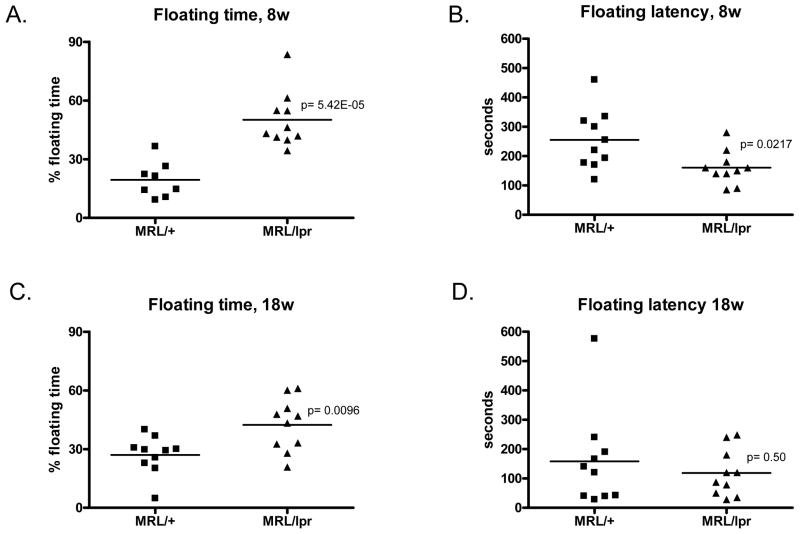
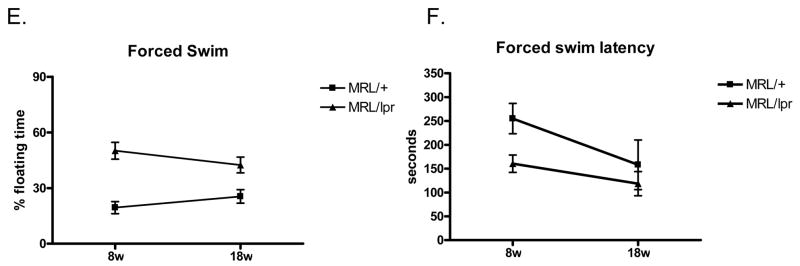
As one of the most prevalent behavioral manifestations of lupus is a major depressive disorder, we assessed depressive like behavior in the forced swim test in lupus-prone female MRL/lpr mice and in age matched MRL/+ mice at early (8 weeks) and later (18 weeks) stages of the disease. In the forced swim test, depression was assessed as increased time spent floating (as opposed to swimming and/or struggling) and shorter latency to begin floating. Lupus-prone MRL/lpr exhibited significantly higher levels of behavioral despair at both time points (8 weeks and 18 weeks old; Fig. 7) than age-matched MRL/+ mice. Lupus-prone MRL/lpr had more than a 100% increase in their % floating time compared to MRL/+ mice, even at the earliest age tested (Fig. 7A; 7C). In Figure 7E, a two-way RM ANOVA (age by genotype) analyzing total floating time in a 9 minute test revealed a main effect of genotype (F(1,16) =25.70; p<0.0001) and no main effect of age (F(1,16) =0.056; p>0.05). In addition to increased total time spent immobile, the MRL/lpr mice also had a shorter latency to begin floating. As shown in Figure 7B, the 8w MRL/lpr mice started floating after 160.5±18 seconds, significantly earlier than the MRL/+ controls (255.0±32 seconds, p<0.03). The 18w MRL/lpr mice started floating at 118.6±20 seconds (Fig. 7D), earlier than in their previous exposure 10 weeks earlier, but not significantly earlier than the MRL/+ controls (158.1±32 seconds, p> 0.05). These results indicate that the 8w and 18w old MRL/lpr mice had distinct depression-like behavior, which was not found in the control strain. In addition, the data are consistent with the suggestion that repeated exposure to the forced swim test tends to increase some indices of behavioral despair (i.e. latency to float) in both control MRL/+ and MRL/lpr female mice. In Figure 7F, a two-way RM ANOVA (age by genotype) analyzing floating latency revealed a main effect of age (F(1,18) =7.855; p<0.05) and no main effect of genotype (F(1,18) =2.596; p>0.05).
Fig. 7.
MRL/lpr mice display depression-like behavior at 8 weeks of age. The forced swim test was used to assess depression-like behavior in mice. % floating time: total floating time converted to percentage of total test time (A, test on 8 weeks of age. C, test on 18 weeks of age). B and D, floating latency is the delay time until mice give up struggling in the water and start to show the ‘still’ floating behavior. Time was recorded in seconds. B, testing 8 week old mice. D, testing 18 week old mice. E, F. Two way ANOVA analysis of the effects of age and genotype on total floating time (E) and floating latency (F) in the tested mice.
