Abstract
Results from the majority of studies show little association between circulating concentrations of vitamin D and prostate cancer risk, a finding that has not been demonstrated in a wider European population, however. The authors examined whether vitamin D concentrations were associated with prostate cancer risk in a case-control study nested within the European Prospective Investigation into Cancer and Nutrition (1994–2000). Serum concentrations of 25-hydroxyvitamin D were measured in 652 prostate cancer cases matched to 752 controls from 7 European countries after a median follow-up time of 4.1 years. Conditional logistic regression models were used to calculate odds ratios for prostate cancer risk in relation to serum 25-hydroxyvitamin D after standardizing for month of blood collection and adjusting for covariates. No significant association was found between 25-hydroxyvitamin D and risk of prostate cancer (highest vs. lowest quintile: odds ratio = 1.28, 95% confidence interval: 0.88, 1.88; P for trend = 0.188). Subgroup analyses showed no significant heterogeneity by cancer stage or grade, age at diagnosis, body mass index, time from blood collection to diagnosis, or calcium intake. In summary, the results of this large nested case-control study provide no evidence in support of a protective effect of circulating concentrations of vitamin D on the risk of prostate cancer.
Keywords: prostatic neoplasms, serum, vitamin D, 25-hydroxyvitamin D 2
In 1990, findings of an inverse association between ultraviolet exposure and prostate cancer mortality rates in the United States led to the hypothesis that vitamin D insufficiency may increase prostate cancer risk (1). Results from experimental studies in animal models and cell lines as well as a phase II clinical trial support the hypothesis that vitamin D may play a role in the etiology of prostate cancer (2–4); however, evidence from prospective studies in the United States and Nordic countries that have assessed the relation between circulating concentrations of 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) and risk of prostate cancer has not clearly supported such an association (5–16). Despite the consistent finding of no association between circulating concentrations of vitamin D and the risk of prostate cancer, there is still considerable support for vitamin D insufficiency as a risk factor for prostate cancer (17, 18). To date, we know of no study that has investigated this association among men from other European countries.
We examine here the relation between prediagnostic serum concentrations of 25(OH)D and risk of prostate cancer among 652 men with incident prostate cancer and 752 matched controls participating in a large, multicenter cohort study: the European Prospective Investigation into Cancer and Nutrition (EPIC). We also evaluate these associations by stage and grade of disease.
MATERIALS AND METHODS
Participants
The methods of recruitment for EPIC and the design of the study have been described in detail elsewhere (19). The present study includes prostate cancer cases diagnosed after blood collection and individually matched to controls from 7 of the 10 participating countries: Germany, Greece, Italy, the Netherlands, Spain, Sweden, and the United Kingdom. France and Norway, and the regional centers in Utrecht, the Netherlands, and Naples, Italy, were not included in the current study because these cohorts included women only. Centers in Denmark and Malmö, Sweden, did not contribute data to the current study. Each case was matched to 1 control, except in Umeå, Sweden, where each case was matched to 2 controls. Matching criteria were study center, age at enrollment (±6 months), time of day of blood collection (±1 hour), and time between blood draw and last consumption of food or drink (<3, 3–6, >6 hours; for Umeå <4, 4–8, >8 hours). A more detailed description of follow-up for cancer incidence and the selection of cases and controls has been published elsewhere (20).
Diet and lifestyle questionnaires
Dietary intake during the year before recruitment was measured by country-specific validated dietary questionnaires designed to capture local dietary patterns. Information on validation of the food questionnaires has been published previously (19, 21). Calcium intakes were calculated by multiplying the calcium content of each food of a specific portion size or quantity (in grams) by the frequency of consumption as stated on the food questionnaire using country-specific national food tables. Intake from supplemental calcium was not included in this analysis.
Blood collection
A 30-mL blood sample was collected from 139,207 (91%) of the 153,457 men participating in EPIC according to a standardized protocol at the time of recruitment. Filled syringes were kept at 5°C–10°C, protected from light, and transferred to a local laboratory for further processing and aliquoting, except for participants recruited through the Oxford center. Here, blood samples were collected throughout the United Kingdom and were transported to a laboratory in Norfolk by mail at ambient temperature. Blood for serum was aliquoted into 0.5-mL straws, which were then heat-sealed at both ends and stored in liquid nitrogen tanks at −196°C, except for Umeå, where samples were stored in 1.5-mL plastic tubes at −70°C.
Laboratory assays
Serum 25(OH)D concentration was determined by enzyme immunoassay (OCTEIA 25-Hydroxy Vitamin D kit; Immunodiagnostic Systems, Limited, Bolton, Tyne and Wear, United Kingdom) in the Medical Research Council Human Nutrition Research laboratories in Cambridge by laboratory personnel blinded to the case or control status of participants. Serum samples from each case-control set were assayed within the same batch. The intraassay and interassay coefficients of variation (unblinded) were 14.8% and 12.0%, respectively, at 20.2 nmol/L and 3.9% and 10.8%, respectively, at 72.7 nmol/L. The lowest limit of detection was 5 nmol/L.
