Abstract
Little is understood regarding the impacts of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure during early development on the health, survival, and reproductive capability of adults. Here we use zebrafish to determine whether early life stage exposure to TCDD induces toxicity in adult zebrafish and their offspring. Zebrafish were exposed to graded concentrations of TCDD (0–400 pg/ml) via waterborne exposure for 1 h/week from 0 to 7 weeks of age. The heart and swim bladder were identified as being most sensitive to TCDD exposure during early development. Subtle developmental toxic responses collectively impaired survival, and only zebrafish in the 0, 25, and 50 pg TCDD/ml groups survived to adulthood. Surviving fish exhibited TCDD toxicity in craniofacial structures (i.e., operculum and jaw), heart, swim bladder, and ovary. Exposure to 25 pg TCDD/ml impaired egg production (40% of control), fertility (90% of control), and gamete quality. TCDD-treated males contributed more than females to impaired reproductive capacity. Transgenerational effects were also discovered in that offspring from parents exposed to TCDD during early life stages showed a 25% increase in mortality compared with the F1 of dimethyl sulfoxide fish, reduced egg production (30–50% of control) and fertility (96% of control). Thus, adverse effects resulting from TCDD exposure during early life stages for one generation of zebrafish were sufficient to cause adverse health and reproductive effects on a second generation of zebrafish. In the environment, transgenerational effects such as these may contribute to population declines for the most TCDD sensitive fish species.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD); developmental toxicity; reproduction; zebrafish; transgenerational; heart; ovary
Halogenated aromatic hydrocarbons (HAHs) constitute a class of ubiquitous and persistent environmental contaminants that include polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is generally considered to be the most potent HAH and is often used as a model compound to assess the toxicity of HAHs. Several studies have demonstrated that TCDD has the potential to modulate early development and reduce reproductive success of fish, and TCDD is a known endocrine disruptor. Because endocrine signaling is important for the regulation of early development and gonad differentiation, exposure to such compounds during critical ontogenetic periods may cause permanent functional changes that result in reduced fitness and reproductive capacity later in life (Bigsby et al., 1999; Guillette et al., 1995; Segner, 2006).
In fish, exposure to TCDD during early development causes cardiovascular dysfunction, edema, hemorrhages, craniofacial malformations, growth arrest, and mortality in larvae (Heideman et al., 2005; Henry et al., 1997; Hill et al., 2005; Peterson et al., 1993; Spitsbergen et al., 1988, 1991; Tanguay et al., 2003; Walker et al., 1991; Walker and Peterson, 1994). The cardiovascular system and craniofacial structures are particularly susceptible to perturbation by TCDD following embryonic exposure (Carney et al., 2006; Xiong et al., 2008). Exposure to TCDD as juveniles or as adults results in reproductive toxicity that is manifested by alterations in gonad development, egg production, reproductive and parental behaviors, and circulating 17β-estradiol concentrations, as well as reduced offspring survival (Giesy et al., 2002; Hutz et al., 1999; King Heiden et al., 2005, 2006; Palstra et al., 2006; Peterson et al., 1993; Wu et al., 2001). Collectively, this work demonstrates the high susceptibility of fish to TCDD during early development that culminates in larval mortality associated with blue sac syndrome, and that exposure of juvenile and adult fish to TCDD results in endocrine disruption and reproductive failure.
Although this body of work has provided great insight into the developmental and reproductive toxicity of TCDD in fish, identifying whether exposure to such compounds contributes to population declines of wild fish species remains unresolved as the long-term effects of exposure to TCDD during early life stages are not known. In order to better understand the population-relevant effects of TCDD-like compounds in fish that are exposed during the sensitive early development period, we transiently exposed zebrafish to graded concentrations of TCDD during early developmental and gonad differentiation stages of development. The goal of this research was to identify threshold concentrations of TCDD for early life stage and gonad differentiation stage exposure that cause in adult zebrafish: (1) reduced survival, (2) craniofacial and cardiac toxicity, and (3) impaired gonad development and reduced reproductive capacity. In addition, we sought to determine in the offspring of adult zebrafish who were exposed during early development and gonad differentiation to graded doses of TCDD: (4) adverse effects on the survival, health, and reproductive capacity of their offspring.
We chose the zebrafish as a model because it has been used for investigating the aryl hydrocarbon receptor (AHR) signaling pathway in fish, identifying endpoints of TCDD developmental toxicity, and understanding mechanisms of TCDD toxicity at the cellular and molecular levels (Carney et al., 2006; Heideman et al., 2005; Henry et al., 1997; Hill et al., 2005). The zebrafish has also been validated for use in both partial and full life-cycle tests for endocrine disrupting compounds (Ankley and Johnson, 2004), and is a member of a large and ecologically important family of fish (Cyprinidae).
MATERIALS AND METHODS
Test species, waterborne exposure of fish to TCDD and fish husbandry.
Fertilized eggs were collected from AB strain zebrafish and were raised according to procedures described by Westerfield et al. (1997). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD, > 99% purity from Chemsyn, Lenexa, KS) was dissolved in dimethyl sulfoxide (DMSO) for preparation of dosing solutions. Figure 1 is a summary of the experimental design for the present study. TCDD exposures were done in two blocks (replicates). Because the data did not differ significantly across blocks; they were combined. Experimental groups (n) were defined as the set of embryos exposed to DMSO or TCDD within a single container; the number of fish within each experimental group is defined for individual experiments. For reproductive capacity experiments, n is defined as a “spawn unit.”
FIG. 1.
Summary of TCDD experimental design for the zebrafish study. Shown are specific times during early development and gonad differentiation when F0 zebrafish were exposed to waterborne TCDD and when endpoints of toxicity were assessed. Abbreviations: F0, parental generation; F1, first filial generation; CYP1A, cytochrome P4501A. See text for specific descriptions for each experiment.
To determine whether early life stage exposure to TCDD induces latent toxicity in adults, zebrafish were exposed to graded concentrations of TCDD once weekly during early developmental stages through sexual differentiation (0–7 weeks of age). The first exposure was shortly after fertilization (4–6 h postfertilization [hpf]; 10 embryos/ml were exposed to 0.1% DMSO [vehicle control] or to graded concentrations of TCDD [25, 50, 100, 200, or 400 pg/ml]). There were a total of six experimental groups (n = 6) for the vehicle control (DMSO) and for each TCDD concentration to be tested; each experimental group (n) consisted of 50 embryos. Following the first vehicle or TCDD exposure, the 50 embryos in each group were rinsed and separated into three subgroups. Experimental subgroup A consisted of 10 fish, and was used to assess early development and TCDD toxicity (n = 6). Experimental subgroups B and C consisted of 20 fish each, and were used for subsequent exposures (described below; n = 12). All exposed fish were transferred to TCDD-free, fresh fish water (60 mg/l Instant Ocean Salts; Aquarium Systems, OH) and maintained at 1 fish/5 ml density for the first week of development.
The next seven static waterborne exposures for the vehicle group (DMSO, control) and five TCDD groups (25, 50, 100, 200, or 400 pg/ml) lasted for 1 h and were conducted at 1, 2, 3, 4, 5, 6, and 7 weeks postfertilization (wpf). There were a total of 12 groups (n = 12) for the vehicle control (DMSO) and 12 groups (n = 12) for each TCDD concentration to be tested (subgroups B and C). Each group (n) consisted of 20 fish. Between the weekly 1 h pulse exposures to TCDD, fish were raised in static beakers of TCDD-free fish water (at a density of one fish per 20 ml) at 26–28°C on a 14-h light and 10-h dark cycle. Because TCDD is a highly persistent contaminant, and the half-life of TCDD in zebrafish is not known, fish were likely continuously exposed to TCDD throughout early development and sexual differentiation, and beyond cessation of waterborne exposures. Fish were fed twice daily with paramecia, brine shrimp and dried food (AP100, Zeigler, Garner, PA), and 50% water changes were performed daily. Fish were observed daily for overall health, signs of toxicity, and mortality.