3.5. Depression scores correlate with anti-dsDNA, anti-NMDAR and anti-cardiolipin autoantibody titers
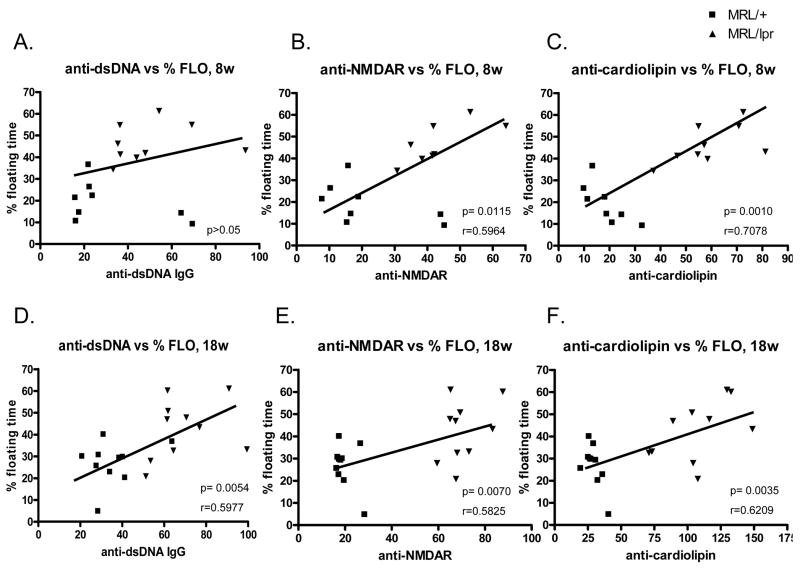
To address a possible pathogenic link of autoantibodies to the development of NPSLE, we correlated the results on the depression test with autoantibody titers at each test age (8w or 18w) in a correlation study. The result of the correlation analysis showed that anti-dsDNA Ab levels in 18w mice significantly correlated with the % floating time in the forced swim test (Fig. 8D, p=0.005, r=0.598), while the correlation was not significant in 8w mice (Fig. 8A, p>0.05).
Fig. 8.
Lupus autoantibody levels correlate with depression behavior. Linear regression correlation of % floating time in forced swim test with serum anti-dsDNA titers (A, 8w; D, 18w), anti-NMDAR antibody titers (B, 8w; E, 18w), or anti-cardiolipin antibody titers (C, 8w; F, 18w) were shown. MRL/+: ■, MRL/lpr: ▼. P values and Pearson’s R values for each correlation are also shown in the graphs.
At both age 8w and 18w, serum anti-NMDAR antibody levels significantly correlated with % floating time in the forced swim test. As shown in Figure 8B, anti-NMDAR Ab titers of 8 week old mice significantly correlated with the % floating time (p=0.015, r=0.596). A similar correlation was also found at 18 weeks of age (Figure 8E, p=0.007, r=0.583). Significant correlations were also found between the % floating time and aCL Ab titers (Fig. 8C, 8 weeks, p=0.001, r=0.708; Fig. 8F, 18 weeks, p=0.004, r=0.621), and anti-chromatin Ab titers (data not shown, p<0.05) at both ages tested.
3.6. Estrous cycle stage was not likely to confound the behavior tests
To exclude the possibility that hormonal influences may have confounded our study, we examined the estrous cycle stages of the mice for two weeks. Table 1 shows the respective stages on the day we tested 8w old mice in the forced swim test. The stages of estrous cycle found in the tested mice were randomly distributed across all the cycle phases, and there was no synchronized or systematic trend in either group of mice. This observation indicated that at least in our study, sex hormones were not likely to have had any systematic effect on the results of the behavior tests.
Table 1.
Estrous cycle stages of tested mice were randomly distributed. Estrous cycle (EC) stages were monitored along the test. The Table shows the phase on the day of forced swim test in 8w mice. P, proestrus; E, Estrous; M, Metestrus; D2, Diestrus II.
| EC phase | P | E | M | D2 |
|---|---|---|---|---|
| MRL/+ | 3 | 4 | 0 | 3 |
| MRL/lpr | 3 | 2 | 2 | 3 |
3.7. MRSI confirms regional brain dysfunction
In addition to the behavioral tests, a MRI examination of the brain was performed on those MRL/lpr and MRL/+ mice which presented behavior differences in the previous depression tests. Mice at 18 weeks of age were tested in pairs, with one lupus mouse and one control mouse in each experimental set (total lupus n=6, control n=6). Both groups appear to display grossly normal brain structure by MRI (data not shown). However, subtle changes in brain anatomy may not have been detectable by this method.