Statistical analyses
Differences between cases and controls regarding age at blood collection, height, weight, body mass index, and serum concentration of vitamin D were investigated by using a weighted version of the paired-sample t test (22). Differences in the categorical baseline characteristics between cases and controls were compared by using conditional logistic regression models. To assess the influence of month of blood draw on serum concentrations of vitamin D, a simple mathematical model of log-transformed serum vitamin D concentration by month of blood collection (as a categorical variable) was fitted. The “standardized” concentrations of 25(OH)D were calculated by adding the residuals from this model to the overall mean log serum vitamin D value and exponentiating these values, and the resulting serum vitamin D concentrations “standardized for month of blood collection” were used for all analyses. The standardized concentrations were also used to calculate the cutpoints for the quintiles and the “low,” “moderate,” and “high” categories of serum 25(OH)D.
Conditional logistic regression models were used to calculate the odds ratios for prostate cancer by quintiles of 25(OH)D concentration, using cutpoints calculated among controls. Tests for linear trend were obtained by replacing the quintiles with the logarithm of serum concentration of 25(OH)D in the model. The effects of potential confounders—body mass index (weight (kg)/height (m)2 in quartiles), smoking status (never, former, current), alcohol intake (<8 g/day, 8–15 g/day, 16–39 g/day, ≥40 g/day), education (primary or none, secondary, degree level), marital status (married and/or cohabiting, not married or cohabiting), and physical activity (inactive, moderately inactive, active)—were examined by including these variables in the logistic regression models. Any missing values were assigned to a separate category. Likelihood ratio chi-squared tests were used to examine the heterogeneity of trends in prostate cancer risk with the logarithm of serum 25(OH)D concentration between localized (tumor, node, metastasis staging score of T0 or T1 or T2 and N0 or NX and M0, or stage coded in the recruitment center as localized; n = 324) or advanced (T3 or T4 and/or N1+ and/or M1, or stage coded in the recruitment center as metastatic; n = 122), low grade (Gleason sum <7 or equivalent (cases coded as well differentiated or moderately differentiated); n = 361) or high grade (Gleason sum ≥7 or equivalent (cases coded as poorly differentiated or undifferentiated); n = 169), age at diagnosis (<60 years or ≥60 years), body mass index (<25 kg/m2 or ≥25 kg/m2), and country of recruitment. We also compared the risk of prostate cancer for men with a diagnosis of prostate cancer less than 4 years (n = 309) with that for those men diagnosed 4 or more years (n = 343) after blood collection.
These analyses were repeated by using predefined cutpoints for concentrations of 25(OH)D of <50, 50–<75 and ≥75 nmol/L to investigate risks associated with low (<50 nmol/L), moderate (50–<75 nmol/L), and high (≥75 nmol/L) concentrations of vitamin D (20). We also used the chi-squared test to assess effect modification of a low (<1,200 mg/day) or high (≥1,200 mg/day) intake of calcium from food sources on the association between low, moderate, and high concentrations of serum vitamin D and risk of prostate cancer.
All statistical analyses were carried out with Stata Statistical Software, release 9 (StataCorp LP, College Station, Texas). All tests of statistical significance were 2-sided, and P values of <0.05 were considered significant.
RESULTS
When characteristics of cases and controls were compared, the groups did not differ appreciably, except that cases reported slightly less physical activity. The mean concentration of 25(OH)D did not differ significantly between cases and controls (P = 0.88) (Table 1).
Table 1.
Baseline Characteristics of Prostate Cancer Cases and Matched Controls in the European Prospective Investigation into Cancer and Nutrition, 1994–2000
| Characteristic | Cases (n = 652) | Controls (n = 752) | P Valuea |
| No. (%)b | |||
| Smokingc | |||
| Never | 217 (34.5) | 243 (34.6) | 0.82 |
| Former | 283 (45.0) | 306 (43.6) | |
| Current | 129 (20.5) | 153 (21.8) | |
| Alcohol consumptionc | |||
| <8 g/day | 260 (41.5) | 321 (45.7) | 0.15 |
| 8–15 g/day | 120 (19.2) | 148 (21.1) | |
| 16–39 g/day | 149 (23.8) | 141 (20.1) | |
| ≥40 g/day | 97 (15.5) | 92 (13.1) | |
| Physical activityc | |||
| Inactive | 103 (17.4) | 85 (13.2) | 0.04 |
| Moderately inactive | 212 (33.8) | 218 (33.8) | |
| Active | 278 (46.9) | 342 (53.0) | |
| Marital statusc | |||
| Married and/or cohabiting | 480 (88.9) | 550 (87.9) | 0.59 |
| Not married or cohabiting | 60 (11.1) | 76 (12.1) | |
| Educational attainmentc | |||
| Primary or equivalent | 245 (39.8) | 290 (41.5) | 0.22 |
| Secondary | 224 (36.4) | 270 (38.6) | |
| Degree | 147 (23.9) | 139 (19.9) | |
| Mean (SD) | |||
| Age at blood collection, years | 61.0 (6.3) | 60.5 (6.2) | 0.15 |
| Weight, kgc | 79.0 (11.1) | 80.0 (11.2) | 0.10 |
| Height, cmc | 172.3 (6.7) | 172.9 (6.9) | 0.10 |
| Body mass index, kg/m2c | 26.6 (3.4) | 26.8 (3.5) | 0.40 |
| Serum 25-hydroxyvitamin D, mean (95% CI), nmol/Ld | 53.6 (52.0, 55.3) | 53.5 (51.9, 55.1) | 0.88 |
| Year of diagnosis, median (range) | 1999 (1994–2005) | ||
| Age at diagnosis, mean (SD), years | 65.4 (6.1) | ||
| Months from blood collection to diagnosis, median (range) | 49 (0–181) | ||
| Prostate cancer stage,e no. (%) | |||
| Localized | 326 (50.0) | ||
| Advanced | 122 (18.7) | ||
| Unknown | 204 (31.3) | ||
| Histologic grade,f no. (%) | |||
| Low | 362 (55.5) | ||
| High | 170 (26.1) | ||
| Unknown | 120 (18.4) | ||
Abbreviations: CI, confidence interval; SD, standard deviation.
