Abstract
The prevalence of obesity, an established epidemiologic risk factor for many chronic diseases including cancer, has been steadily increasing in the US over several decades. The mechanisms used to regulate energy balance and adiposity and the relationship of these factors to cancer are not completely understood. Here we have used knockout mice to examine the roles of the transcription factors CCAAT/enhancer-binding protein (C/EBP) β and C/EBPδ in regulating body composition and systemic levels of hormones such as insulin-like growth factor-1 (IGF-1), leptin and insulin that mediate energy balance. Dual-energy X-ray absorptiometry showed that C/EBPβ, either directly or indirectly, modulated body weight, fat content and bone density in both males and females, while the effect of C/EBPδ was minor and only affected adiposity and body weight in female animals. Levels of IGF-1, leptin and insulin in the serum were decreased in both male and female C/EBPβ−/− mice, and C/EBPβ was associated with their promoters in vivo. Moreover, colon adenocarcinoma cells displayed reduced tumorigenic potential when transplanted into C/EBPβ-deficient animals, especially males. Thus, C/EBPβ contributes to endocrine expression of IGF-1, leptin and insulin, which modulate energy balance and can contribute to cancer progression by creating a favorable environment for tumor cell proliferation and survival.
Introduction
Obesity is a growing epidemic in our country, with >32% of the US adult population categorized as obese (1). In addition to numerous other health hazards, obesity is a risk factor for many types of cancer. In fact, ∼20% of cancer deaths in women and 14% of those in men have been attributed to excess weight (2). Thus, it is essential to gain a better understanding of the mechanisms underlying the obesity–cancer link to guide the development of effective preventive and therapeutic strategies.
Several hormones serve as intermediate and long-term communicators of nutritional state and have been implicated in both energy balance and carcinogenesis. These hormones include insulin-like growth factor-1 (IGF-1), insulin and leptin, which play interactive roles in endocrine, paracrine and autocrine signaling networks controlling body composition, energy metabolism and cancer cell growth (3). Numerous studies have supported an association between elevated levels of IGF-1 and proliferation of cancer cells in vitro and in vivo (4). IGF-1 enhances the survival of several cell lines, such as human colon cancer cells (5,6). Furthermore, the risk of many types of human cancers is associated with elevated serum levels of IGF-1, including breast, colon, prostate, bladder, pancreas and lung (7,8).
Experiments with calorie restriction, which reduces circulating IGF-1 (4), liver-specific IGF-1-deficient mice (9), or antisense oligonucleotides reveal that blocking IGF-1 signaling can inhibit tumor growth in several animal models of cancer (10,11). IGF-1 signaling also suppresses apoptosis in a variety of cells (12–14). Because IGF-1 signaling promotes proliferation and metastasis in many cancer cells, strategies to disrupt IGF-1-signaling pathways have emerged as a potential means of both chemoprevention (15) and cancer therapy (16).
Chronic hyperinsulinemia and insulin resistance increase risk for several cancers (17,18). The tumor-enhancing effects of insulin may be due to direct effects via the insulin receptor or to indirect effects via stimulation of IGF-1 or other hormones. High circulating levels of insulin promote the hepatic synthesis of IGF-1 and decrease the production of insulin-like growth factor binding protein-1, consistent with enhanced IGF-1 signaling (17,18). In addition, both insulin and IGF-1 act in vitro as growth factors to promote cancer cell proliferation and decrease apoptosis (19). Epidemiologic evidence suggests that Type 2 diabetes, which is usually characterized by hyperinsulinemia, elevated IGF-1 and insulin resistance, is associated with increased risks of endometrial, colon, pancreas, kidney, pancreatic and post-menopausal breast cancers (17,18).
Leptin is an adipokine involved in appetite control and energy metabolism. The obese state is associated with high systemic leptin levels and leptin resistance (20–22). An association between circulating leptin levels and cancer risk has been reported for several cancer types, notably colon (23) and prostate cancer (24,25). Leptin stimulates proliferation of multiple types of preneoplastic and neoplastic cells but not ‘normal’ cells (26), and in animal models it appears to promote tumor invasion and angiogenesis (27).
There is substantial evidence suggesting that specific transcription factors may integrate the hormonal signals underlying the obesity–cancer link. In particular, members of the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors are important regulators of adipogenesis, glucose metabolism and IGF-1 expression (28). Several studies indicate that C/EBPδ regulates the IGF-1 promoter in cells of the skeletal system (29,30). The role of C/EBPβ has been less studied, although we previously reported that IGF-1 gene expression in macrophage tumor cells is strongly dependent on C/EBPβ (31). Transformed C/EBPβ−/− macrophages failed to survive in the absence of exogenous hematopoietic growth factors due to loss of autocrine/paracrine IGF-1 signaling, and these cells also displayed a markedly diminished capacity to form tumors in nude mice (31). IGF-1 promoter–reporter assays indicated that IGF-1 is a direct transcriptional target of C/EBPβ. These and other studies suggest an important role for C/EBPβ in modulating tumorigenesis and for C/EBPβ and C/EBPδ in regulating expression of IGF-1 in specific cellular contexts.
In the present study, we have used knockout mice to investigate the relative importance of C/EBPβ and C/EBPδ in regulating body composition, systemic IGF-1 and other energy balance-related hormones and colon cancer cell growth.
Materials and methods
Animals
C57BL/6 C/EBPδ−/− mice (32) were mated to 129Sv C/EBPβ+/− animals (33) and F1 progeny heterozygous for both loci were intercrossed to create an F2 generation of mixed C57Bl/6;129Sv background. The resulting animals representing all nine possible genotypes (minimum of five animals per gender and genotype) were singly housed and analyzed at 10 weeks of age. Weights and blood samples were taken immediately prior to killing by CO2 asphyxiation, and a portion of the liver was removed following killing and used for RNA extraction. All animals were maintained in accordance with the National Institutes of Health animal guidelines.