At 8 weeks postfertilization (wpf), surviving fish from all groups within a particular treatment (i.e., DMSO vehicle control, 25 or 50 pg TCDD/ml) were combined and moved to re-circulating aquaria where the water temperature was maintained at 25–27°C with the same light/dark cycle. Zebrafish in the re-circulating aquaria were maintained at a density of one fish per gallon of water. Fish were fed fresh brine shrimp twice and trout chow or Aquatox food (Aquatic Eco-Systems, Apopka, FL) once daily. Fish were observed daily for overall health, signs of toxicity, and mortality through adulthood.
Dose-dependent developmental toxicity.
Embryos/larvae within subgroup A were evaluated daily under a dissecting microscope (0–7 days postfertilization, dpf) to assess TCDD toxicity including: pericardial or yolk sac edema, shortened jaw, and failure to inflate the swim bladder. At 5 dpf, the percent of larvae exhibiting either blue sac syndrome (defined as embryos having a combination of yolk sac edema, pericardial edema, and overt craniofacial malformations) or failure to inflate the swim bladder was recorded (n = 6/treatment where n = 1 is defined as a group of 10 larvae).
Growth and severity of the toxic effects were quantified by measuring pericardial and yolk sac edema, as well as length of the lower jaw of three representative larvae from each group (n = 6; where n = 1 is defined as a set of three larvae; results averaged). Larvae were immobilized in 3% methylcellulose and lateral images were acquired using an Optronics MicroFire camera mounted on a Leica MZ16 stereomicroscope (Bannockburn, IL). Micrographs were analyzed using Scion Image software to measure total body length as a measure of growth, and lower jaw length, as well as pericardial and yolk sac area as a measure of edema. The percentage of 5 dpf larvae (incidence of effect) with uninflated swim bladders, overt jaw malformations, pericardial edema, and yolk sac edema was calculated for each treatment group.
Dose-response effects on heart function.
Because the heart is a key target organ for TCDD-induced developmental toxicity, we sought to determine the dose-dependent effects of TCDD on heart function. In separate experiments, 10 embryos/ml (n = 5/treatment) were exposed to 0.1% DMSO (vehicle control) or 25, 50, 100, 200, or 400 pg TCDD/ml via 1-h static waterborne exposure shortly after fertilization (4–6 h postfertilization). At the end of the 1 h exposure, fish were transferred to fresh water. At 24 hpf, 0.003% 1-phenyl-2-thiourea (PTU) was added to the fish water to prevent pigment formation. At 96 hpf, three representative embryos from each treatment group (n = 5 where n = 1 is defined as a set of three larvae; results averaged) were used to assess heart function. The entire experiment was run in duplicate. Embryos were immobilized in 3% methylcellulose and photographed ventrally to visualize the heart. For assessing heart function, embryos were oriented laterally and images were acquired using an using an Optronics MicroFire camera mounted on a Nikon TE300 inverted microscope with a Princeton Instruments Micromax charge-coupled, high-speed Motion Scope camera. Incidence of ventricular standstill and the heart rate, stroke volume, and cardiac output were determined from time-lapse recordings (250 frames/s) as previously described (Antkiewicz et al., 2005, 2006). The proportion of these larvae (incidence) with reduced heart function (cardiac output < 1 SD of the mean for DMSO fish) was calculated for each treatment group. These fish were euthanized at the completion of experiments, and were not part of the population used in latent toxicity experiments.
Toxicity in adults prior to spawning.
Once fish exposed to 0, 25, and 50 pg/ml TCDD from 0 to 7 wpf reached sexual maturity (3 months), sex was determined via external morphological characteristics (confirmed later by histology), and males and females were placed in separate tanks. A subsample of fish (n = 5 or 6 fish per treatment) were euthanized with 3-aminobenzoic acid ethyl ester (MS-222, Sigma, St. Louis, MO) and processed for histopathology. Wet weight and total length were recorded and condition factor (CF = length/weight3 × 100) was calculated for each fish. Fish were fixed with 10% Zn formalin, decalcified with Cal-ExII, dehydrated in a graded series of ethanol, cleared in xylene, and embedded in paraffin. During paraffin embedding, fish were bisected laterally (to minimize the number of sections required for analysis of several organ systems) into dorsal and ventral halves using the center of the eye as a landmark. Fish were embedded cut side down and sectioned at 5 μm, mounted on slides, and stained with hematoxylin and eosin. Histopathologic lesions were evaluated from four sections at two separate levels within each fish.
Reproductive capacity.
Evaluations of reproductive capacity were initiated when remaining DMSO- and 25 pg TCDD/ml-treated fish reached 6 months of age; each experiment consisted of four spawn events (once per week). For all experiments, fish were allowed to spawn for approximately 3 h after the lights came on in the morning, following standard spawn procedures. Released eggs were collected and maintained at 28°C, and both fertilized and unfertilized eggs were counted approximately 2–3 h later.
To assess reproductive capacity of the population (i.e., the treatment group as a whole in group spawns), fish were spawned in two groups (population effects, 2 females:1 male) and these fish were used in a total of four spawn events. It is possible that exposure to TCDD could cause reproductive failure in some fish, whereas not affecting others. Alternatively, reproduction could be impaired in all fish. To determine if exposure to 25 pg TCDD/ml caused complete reproductive failure for some of the fish within the population, fish also were spawned in pairs (individual effects, 1 female:1 male). For these experiments, there were seven spawning pairs (units) of zebrafish per treatment and four spawn events. Finally, to determine whether exposure to TCDD had “sex-dependent” impacts on reproduction, TCDD-treated females were spawned individually with vehicle control males [sex-dependent effects, one TCDD-treated female (1TF) x one DMSO male (1DM)], and vehicle control females were individually spawned with TCDD-treated males [sex-dependent effects, one DMSO female (1DF) x one TCDD-treated male (1TM)]. There were seven spawning pairs (units) of zebrafish per treatment and a total of four spawn events. For all three experiments, egg production and percent of fertilized eggs were not different across spawn events within a treatment group. Therefore, we averaged outcomes of all spawn events for each type of experiment as an estimate of reproductive capacity.
To assess impacts of TCDD exposure on gamete quality (survival through 24 hpf) and early embryonic development in all three experiments, four subsets of fertilized eggs per spawn (10 eggs per subset) from each spawn unit (group or pair) were raised in fresh fish water through 5 dpf (n = 2 or 7 per treatment) for each spawn event. Mortality and early development of offspring were monitored as described above through 5 dpf.
Maternal transfer of TCDD.
To determine whether offspring were exposed to TCDD via maternal transfer, CYP1A enzyme activity was assessed as a marker of TCDD exposure using the in vivo ethoxyresorufin-O-deethylase (EROD) assay as previously described (Nacci et al., 1998). Live embryos (F1) from TCDD- and DMSO-treated fish were collected from the second, third, and fourth population spawn experiments described above. Some eggs from DMSO-treated fish were exposed to 400 pg TCDD/ml via waterborne exposure at 4 hpf for 1 h as a positive control (n = 5; 10 embryos/ml). At 96 hpf, embryos were incubated individually for 5 min in 7-ethoxyresorufin, which is O-deethylated by CYP1A to resorufin. Fish were rinsed with fish water and after allowing an additional 5 min for the reaction to occur, embryos were mounted in 3% methylcellulose for observation and imaging with epifluorescence microscopy (excitation λ, 577 nm; emission λ, 620 nm). For these experiments, five embryos per treatment (DMSO F1, TCDD F1, and DMSO F1 treated with TCDD) were individually exposed to 7-ethoxyresorufin, and n = 1 is defined as a single embryo. The experiment was repeated three times for a total n = 15 for each treatment group.
Toxicity in adults after spawning.
Following all spawn trials, 10 males and 10 females from each treatment group were euthanized. The only exception was the 25 pg TCDD/ml group where seven females were euthanized. Wet weight and total length were recorded, and used to calculate CF for each fish as described above. Fish were then fixed and processed for histopathology as described above. One female from the 25 pg TCDD/ml group was unable to be analyzed for histopathology. Additionally, 10 separate male fish from each treatment group were photographed in the lateral orientation for gross microscopic observation. Micrographs were analyzed using Scion Image software to measure total body length, snout length, head size (area of head from snout to edge of operculum), and operculum length. Snout length, head size, and operculum length were expressed relative to total body length.
Transgenerational effects.