Weighted tests of mean difference between cases and controls in each matched set or tests of association between category and case-control status using conditional logistic regression, as appropriate.
Percentages may not add to 100 because of rounding.
Unknown for some subjects; calculations of percentages exclude missing values.
Serum concentrations of vitamin D were standardized for month of blood collection.
Tumor, node, metastasis (TNM) staging score of T0 or T1 or T2 and N0 or NX and M0 (localized); TNM staging score of T3 or T4 and/or N1+ and/or M1 (advanced).
Gleason sum <7 or equivalent or well or moderately differentiated (low grade); Gleason sum ≥7 or poorly differentiated or undifferentiated (high grade).
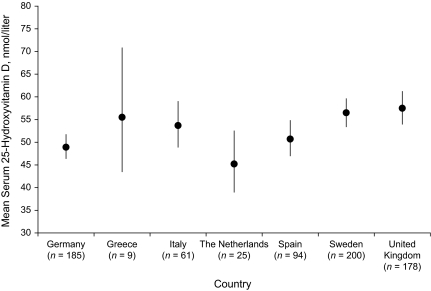
After we adjusted for age at blood collection, serum concentrations of 25(OH)D varied significantly by month of blood collection among the cases and controls (Figure 1), but there was no significant difference in the mean month of blood collection between the matched sets (P = 0.643). Mean concentrations were lowest in cases in January (44.7 nmol/L) and in controls in March (42.9 nmol/L), and they were highest in August (72.4 nmol/L) and September (78.4 nmol/L), respectively. Following standardization of 25(OH)D concentrations for month of blood collection and adjustment for age at blood collection, concentrations varied significantly by country of recruitment (P < 0.001) among the controls, with the highest mean concentrations in men in the United Kingdom (57.5 nmol/L) and Sweden (56.5 nmol/L) and the lowest in the Dutch men (45.2 nmol/L) (Figure 2). Following standardization of 25(OH)D concentrations for month of blood collection and adjustment for study center, we found a significant inverse association between 25(OH)D concentrations and age at blood collection; compared with men older than age 70 years, men aged 50–54 years had a vitamin D concentration that was 12.5 nmol/L higher (P = 0.001, results not shown).
Figure 1.
Geometric mean serum 25-hydroxyvitamin D concentrations, by month of blood draw, in cases and controls in the European Prospective Investigation into Cancer and Nutrition, 1994–2000. Means are geometric means and their corresponding 95% confidence intervals adjusted for age at blood draw, study center, and their interaction. Solid circles, means for cases; open circles, means for controls. Jan, January; Feb, February; Mar, March; Apr, April; Jun, June; Jul, July; Aug, August; Sep, September; Oct, October; Nov, November; Dec, December.
Figure 2.
Geometric mean (and 95% confidence interval) serum 25-hydroxyvitamin D concentrations, by country, in controls in the European Prospective Investigation into Cancer and Nutrition, 1994–2000. Means are geometric means and their corresponding 95% confidence intervals standardized for month of blood collection and adjusted for age at blood draw.