RNA isolation and IGF-1 RNase protection assays
Liver RNA was isolated using ToTALLY RNA (Applied Biosystems/Ambion, Austin, TX) or MELT Total RNA (Ambion), essentially as recommended by the manufacturer. RNA was stored at −70°C. Total RNA (10 μg) was analyzed for IGF-1 expression using Riboquant Ribonuclease Protection Assay (BD-Biosciences, San Jose, CA). A custom probe set containing mouse IGF-1 and glyceraldehyde 3-phosphate hydrogenase (GAPDH) and L-32 controls (BD-Biosciences) was labeled with [α-32P]uridine triphosphate (Amersham/GE Healthcare, Piscataway, NJ) using the Riboquant in vitro assay kit (BD-Biosciences) as recommended by the manufacturer. Ten microliters of hybridization buffer/probe mixture was added to each sample and incubated overnight at 57°C. The samples were RNAse treated and precipitated before loading onto a 6% denaturing polyacrylamide gel. IGF-1 transcripts were quantitated using a STORM 860 PhosphorImager (Molecular Dynamics, Inc., Sunnyvale, CA). IGF-1 expression data were analyzed using ImageQuant 5.2 software (Molecular Dynamics) and normalized to GAPDH.
Serum collection and hormone assays
Serum was obtained from blood samples taken just prior to animal killing at 10 weeks of age and stored at −80°C. IGF-1 levels were measured using an radioimmunoassay kit for mouse/rat serum (Diagnostic Systems Laboratories, Inc., Webster, TX) according to the manufacturer’s protocol. The assay was normalized based on internal controls of both low and high mouse/rat IGF-1 levels. Serum leptin and insulin levels were determined using the Mouse Endocrine kit (Lincoplex) containing insulin and leptin antibody-immobilized beads. Samples (25 μl) were placed in duplicate wells that were previously blocked using assay buffer. The endocrine antibody-immobilized beads were then added to the samples and mixed on a plate shaker overnight at 4°C. Samples were washed and a secondary antibody cocktail was used to bind the insulin and leptin antibodies. Streptavidin–phycoerthrin was added to each well, incubated and washed. Sheath fluid was added to each well and the samples were read using an array reader (Bio-Plex). The data were analyzed using Bio-Plex manager software.
Dual-energy X-ray absorptiometry scans
Fat weight, lean weight and bone mineral density (BMD) were determined using dual-energy X-ray absorptiometry (GE Lunar Piximus II) following the methods of Nagy et al. (34) and Berrigan et al. (35). Briefly, frozen mouse carcasses were thawed for 24 h at 4°C and placed on a specimen tray in the prone position with limbs and tail outstretched and repositioned after each scan. Carcasses were weighed and scanned individually. After scanning, GE-supplied software (version 1.46) was used to exclude heads from the image area and then estimates of total, lean and fat weight were obtained directly from the dual-energy X-ray absorptiometry instrument output. This procedure was used because some of the mice were longer than the scan window. Samples sizes ranged from 12 to 24 per group.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assay was performed as described previously (36). Briefly, livers from C/EBPβ+/+ and C/EBPβ−/− female mice were isolated and cross-linked in phosphate-buffered saline containing 1% formaldehyde for 10 min at room temperature. Tissues were disintegrated by Dounce homogenization, washed with phosphate-buffered saline, resuspended in lysis buffer (0.1% sodium dodecyl sulfate, 0.5% Triton X-100, 150 mM NaCl and 20 mM Tris–HCl, pH 8.1) and sonicated to obtain DNA fragments of 500–1000 bp. Immunoprecipitation was performed using 1 μg antibody against C/EBPβ (C-19, Santa Cruz Biotechnology, Santa Cruz, CA) or C/EBPδ (C-22, Santa Cruz). In control reactions, antibodies were preincubated with their respective blocking peptides. Samples were incubated with antibodies overnight at 4°C and preblocked StaphA cells (Calbiochem, Gibbstown, NJ) were added and incubated for 30 min at 4°C. Precipitates were washed and processed for DNA purification. DNA was amplified by polymerase chain reaction using sequence-specific primers for 30–35 cycles (primer sequences are available upon request). Bands were imaged and quantitated using a Kodak Gel Logic 100 Imaging system; band densities were corrected by subtracting background values.
Tumor transplantation
C/EBPβ−/− and C/EBPβ+/+ mice were used as recipients for tumor cells. MC-38 mouse colon adenocarcinoma cells (37) derived from C57Bl/6 mice were obtained originally from Dr J.Helman, National Cancer Institute. Approximately, 500 000 cells in 100 μl phosphate-buffered saline were injected into each flank of male and female C/EBPβ−/− and C/EBPβ+/+ mice at 6–10 weeks of age. Twenty-six animals (14 females and 12 males) were used in this study. Tumor growth was assessed through daily observation and measurement of tumor diameter for 3–4 weeks. Animals were killed by CO2 asphyxiation when the animal showed signs of morbidity and/or tumors reached 2 cm in diameter.
Statistical analysis
Body composition data were analyzed using analysis of variance and analysis of covariance with body weight or lean weight as covariates. Note that for body composition and IGF-1 levels, all possible combinations of C/EBPβ and C/EBPδ mutant genotypes were measured. Thus, a complete factorial approach was possible, allowing robust estimation of the presence of interactions between C/EBPβ and C/EBPδ. We report estimates of the main effects of each genotype and discuss interactions in the text. Differences in body weight were examined using analysis of variance and analysis of covariance with body weight or lean weight as covariates to test for treatment effects. Then, to examine the difference between group means, comparisons were performed using Tukey's honestly significant differences (HSD) test or linear contrasts. For analysis of serum insulin and leptin, only a subset of genotypic combinations was measured, precluding the estimation of interaction effects. Serum levels of IGF-1, insulin and leptin and liver IGF-1 messenger RNA levels were analyzed by analysis of covariance or analysis of variance on log-transformed values. Mortality data were analyzed using a Kaplan–Meier survival test. Means comparisons were performed using Tukey's HSD test, linear contrasts or t-tests. Tables I and II include superscript numerals identifying significantly different means. In Table I, these tests are performed within a genotype (β or δ) and within a gender (male or female). Therefore, different superscript numerals refer to the three means reported for each gender and genotypic combination. In Table II, superscript numerals refer to contrasts between the four genotypic combinations within each gender. All analyses were performed in SAS JMP (Cary, NC). Data represented graphically compared each genotype with C/EBPβ+/+;C/EBPδ+/+ or control animals using a t-test assuming unequal variances. Significance values of <0.05 are denoted with a single asterisk (*) and <0.01 are denoted with a double asterisk (**).
Table I.