After the completion of all spawn trials, DMSO- and TCDD-treated fish were spawned (n = 2 population spawns/treatment) and five cohorts of offspring (n = 5 groups of F1 fish per spawn group for a total of n = 10 cohorts of F1/dose; 20 fish per cohort) were raised to adulthood as described above. Fish were observed daily for overall health and mortality as described above. Once F1 fish reached maturity, sex ratio was determined via external morphological characteristics. Evaluations of reproductive capacity (population, individual and sex-dependent effects) were initiated when F1 fish reached 6 months of age as described above (n = 10 spawn pairs for the individual and sex-dependent spawn trials). Wet weight, total length, and jaw length were recorded, and CF was calculated for 10 males and females.
Data analysis.
Statistical analyses were performed using Sigma-Stat software 2.0 (Ashburn, VA) and results are presented as mean ± SE. Data were evaluated for homoscedasticity (Leven Median test), and one-way ANOVA; pair-wise multiple comparisons were conducted using Tukey's test. The Fisher's Exact Probability test was used to determine whether TCDD-exposed larvae showed altered sex ratios compared with DMSO vehicle control. The level of significance for all analyses was p < 0.05.
RESULTS
Dose-Dependent Developmental and Cardiac Toxicity
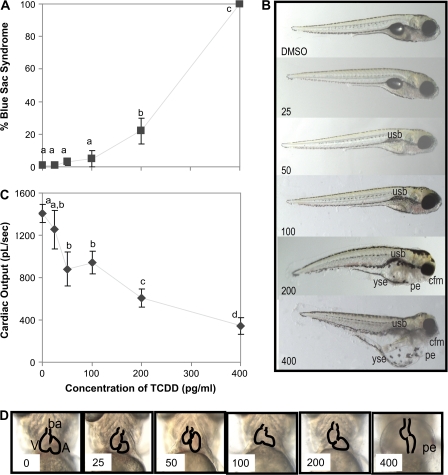
Following 1-h pulse exposure to ≥200 pg TCDD/ml at 4 hpf, 120 hpf larvae showed increased incidence of blue sac syndrome (Fig. 2A), a hallmark of TCDD-induced developmental toxicity in fishes. However, the severity of blue sac syndrome and individual endpoints of toxicity (i.e., degree of edema, Fig. 2B) was minimal, and only those in the 400 pg TCDD/ml-treatment group showed significant increases in grossly evident pericardial or yolk sac edema, or shorter jaws. Gross morphology of those exposed to <200 pg TCDD/ml appeared similar to control larvae (Fig. 2B) except for a decreased incidence of swim bladder inflation (results not shown). Developmental toxicity was subtle, and although the swim bladder was not inflated in these fish, larvae did not appear emaciated (Fig. 2B). Larval growth; however, was affected in zebrafish exposed to ≥100 pg TCDD/ml, as their total length was 92–97% of control (p < 0.05).
FIG. 2.
Developmental toxicity in zebrafish following exposure to graded concentrations of TCDD for 1 h at 4–6 hpf. (A) Percent larvae with blue sac syndrome at 120 hpf. Values are mean ± SEM of n = 6 (10 larvae per n); letters denote significant differences (p < 0.05). (B) Representative micrographs of larvae at 120 hpf following exposure to TCDD. Abbreviations: usb, uninflated swim bladder; cfm, craniofacial malformations; yse, yolk sac edema; pe, pericardial edema. (C) Cardiac output at 96 hpf following exposure to TCDD for 1 h at 4–6 hpf. Values are mean ± SEM of n = 10 (three larvae per n); letters denote significant differences (p < 0.05). (D) Representative micrographs of hearts in 96 hpf embryos. Abbreviations: V, ventrical; ba, bulbous arteriosis; A, atrium; pe, pericardial edema.
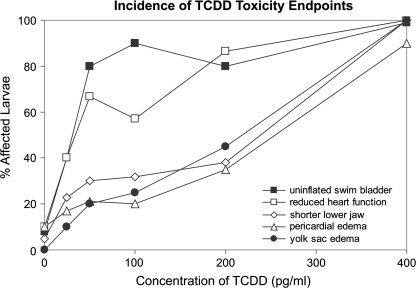
Although heart rate was not altered, exposure to TCDD caused dose-dependent decreases in stroke volume (results not presented) and cardiac output (Fig. 2C), even when no significant gross heart malformations or pericardial edema were observed (Fig. 2D). Of the developmental toxicity endpoints observed following exposure to sublethal concentrations of TCDD, failure to inflate the swim bladder and an increased incidence of reduced heart function were most common, whereas effects on jaw length and pericardial and yolk sac edema were less common (Fig. 3).
FIG. 3.
Incidence of the individual endpoints of toxicity at 5 dpf following exposure to graded concentrations of TCDD for 1 h at 4–6 hpf. Percent of fish that were affected was determined from representative larval photomicrographs (n = 18 fish per treatment).
Impacts on Survival and Latent Toxicity
Weekly 1 h pulse exposures to TCDD for 7 wpf resulted in a dose-dependent increase in mortality for all treatment groups (Fig. 4). Only those exposed to 25 and 50 pg TCDD/ml survived to 8 wpf. Between 2 and 3 months, three fish within the 50 pg/ml treatment group had fin necrosis and slightly darker pigmentation and were euthanized because they demonstrated signs of deformities or severe stress (i.e., severely bent spines and/or lethargic swimming behavior). Those in the 25 pg TCDD/ml group did not show signs of overt toxicity other than shortened snouts. Once fish reached adulthood (3 months), percent mortality normalized to control for the 25 and 50 pg TCDD/ml groups was 44 ± 4 and 90 ± 2%, respectively. Sex ratio was significantly shifted towards males in a dose-dependent manner (Table 1). Overall, those that survived appeared healthy with CFs of 0.009 ± 0.0007, 0.009 ± 0.0008, and 0.0102 ± 0.0006 at 3 months of age in the DMSO and 25 and 50 pg TCDD/ml-treatment groups, respectively. Fish appeared to remain healthy at 11 months, with CFs of 0.009 ± 0.0003 and 0.010 ± 0.006 in the DMSO and 25 pg TCDD/ml groups, respectively.
FIG. 4.
Survival following early life stage exposure to TCDD. Exposure to all concentrations of TCDD during early life stages caused increased mortality (n = 12; percent mortality is normalized to control). Arrows represent the times at which fish were pulse-exposed to waterborne TCDD; however, it should be noted that because TCDD is highly persistent, exposure was likely continuous during these stages, and may have continued past cessation of waterborne exposures.
TABLE 1.
Effects of TCDD Exposure during Early Development and Gonad Differentiation on Prespawning and Postspawning Zebrafish
| Prespawning (3 months) TCDD (pg/ml) |
Postspawning (11 months) TCDD (pg/ml) |
||||
| 0 | 25 | 50 | 0 | 25 | |
| Heart | |||||
| Pericardial edema (mild) | 0/6 | 0/6 | 3/5 | 0/20 | 1/16 |
| Hyperplasia of ventriclar epicardium | 0/6 | 0/6 | 2/5 | 0/20 | 0/16 |
| Head | |||||
| Submucosal edema of operculum (mild) | 0/6 | 0/6 | 4/5 | 0/20 | 0/16 |
| Foreshortened maxialla; mandible (severe) | 0/6 | 0/6 | 5/5 | 0/20 | 0/16a |
| Underdeveloped gill holobranchs, hemibranchs, and filaments | 0/6 | 0/6 | 3/5 | 0/20 | 0/16 |
| Underdeveloped nasal neurosensory epithelium | 0/6 | 0/6 | 3/5 | 0/20 | 0/16 |
| Swim bladder | |||||
| Underinflated anterior compartment | 0/6 | 0/6 | 1/5 | 0/20 | 0/16 |
| Mxyomatous materialb | 0/6 | 0/6 | 2/5 | 0/20 | 0/16 |
| Edema surrounding swim bladder | 0/6 | 0/6 | 3/5 | 0/20 | 0/16 |
| Liver | |||||
| Reduced hepatocyte glycogen (mild to severe) | 0/6 | 0/6 | 0/5 | 0/20 | 6/16 |
| Female gonad | |||||
| Increased # atretic follicles | 0/5 | 1/1 | n/ac | 1/10 | 2/6 |
| Amorphous yolk and egg debris | 0/5 | 1/1 | n/ac | 2/10 | 2/6 |
| Reduced # vitellogenic follicles | 0/5 | 0/1 | n/ac | 1/10 | 3/6 |
| Increased macrophages | 0/5 | 0/1 | n/ac | 1/10 | 2/6 |
| Sex ratio | |||||
| # Females/# males | 1.96d | 0.33 | 0.17 | ||
Histological craniofacial malformations were more difficult to assess in 11-month-old fish due to variations in the plane of section across fish. However, gross assessment did identify craniofacial malformations in males (as described in the text).