The odds ratios for prostate cancer by quintiles of 25(OH)D from conditional logistic regression models conditioned on the matching variables and adjusted for lifestyle variables are shown in Table 2. Results showed no significant association between concentrations of 25(OH)D and overall risk of prostate cancer, with or without adjustment for covariates. In the unadjusted results, the risk of prostate cancer for the highest versus the lowest quintile of serum concentrations of vitamin D was 1.24 (95% confidence interval: 0.87, 1.79; P for trend = 0.265). After additional adjustment for body mass index, smoking status, alcohol intake, education, marital status, and physical activity, the odds ratio for the highest in comparison to the lowest quintile of vitamin D changed very little (odds ratio = 1.28, 95% confidence interval: 0.88, 1.88; P for trend = 0.188). There was no evidence of significant heterogeneity in the trends for the risk of prostate cancer with vitamin D concentration by stage or grade or by the age of men at diagnosis (Table 2). However, when the associations between prostate cancer risk and 25(OH)D were examined separately by age, a borderline significant positive association was observed for men diagnosed at less than age 60 years (odds ratio for the highest compared with the lowest quintile = 2.90, 95% confidence interval: 0.85, 9.88; P for trend = 0.046), and this association was not significant for men aged 60 years or older (P for trend = 0.594); however, neither was the test for heterogeneity between the trends statistically significant (P for heterogeneity = 0.160).
Table 2.
Odds Ratios (and 95% Confidence Intervals) for Prostate Cancer by Quintile of 25-Hydroxyvitamin D Concentration in the European Prospective Investigation into Cancer and Nutrition, 1994–2000
| Quintile of 25-Hydroxyvitamin D Concentrationa |
Doubling of Concentration |
P for Trendb | P for Heterogeneityc | ||||||||||
| 1d | 2 |
3 |
4 |
5 |
|||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| Range, nmol/L | 2.5−40.4 | 40.5−50.4 | 50.5−59.1 | 59.2−70.8 | 70.9−163.7 | ||||||||
| No. of cases | 125 | 143 | 128 | 114 | 142 | ||||||||
| No. of controls | 151 | 150 | 151 | 150 | 150 | ||||||||
| Odds ratioe | 1 | 1.24 | 0.88, 1.74 | 1.15 | 0.82, 1.62 | 1.01 | 0.71, 1.45 | 1.24 | 0.87, 1.79 | 1.13 | 0.91, 1.41 | 0.265 | |
| Adjusted odds ratiof | 1 | 1.27 | 0.89, 1.81 | 1.23 | 0.85, 1.76 | 1.06 | 0.73, 1.55 | 1.28 | 0.88, 1.88 | 1.17 | 0.93, 1.47 | 0.188 | |
| Prostate cancer stagef | |||||||||||||
| Localized (n = 326) | 1 | 1.28 | 0.76, 2.16 | 1.20 | 0.72, 1.97 | 1.09 | 0.65, 1.83 | 1.15 | 0.67, 1.97 | 1.12 | 0.80, 1.56 | 0.508 | 0.577 |
| Advanced (n = 122) | 1 | 1.61 | 0.71, 3.65 | 1.10 | 0.40, 2.99 | 0.83 | 0.32, 2.12 | 1.13 | 0.37, 3.43 | 1.31 | 0.73, 2.37 | 0.364 | |
| Histologic gradef | |||||||||||||
| Low (n = 362) | 1 | 1.45 | 0.89, 2.37 | 1.37 | 0.85, 2.21 | 1.30 | 0.79, 2.15 | 1.52 | 0.91, 2.56 | 1.29 | 0.95, 1.74 | 0.097 | 0.108 |
| High (n = 170) | 1 | 0.94 | 0.42, 2.10 | 0.63 | 0.26, 1.49 | 0.86 | 0.36, 2.05 | 0.83 | 0.34, 2.07 | 0.94 | 0.52, 1.70 | 0.846 | |
| Age at blood collectionf | |||||||||||||
| <60 years (n = 112) | 1 | 2.02 | 0.59, 6.90 | 1.87 | 0.65, 5.37 | 3.73 | 1.06, 13.17 | 2.90 | 0.85, 9.88 | 1.90 | 0.99, 3.64 | 0.046 | 0.160 |
| ≥60 years (n = 540) | 1 | 1.30 | 0.89, 1.90 | 1.19 | 0.79, 1.77 | 0.93 | 0.62, 1.40 | 1.19 | 0.78, 1.80 | 1.07 | 0.83, 1.39 | 0.594 | |
Abbreviations: CI, confidence interval; OR, odds ratio.
Serum concentrations of vitamin D were standardized for month of blood collection.
Values were obtained with the logarithm of plasma 25-hydroxyvitamin D replacing the categorical plasma 25-hydroxyvitamin D variable in the model.
Values relate to likelihood ratio chi-squared tests of heterogeneity between trends for localized and advanced-stage and low- and high-grade prostate cancer, and for age <60 years and ≥60 years at blood collection.
All values of 1 are odds ratios.
Conditioned on matching variables by using conditional logistic regression.
Conditioned on matching variables and adjusted for body mass index, smoking, alcohol intake, education, marital status, and physical activity by using conditional logistic regression.
Table 3 results show that the risk of prostate cancer was not significantly different for men with a low (<50 nmol/L) or a high (≥75 nmol/L) concentration of vitamin D in comparison to men with a moderate (50–<75 nmol/L) concentration. There was also no association between these predefined categories of vitamin D concentrations and risk of prostate cancer subdivided by stage, grade, or age at diagnosis. In addition, we found no association between the categories of vitamin D and risk of prostate cancer for men with a high (≥1,200 mg/day) or a low (<1,200 mg/day) intake of calcium (Table 4).