Effects of genotype on body composition by gender
| Massa (g), mean (SE)b | BMD 1000 (g/cm2), mean (SE) | Adjusted BMDc 1000 (g/cm2), mean (SE) | Fat mass (g), mean (SE) | ||
| Females | |||||
| β | +/+ | 21.91 (0.4) | 48.831 (0.06) | 47.701 (0.06) | 5.21 (0.3) |
| +/− | 20.81 (0.4) | 48.661 (0.06) | 48.291 (0.06) | 4.02 (0.3) | |
| −/− | 17.72 (0.5) | 43.652 (0.06) | 45.362 (0.07) | 3.42 (0.4) | |
| δ | +/+ | 19.72 (0.5) | 46.411 (0.06) | 46.791 (0.06) | 3.82 (0.3) |
| +/− | 21.51 (0.4) | 48.171 (0.06) | 47.271 (0.06) | 5.11 (0.3) | |
| −/− | 19.12 (0.4) | 46.561 (0.07) | 47.291 (0.06) | 3.72 (0.3) | |
| P (β) | <0.0001 | <0.0001 | 0.0127 | 0.0001 | |
| P (δ) | 0.0006 | 0.1025 | 0.8021 | 0.0010 | |
| P (β × δ) | 0.034 | 0.0117 | 0.1727 | 0.4697 | |
| Males | |||||
| β | +/+ | 26.61 (0.6) | 46.861 (0.06) | 45.961 (0.05) | 7.11 (0.3) |
| +/− | 26.61 (0.6) | 48.631 (0.06) | 47.721 (0.06) | 6.91 (0.3) | |
| −/− | 21.12 (0.5) | 43.272 (0.06) | 45.222 (0.06) | 3.92 (0.4) | |
| δ | +/+ | 24.81 (0.6) | 46.411 (0.06) | 46.451 (0.05) | 6.11 (0.3) |
| +/− | 24.71 (0.6) | 46.401 (0.05) | 46.391 (0.05) | 6.01 (0.3) | |
| −/− | 24.81 (0.6) | 45.971 (0.06) | 46.061 (0.05) | 5.91 (0.3) | |
| P (β) | <0.0001 | <0.0001 | 0.0106 | <0.0001 | |
| P (δ) | 0.9719 | 0.8221 | 0.8346 | 0.9037 | |
| P (β × δ) | 0.6395 | 0.3431 | 0.6395 | 0.5425 |
Fat weight, lean weight and BMD were determined using dual-energy X-ray absorptiometry. Samples sizes range from 12 to 24 per group. Each genotypic grouping actually represents three genotypes. For example, the C/EBPβ+/+ group is the mean of all animals that were wild-type for C/EBPβ, which included C/EBPβ+/+;δ+/+, C/EBPβ+/+;δ+/− and C/EBPβ+/+;δ−/−. Statistical significance was assessed using Tukey’s HSD test.
Different superscript numerals indicate means that are significantly different within a gender and genotype based on Tukey’s HSD test. For example, β−/− females are significantly lighter than +/+ or +/− females.
The means and standard errors (SEs) were obtained from analysis of variance and covariance.
Bone density was adjusted for total mass using analysis of covariance.
Table II.
Effects of genotype on serum leptin and insulin levels
| β | δ | Female |
Male |
||
| Serum insulin | Serum leptin | Serum insulin | Serum leptin | ||
| +/+ | +/+ | 4.61 (0.1) | 4.21 (0.3) | 5.21 (0.2) | 5.11 (0.4) |
| +/+ | −/− | — | 4.41 (0.2) | — | 4.91 (0.2) |
| −/− | +/+ | 4.02 (0.1) | 3.12 (0.1) | 4.71,2 (0.1) | 3.62 (0.1) |
| −/− | −/− | 3.63 (0.1) | 1.73 (0.1) | 4.52 (0.1) | 2.53 (0.1) |
| P (β) | — | 0.0001 | — | 0.0001 | |
| P (δ) | — | 0.0237 | — | 0.0526 | |
| P (β × δ) | — | 0.0039 | — | 0.2214 | |
Serum obtained from animals prior to killing was used to analyze insulin or leptin levels. Levels of insulin and leptin are depicted in male and female mice by genotype and are expressed as natural log-transformed mean ng/ml (standard error). Means and standard errors were obtained from one-way analysis of variance treating each group as an independent variable. Statistical significance was analyzed using the Tukey’s HSD test. Different superscript numerals indicate means that are significantly different based on Tukey’s HSD test. For example, serum insulin levels in β−/−;δ−/− females are significantly lower than the other female genotypes.
Results
Effects of C/EBPβ and C/EBPδ deficiency on body composition
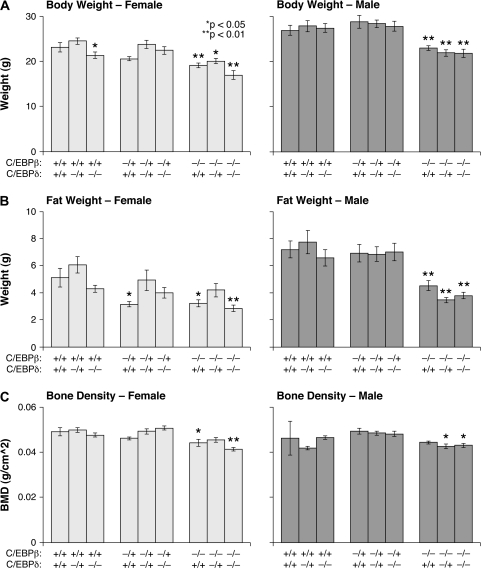
Singly and doubly mutant C/EBPβ and C/EBPδ knockout mice were subjected to various measurements to assess whether these two transcription factors modulate physiological parameters related to energy metabolism. Following necropsy, total body weight was measured and then dual-energy X-ray absorptiometry scans were used to assess fat weight and bone density in male and female animals for each of the nine possible genotypes. Figure 1 shows the effects of deleting C/EBPβ, C/EBPδ or both on body composition, whereas Table I provides information about the interaction between C/EBPβ and C/EBPδ and the effect of that interaction on various aspects of body composition. It is important to note that for the analysis depicted in Table I, each grouping represents three genotypes in Figure 1. For example, the C/EBPβ+/+ group is the mean of all animals wild-type for C/EBPβ, which included β+/+;δ+/+, β+/+;δ+/− and β+/+;δ−/− mice.
Fig. 1.