Myxomatous material was found between tunica interna and tunica muscularis.
Remaining fish in the 50 pg/ml TCDD group were all males. Histolologic lesions were not observed in these males, or in males (n = 10) sampled at 11 months.
Under these rearing conditions, we typically see a female:male sex ratio ranging from 1:1 to 2:1. Sex was determined via external morphology, and confirmed by histology at 3 or 11 months.
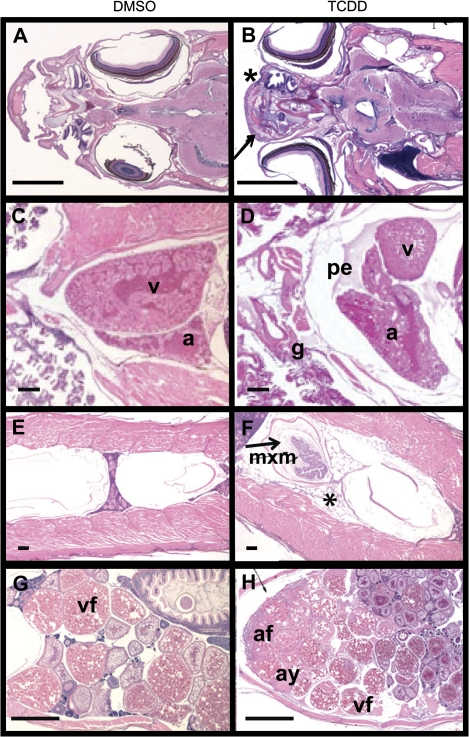
Histologic study conducted on DMSO (vehicle control) and TCDD-exposed zebrafish at the prespawning stage of development (12 wpf) revealed that bony structures such as the bone in the operculum, maxilla, and mandible, and soft tissues including the heart, swim bladder, and ovary showed signs of dose-dependent TCDD toxicity (results summarized in Table 1; representative micrographs shown in Fig. 5). At the prespawning sampling time point, histologic lesions were evident in tissues other than gonad only in the 50 pg/ml treatment group. These fish showed underdeveloped cartilaginous and/or bony structures of the skull such as maxilla and mandible. The gill was also underdeveloped in this group compared with control fish, with smaller holobranchs, hemibranchs and gill filaments than those of controls. Pericardial edema was evident in 3/5 fish and mild hyperplasia the ventricular epicardium occurred in 2/5 fish in this group, respectively. Clear or proteinaceous edema fluid was evident in the peritoneum surrounding the gas bladder in 3/5 fish in the 50 pg/ml group, and in 2/5 of these fish gas bladders were underdeveloped with retention of myxomatous material between the tunica interna and the tunica muscularis, as typically occurred in zebrafish of an earlier developmental stage (Henry et al., 1997). In 1/5 of these fish the anterior chamber of the gas bladder was underinflated. Unfortunately just one female fish was available for histologic examination in the 25 pg/ml treatment group, and no females were available in the 50 pg/ml group at this sampling time point. Thus, definitive statements regarding effects of TCDD on ovarian histology at this stage of development cannot be made. However, in comparison to control females, the single TCDD-treated female examined showed showed an increase in the proportion of atretic and ovulated mature oocytes undergoing degeneration with basophilic material in the cytoplasm. No lesions were identified in the testis of TCDD-treated male zebrafish prior to spawning.
FIG. 5.
Representative photomicrographs demonstrating lesions in prespawning zebrafish. (A, B) Exposure to TCDD during early life stages of zebrafish induced malformations of the head such as a shortened maxilla (arrow) and underdeveloped nasal mucosa compared with control (*). (C, D) Lesions in the gills (g, underdeveloped compared with control) and heart (pe, pericardial edema with proteinaceous fluid) were also noted. (E, F) Fish exposed to TCDD had an uninflated anterior swim bladder (arrow) containing myxomatous material (mxm) and excess clear fluid surrounding the swim bladder (*). (G, H) The ovary of TCDD-treated fish showed an increased proportion of atretic follicles (af) and amorphous yolk material (ay), as well as reduced numbers of vitellogenic follicles (v). See Table 1 for a full summary of the histopathology report. Abbreviations: v, ventricle; a, atrium; pe, pericardial edema; g, gills; mxm, myxomatous material; af, atretic follicles; ay, amorphous yolk material; vf, vitellogenic follicle. Scale bars in A, B, G, H = 1000 μm; C, D, E, F = 100 μm.
Postspawn, the only gross malformations observed in the 25 pg TCDD/ml-treatment group were effects on craniofacial structures in male fishes. These fish showed significantly reduced snout length (60% of control), smaller head (87% of control), and smaller operculum length (89% of control). As at 3 months, few histologic lesions were found in fish that reached 11 months of age (Table 1). In males, the most pronounced lesions were identified in the liver, with 6/10 TCDD-treated males showing reduced glycogen stores in the hepatocyte cytoplasm. Based on our extensive previous published studies of glycogen depletion in TCDD-treated zebrafish, trout, and other fish species, the loss of characteristic glycogen clefts does not require special stains for confirmation (Henry et al., 1997; Spitsbergen et al., 1991). No lesions were identified in the testes of males sampled at 11 months. Four of the six 25 pg TCDD/ml-treated females showed significant lesions in the ovary, including fewer vitellogenic follicles, presence of amorphous yolk and egg debris, increased atretic follicles, and 20% of ovarian tissue occupied by macrophages. Two of the ten control females showed similar, but less severe phenotypes, so the significance of these lesions remains unclear. Additional larger scale histologic studies incorporating morphometric analysis of the gonad that also include the F1 generation will be required to clarify these issues, and to draw definitive conclusions regarding the subtle latent effects of TCDD on the developing ovary of zebrafish.
Impacts on Reproductive Capacity
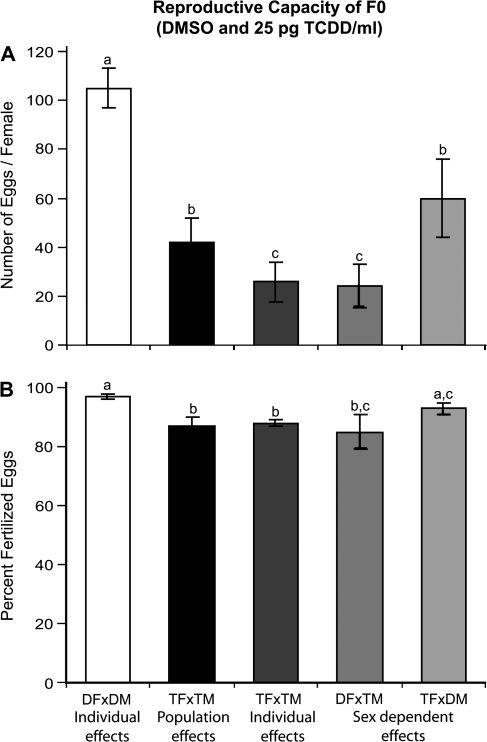
Fish exposed for one hour to 25 pg TCDD/ml weekly from 0 to 7 wpf showed reduced egg production (30–50% of control, Fig. 5A). On average, only 43 ± 10% of TCDD-treated fish successfully spawned (released > 10 eggs per spawn event), whereas 85 ± 7% of control fish successfully spawned based on the individual spawn assays (p < 0.05). TCDD-treated fish that successfully spawned still produced significantly fewer eggs than control fish (∼60% of control, p < 0.05). Males contributed slightly more than females to reduced egg production following exposure to TCDD. Control females spawned with TCDD-treated males (DF × TM) showed a marked reduction in egg production, producing fewer eggs (23% of control) than when spawned with control males (p < 0.05). When TCDD-treated females spawned with control males (TF × DM), egg production increased 34% relative to the number of eggs produced when control females were spawned with TCDD-treated males (DF × TM, p < 0.05); however, egg production was still significantly less than control (60% of DF × DM, p < 0.05).