Table 3.
Odds Ratios (and 95% Confidence Intervals) for Prostate Cancer by Predefined Categories of 25-Hydroxyvitamin D Concentration in the European Prospective Investigation into Cancer and Nutrition, 1994–2000
| Category of 25-Hydroxyvitamin D Concentrationa |
P Valueb | |||||
| 1 |
2c | 3 |
||||
| OR | 95% CI | OR | 95% CI | |||
| Range, nmol/L | <50 | 50−74.9 | ≥75 | |||
| No. of cases | 258 | 283 | 111 | |||
| No. of controls | 286 | 353 | 113 | |||
| Odds ratiod | 1.04 | 0.82, 1.32 | 1 | 1.17 | 0.85, 1.62 | 0.265 |
| Adjusted odds ratioe | 1.00 | 0.78, 1.28 | 1 | 1.14 | 0.82, 1.58 | 0.188 |
| Prostate cancer stagee | ||||||
| Localized | 1.06 | 0.75, 1.49 | 1 | 1.02 | 0.64, 1.62 | 0.508 |
| Advanced | 1.22 | 0.66, 2.25 | 1 | 1.17 | 0.44, 3.13 | 0.364 |
| Histologic gradee | ||||||
| Low | 0.95 | 0.69, 1.30 | 1 | 1.19 | 0.76, 1.88 | 0.097 |
| High | 1.25 | 0.71, 2.19 | 1 | 1.29 | 0.62, 2.70 | 0.846 |
| Age at blood collectione | ||||||
| <60 years | 1.08 | 0.74, 1.58 | 1 | 1.00 | 0.62, 1.62 | 0.046 |
| ≥60 years | 0.83 | 0.58, 1.19 | 1 | 1.33 | 0.81, 2.18 | 0.594 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Serum concentrations of vitamin D were standardized for month of blood collection.
Values were obtained with the logarithm of plasma 25-hydroxyvitamin D replacing the categorical plasma 25-hydroxyvitamin D variable in the model.
All values of 1 are odds ratios.
Conditioned on matching variables by using conditional logistic regression.
Conditioned on matching variables and adjusted for body mass index, smoking, alcohol intake, education, marital status, and physical activity by using conditional logistic regression.
Table 4.
Odds Ratios (and 95% Confidence Intervals) for Prostate Cancer by Predefined Categories of 25-Hydroxyvitamin D Concentration and Intake of Dietary Calcium in the European Prospective Investigation into Cancer and Nutrition, 1994–2000
| Calcium Intake, mg/day | Category of 25-Hydroxyvitamin D Concentrationa (Range, nmol/L) |
P Valueb | |||||||||||
| 1 (<50) |
2 (50–74.9) |
3 (≥75) |
|||||||||||
| No. of Casesc | No. of Controlsc | OR | 95% CI | No. of Casesc | No. of Controlsc | OR | 95% CI | No. of Casesc | No. of Controlsc | OR | 95% CI | ||
| <1,200 | 196 | 186 | 183 | 216 | 75 | 70 | |||||||
| ≥1,200 | 39 | 59 | 63 | 61 | 24 | 24 | |||||||
| Unadjustedd | |||||||||||||
| <1,200 | 1.19 | 0.89, 1.60 | 1 | 1.25 | 0.84, 1.86 | 0.305 | |||||||
| ≥1,200 | 0.73 | 0.46, 1.16 | 1.20 | 0.79, 1.82 | 1.15 | 0.63, 2.11 | |||||||
| Adjustede | |||||||||||||
| <1,200 | 1.15 | 0.80, 1.68 | 1 | 1.20 | 0.80, 1.81 | 0.333 | |||||||
| ≥1,200 | 0.69 | 0.43, 1.11 | 1.19 | 0.78, 1.83 | 1.09 | 0.58, 2.05 | |||||||
Abbreviations: CI, confidence interval; OR, odds ratio.
Serum concentrations of vitamin D were standardized for month of blood collection.
Values were obtained by using chi-squared tests.
For 72 cases and 136 controls, information on dietary calcium intake was missing.
Conditioned on matching variables by using conditional logistic regression.
Conditioned on matching variables and adjusted for body mass index, smoking, alcohol intake, education, marital status, and physical activity by using conditional logistic regression.
There was no evidence for significant heterogeneity regarding the association between serum 25(OH)D and risk of prostate cancer between men with an early (<4 years) versus a later (≥4 years) diagnosis (P for heterogeneity = 0.857), a high (≥25 kg/m2) versus a low (<25 kg/m2) body mass index (P for heterogeneity = 0.825), or by country (P for heterogeneity = 0.166, results not shown).