Comparison of total body weight, fat weight and bone density in male and female animals by genotype. Fat weight, lean weight and BMD were each determined using dual-energy X-ray absorptiometry. Samples sizes range from 12 to 24 per group. Data represent the average ± standard error sorted by genotype. The male and female body weight (A) and fat weight (B) are measured in grams, whereas bone density (C) is measured in grams per square centimeter. Differences in body weight and bone density were examined using analysis of variance to test for treatment effects and Student’s unpaired t-test for comparisons with +/+;+/+ animals (*P < 0.05; **P < 0.01).
β−/− female mice were significantly lighter (Figure 1A) and had lower fat content (Figure 1B) and lower BMD (Figure 1C) than their wild-type counterparts. There was some evidence for a C/EBPβ dose effect, as female heterozygous mice had lower body weights (Table I) and had less fat than wild-type mice but these differences were only significant for fat content. Similar results were obtained for fat mass after adjustment for lean mass. BMD values were positively correlated with total body mass. However, the reduced bone density observed in β−/−;δ−/− mice persisted after adjustment for total body mass. Although bone density is highly influenced by changes in biomass, this result indicates that C/EBPβ has a direct effect on bone physiology in addition to its influence on body mass. The effects of C/EBPβ loss in male mice were similar to those in females, with β−/− mice being smaller (Figure 1A), having less fat (Figure 1B) and lower BMD (Figure 1C) than their wild-type littermates.
In both genders, the effects of C/EBPδ differed from those of C/EBPβ. Heterozygous δ+/− females were slightly larger and had increased adiposity compared with either wild-type (δ+/+) or δ−/− groups (Table I), suggesting that C/EBPδ may contribute to the effects of C/EBPβ on body weight and fat content. However, C/EBPδ had no observable effect on bone density for either gender. In addition, in female animals, there was a small but statistically significant interaction term between C/EBPβ and C/EBPδ (Table I). This results from C/EBPδ-deficient females being larger than expected based on the additive effects of C/EBPβ and δ absence. In contrast, C/EBPδ deficiency had no influence on body composition in males and consequently there was no detectable interaction observed between C/EBPβ and C/EBPδ in these mice. Collectively, these data suggest that while C/EBPδ may contribute to the ability of C/EBPβ to modulate body size and fat content in females, only C/EBPβ mediates these parameters in male mice. Furthermore, C/EBPβ, but not C/EBPδ, is a critical regulator of bone density.
Systemic IGF-1 levels are reduced in β−/− and β−/−;δ−/− mice
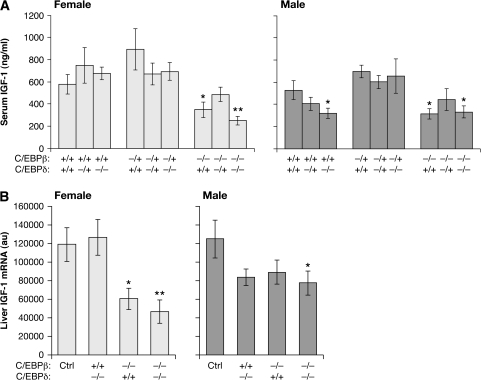
To investigate the role of these two transcription factors in determining IGF-1 levels, we analyzed serum IGF-1 titers and IGF-1 messenger RNA expression in liver (Figure 2). In males and females, both wild-type and β+/− mice had higher levels of serum IGF-1 than either β−/− or β−/−;δ−/− mice (Figure 2A). In animals lacking C/EBPβ, there was an ∼50% reduction in circulating levels of IGF-1. As predicted, the liver IGF-1 messenger RNA expression data (Figure 2B) were similar, with both male and female C/EBPβ and C/EBPβ/δ-deficient mice showing a substantial reduction. However, in females, the decrease in IGF-1 expression was more pronounced, similar to the 50% reduction seen in serum IGF-1. In contrast to the dramatic effects of C/EBPβ on IGF-1 expression, loss of C/EBPδ had no observable effect on either IGF-1 serum or liver RNA for either males (P = 0.14 and P = 0.17) or females (P = 0.23 and P = 0.84). These results could be related to the decrease in body size seen in female C/EBPβ knockouts (Figure 1 and Table I). In addition, for female animals, there was a small interaction effect observed between C/EBPβ and C/EBPδ for both serum and liver IGF-1 (P = 0.077 and P = 0.023, respectively). Thus, loss of C/EBPβ but not C/EBPδ has a significant impact on IGF-1 expression in adult mice.
Fig. 2.
Diminished circulating IGF-1 levels in the absence of C/EBPβ/δ. (A) Blood was collected from both male and female mice by genotype upon sacrifice and serum was isolated by centrifugation. IGF-1 levels in the serum were assayed by radioimmunoassay. (B) RNA was isolated from mouse livers and IGF-1 messenger RNA levels were determined by RNase protection assay. Results are normalized to GAPDH (au, arbitrary units). Data show the average ± standard error and are sorted by gender and genotype. To increase the sample size of the control (wild-type) group, data from β+/+;δ+/+ and β+/−;δ+/+ animals were combined and are labeled ‘Ctrl’. Statistical analysis in comparison with the control group was performed using Student’s unpaired t-test (*P < 0.05; **P < 0.01).
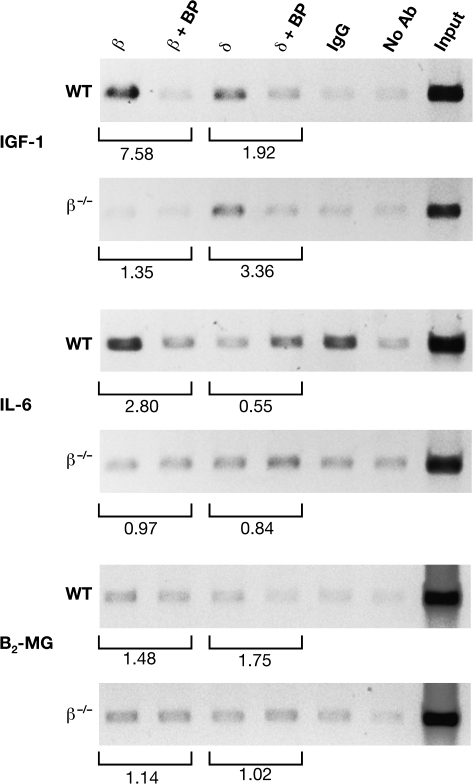
C/EBPβ interacts with the IGF-1 promoter in vivo
Promoter 1 of the IGF-1 gene contains a C/EBP site located in the non-coding region of exon 1 that mediates transcriptional activation by C/EBPδ in osteoblasts (30). Therefore, we used ChIP assays to examine whether C/EBPβ and C/EBPδ bind to this region of the IGF-1 gene in mouse liver (Figure 3). C/EBPβ showed significant interaction and C/EBPδ binding was also detectable, but weaker. The specificity of the interactions was demonstrated by decreased ChIP signals when the appropriate blocking peptides were included in the immunoprecipitations. As a further control, C/EBPβ binding was not evident when liver tissue from β−/− mice was used. Positive signals were obtained for C/EBPβ binding to the interleukin (IL)-6 promoter, which has a well-characterized C/EBP site (38), whereas no binding was detected using a negative control region from the β2-microglobulin gene (36). Thus, C/EBPβ and, to a lesser extent, C/EBPδ are associated with the IGF-1 C/EBP site in vivo.