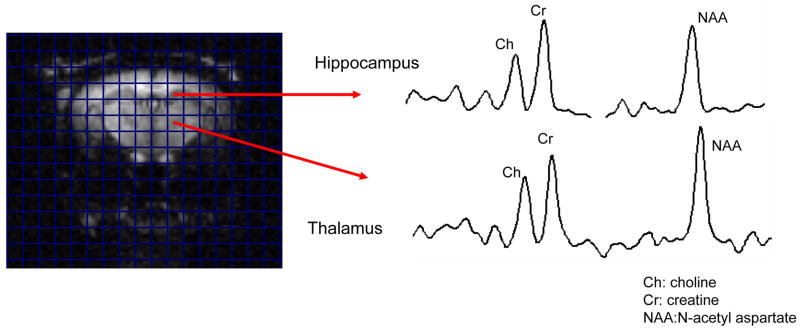
To analyze major brain metabolites which reflect brain cell activity, including total creatine (Cr), choline-containing compounds (Ch), and neuron-specific product N-acetyl aspartate (NAA), magnetic resonance spectroscopic imaging (MRSI) was performed (Fig. 9). These metabolites are traditionally standardized by creatine, a stable marker, and are expressed as ratios of each metabolite to the creatine standard (e.g. NAA/Cr). Furthermore, choline levels reflect membrane turnover or genesis, and are expressed as Ch/Cr. These metabolic ratios have been extensively used to identify regional brain dysfunction or damage in human brain studies (Brooks et al., 1997; Sibbitt et al., 1994; Sibbitt et al., 1997; Sibbitt and Sibbitt, 1993) as well as in studies of the neuronal pathogenesis of anti-dsDNA autoantibodies (Kowal et al., 2004). As shown in Table 2, MRL/lpr mice had significantly lower Ch/Cr ratios in the hippocampus (0.61± 0.010 vs. 0.66± 0.014, p=0.044) and cortical regions (0.67± 0.020 vs 0.74± 0.016, p=0.039) than the control group. As compared to non-autoimmune mice with induced lupus (Kowal et al., 2004), MRL/lpr mice with spontaneous disease did not however display decreased hippocampal NAA/Cr ratios (Table 2). In contrast, a higher NAA/Ch ratio was found in the cortex of MRL/lpr mice than in the MRL/+ controls (1.39± 0.056 vs. 1.24± 0.055, p=0.027), indicating that cortical brain cells (neurons and/or glia) in MRL/lpr mice were more active than those in MRL/+ mice. These results suggest that brain cell activity changes in the hippocampal and cortical regions may be important in regulation of emotion in brains of MRL/lpr lupus mice.
Fig. 9.
MRI/MRSI analyses show differences in regional brain metabolism between control and lupus mice. The figure illustrates the type of spectra obtained from the hippocampus (top) and thalamus (bottom), with a corresponding anatomical image of a lupus mouse brain. Data from the comparative analysis of lupus and control mouse brains is provided in Table 2.
Table 2.
Mouse brain regional NAA/Ch, NAA/creatine and Ch/Cr ratios. N=6 for each group.
| Hippocampus |
Thalamus |
Cortex |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| NAA/Cr | NAA/Ch | Ch/Cr | NAA/Cr | NAA/Ch | Ch/Cr | NAA/Cr | NAA/Ch | Ch/Cr | |
| MRL/+ | 0.92±0.03 | 1.40±0.05 | 0.66±0.01 | 1.25±0.02 | 1.73±0.04 | 0.73±0.02 | 0.90±0.03 | 1.24±0.06 | 0.74±0.02 |
| MRL/lpr | 0.91±0.03 | 1.50±0.05 | 0.61±0.01 | 1.24±0.03 | 1.76±0.05 | 0.71±0.02 | 0.92±0.04 | 1.39±0.06 | 0.67±0.02 |
| t test | ns | ns | 0.0438* | ns | ns | ns | ns | 0.0273* | 0.0393* |
4. Discussion
Major depression is one of the most common neuropsychiatric manifestations in SLE patients (Wekking, 1993). In our study, female MRL/lpr mice showed depressive behavior as early as 8 weeks of age, the onset of the lupus-like disease. This significant increase in behavioral despair in the forced swim test was not accompanied by abnormal behavior in tests of general activity, motor activity, anxiety, social activity and visual memory. Excessive floating and early cessation of attempts to escape have been used to characterize the depression in the forced swim test model (Porsolt et al., 1977a; Porsolt et al., 1977b). Both 8w old and 18w old MRL/lpr mice were immobile for a significantly longer period of time, and the 8w old MRL/lpr mice stopped struggling much earlier than the MRL/+ congenic controls. Surprisingly, MRL/lpr mice actually displayed significantly less anxiety behaviors as compared to the background matched MRL/+ mice, despite more severe depression. Finally, the fact that the mice did not have severe nephritis while displaying depressive behavior indicates that neuropsychiatric outcomes of SLE are dissociable from kidney pathology. Since in this study we compared MRL/lpr lupus mice to background matched MRL/+ mice, any relative differences observed between these mice strains are solely attributable to disease acceleration mediated by the lpr trait. However, it is important to point out that the MRL background, which itself promotes autoimmune manifestations late in life, may also have contributed to the absolute severity of neurobehavioral abnormalities described above.