DISCUSSION
The findings from this large, prospective study show that the risk of prostate cancer did not vary significantly by serum concentration of 25(OH)D and do not support the hypothesis that circulating 25(OH)D plays a major role in the pathogenesis of prostate cancer. Despite the widespread notion that vitamin D insufficiency is an important risk factor for prostate cancer (17, 18), this theory has not been substantiated by results from the majority of published prospective studies (5, 7, 8, 10–14). Results from 2 studies have shown support for lower concentrations of 25(OH)D and increased risk of prostate cancer. Ahonen et al. (6) demonstrated a greater risk of prostate cancer for Nordic men with a 25(OH)D concentration of ≤40 nmol/L in comparison to men with concentrations of >40 nmol/L; a second Nordic study reported a U-shaped relation, with a higher risk for men with low (≤19 nmol/L) and high (≥80 nmol/L) concentrations of 25(OH)D in comparison to men with moderate concentrations (15).
Some studies have suggested that the inconsistent results regarding an association between vitamin D and risk of prostate cancer may be due to the variation in vitamin D concentrations between populations. For instance, the proportion of men with low 25(OH)D concentrations (<50 nmol/L) was higher among the Nordic populations (6, 9, 15) than in most of the US cohorts, where sun exposure is likely to be greater (5, 11, 13), and/or study populations were drawn from health-conscious populations (12, 14) whose intake of vitamin D may be higher. Our data on serum 25(OH)D concentrations suggest a moderate prevalence of vitamin D insufficiency in men across Europe; 39% of the cases and controls had 25(OH)D concentrations of <50 nmol/L. Our finding of a null association was also similar to a study in a Finnish population in which 50% of participants had 25(OH)D concentrations of <50 nmol/L (9).
Several studies have evaluated the risk of prostate cancer associated with concentrations of 1,25(OH)2D, the hormonal biologically active form of vitamin D, which has been shown in experimental studies to reduce the degree of cell proliferation in the prostate (4, 23, 24). In 2 studies, the investigators reported a nonsignificant decreased risk for men with high concentrations of both vitamin D metabolites (8, 13), whereas several others have reported no association (7, 10–12, 14). We chose to assess the association between prostate cancer risk and serum 25(OH)D and not 1,25(OH)2D because circulating 25(OH)D (from diet, supplementation, and sun exposure) is probably a better marker of an individual's vitamin D exposure than circulating concentrations of 1,25(OH)2D, which are homeostatically controlled, have a half life of 4 hours, and most probably reflect the production of 1,25(OH)2D in the kidneys (25). However, a potential limitation of the current study and all previous epidemiologic studies is that it is not clear to what extent circulating 25(OH)D reflects intraprostatic vitamin D concentrations because 1,25(OH)2D is produced locally in the prostate by cells expressing the enzyme 25(OH)-1α-hydroxylase (26).
A potential effect modifier of the association between vitamin D and prostate cancer risk is calcium. A high intake of calcium coupled with low vitamin D status may increase the risk of prostate cancer by reducing the amount of 1,25(OH)2D synthesized (27). High levels of calcium may suppress the release of parathyroid hormone, and the action of this hormone tightly regulates conversion of 25(OH)D to 1,25(OH)2D in renal cells (28). Nevertheless, these results showed no evidence that the association between concentrations of 25(OH)D and the risk of prostate cancer varied according to calcium intake, which is consistent with findings reported by other studies that have examined the effect of calcium intake (5, 12, 14) or status (13, 29).
We found no support for a difference in the relation between vitamin D and prostate cancer by stage or histologic grade of the disease, which is consistent with others (7, 8, 12–14) apart from Ahn et al. (5), whose results showed a positive association between 25(OH)D and risk of aggressive disease (Gleason sum ≥7 or clinical stage III or IV). These results also showed no heterogeneity in the association between 25(OH)D and prostate cancer risk by age at diagnosis; however, there was a positive association between serum concentrations of vitamin D and risk of disease for men diagnosed at less than age 60 years. The majority of recent studies have reported no substantial difference in the relation between vitamin D and prostate cancer risk by age (7, 11–14), with the exception of 2 studies (6, 8). Ahonen et al. (6) demonstrated an inverse association between 25(OH)D concentrations and prostate cancer risk for men aged than age 52 years, and Corder et al. (8) reported a similar inverse association but only for men older than age 57 years. Notwithstanding the possibility of a differential effect by age, both of these subgroup analyses and our own were based on a small number of cases (n = 67, n = 91, and n = 112 men, respectively), and, because a number of comparisons were made in our analyses, the role of chance cannot be excluded.
Others have also demonstrated that the influence of low concentrations of 25(OH)D on the risk of prostate cancer differed according to several polymorphisms located on the vitamin D receptor gene, including Cdx2, Fok1, and Bsm1 (12, 30). Moreover, evidence also suggests that polymorphisms in the vitamin D binding protein affect circulating concentrations of 25(OH)D (31). Given that the genotype of vitamin D receptor or vitamin D binding protein was not determined for the men in this study, our results do not rule out the possibility that low levels of circulating 25(OH)D may be associated with a greater risk of prostate cancer for certain individuals with a specific genotype or haplotype.