Fig. 3.
C/EBPβ and C/EBPδ bind to the IGF-1 promoter in liver. ChIP assays from C/EBPβ+/+ and C/EBPβ−/− liver tissue. Chromatin was immunoprecipitated with the indicated antibodies and the recovered DNA was analyzed by polymerase chain reaction using primers corresponding to the indicated genes. Normal rabbit IgG and no antibody (Ab) were used as controls. Specific binding of the antibodies was determined by preincubating the antibodies with their respective blocking peptides (BP) prior to the immunoprecipitation reaction. Input represents 2% of the total chromatin. β2-Microglobulin (B2-MG) is a negative control and IL-6 is a positive control for C/EBP binding. Bands were quantitated by densitometry; Ab:Ab+BP ratios are indicated beneath each pair of lanes.
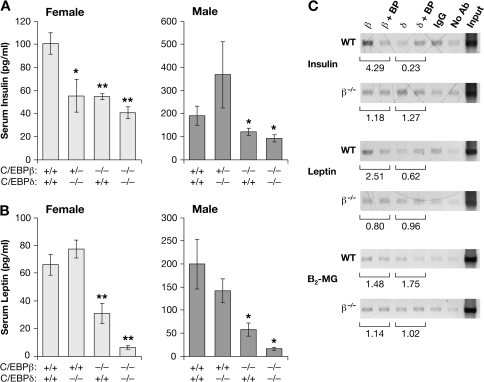
Effect of C/EBPβ and C/EBPδ deficiency on serum insulin and leptin levels
Insulin and leptin are also important hormones that mediate energy balance and contribute to body weight and bone mass. Therefore, we analyzed levels of serum insulin and leptin in β−/−, δ−/−, β−/−;δ−/− and wild-type control mice (Figure 4 and Table II). In females, all three mutant genotypes showed reduced insulin, with lowest levels in β−/−;δ−/− mice. In males, only animals lacking C/EBPβ (β−/− and β−/−;δ−/−) had decreased levels of insulin. These effects of genotype were significant for both genders (P < 0.05). Serum leptin was also diminished in male and female β−/− animals, whereas β−/−;δ−/− mice exhibited even lower leptin levels. Note that the overall insulin and leptin values are higher in males than females, as reflected in previous reports (39–41). While female δ−/− animals also showed a decrease in circulating insulin, the deletion of C/EBPδ alone had no observable effect on leptin levels. In contrast, the combined effect of C/EBPβ and δ on leptin levels is much more dramatic than for insulin, with little leptin detected in the β−/−;δ−/− mice. Table II supports an interaction between the two genes in determining leptin levels in female animals [P(interaction) = 0.02]. There was no significant interaction observed between C/EBPβ and δ in male animals [P(interaction) > 0.1]. However, from a statistical standpoint, it is important to note that this is a weaker test than the analyses of IGF-1 because measurements of several of the heterozygous genotypes were lacking due to sample availability.
Fig. 4.
Role of C/EBPβ in mediating the levels of other hormonal regulators of energy homeostasis. Serum was assayed for insulin and leptin content. Levels of insulin (A) and leptin (B) are depicted for male and female mice by genotype. Statistical significance in comparison with control animals was evaluated using Student’s unpaired t-test (*P < 0.05; **P < 0.01). (C) ChIP analysis of C/EBPβ and C/EBPδ binding to the insulin and leptin promoters. Chromatin from C/EBPβ+/+ and C/EBPβ−/− liver tissue was immunoprecipitated with the indicated antibodies and the DNA was analyzed by polymerase chain reaction using primers corresponding to the indicated genes. The experiment was performed as described in Figure 3.
In Table II and Figure 4A, serum insulin measurements on β+/+;δ−/− mice were not available due to a shortage of serum, but we were able to measure serum insulin on a small sample of β+/−;δ−/− females (n = 5) and males (n = 3). Female β+/−;δ−/− serum insulin levels [mean ln level = 3.8 (SE = 0.16)] were significantly lower than those in β+/+; δ+/+ females but were indistinguishable from the other genotypes. In contrast, serum insulin levels of male β+/−;δ−/− mice were indistinguishable from those of β+/+;δ+/+ males but were significantly higher than in the other two genotypes.
Because loss of C/EBPβ and/or C/EBPδ decreased insulin and leptin expression, we examined binding of these proteins to the respective promoters using ChIP assays (Figure 4C). C/EBPβ binding to the insulin promoter was evident, as the ChIP signal was diminished by the C/EBPβ antibody-blocking peptide and this reduction was not observed for β−/− tissue. Weak C/EBPδ binding was only apparent in the C/EBPβ−/− background, possibly reflecting competition for binding between these two transcription factors when both are present. C/EBPβ association with the leptin gene was also observed, but there was no evidence for C/EBPδ binding. This parallels the serum leptin results, as there was no significant decrease in leptin levels in C/EBPδ−/− animals.