TCDD-treated fish also showed significant reductions in the proportion of eggs that were fertilized; fertility for the population was approximately 90% of control (Fig. 5B, p < 0.05). When control females were spawned with treated males (DF × TM), fertility was not significantly improved; however, when treated females were spawned with control males (TF × DM), the proportion of fertilized eggs increased by 5% (compared with TF × TM, p < 0.05).
The ability of embryos to progress through the earliest stages of development is one measure of gamete quality. In TCDD-treated fish, mortality of offspring at 24 hpf was 3–5% greater than control (p < 0.05) suggesting that gamete quality of these fish was also reduced; these effects were not sex-dependent (i.e., no differences between offspring from DF × TM or TF × DM crosses; results not shown).
Transgenerational Effects
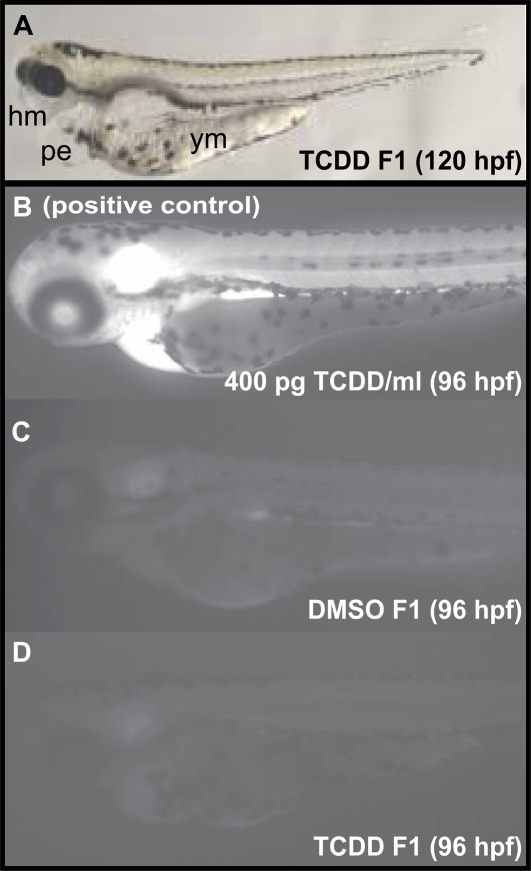
Unexposed offspring, from fish exposed to 25 pg TCDD/ml from 0 to 7 wpf (F1), showed reduced health and survival, with a 15% increase in mortality at 120 hpf compared with offspring from control fish (p < 0.05). Approximately 30% of F1 fish that survived through 120 hpf showed severe developmental anomalies including edema around the heart, eyes and jaws, as well as malformed yolk sacs due to incomplete yolk absorption (Fig. 6A). Eighty percent of surviving F1 larvae failed to inflate their swim bladders and overall the fish showed reduced embryonic growth that was about 92% of control (p = 0.05). Embryos did not show increased CYP1A activity (Figs. 6B–D). F1 fish continued to show marked increases in mortality through adulthood (mortality was ∼10% higher during larval and juvenile stages compared with control); condition factor and sex ratios were not different from control (results not shown). However, the jaws of male F1 fish from TCDD-treated fish were significantly shorter (74% of control males, p < 0.05).
FIG. 6.
TCDD exposure during early development and gonad differentiation of zebrafish reduces egg production (no. of fertilized eggs, A) and fertility (% fertilized, B) of adults. Values represent the mean ± SE of n = 7 averaged across four spawn events; letters denote significant differences (p < 0.05). Individual effects (DF × DM and TF × TM) represent egg production and fertility from successful spawns only (if spawns resulted in < 10 eggs/female, data was excluded). In control fish, egg production and fertilization success was not different between population and individual spawn experiments; therefore, only results for individual spawns are presented. Abbreviations: DF, DMSO control female; DM, DMSO control male; TF, TCDD-treated female; TM, TCDD-treated male; individual effects, pair spawns; population effects, group spawns; sex-dependent effects, pair spawns of control × TCDD-exposed fish.
In addition to reduced survival, offspring from zebrafish exposed to TCDD during early life stages and gonad differentiation also showed reduced reproductive capacity. Egg production was 30–50% of control (Fig. 7A). On average, only 66 ± 5% of the F1 female offspring from TCDD-treated fish successfully spawned (released > 10 eggs per spawn event), whereas 83 ± 9% of F1 female offspring from control fish successfully spawned based on results of individual spawn experiments (p < 0.05). The F1 offspring from TCDD-treated fish that successfully spawned still released significantly fewer eggs than control fish (50% fewer eggs than control, p < 0.05). No sex-dependent effects on egg production were found. Fertility for the population of F1 offspring derived from TCDD-treated fish was 96% of control (Fig. 7B, p = 0.05); however, no sex-dependent effects were found. Mortality of offspring at 24 hpf was not significantly greater (2–3% increase, p = 0.06), suggesting that gamete quality of F1 fish was not affected (results not shown).
FIG. 7.
Exposure to TCDD during early development and gonad differentiation of zebrafish affects the quality of their offspring. Approximately 30% of offspring showed developmental anomalies (A, n = 56). An in vivo EROD assay was performed at 96 hpf to assess TCDD-induced CYP1A enzyme activity (n = 15). Representative TCDD-exposed fish (400 pg/ml, B), vehicle-exposed (DMSO, C), and F1 fish from fish exposed to 25 pg/ml TCDD during early life stages (D) are shown. Abbreviations: hm, head malformations including submandibular and perioccular edema, shortened snout, and smaller eyes; pe, pericardial edema; ym, yolk malabsorption (yolk not completely resorbed).
FIG. 8.
Both egg production (no. fertilized eggs, A) and fertility (% fertilized, B) of the F1 fish were also decreased. Values represent the mean ± SE of n = 10 averaged across four spawn events; letters denote significant differences (p < 0.05). Individual effects (DF1 × DM1 and TF1 × TM1) represent egg production and fertility from successful spawns only (if spawns resulted in < 10 eggs/female, data was excluded). In F1 from control fish, egg production and fertilization success was not different between population and individual spawn experiments; therefore, only results for individual spawns are presented. Abbreviations: DF1, F1 from DMSO control female; DM1, F1 from DMSO control male; TF1, F1 from TCDD-treated female; TM1, F1 from TCDD-treated male; individual effects, pair spawns; population effects, group spawns; sex-dependent effects, pair spawns of control × TCDD-exposed fish.
DISCUSSION
Although zebrafish are less sensitive to TCDD toxicity than other fish species, when exposed to sufficient concentrations of TCDD, endpoints of toxicity are essentially the same as in other fish species (Carney et al., 2006). Nevertheless much of what we have learned thus far about the developmental toxicity of TCDD in zebrafish has come from studies using lethal doses of TCDD. Here we demonstrate for the first time in zebrafish, that exposure of embryos and larvae to concentrations of waterborne TCDD that are too low to cause larval mortality secondary to blue sac syndrome, still induce multiple, subtle, toxic responses that culminate in reduced health and survival. To our knowledge, this is the first work to demonstrate that transient exposure of zebrafish to TCDD during early development and sexual differentiation is capable of inducing latent TCDD toxicity in adults, as well as in their offspring whose only exposure to TCDD was as a gamete. Recent evidence suggests that exposure to endocrine disrupting compounds during early development may influence developmental programming, resulting in permanent functional changes not realized until later in life (Anway et al., 2006a; Chang et al., 2006; Heindel, 2005, 2007; Jefferson et al., 2007a,b; Nayyar et al., 2007; Uzumcu and Zachow, 2007). This work provides the framework for further investigations into the mechanisms by which early life stage exposure to endocrine disruptors such as TCDD induces cardiac malformations and reproductive dysfunction later in life.