Strengths of this study include the large number of cases, prospective design, and inclusion of men from a number of European countries. One of the limitations is that participants were not matched on month of blood collection. Because serum 25(OH)D concentrations varied according to the month in which the blood sample was collected, vitamin D values were standardized for month of blood collection before we assessed the association between vitamin D and risk of prostate cancer so as to minimize the risk of confounding by season of blood collection. The mean preclinical duration of prostate cancer has been estimated to be 10 years (32); in this study, the median time between blood collection and diagnosis of cases was 4 years. Thus, a potential limitation of this analysis was that serum 25(OH)D concentrations in the cases reflected vitamin D status at a time when early preclinical tumors were present rather than prior to initiation of tumor growth. It is possible that any subclinical tumors present at the time of blood collection influenced serum concentrations of 25(OH)D. Notwithstanding these limitations, exclusion of cases diagnosed in the first 4 years of follow-up did not alter the main findings.
In conclusion, the findings from this large prospective study of European men showed no strong association between serum concentrations of 25(OH)D and risk of total prostate cancer. Taken together with the results from other prospective studies, the totality of evidence indicates that low vitamin D concentrations do not have an important role in the etiology of prostate cancer. Further pooled analyses would help to determine whether there are any subgroup associations or an effect of the exposure distribution and range of vitamin D concentrations on the risk of prostate cancer.
Acknowledgments
Author affiliations: Cancer Epidemiology Unit, University of Oxford, Oxford, United Kingdom (Ruth C. Travis, Francesca L. Crowe, Naomi E. Allen, Paul N. Appleby, Andrew W. Roddam, Timothy J. Key); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (Anne Tjønneland, Anja Olsen); Unit of Nutritional Epidemiology, Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (Jakob Linseisen, Rudolf Kaaks); German Institute of Human Nutrition Potsdam-Rehbrücke, Nuthetal, Germany (Heiner Boeing, Janine Kröger); Department of Hygiene and Epidemiology, University of Athens Medical School, Athens, Greece (Antonia Trichopoulou, Vardis Dilis); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Dimitrios Trichopoulos); Department of Epidemiology and Public Health, Imperial College, London, United Kingdom (Paolo Vineis, Elio Riboli); Division of Epidemiology and Life Sciences, Institute for Scientific Interchange Foundation, Torino, Italy (Paolo Vineis); Molecular and Nutritional Epidemiology Unit, ISPO-Scientific Institute of Tuscany, Florence, Italy (Domenico Palli); Cancer Registry, Azienda Ospedaliera “Civile M.P. Arezzo,” Ragusa, Italy (Rosario Tumino); Nutritional Epidemiology Unit, National Cancer Institute, Milan, Italy (Sabina Sieri); National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands (H. Bas Bueno-de-Mesquita, Fränzel J. B. van Duijnhoven); Epidemiology Department, Murcia Health Council, Murcia, Spain (María-Dolores Chirlaque); CIBER Epidemiología y Salud Pública (CIBERESP), Spain (María-Dolores Chirlaque, Aurelio Barricarte, Nerea Larrañaga, Maria-José Sánchez); Public Health Institute of Navarra, Pamplona, Spain (Aurelio Barricarte); Public Health Department of Gipuzkoa, Basque Government, Donostia-San Sebastián, Spain (Nerea Larrañaga); Unit of Nutrition, Environment and Cancer, Catalan Institute of Oncology (ICO), Barcelona, Spain (Carlos A. González); Public Health and Participation Directorate, Health and Health Care Services Council, Asturias, Spain (Marcial V. Argüelles); Andalusian School of Public Health, Granada, Spain (Maria-José Sánchez); Department of Surgical and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden (Pär Stattin); Department of Public Health and Clinical Medicine, Nutritional Research, Umeå University, Umeå, Sweden (Göran Hallmans); Department of Gerontology, University of Cambridge, Cambridge, United Kingdom (Kay-Tee Khaw); Medical Research Council Dunn Human Nutrition Unit and Medical Research Council Center for Nutritional Epidemiology in Cancer Prevention and Survival, Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom (Sheila Bingham); and International Agency for Research on Cancer (IARC-WHO), Lyon, France (Sabina Rinaldi, Nadia Slimani, Mazda Jenab).
The EPIC study is supported by Cancer Research UK; European Commission: Public Health and Consumer Protection Directorate 1993–2004; Research Directorate-General 2005; German Cancer Aid; German Cancer Research Center; German Federal Ministry of Education and Research; Health Research Fund (FIS) of the Spanish Ministry of Health; ISCIII RETIC (RD06/0020) Spain; the participating regional governments and institutions of Spain; Medical Research Council, United Kingdom; the Stroke Association, United Kingdom; British Heart Foundation; Department of Health, United Kingdom; Food Standards Agency, United Kingdom; Greek Ministry of Health; Greek Ministry of Education; Italian Association for Research on Cancer; Italian National Research Council; Dutch Ministry of Public Health, Welfare and Sports; Dutch Ministry of Health; Dutch Prevention Funds; LK Research Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); Swedish Cancer Society; Swedish Scientific Council; and the regional government of Skane, Sweden. This research was conducted during tenure of a Girdlers’ New Zealand Health Research Council Fellowship.