Decreased tumorigenicity of colon adenocarcinoma cells in C/EBPβ deficient mice
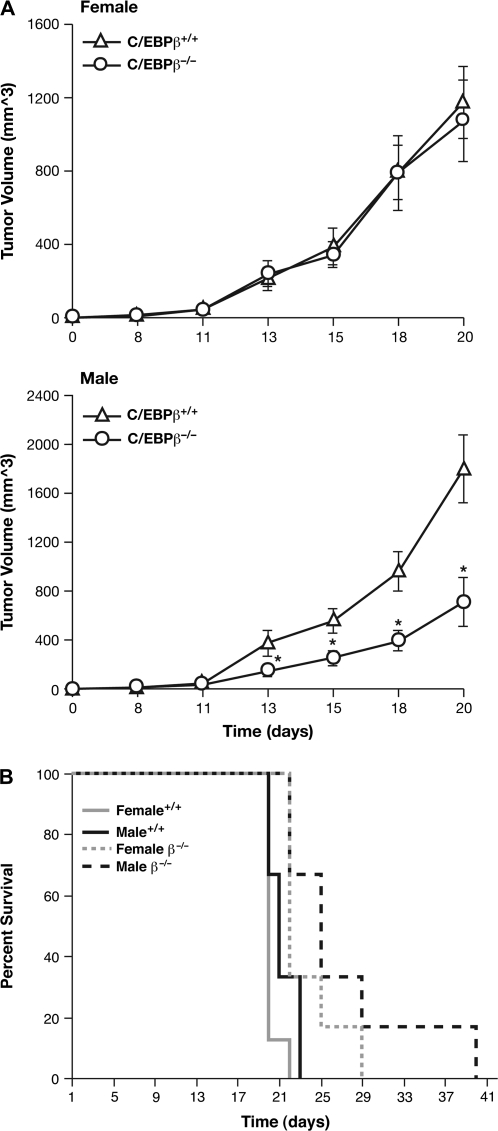
Given the importance of C/EBPβ in regulating IGF-I expression and the relationship between reduced IGF-I levels and diminished malignancy of numerous cancers, as observed with calorie restriction and IGF-1-deficient mice (4,42), we asked whether the tumorigenic potential of transplanted tumor cells is altered in C/EBPβ-deficient host animals. MC-38 mouse colon adenocarcinoma cells (37) were injected subcutaneously into β−/− mice or their wild-type littermates and the animals were monitored for mortality and tumor growth over time (Figure 5). In female mice, the tumor growth rate was similar for wild-type and β−/− recipients (Figure 5A). In contrast, male mutant mice showed a substantially slower rate of tumor growth between days 13 and 20 compared with wild-type. For example, on day 20, the average tumor volume in male β−/− mice was 711 versus 1801 mm3 for wild-type animals.
Fig. 5.
Reduced tumorigenicity of transplanted colon adenocarcinoma cells in C/EBPβ−/− mice. Mouse MC-38 colon adenocarcinoma cells were injected into each flank of male and female C/EBPβ+/+ and C/EBPβ−/− mice. The animals were monitored for tumor development and were sacrificed upon onset of severe tumor burden. Six to eight animals per group were analyzed. (A) Average daily tumor volume is shown in cubic millimeter ± standard error. Statistical significance in comparison with control animals was evaluated using Student’s unpaired t-test (*P < 0.05; **P < 0.01). (B) Survival data. Mortality data were analyzed using the Kaplan–Meier survival test. Mean comparisons were performed using Tukey’s HSD test or linear contrasts.
The Kaplan–Meier plots shown in Figure 5B illustrate that the survival of β−/− mice injected with tumor cells is enhanced compared with wild-type animals. The average survival times for wild-type males and females calculated from the data of Figure 5B were 20.1 (1.4) and 21.8 (1.2) days, respectively, versus 26.7 (1.4) and 23.8 (1.4) days for β−/− males and females. The differences between the wild-type and β−/− groups were significant (P = 0.0034) and these differences persisted even after exclusion of a long-lived knockout male mouse. Collectively, these data indicate that the presence of C/EBPβ provides a more permissive tumor environment that promotes tumor development and malignancy, at least for colon adenocarcinoma cells. Moreover, this effect is more pronounced in male animals.
Discussion
Previous work supports the notion that C/EBPα, β and δ are important regulators of adipocyte terminal differentiation and gene expression (43–46). In addition, the absence of C/EBPβ reduces triglyceride content and lipogenic enzyme activity and decreases adiposity (47). Our study is the first to comprehensively investigate the in vivo roles of C/EBPβ and δ in body weight, fat weight and bone density as well as the hormonal regulators involved in modulating energy homeostasis and body composition. C/EBPβ is the most critical regulator of body weight, fat and bone density. Whereas C/EBPβ is involved in regulating all three of these biological parameters in both male and female mice, C/EBPδ has minimal effects on these properties in either sex. The difference in importance of these two C/EBPs may be partially explained by the fact that C/EBPδ is primarily an inducible factor, whereas C/EBPβ expression is constitutive in many tissues. For example, normal unstimulated hepatocytes contain abundant C/EBPβ but express only low levels of C/EBPδ.
In some tissues, such as bone, C/EBPδ may also be constitutively expressed. Most of the studies on C/EBPs and bone mass have focused on the role of C/EBPδ in regulating IGF-1 expression in osteoblasts and osteoclasts. In a normal physiologic setting, parathyroid hormone and prostaglandin E2 cause osteoblasts to produce IGF-1 and ultimately stimulate bone growth (48,49). Exposure to these hormones stimulates cyclic adenosine 3′,5′-monophosphate synthesis and activates protein kinase A. These stimuli result in translocation of constitutively expressed C/EBPδ from the cytoplasm to the nucleus where it can activate expression of IGF-1 (50,51). Although numerous reports implicate C/EBPδ as a key regulator of IGF-1 and, by extension, growth of osteoblasts and bone formation, our data clearly identify C/EBPβ as more critical for bone density in vivo. Whether this phenotype of C/EBPβ−/− mice results from decreased circulating IGF-1 or from impaired local IGF-1 production, or both, is presently unclear.
C/EBPβ was shown previously to regulate IGF-1 expression in various cells, including myeloid tumor cells, normal bone marrow macrophages and HepG2 hepatocarcinoma cells (31,52). However, to date, there has been no evidence that C/EBPβ controls endocrine expression of IGF-1, which is thought to mediate physiological parameters such as bone density and body weight regulation. Since there is a substantial decrease in circulating IGF-1 in C/EBPβ-deficient mice, C/EBPβ clearly plays an important role in regulating IGF-1 produced by the liver and secreted into the circulation. Thus, in addition to controlling an autocrine IGF-1 pro-survival pathway (31), C/EBPβ modulates systemic IGF-1 levels.