Dose-Response Developmental Toxicity
Although exposure to sublethal concentrations of TCDD did not induce the hallmark signs of blue sac disease in zebrafish larvae (Henry et al., 1997), exposure to TCDD caused subtle malformations and reductions in growth that, in combination, likely influenced their poor survival following subsequent exposure. In fishes, survival and growth through the transitional period from endogenous feeding (yolk sac larvae, 0–4 dpf in zebrafish) to exogenous feeding (free-swimming larvae, 5–6 dpf in zebrafish) is directly correlated with recruitment success (Hjort's Critical Period Hypothesis, reviewed by [Cowan and Shaw, 2002]). Therefore, subtle reductions in size and fitness of free-swimming larvae can have an impact on whether or not a population of fishes is able to sustain itself.
In larvae, both the heart and craniofacial structures are known to be sensitive to TCDD following exposure to lethal concentrations (Antkiewicz et al., 2005; Elonen et al., 1998; Heideman et al., 2005; Hill et al., 2005; Hornung et al., 1999; Peterson et al., 1993; Teraoka et al., 2002). Here, we show that exposure to sublethal concentrations of TCDD impacts heart function in larvae, despite a lack of gross morphologic effects (pericardial edema or unlooped heart). Because cardiovascular health is directly correlated with swimming ability and overall health and survival of fishes (Claireaux et al., 2005; Gamperl et al., 2002; Shingles et al., 2005), it is likely that reduced heart function also contributes to their poor survival following subsequent exposure. Similarly, sublethal TCDD exposure did not induce significant gross craniofacial malformations. However, preliminary experiments by our group (King Heiden et al., 2008a) and others (Hill et al., 2004) show that exposure to low concentrations of waterborne TCDD does in fact induce subtle malformations in the lower jaw. Additional experiments are needed to determine whether such malformations in jaw structure result in reduced feeding capabilities.
A sensitive endpoint of developmental TCDD toxicity identified by this study was the effect on the swim bladder (lack of inflation) observed in free-swimming larvae. It has been suggested that absence of an inflated swim bladder can impair sensory capability and orientation of fishes, particularly in species such as cyprinids that utilize a Weberian apparatus for detecting and transmitting sensory information (Goolish and Okutake, 1999). This could account for abnormal swimming behaviors seen in fish with overt TCDD toxicity. Furthermore, some studies suggest that failure to inflate the swim bladder can result in skeletal malformations (Chatain, 1994), altered metabolic rates (Marty et al., 1995), reduced growth (Chatain, 1987), and increased stress-induced mortality (Chatain and Dewvrin, 1989) in free-swimming larvae and juveniles. Because zebrafish are physostomous fishes (Finney et al., 2006), it is unclear whether or not impacts of TCDD exposure on inflation of the swim bladder of larval fishes would be permanent and impact overall fitness. It is likely that lack of swim bladder inflation contributed to the increased mortality of larval and juvenile fish by, for example, reducing their ability to catch prey. Alternatively, affected fish could have later filled their swim bladders (by gulping air), and mortality could be related to other developmental toxicities.
Once TCDD-treated fish reached adulthood, effects on the swim bladder were found in only in those treated with 50 pg TCDD/ml, and were restricted to the anterior swim bladder (used primarily for acoustic resonation in physostomous cyprinids; Finney et al., 2006 and references therein). However, fish did not show reduced condition factor suggesting that in a laboratory setting, surviving fish were able to eventually compensate for any effects on the swim bladder. Long-term effects of TCDD on inflation of the swim bladder with regard to survival through larval and juvenile stages and overall fitness warrant further clarification. From this work one can hypothesize that collectively, subtle developmental adverse effects on the swim bladder, heart, and jaw caused by TCDD exposure in zebrafish could account for observed reductions in larval growth rates and poor survival through early larval and juvenile stages.
Latent TCDD Toxicity in Adults
An important result of the present study was our discovery that exposure to TCDD from early development through sexual differentiation caused latent endpoints of toxicity that persisted in adult zebrafish. Latent toxic responses were found primarily in fish exposed to 50 pg TCDD/ml, and most of the lesions observed were likely the result of residual cardiovascular toxicity or maturation arrest. Only at the lowest concentration of TCDD, 25 pg/ml, did significant numbers of fish survive through adulthood; of those that survived, histopathologic responses were minimal. Overall, only the craniofacial structures and the gonad were significantly affected by exposure to 25 pg TCDD/ml during early life stages, with fish showing shortened jaws and smaller heads, male-biased sex ratios, and altered ovarian development.
Although sex is determined genetically for most fish species, sex differentiation is labile and can be modulated, for example, by exposure to androgenic and estrogenic compounds (Hunter and Denton, 1983). Potential implications of TCDD exposure on sex ratio, whereas interesting, remain unclear as several factors can affect sex differentiation in zebrafish (Shang et al., 2006; Slanchev et al., 2005), and with the high mortality during juvenile stages, we cannot rule out sex-dependent mortality as a factor that influenced sex ratios. However, this work illustrates the clear need for further investigation into the possibility that HAHs such as TCDD may influence sex differentiation in fishes.
Interestingly, early life stage exposure to TCDD resulted in similar, but less severe, ovarian toxicity as previously described in adult female zebrafish exposed to TCDD (King Heiden et al., 2006; Wannemacher et al., 1992) and adult carp living in TCDD-contaminated waters (Sakamoto et al., 2003). Previous work suggests that exposure of sexually mature females to TCDD may inhibit follicular development by down-regulating estrogen-responsive genes (King Heiden et al., 2008b), resulting in impaired reproduction. Although our findings do not conclusively demonstrate that such subtle impacts on ovarian development underlie observed reduced egg production, they demonstrate the clear need for further investigations into the mechanisms by which early life stage exposure to AHR ligands such as TCDD influence ovarian development, including a quantitative assessment of follicular development, serum hormone levels, and ovarian gene expression.
Less is known regarding the effects of TCDD on the testes. Although TCDD exposure to sexually mature males has been demonstrated to induce lesions in the testes of medaka characterized by a disorganization of spermatogenesis at the testis periphery, disruption of the interstitium, leydig cell swelling, and sertoli cell vacuolation (Volz et al., 2005), no lesions were identified in the testes of zebrafish following early life stage exposure to TCDD. Differences in the response of the testes between these fish species could be attributed to several factors including age at exposure, dose and route of exposure, or species-specific differences in tissue distribution and susceptibility to TCDD.
Impacts on Reproductive Capacity
Although the reproductive toxicity of TCDD in adult female fishes has been well established (Giesy et al., 2002; King Heiden et al., 2005, 2006; Palstra et al., 2006; Walker and Peterson, 1994; Wannemacher et al., 1992), this is the first report in fishes with reduced reproductive capacity following exposure to TCDD during early life stages. Exposure to TCDD at this time reduced egg production, fertility and gamete quality of adult zebrafish. Altered follicular development following exposure to TCDD may contribute to observed reductions in egg production. Although a 10% reduction in fertility alone may not cause population-level effects (Gurney, 2006), in conjunction with reduced egg production and poor offspring survival, impacts on reproductive capacity following exposure to TCDD during early life stages may pose a threat to sustainability of fish populations. It was interesting that even though the testes were devoid of lesions, males appear to contribute more to TCDD-induced reductions in egg production and fertility. Several factors could contribute to this including effects of TCDD on the quality of sperm produced or altered reproductive behavior, and warrant further study.
Finally, these studies suggest that exposure to TCDD during early life impairs the quality of gametes produced (as measured here by survival through the earliest stages of development), as has been previously demonstrated following exposure to adult females (King Heiden et al., 2005). Impacts on gamete quality are thought to have profound effects on natural populations of fishes (Kime and Nash, 1999). Further studies evaluating the mechanisms by which TCDD reduces gamete quality are necessary to determine which component of the reproductive system is most sensitive to TCDD-like compounds.
Transgenerational Effects
Probably the most striking results from these studies were impacts on the F1 generation. Recruitment and reproductive success of offspring are another important aspect of reproductive capacity, and even within a laboratory setting, survival of F1 fish through adulthood was reduced by approximately 25%. It is likely that this would be increased if these fish had to compete for resources and deal with additional pressures such as predation or disease, and suggests that early life stage exposure to AHR ligands such as TCDD can impair recruitment even if the offspring are not additionally exposed to the toxicant (other than as gametes). Thus, for the first time in fish, we show that not only is survival of F1 fish derived from fish exposed to TCDD from early development through gonad differentiation reduced, but so is their reproductive capacity. Histopathology of these fish has not been completed; therefore, at this time we cannot comment on whether gonad development of F1 fish was impaired.