SLE is much more highly prevalent in women, with a 9:1 female:male ratio in human patients. It is thought that sex hormones (such as estrogens and prolactin) have a potent role in disease development (Cohen-Solal et al., 2006; Grimaldi, 2006; Lahita, 1999). This sex difference is also evident in several rodent models of lupus including MRL/lpr; however, female mice have rarely been systematically studied (Sakic et al., 1997a; Sakic et al., 1993; Sakic et al., 1992; Sakic et al., 1996). As one of the very few studies using female MRL/lpr mice for behavioral tests, our results demonstrate that female MRL/lpr mice develop depression at a very young age, much earlier than the 12–16 weeks of age time point previously reported in studies of MRL/lpr males (Sakic et al., 1994). Nevertheless, the fact that female MRL/lpr mice were in different stages of the estrous cycle at the time of testing in the forced swim test, indicates that there is not a simple relationship between circulating sex hormones and manifestations of depression-like behavior.
There is debate in the literature whether neuropsychiatric symptoms of lupus are a secondary complication of peripheral organ pathology (such as severe nephritis) or reflect a primary CNS etiology. Though renal dysfunction in lupus may indeed affect neuropsychiatric manifestations through metabolic disturbances, our results suggested that CNS lupus can occur long before kidney insufficiency in female lupus-prone mice, since severe nephritis (≥ 300mg/dL proteinuria) was not found at the age that depression was demonstrated. This finding is consistent with previous studies in another lupus-prone strain, NZM88 mice, which developed behavioral changes before the onset of nephritis (Rudofsky et al., 1993; Rudofsky and Lawrence, 1999). In our study of MRL/lpr mice, we found that development of depression does not require end-organ injury such as renal failure. Moreover, other target organ involvement observed in MRL/lpr mice including rash, arthritis, and lymphadenopathy were also not present when marked depression was already manifest (data not shown). Taken together, our data suggest that the brain is a primary target organ in lupus, and that depression-like behavior may be an early indicator of lupus target organ involvement.
It is interesting to note that we did not find increased anxiety in the MRL/lpr mice, in either the EPM or the social preference tests. Anxiety is often co-morbid with depression in lupus patients (Nery et al., 2008). Previous studies have demonstrated increased anxiety in lupus models: 12–16 week old MRL/lpr male mice that floated extensively in the forced swim test had higher levels of anxiety in the EPM test (Sakic et al., 1994), while increased anxiety was found in lupus-prone NZB/W F1 mice as well (Schrott and Crnic, 1996). In contrast, we found in the current study that MRL/lpr female mice were depressed but not anxious, raising the possibility that at least in female MRL/lpr mice there are separate pathways contributing to the development of depression and anxiety.
Since behavioral changes can occur before other disease manifestations in SLE patients, and lupus-prone NZM88 (Rudofsky and Lawrence, 1999) and MRL/lpr mice (current study) as well develop behavioral deficits before the onset of nephritis, there has been a great deal of interest in possible underlying mechanisms for the lupus associated behavioral syndrome. It has been suggested that certain brain antigen reactive antibodies can independently cause or accelerate the development of NPSLE-like manifestations in mice (Lawrence et al., 2007). To be sure, several previous studies have shown that lupus associated autoantibodies can promote behavioral changes. Inducing high titers of anti-phospholipid antibodies in non-autoimmune mice led to a loss of reflexes and gripping potential (Ziporen et al., 1997), while mice injected with anti-ribosomal P autoantibodies from human patients exhibited depression that was reversible with anti-depressant treatment (Katzav et al., 2007). In the current study we found that the depression-like behavior of lupus-prone MRL/lpr mice correlated with autoantibodies against dsDNA, chromatin, NMDA receptors, and cardiolipin, further supporting a mechanistic link between the depression syndrome and circulating autoantibodies. While some authors have reported that behavioral deficits such as cognitive decline do not parallel lupus activity (Leritz et al., 2000) and that autoantibody titers do not linearly correlate with CNS pathology (Kowal et al., 2004), there is also convincing evidence that cognitive deficits (reduced novel object exploration) in MRL/lpr mice do correlate with ANA titers. Moreover, this behavioral abnormality in lupus mice was significantly improved by immunosuppressive drug therapy which dramatically reduced ANA titers (Sakic et al., 1994). In our study, we found a significant correlation between depression and anti-NMDAR autoantibody titers in MRL/lpr female mice, consistent with the previous finding that high serum anti-NMDAR antibody titers were associated with depression, but not cognitive deficits, in SLE patients (Lapteva et al., 2006). It is possible that different types of pathogenic autoantibodies affect certain brain regions separately, according to their antigen specificity. Interestingly, female MRL/lpr mice already showed definite depression-like behavior even at the age when only subtle or insignificant increases of autoantibodies were detected. This raises the question whether lowering the currently accepted threshold for detection of anti-nuclear antibodies (i.e. making the test more sensitive) would result in an earlier clinical diagnosis of NPSLE.