Leptin regulates body weight by decreasing appetite and food intake and increasing energy output. Leptin is secreted primarily by adipocytes and acts as part of a feedback mechanism to provide the hypothalamus with information on fat stores in the body (53,54). Studies in leptin-deficient ob/ob mice, which are obese, insulin resistant and infertile (55–58), in addition to clinical studies showing that the ob gene is markedly upregulated in obese subjects (53), demonstrate the importance of this hormone to maintaining body weight and adiposity (59). We found that leptin levels are modulated predominantly by C/EBPβ. Nonetheless, leptin is further reduced when both C/EBPβ and C/EBPδ are absent, indicating that C/EBPδ also contributes to leptin gene regulation. C/EBPβ interacts with the leptin promoter in vivo (Figure 4C), consistent with previous observations showing that there is a C/EBP motif within the leptin gene and C/EBPα, β and δ are capable of transactivating the leptin promoter (60,61). Although it is probable that C/EBPβ regulates leptin gene transcription, serum leptin is also determined by fat content (17). Therefore, the reduction in leptin levels in C/EBPβ-deficient mice could be partly due to their decreased adiposity. Serum leptin levels are higher in women than men (62), but leptin levels in male mice (39) and rats (40,41) are higher than in females. Such reverse sexual dimorphism may be explained by the higher levels body fat found in males of some rodent species. Male mice in our study had greater absolute levels of body fat (Table I) and significantly higher percent fat (23.5% in males versus 20.4% in females), and this difference may account for the much higher serum leptin levels found in males (Figure 4B).
Interestingly, Schroeder-Gloeckler et al. (47) recently reported that crossing leptin receptor-deficient (db/db) mice to C/EBPβ knockout animals attenuates the obesity, fatty liver and diabetic phenotype conferred by absence of the leptin receptor. Together with our data showing that C/EBPβ deficiency severely reduces leptin levels, these findings suggest a complex interaction between C/EBPβ and leptin signaling in regulating adiposity and energy metabolism.
Insulin is a major anabolic hormone that mediates the breakdown of protein, fat and carbohydrates to produce energy. Much like leptin, insulin has been shown to modulate neuropeptide expression in the hypothalamus, leading to decreased appetite and food intake (63,64). In addition, mice lacking a brain-specific insulin receptor display a phenotype similar to the ob/ob mice, exhibiting obesity and impaired fertility (65). Our results show that C/EBPβ is an important regulator of serum insulin in male and female animals, whereas C/EBPδ deficiency affects circulating insulin levels only in females. ChIP studies indicate that C/EBPβ and C/EBPδ associate with the insulin promoter (Figure 4C), which has been shown to contain a C/EBP site (66,67). These observations suggest that C/EBPβ may directly regulate insulin gene transcription. However, since serum insulin is also determined by fat content, the reduction in insulin levels in C/EBPβ-deficient as well as female C/EBPδ-deficient mice may be partially due to their decreased adiposity.
C/EBPβ influences cellular transformation and tumorigenesis in a variety of mouse and human cell types (68). For example, C/EBPβ-deficient mice are completely resistant to carcinogen-induced skin tumors (69), and conditional ablation of C/EBPβ in keratinocytes has established that C/EBPβ acts cell autonomously to promote cell survival and papillomagenesis in the skin tumor model (70). Furthermore, C/EBPβ regulates autocrine release of IGF-1 and survival of Myc/Raf-transformed macrophages (31) and has a pro-survival role in metastatic Wilms tumor cells (71) and anaplastic large-cell lymphomas (72). C/EBPβ can also act in a cell non-autonomous manner to facilitate tumorigenesis since we found that transplanted mouse colon adenocarcinoma cells display a reduced ability to generate tumors in C/EBPβ−/− mice as evidenced by diminished tumor growth rate and delayed mortality of these animals (Figure 5).
The ability of C/EBPβ to regulate IGF-1 and leptin expression may contribute to the diminished tumorigenicity observed in C/EBPβ-deficient mice In addition to their roles in maintaining energy homeostasis, IGF-1 and leptin have been shown to be involved in tumorigenesis. IGF-1 promotes survival of many types of human cancer cells in vitro (8,73,74), and increased levels of IGF-1 and its receptor are found in many tumor cells (75). Moreover, calorie restriction experiments suggest that reductions in systemic IGF-1 contribute to the antiproliferative and anticancer effects of reduced caloric intake (4,76,77). Recently, it has been observed that leptin stimulates proliferation of preneoplastic and cancer cell lines (26), and leptin and its receptor are both expressed at higher levels in human primary breast tumors (78) and lymph node metastases (79) than in normal breast tissue. Thus, it is plausible that decreased levels of insulin, IGF-1 and leptin create a less favorable tumor environment in C/EBPβ-deficient animals.
The effect of C/EBPβ deficiency on tumor growth was more prominent in males than females. At present, we do not have a molecular explanation for this gender difference. Recent studies demonstrated that male mice display increased susceptibility to diethylnitrosamine-induced hepatocellular carcinoma, which was attributed to enhanced IL-6 production by Kupffer cells (liver macrophages) in males and increased pro-oncogenic inflammation (80). The gender disparity in liver carcinogenesis disappeared in mice lacking IL-6 or the adapter protein MyD88. Decreased hepatocellular carcinoma incidence in females stems from their elevated estrogen levels, which suppress nuclear factor-κB activity and inhibit IL-6 production. Since C/EBPβ has a role in regulating inflammatory cytokines and other mediators such as cyclooxygenase 2 (81,82), it is possible that this function of C/EBPβ also contributes to a permissive tumor microenvironment in a sex-specific manner.
Much effort has been focused on understanding the mechanism by which obesity contributes to cancer. The present work and previous studies establish that C/EBPβ is a critical regulator of body weight, adiposity and tumorigenesis in mice. Future analysis of the mechanism by which C/EBPβ regulates expression of IGF-1, insulin and leptin may suggest novel approaches to cancer prevention via energy balance-related pathways.
Funding
Intramural Research Program of the National Institutes of Health; National Cancer Institute, Center for Cancer Research.