Based upon the lack of observable CYP1A1 activity in F1 larvae, the observed increase in mortality and reduction in reproductive capacity of offspring from fish exposed to TCDD during early life stages is not likely the result of maternal transfer of TCDD. The only time that F1 fish could potentially have been exposed to TCDD was as gametes. Therefore, the mechanisms that underlie the reduced quality of offspring have yet to be determined. Some suggest that exposure to endocrine disrupting chemicals during early stages of development can affect the germ line via epigenetic mechanisms, resulting in increased susceptibility to disease or reduced fitness later in life (Anway and Skinner, 2006; Anway et al., 2006; Chang et al., 2006; Heindel, 2005, 2007; Jirtle and Skinner, 2007). It is important that these findings be replicated to provide sufficient evidence for the hypothesis that epigenetic alterations may underlie transgenerational effects of TCDD on reproductive capacity.
In summary, this work suggests that exposure to TCDD during early life stages may have a profound effect on the ability of a fish population to sustain itself, even when no gross malformations or histologic lesions are present in adults. Semelparous and iteroparous fish species with long life spans, no parental guarding behaviors, and increased age to maturation (i.e., trout and salmon species) are thought to be more sensitive to chronic stressors (Spromberg and Birge, 2005a,b), and are considerably more sensitive to the developmental toxic effects of TCDD (Elonen et al., 1998; Hahn, 2001; Peterson et al., 1993; Spitsbergen et al., 1991; Tanguay et al., 2003; Walker et al., 1991). Therefore, it is not difficult to hypothesize that exposure to TCDD and related AHR agonists could greatly impair sustainability of sensitive wild fish populations. It is thought that exposure to HAHs such as TCDD contributed to the population declines of lake trout in Lake Ontario during the 20th century (Cook et al., 2003; Spitsbergen et al., 1991; Tillitt et al., 2008; Walker et al., 1991) and to the collapse of current silver eel populations in Europe (Palstra et al., 2006). Our work supports this hypothesis and illustrates the usefulness of the zebrafish as a model to evaluate population-relevant effects of TCDD exposure in wild fish and their offspring, and to investigate the molecular mechanisms that underlie such effects.
FUNDING
University of Wisconsin Sea Grant Institute, National Sea Grant College Program, National Oceanic and Atmospheric Administration, Sea Grant Project number R/BT-20; and Molecular and Environmental Toxicology Postdoctoral Training Grant number (T32 ES007015) from the National Institute of Environmental Health Sciences (NIEHS) and more recently by a Ruth L. Kirchstein National Research Service Award from NIEHS (F32 ES016714-01) funded T.C.K.H.
Acknowledgments
We thank Emelyne Dengler, Douchi Yang, Dorothy Nesibt and Dagmara Antkiewicz for technical assistance and training. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. This manuscript is contribution # 372 to the NIEHS Molecular and Environmental Toxicology Postdoctoral Training Grant.
References
- Ankley GT, Johnson RD. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR. J. 2004;45:469–483. doi: 10.1093/ilar.45.4.469. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006a;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006b;147:S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- Bigsby R, Chapin RE, Daston GP, Davis BJ, Gorski J, Gray LE, Howdeshell KL, Zoeller RT, vom Saal FS. Evaluating the effects of endocrine disruptors on endocrine function during development. Environ. Health Perspect. 1999;107(Suppl. 4):613–618. doi: 10.1289/ehp.99107s4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. A Clin. Mol. Teratol. 2006;76:7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- Chang HS, Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination. Endocrinology. 2006;147:5524–5541. doi: 10.1210/en.2006-0987. [DOI] [PubMed] [Google Scholar]
- Chatain B. La vessie natatoire chez Dicentraarchus labrax et Sparus auratus II. Influence des anomalies de dévéloppement sur la croissance de la larve. Aquaculture. 1987;65:175–181. [Google Scholar]
- Chatain B. Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus) Aquaculture. 1994;119:371–379. [Google Scholar]
- Chatain B, Dewavrin G. Influence des anomalies de dévéloppment de la vessie natatoire sur la mortalité de Dicentrarchus labrax au cours du sevrate. Aquaculture. 1989;78:55–61. [Google Scholar]
- Claireaux G, McKenzie DJ, Genge AG, Chatelier A, Aubin J, Farrell AP. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J. Exp. Biol. 2005;208:1775–1784. doi: 10.1242/jeb.01587. [DOI] [PubMed] [Google Scholar]
- Cook PM, Robbins JA, Endicott DD, Lodge KB, Guiney PD, Walker MK, Zabel EW, Peterson RE. Effects of aryl hydrocarbon receptor-mediated early life stage toxicity on lake trout populations in Lake Ontario during the 20th century. Environ. Sci. Technol. 2003;37:3864–3877. doi: 10.1021/es034045m. [DOI] [PubMed] [Google Scholar]
- Cowan JH, Jr, Shaw RF. Recruitment. In: Fuiman LA, Werner RG, editors. Fisheries Science: The Unique Contributions of Early Life Stages. Malden, MA: Blackwell Science, Ltd.; 2002. pp. 88–111. [Google Scholar]
- Elonen GE, Spehar RL, Holcombe GW, Johnson RD, Fernandez JD, Erickson RJ, Tietge JE, Cook PM. Comparative toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to seven freshwater fish species during early life-stage development. Environ. Toxicol. Chem. 1998;17:472–483. [Google Scholar]
- Finney JL, Robertson GN, McGee CA, Smith FM, Croll RP. Structure and autonomic innervation of the swim bladder in the zebrafish (Danio rerio) J. Comp. Neurol. 2006;495:587–606. doi: 10.1002/cne.20948. [DOI] [PubMed] [Google Scholar]
- Gamperl AK, Rodnick KJ, Faust HA, Venn EC, Bennett MT, Crawshaw LI, Keeley ER, Powell MS, Li HW. Metabolism, swimming performance, and tissue biochemistry of high desert redband trout (Oncorhynchus mykiss ssp.): Evidence for phenotypic differences in physiological function. Physiol. Biochem. Zool. 2002;75:413–431. doi: 10.1086/343139. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Jones PD, Kannan K, Newsted JL, Tillitt DE, Williams LL. Effects of chronic dietary exposure to environmentally relevant concentrations to 2,3,7,8-tetrachlorodibenzo-p-dioxin on survival, growth, reproduction and biochemical responses of female rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 2002;59:35–53. doi: 10.1016/s0166-445x(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Goolish EM, Okutake K. Lack of gas bladder inflation by the larvae of zebrafish in the absence of an air-water interface. J. Fish Biol. 1999;55:1054–1063. [Google Scholar]
- Guillette LJ, Jr, Crain DA, Rooney AA, Pickford DB. Organization versus activation: The role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ. Health Perspect. 1995;103(Suppl. 7):157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney WS. Modeling the demographic effects of endocrine disruptors. Environ. Health Perspect. 2006;114(Suppl. 1):122–126. doi: 10.1289/ehp.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME. Dioxin toxicology and the aryl hydrocarbon receptor: Insights from fish and other non-traditional models. Mar. Biotechnol. 2001;3:S224–S238. doi: 10.1007/s10126-001-0045-y. [DOI] [PubMed] [Google Scholar]
- Heideman W, Antkiewicz DS, Carney SA, Peterson RE. Zebrafish and cardiac toxicology. Cardiovasc. Toxicol. 2005;5:203–214. doi: 10.1385/ct:5:2:203. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. The fetal basis of adult disease: Role of environmental exposures—Introduction. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:131–132. doi: 10.1002/bdra.20119. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod. Toxicol. 2007;23:257–259. doi: 10.1016/j.reprotox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Hill A, Howard V, Cossins A. Characterization of TCDD-induced craniofacial malformations and retardation of zebrafish growth. J. Fish Biol. 2004;64:911–922. [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Spitsbergen JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss) Toxicol. Sci. 1999;47:40–51. doi: 10.1093/toxsci/47.1.40. [DOI] [PubMed] [Google Scholar]