Human SLE and murine models of the disease are characterized serologically by a broad array of autoantibodies against nuclear antigens and other cellular components. Of these, several autoantibody specificities have been associated with focal and diffuse neuropsychiatric syndromes affecting both adult and pediatric lupus populations (recently reviewed in Muscal and Myones, 2007; Stojanovich et al., 2007; Zandman-Goddard et al., 2007; Toubi and Shoenfeld, 2007). Autoantibodies linked to neuropsychiatric lupus include autoantibodies to brain components (e.g. anti-neuronal antibodies, brain reactive antibodies, anti-microtubule-associated protein 2 (MAP-2) antibodies), and systemic antigens (e.g. anti-cardiolipin/anti-phospholipid antibodies, anti-ribosomal P antibodies, anti-endothelial cell antibodies). However, no autoantibody specificity for any brain specific or systemic antigen has been linked with a single neuropsychiatric manifestation (Zandman-Goddard et al., 2007).
Of the systemic autoantibodies related to neuropsychiatric lupus, antibodies to ribosomal P have been particularly well studied and linked in several studies in lupus patients to psychosis and depression (Eber et al., 2005; Schneebaum et al., 1991; Zandman-Goddard et al., 2007; Toubi and Shoenfeld, 2007). Anti-ribosomal P antibodies have the ability to penetrate into live cells, induce apoptosis and modulate cytokine secretion (Shoenfeld, 2007). In addition, recent studies by Shoenfeld and colleagues (Katzav et al., 2007) have implicated a novel mechanism by which anti-ribosomal P antibodies may contribute to depression. It was previously shown that defects of the smell apparatus can lead to depression in mice (Zueger et al., 2005) and rats (Pause et al., 2001). Interestingly, human anti-ribosomal P antibodies were shown to bind to olfactory-related brain structures including the piriform and cingulate cortex, while injection of anti-ribosomal P antibodies intracerebroventricularly in non-autoimmune C3H mice directly caused depression-like behavior (Katzav et al., 2007). While any possible effect on smell capabilities was not directly evaluated in injected mice, these studies raise an interesting possible connection between anti-ribosomal P antibodies, disturbed olfaction, and depressive behavior. However, MRL/lpr mice display a relatively low incidence (<15%) of this particular autoantibody specificity (Bonfa et al., 1988; Elkon et al., 1989), and therefore we did not measure anti-ribosomal P antibodies in our studies. The severe depression we found in young MRL/lpr mice, despite low titers of anti-ribosomal P antibodies described in this strain, indicates that several distinct pathways may contribute to depression in SLE.
The mechanism by which cross-reactive autoantibodies pass through the blood brain barrier to affect brain cell function is a matter of great interest. It was previously reported that 33% of 8 week old MRL/lpr mice had inflammation and perivascular leakage of the BBB, which becomes more prevalent as the mice age (Vogelweid et al., 1991). The early disruption of the BBB presumably can provide an entry for circulating factors such as inflammatory cytokines and pathogenic autoantibodies to the inner brain regions in lupus-prone mice even early on in the disease. Thus, one would expect a positive correlation between brain-reactive pathogenic antibodies and brain dysfunction as observed in behavior tests, as we did find in our study. There is indeed convincing experimental evidence that a breach of the blood brain barrier can result in circulating autoantibodies gaining access to the brain, leading to manifestations of NPSLE. In a series of elegant studies, Diamond and colleagues have shown that in normal BALB/c mice, NMDAR-reactive anti-DNA antibodies can penetrate into the mouse brain following an induced breach of the BBB and cause apoptotic death of hippocampal neurons, leading to significant cognitive deficits in hippocampal-dependent functions of learning and memory (Huerta et al., 2006; Kowal et al., 2006; Kowal et al., 2004). Moreover, treatment with a NMDA receptor antagonist prevents neuronal cell death. Interestingly, the agent used to open the BBB determines which brain region is made vulnerable to these neurotoxic autoantibodies. Using lipopolysaccharide to breach the BBB, induction of high titer anti-NMDAR Abs resulted in neuronal death in the hippocampus, cognitive dysfunction and altered hippocampal metabolism (Kowal et al., 2004), while a combination of epinephrine plus autoantibodies resulted in neuron loss in the lateral amygdala and emotional dysfunction characterized by defective fear conditioning (Huerta et al., 2006). The observation that floating time and latency were not increased in older MRL/lpr mice as compared to younger mice of this strain, together with these other studies, suggests that BBB breach and the early exposure to autoantibodies could indeed be the key to trigger NPSLE. However, what factors increase BBB permeability in young lupus mice is not currently known.