The authors thank Steve Austin and Christine Clewes at the Medical Research Council Human Nutrition Research Laboratory (Cambridge, United Kingdom) for conducting the laboratory analysis and Bertrand Hémon and colleagues at IARC for their expertise in handling the data. They further acknowledge the Dutch National Cancer Registry and the Regional Cancer Registries Amsterdam, East and Limburg for providing data on cancer incidence.
Conflict of interest: none declared.
Glossary
Abbreviations
- EPIC
European Prospective Investigation into Cancer and Nutrition
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
References
- 1.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10(5A):1307–1311. [PubMed] [Google Scholar]
- 2.Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Wilding G, Staab MJ, et al. Phase II study of 1α-hydroxyvitamin D2 in the treatment of advanced androgen-independent prostate cancer. Clin Cancer Res. 2003;9(11):4077–4083. [PubMed] [Google Scholar]
- 4.Peehl DM, Skowronski RJ, Leung GK, et al. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54(3):805–810. [PubMed] [Google Scholar]
- 5.Ahn J, Peters U, Albanes D, et al. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100(11):796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahonen MH, Tenkanen L, Teppo L, et al. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11(9):847–852. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 7.Braun MM, Helzlsouer KJ, Hollis BW, et al. Prostate cancer and prediagnostic levels of serum vitamin D metabolites (Maryland, United States) Cancer Causes Control. 1995;6(3):235–239. doi: 10.1007/BF00051795. [DOI] [PubMed] [Google Scholar]
- 8.Corder EH, Guess HA, Hulka BS, et al. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev. 1993;2(5):467–472. [PubMed] [Google Scholar]
- 9.Faupel-Badger JM, Diaw L, Albanes D, et al. Lack of association between serum levels of 25-hydroxyvitamin D and the subsequent risk of prostate cancer in Finnish men. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2784–2786. doi: 10.1158/1055-9965.EPI-07-0672. [DOI] [PubMed] [Google Scholar]
- 10.Gann PH, Ma J, Hennekens CH, et al. Circulating vitamin D metabolites in relation to subsequent development of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1996;5(2):121–126. [PubMed] [Google Scholar]
- 11.Jacobs ET, Giuliano AR, Martínez ME, et al. Plasma levels of 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and the risk of prostate cancer. J Steroid Biochem Mol Biol. 2004;89–90(1–5):533–537. doi: 10.1016/j.jsbmb.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4(3):562–571. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura AM, Stemmermann GN, Lee J, et al. Serum vitamin D metabolite levels and the subsequent development of prostate cancer (Hawaii, United States) Cancer Causes Control. 1998;9(4):425–432. doi: 10.1023/a:1008875819232. [DOI] [PubMed] [Google Scholar]
- 14.Platz EA, Leitzmann MF, Hollis BW, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 15.Tuohimaa P, Tenkanen L, Ahonen M, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108(1):104–108. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 16.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 17.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Medical progress: vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 20.Key TJ, Appleby PN, Allen NE, et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr. 2007;86(3):672–681. doi: 10.1093/ajcn/86.3.672. [DOI] [PubMed] [Google Scholar]
- 21.Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol. 1997;26(suppl 1):S1–S5. doi: 10.1093/ije/26.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- 22.Rosner B. A generalization of the paired t-test. Appl Stat. 1982;31(1):9–13. [Google Scholar]
- 23.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132(5):1952–1960. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz GG, Wang MH, Zhang M, et al. 1 alpha,25-dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 1997;6(9):727–732. [PubMed] [Google Scholar]
- 25.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35(5):290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GG, Whitlatch LW, Chen TC, et al. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25- hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7(5):391–395. [PubMed] [Google Scholar]
- 27.Chan JM, Giovannucci EL. Dairy products, calcium, and vitamin D and risk of prostate cancer. Epidemiol Rev. 2001;23(1):87–92. doi: 10.1093/oxfordjournals.epirev.a000800. [DOI] [PubMed] [Google Scholar]
- 28.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Corder EH, Friedman GD, Vogelman JH, et al. Seasonal variation in vitamin D, vitamin D-binding protein, and dehydroepiandrosterone: risk of prostate cancer in black and white men. Cancer Epidemiol Biomarkers Prev. 1995;4(6):655–659. [PubMed] [Google Scholar]
- 30.Mikhak B, Hunter DJ, Spiegelman D, et al. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate. 2007;67(9):911–923. doi: 10.1002/pros.20570. [DOI] [PubMed] [Google Scholar]
- 31.Lauridsen AL, Vestergaard P, Hermann AP, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77(1):15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 32.Etzioni R, Cha R, Feuer EJ, et al. Asymptomatic incidence and duration of prostate cancer. Am J Epidemiol. 1998;148(8):775–785. doi: 10.1093/oxfordjournals.aje.a009698. [DOI] [PubMed] [Google Scholar]