- Hunter GA, Denton TE. Hormonal sex control and its application to fish culture. In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish Physiology. Vol. IX. New York, NY: Academic Press; 1983. pp. 223–303. [Google Scholar]
- Hutz RJ, Wimpee BAB, Dasmahapatra A, Weber DN, Heimler I, Chaffin CL. Differential modulation by aromatic hydrocarbon receptor agonist of circulating estradiol-17beta and estrogen-receptor DNA-binding capability in female rainbow trout (Oncorhynchus mykiss) Zool. Sci. 1999;16:161–166. [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the developing female reproductive system by phytoestrogens: Genistein as an example. Mol. Nutr. Food Res. 2007a;51:832–844. doi: 10.1002/mnfr.200600258. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod. Toxicol. 2007b;23:308–316. doi: 10.1016/j.reprotox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kime DE, Nash JP. Gamete viability as an indicator of reproductive endocrine disruption in fish. Sci. Total Environ. 1999;233:123–129. [Google Scholar]
- King Heiden T, Carvan MJ, III, Hutz RJ. Inhibition of follicular development, vitellogenesis, and serum 17{beta}-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;90:490–499. doi: 10.1093/toxsci/kfj085. [DOI] [PubMed] [Google Scholar]
- King Heiden T, Hutz RJ, Carvan MJ., III Accumulation, tissue distribution, and maternal transfer of dietary 2,3,7,8,-tetrachlorodibenzo-p-dioxin: Impacts on reproductive success of zebrafish. Toxicol. Sci. 2005;87:497–507. doi: 10.1093/toxsci/kfi201. [DOI] [PubMed] [Google Scholar]
- King Heiden TC, Spitsbergen J, Xiong KM, Heideman W, Peterson RE. Sublethal TCDD exposure during early stages of development induces craniofacial, cardiac and reproductive toxicity in adult fish. The Toxicologist. 2008a;102:239. [Google Scholar]
- King Heiden TC, Struble CA, Rise ML, Hessner MJ, Hutz RJ, Carvan MJ., III Molecular targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) within the zebrafish ovary: Insights into TCDD-induced endocrine disruption and reproductive toxicity. Reprod. Toxicol. 2008b;25:47–57. doi: 10.1016/j.reprotox.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty GD, Hinton DE, Cech JJ. Oxygen consumption by larval Japanese medaka with inflated and uninflated swimbladders. Trans. Am. Fish. Soc. 1995;124:623–627. [Google Scholar]
- Nacci D, Coiro L, Kuhn A, Champlin D, Munns W, Specker J, Cooper K. Nondestructive indicator of ethoxyresorufin-O-deethylase activity in embryonic fish. Environ. Toxicol. Chem. 1998;17:2481–2486. [Google Scholar]
- Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod. Toxicol. 2007;23:326–336. doi: 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palstra AP, van Ginneken VJ, Murk AJ, van den Thillart GE. Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften. 2006;93:145–148. doi: 10.1007/s00114-005-0080-z. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: Cross-species comparisons. Crit. Rev. Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Sakamoto KQ, Nakai K, Aoto T, Yokoyama A, Ushikoshi R, Hirose H, Ishizuka M, Kazusaka A, Fujita S. Cytochrome p450 induction and gonadal status alteration in common carp (Cyprinus carpio) associated with the discharge of dioxin contaminated effluent to the Hikiji River, Kanagawa Prefecture, Japan. Chemosphere. 2003;51:491–500. doi: 10.1016/S0045-6535(03)00005-5. [DOI] [PubMed] [Google Scholar]
- Segner H. Comment on “Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment”. Environ. Sci. Technol. 2006;40:1084–1085. doi: 10.1021/es051791d. [DOI] [PubMed] [Google Scholar]
- Shang EH, Yu RM, Wu RS. Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio) Environ. Sci. Technol. 2006;40:3118–3122. doi: 10.1021/es0522579. [DOI] [PubMed] [Google Scholar]
- Shingles A, McKenzie DJ, Claireaux G, Domenici P. Reflex cardioventilatory responses to hypoxia in the flathead gray mullet (Mugil cephalus) and their behavioral modulation by perceived threat of predation and water turbidity. Physiol. Biochem. Zool. 2005;78:744–755. doi: 10.1086/432143. [DOI] [PubMed] [Google Scholar]
- Slanchev K, Stebler J, Cueva-Mendez G, Raz E. Development without germ cells: The role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4074–4079. doi: 10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Kleeman JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in yellow perch (Perca flavescens) J. Toxicol. Environ. Health. 1988;23:359–383. doi: 10.1080/15287398809531120. [DOI] [PubMed] [Google Scholar]
- Spitsbergen J, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2, 3, 7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat. Toxicol. 1991;19:41–72. [Google Scholar]
- Spromberg JA, Birge WJ. Modeling the effects of chronic toxicity on fish populations: The influence of life-history strategies. Environ. Toxicol. Chem. 2005a;24:1532–1540. doi: 10.1897/04-160.1. [DOI] [PubMed] [Google Scholar]
- Spromberg JA, Birge WJ. Population survivorship index for fish and amphibians: Application to criterion development and risk assessment. Environ. Toxicol. Chem. 2005b;24:1541–1547. doi: 10.1897/04-159.1. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Andreasen E, Walker MK, Peterson RE. Dioxin toxicity and aryl hydrocarbon receptor signaling in fish. In: Schecter A, Gasiewicz TA, editors. Dioxins and Health. New Jersey: John Wiley & Sons; 2003. pp. 603–628. [Google Scholar]
- Teraoka H, Dong W, Ogawa S, Tsukiyama S, Okuhara Y, Niiyama M, Ueno N, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002;65:192–199. doi: 10.1093/toxsci/65.2.192. [DOI] [PubMed] [Google Scholar]
- Tillitt DE, Cook PM, Giesy JP, Heideman W, Peterson RE. Reproductive impairment of great lakes trout by dioxin-like compounds. In: Di Giulio RT, Hinton DE, editors. Toxicology of Fishes. Boca Raton, FL: CRC Press; 2008. pp. 819–875. [Google Scholar]
- Uzumcu M, Zachow R. Developmental exposure to environmental endocrine disruptors: Consequences within the ovary and on female reproductive function. Reprod. Toxicol. 2007;23:337–352. doi: 10.1016/j.reprotox.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz DC, Bencic DC, Hinton DE, Law JM, Kullman SW. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces organ-specific differential gene expression in male Japanese medaka (Oryzias latipes) Toxicol. Sci. 2005;85:572–584. doi: 10.1093/toxsci/kfi109. [DOI] [PubMed] [Google Scholar]
- Walker MK, Peterson RE. Aquatic toxicity of dioxins and related chemicals. In: Schecter A, editor. Dioxins and Health. New York, NY: Plenum Press; 1994. pp. 347–387. [Google Scholar]
- Walker MK, Spitsbergen JM, Olson JR, Peterson RE. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity during early life stage development of lake trout (Salvelinus namaycush) Can. J. Fish Aquat. Sci. 1991;48:875–883. [Google Scholar]
- Wannemacher R, Rebstock A, Kulzer E, Schrenk D, Bock KW. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproduction and oogenesis in zebrafish (Brachydanio rerio) Chemosphere. 1992;24:1361–1368. [Google Scholar]
- Westerfield M, Doerry E, Kirkpatrick AE, Driever W, Douglas SA. An on-line database for zebrafish development and genetics research. Semin. Cell Dev. Biol. 1997;8:477–488. doi: 10.1006/scdb.1997.0173. [DOI] [PubMed] [Google Scholar]
- Wu WZ, Li W, Xu Y, Wang JW. Long-term toxic impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the reproduction, sexual differentiation, and development of different life stages of Gobiocypris rarus and Daphnia magna. Ecotoxicol. Environ. Saf. 2001;48:293–300. doi: 10.1006/eesa.2000.2013. [DOI] [PubMed] [Google Scholar]
- Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor-mediated downregulation of Sox9b causes jaw malformations in zebrafish embryos. Mol. Pharmacol. 2008;74:1544–1553. doi: 10.1124/mol.108.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]