In human brain studies, MRSI is used to detect inflammation, gross pathology, and for localization of abnormalities in regional brain function. We applied this method to mouse studies and found significant metabolic differences between the normal control mice and lupus mice in both the hippocampus and cortex regions, which are involved in cognition and regulation of emotion. These metabolic abnormalities reflected abnormal neuronal (and/or glial) activity, but had no structural correlate detectable by MRI. The results in our studies suggest that these brain regions may be particularly susceptible to the effects of SLE autoimmunity. Moreover, no gross structural abnormalities (such as cerebral infarction, increased ventricular volume or hippocampal or cortical atrophy) were found in MRL/lpr mice despite high aCL autoantibody titers, similar to results reported in a previous study (Hess et al., 1993).
In conclusion, we found that female lupus mice developed depression-like behavior as early as 8 weeks of age, the onset of the disease, and that the behavior deficits correlated with autoantibody levels. Moreover, at least in female MRL/lpr mice, anxiety and depression were dissociated. Our results indicate that NPSLE can develop at a very early stage of the disease when only subtle changes of autoimmunity are present in MRL/lpr mice, and encourage additional study into further understanding the important role of autoantibodies in the pathogenesis of neuropsychiatric SLE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bessa JM, Oliveira M, Cerqueira JJ, Almeida OF, Sousa N. Age-related qualitative shift in emotional behaviour: paradoxical findings after re-exposure of rats in the elevated-plus maze. Behav Brain Res. 2005;162:135–142. doi: 10.1016/j.bbr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bluestein HG. The central nervous system in systemic lupus erythematosus. In: Lahita RG, editor. Systemic lupus erythematosus. Churchill Livingstone; New York: 1992. pp. 639–655. [Google Scholar]
- Bonfa E, Marshak-Rothstein A, Weissbach H, Brot N, Elkon K. Frequency and epitope recognition of anti-ribosome P antibodies from humans with systemic lupus erythematosus and MRL/lpr mice are similar. J Immunol. 1988;140:3434–3437. [PubMed] [Google Scholar]
- Brey RL, Abbott RD, Curb JD, Sharp DS, Ross GW, Stallworth CL, Kittner SJ. beta(2)-Glycoprotein 1-dependent anticardiolipin antibodies and risk of ischemic stroke and myocardial infarction: the honolulu heart program. Stroke. 2001;32:1701–1706. doi: 10.1161/01.str.32.8.1701. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Sabet A, Sibbitt WL, Jr, Barker PB, van Zijl PC, Duyn JH, Moonen CT. Neurochemistry of brain lesions determined by spectroscopic imaging in systemic lupus erythematosus. J Rheumatol. 1997;24:2323–2329. [PubMed] [Google Scholar]
- Chu JL, Drappa J, Parnassa A, Elkon KB. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. J Exp Med. 1993;178:723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Solal JF, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex hormones and SLE: influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- Deocharan B, Qing X, Beger E, Putterman C. Antigenic triggers and molecular targets for anti-double-stranded DNA antibodies. Lupus. 2002;11:865–871. doi: 10.1191/0961203302lu308rr. [DOI] [PubMed] [Google Scholar]
- Deocharan B, Zhou Z, Antar K, Siconolfi-Baez L, Angeletti RH, Hardin J, Putterman C. Alpha-actinin immunization elicits anti-chromatin autoimmunity in nonautoimmune mice. J Immunol. 2007;179:1313–1321. doi: 10.4049/jimmunol.179.2.1313. [DOI] [PubMed] [Google Scholar]
- Eber T, Chapman J, Shoenfeld Y. Anti-ribosomal P-protein and its role in psychiatric manifestations of systemic lupus erythematosus: myth or reality? Lupus. 2005;14:571–575. doi: 10.1191/0961203305lu2150rr. [DOI] [PubMed] [Google Scholar]
- Elkon KB, Bonfa E, Llovet R, Eisenberg RA. Association between anti-Sm and anti-ribosomal P protein autoantibodies in human systemic lupus erythematosus and MRL/lpr mice. J Immunol. 1989;143:1549–1554. [PubMed] [Google Scholar]
- Emmer BJ, van der Grond J, Steup-Beekman GM, Huizinga TW, van Buchem MA. Selective involvement of the amygdala in systemic lupus erythematosus. PLoS Med. 2006;3:e499. doi: 10.1371/journal.pmed.0030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fragoso-Loyo H, Richaud-Patin Y, Orozco-Narvaez A, Davila-Maldonado L, Atisha-Fregoso Y, Llorente L, Sanchez-Guerrero J. Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis Rheum. 2007;56:1242–1250. doi: 10.1002/art.22451. [DOI] [PubMed] [Google Scholar]
- Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153:155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- Gorelick PB. Stroke prevention therapy beyond antithrombotics: unifying mechanisms in ischemic stroke pathogenesis and implications for therapy: an invited review. Stroke. 2002;33:862–875. doi: 10.1161/hs0302.103657. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr Opin Rheumatol. 2006;18:456–461. doi: 10.1097/01.bor.0000240354.37927.dd. [DOI] [PubMed] [Google Scholar]
- Hermosillo-Romo D, Brey RL. Diagnosis and management of patients with neuropsychiatric systemic lupus erythematosus (NPSLE) Best Pract Res Clin Rheumatol. 2002;16:229–244. doi: 10.1053/berh.2001.0223. [DOI] [PubMed] [Google Scholar]
- Hess DC, Taormina M, Thompson J, Sethi KD, Diamond B, Rao R, Chamberlain CR, Feldman DS. Cognitive and neurologic deficits in the MRL/lpr mouse: a clinicopathologic study. J Rheumatol. 1993;20:610–617. [PubMed] [Google Scholar]
- Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav A, Solodeev I, Brodsky O, Chapman J, Pick CG, Blank M, Zhang W, Reichlin M, Shoenfeld Y. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007;56:938–948. doi: 10.1002/art.22419. [DOI] [PubMed] [Google Scholar]
- Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, Diamond B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352–356. doi: 10.1097/00002281-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Lapteva L, Nowak M, Yarboro CH, Takada K, Roebuck-Spencer T, Weickert T, Bleiberg J, Rosenstein D, Pao M, Patronas N, Steele S, Manzano M, van der Veen JW, Lipsky PE, Marenco S, Wesley R, Volpe B, Diamond B, Illei GG. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–2514. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- Lawrence DA, Bolivar VJ, Hudson CA, Mondal TK, Pabello NG. Antibody induction of lupus-like neuropsychiatric manifestations. J Neuroimmunol. 2007;182:185–194. doi: 10.1016/j.jneuroim.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz E, Brandt J, Minor M, Reis-Jensen F, Petri M. “Subcortical” cognitive impairment in patients with systemic lupus erythematosus. J Int Neuropsychol Soc. 2000;6:821–825. doi: 10.1017/s1355617700677093. [DOI] [PubMed] [Google Scholar]
- Miyasaka N, Takahashi K, Hetherington HP. 1H NMR spectroscopic imaging of the mouse brain at 9.4 T. J Magn Reson Imaging. 2006;24:908–913. doi: 10.1002/jmri.20709. [DOI] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat. 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Muscal E, Myones BL. The role of autoantibodies in pediatric neuropsychiatric systemic lupus erythematosus. Autoimmun Rev. 2007;6:215–217. doi: 10.1016/j.autrev.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Nery FG, Borba EF, Viana VS, Hatch JP, Soares JC, Bonfa E, Neto FL. Prevalence of depressive and anxiety disorders in systemic lupus erythematosus and their association with anti-ribosomal P antibodies. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:695–700. doi: 10.1016/j.pnpbp.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Pan JW, Kim JH, Cohen-Gadol A, Pan C, Spencer DD, Hetherington HP. Regional energetic dysfunction in hippocampal epilepsy. Acta Neurol Scand. 2005;111:218–224. doi: 10.1111/j.1600-0404.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- Pause BM, Miranda A, Goder R, Aldenhoff JB, Ferstl R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 2001;35:271–277. doi: 10.1016/s0022-3956(01)00029-2. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145–151. doi: 10.1006/jaut.2000.0409. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977a;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977b;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Putterman C. New approaches to the renal pathogenicity of anti-DNA antibodies in systemic lupus erythematosus. Autoimmun Rev. 1994;3:7–11. doi: 10.1016/S1568-9972(03)00082-X. [DOI] [PubMed] [Google Scholar]
- Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest. 1993;68:419–426. [PubMed] [Google Scholar]
- Rudofsky UH, Lawrence DA. New Zealand mixed mice: a genetic systemic lupus erythematosus model for assessing environmental effects. Environ Health Perspect. 1999;107(Suppl 5):713–721. doi: 10.1289/ehp.99107s5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Braciak T, Richards C, Gauldie J, Denburg JA. Reduced preference for sucrose in autoimmune mice: a possible role of interleukin-6. Brain Res Bull. 1997a;44:155–165. doi: 10.1016/s0361-9230(97)00107-x. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Denburg JA. Neurobehavioral alterations in autoimmune mice. Neurosci Biobehav Rev. 1997b;21:327–340. doi: 10.1016/s0149-7634(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Denburg S, Carbotte R, Denburg JA. Spatial learning during the course of autoimmune disease in MRL mice. Behav Brain Res. 1993;54:57–66. doi: 10.1016/0166-4328(93)90048-u. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Keffer M, Talangbayan H, Stead R, Denburg JA. A behavioral profile of autoimmune lupus-prone MRL mice. Brain Behav Immun. 1992;6:265–285. doi: 10.1016/0889-1591(92)90048-s. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Stead RH, Denburg JA. Joint pathology and behavioral performance in autoimmune MRL-lpr Mice. Physiol Behav. 1996;60:901–905. doi: 10.1016/0031-9384(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Talangbayan H, Denburg SD, Carbotte RM, Denburg JA. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol Behav. 1994;56:609–617. doi: 10.1016/0031-9384(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Schneebaum AB, Singleton JD, West SG, Blodgett JK, Allen LG, Cheronis JC, Kotzin BL. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am J Med. 1991;90:54–62. doi: 10.1016/0002-9343(91)90506-s. [DOI] [PubMed] [Google Scholar]
- Schrott LM, Crnic LS. Anxiety behavior, exploratory behavior, and activity in NZB x NZW F1 hybrid mice: role of genotype and autoimmune disease progression. Brain Behav Immun. 1996;10:260–274. doi: 10.1006/brbi.1996.0023. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y. To smell autoimmunity: anti-P-ribosomal autoantibodies, depression, and the olfactory system. J Autoimmun. 2007;28:165–9. doi: 10.1016/j.jaut.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Nahum A, Korczyn AD, Dano M, Rabinowitz R, Beilin O, Pick CG, Leider-Trejo L, Kalashnikova L, Blank M, Chapman J. Neuronal-binding antibodies from patients with antiphospholipid syndrome induce cognitive deficits following intrathecal passive transfer. Lupus. 2003;12:436–442. doi: 10.1191/0961203303lu409oa. [DOI] [PubMed] [Google Scholar]
- Sibbitt WL, Jr, Haseler LJ, Griffey RH, Hart BL, Sibbitt RR, Matwiyoff NA. Analysis of cerebral structural changes in systemic lupus erythematosus by proton MR spectroscopy. AJNR Am J Neuroradiol. 1994;15:923–928. [PMC free article] [PubMed] [Google Scholar]
- Sibbitt WL, Jr, Haseler LJ, Griffey RR, Friedman SD, Brooks WM. Neurometabolism of active neuropsychiatric lupus determined with proton MR spectroscopy. AJNR Am J Neuroradiol. 1997;18:1271–1277. [PMC free article] [PubMed] [Google Scholar]
- Sibbitt WL, Jr, Sibbitt RR. Magnetic resonance spectroscopy and positron emission tomography scanning in neuropsychiatric systemic lupus erythematosus. Rheum Dis Clin North Am. 1993;19:851–868. [PubMed] [Google Scholar]
- Sidor MM, Sakic B, Malinowski PM, Ballok DA, Oleschuk CJ, Macri J. Elevated immunoglobulin levels in the cerebrospinal fluid from lupus-prone mice. J Neuroimmunol. 2005;165:104–113. doi: 10.1016/j.jneuroim.2005.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovich L, Zandman-Goddard G, Pavlovich S, Sikanich N. Psychiatric manifestations in systemic lupus erythematosus. Autoimmun Rev. 2007;6:421–426. doi: 10.1016/j.autrev.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN. Murine models of lupus. In: Lahita RG, editor. Systemic lupus erythematosus. Churchill Livingstone; New York: 1992. pp. 121–194. [Google Scholar]
- Toubi E, Shoenfeld Y. Clinical and biological aspects of anti-P-ribosomal protein autoantibodies. Autoimmun Rev. 2007;6:119–125. doi: 10.1016/j.autrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Twieg DB, Meyerhoff DJ, Hubesch B, Roth K, Sappey-Marinier D, Boska MD, Gober JR, Schaefer S, Weiner MW. Phosphorus-31 magnetic resonance spectroscopy in humans by spectroscopic imaging: localized spectroscopy and metabolite imaging. Magn Reson Med. 1989;12:291–305. doi: 10.1002/mrm.1910120302. [DOI] [PubMed] [Google Scholar]
- Vogelweid CM, Johnson GC, Besch-Williford CL, Basler J, Walker SE. Inflammatory central nervous system disease in lupus-prone MRL/lpr mice: comparative histologic and immunohistochemical findings. J Neuroimmunol. 1991;35:89–99. doi: 10.1016/0165-5728(91)90164-3. [DOI] [PubMed] [Google Scholar]
- Wekking EM. Psychiatric symptoms in systemic lupus erythematosus: an update. Psychosom Med. 1993;55:219–228. doi: 10.1097/00006842-199303000-00011. [DOI] [PubMed] [Google Scholar]
- Zandman-Goddard G, Chapman J, Shoenfeld Y. Autoantibodies involved in neuropsychiatric SLE and antiphospholipid syndrome. Semin Arthritis Rheum. 2007;36:297–315. doi: 10.1016/j.semarthrit.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ziporen L, Shoenfeld Y, Levy Y, Korczyn AD. Neurological dysfunction and hyperactive behavior associated with antiphospholipid antibodies. A mouse model. J Clin Invest. 1997;100:613–619. doi: 10.1172/JCI119572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zueger M, Urani A, Chourbaji S, Zacher C, Roche M, Harkin A, Gass P. Olfactory bulbectomy in mice induces alterations in exploratory behavior. Neurosci Lett. 2005;374:142–146. doi: 10.1016/j.neulet.2004.10.040. [DOI] [PubMed] [Google Scholar]