Acknowledgments
We thank Angie Hackley and Barbara Shankle for animal handling and genotyping and Nicole Smith for technical assistance. We dedicate this paper to the memory of Heather Leigh Hill, a talented technician who conducted the radioimmunoassays and hormone assays in this study and who lost her valiant fight with ovarian cancer in 2005, and to Barbara Shankle, a dedicated and beloved coworker who succumbed to cervical cancer in September 2008.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BMD
bone mineral density
- C/EBP
CCAAT/enhancer-binding protein
- ChIP
chromatin immunoprecipitation
- HSD
honestly significant differences
- IGF-1
insulin-like growth factor-1
- IL
interleukin
References
- 1.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Hursting SD, et al. Energy balance and carcinogenesis: underlying pathways and targets for intervention. Curr. Cancer Drug Targets. 2007;7:484–491. doi: 10.2174/156800907781386623. [DOI] [PubMed] [Google Scholar]
- 4.Hursting SD, et al. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu. Rev. Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 5.Singh P, et al. Proliferation and differentiation of a human colon cancer cell line (CaCo2) is associated with significant changes in the expression and secretion of insulin-like growth factor (IGF) IGF-II and IGF binding protein-4: role of IGF-II. Endocrinology. 1996;137:1764–1774. doi: 10.1210/endo.137.5.8612513. [DOI] [PubMed] [Google Scholar]
- 6.LeRoith D, et al. Insulin-like growth factors and cancer. Ann. Intern. Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, et al. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. J. Urol. 2003;169:714–717. doi: 10.1097/01.ju.0000036380.10325.2a. [DOI] [PubMed] [Google Scholar]
- 8.Furstenberger G, et al. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–4388. [PubMed] [Google Scholar]
- 10.Resnicoff M, et al. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 1994;54:4848–4850. [PubMed] [Google Scholar]
- 11.Trojan J, et al. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc. Natl Acad. Sci. USA. 1992;89:4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshman E, et al. Insulin-like growth factors and insulin-like growth factor binding proteins in mammary gland function. Breast Cancer Res. 2002;4:231–239. doi: 10.1186/bcr535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason JL, et al. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J. Neurosci. 2000;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrizas M, et al. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Adhami VM, et al. Insulin-like growth factor-I axis as a pathway for cancer chemoprevention. Clin. Cancer Res. 2006;12:5611–5614. doi: 10.1158/1078-0432.CCR-06-1564. [DOI] [PubMed] [Google Scholar]
- 16.Sachdev D, et al. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol. Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 17.Calle EE, et al. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 18.Bianchini F, et al. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 19.Yakar S, et al. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Woods SC, et al. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 21.Lonnqvist F, et al. Relationship between circulating leptin and peripheral fat distribution in obese subjects. Int. J. Obes. Relat. Metab. Disord. 1997;21:255–260. doi: 10.1038/sj.ijo.0800394. [DOI] [PubMed] [Google Scholar]
- 22.Montague CT, et al. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–347. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- 23.Stattin P, et al. Obesity and colon cancer: does leptin provide a link? Int. J. Cancer. 2004;109:149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 24.Chang S, et al. Leptin and prostate cancer. Prostate. 2001;46:62–67. doi: 10.1002/1097-0045(200101)46:1<62::aid-pros1009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Saglam K, et al. Leptin influences cellular differentiation and progression in prostate cancer. J. Urol. 2003;169:1308–1311. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- 26.Fenton JI, et al. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol. Biomarkers Prev. 2005;14:1646–1652. doi: 10.1158/1055-9965.EPI-04-0916. [DOI] [PubMed] [Google Scholar]
- 27.Bouloumie A, et al. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 28.Ramji DP, et al. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umayahara Y, et al. CCAAT/enhancer-binding protein delta activates insulin-like growth factor-I gene transcription in osteoblasts. Identification of a novel cyclic AMP signaling pathway in bone. J. Biol. Chem. 1997;272:31793–31800. doi: 10.1074/jbc.272.50.31793. [DOI] [PubMed] [Google Scholar]
- 30.Umayahara Y, et al. CCAAT/enhancer-binding protein delta is a critical regulator of insulin-like growth factor-I gene transcription in osteoblasts. J. Biol. Chem. 1999;274:10609–10617. doi: 10.1074/jbc.274.15.10609. [DOI] [PubMed] [Google Scholar]
- 31.Wessells J, et al. Critical prosurvival roles for C/EBP beta and insulin-like growth factor I in macrophage tumor cells. Mol. Cell. Biol. 2004;24:3238–3250. doi: 10.1128/MCB.24.8.3238-3250.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterneck E, et al. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc. Natl Acad. Sci. USA. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterneck E, et al. An essential role for C/EBPβ in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagy TR, et al. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes. Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 35.Berrigan D, et al. Phenotypic effects of calorie restriction and insulin-like growth factor-1 treatment on body composition and bone mineral density of C57BL/6 mice: implications for cancer prevention. In Vivo. 2005;19:667–674. [PubMed] [Google Scholar]
- 36.Sebastian T, et al. C/EBPbeta cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 2005;24:3301–3312. doi: 10.1038/sj.emboj.7600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbett TH, et al. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
- 38.Akira S, et al. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol. Rev. 1992;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 39.Gui Y, et al. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes. Res. 2004;12:1481–1491. doi: 10.1038/oby.2004.185. [DOI] [PubMed] [Google Scholar]
- 40.Landt M, et al. Radioimmunoassay of rat leptin: sexual dimorphism reversed from humans. Clin. Chem. 1998;44:565–570. [PubMed] [Google Scholar]
- 41.Morash BA, et al. Pituitary leptin gene expression is reduced by neonatal androgenization of female rats. Pituitary. 2001;4:63–70. doi: 10.1023/a:1012938911380. [DOI] [PubMed] [Google Scholar]
- 42.Yakar S, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147:5826–5834. doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- 43.Cao Z, et al. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 44.Lin FT, et al. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- 45.Yeh WC, et al. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka T, et al. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder-Gloeckler JM, et al. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J. Biol. Chem. 2007;282:15717–15729. doi: 10.1074/jbc.M701329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarthy TL, et al. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124:1247–1253. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy TL, et al. Prostaglandin E2 stimulates insulin-like growth factor I synthesis in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1991;128:2895–2900. doi: 10.1210/endo-128-6-2895. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy TL, et al. Local IGF-I expression and bone formation. Growth Horm. IGF Res. 2001;11:213–219. doi: 10.1054/ghir.2001.0236. [DOI] [PubMed] [Google Scholar]
- 51.Billiard J, et al. Regulated nuclear-cytoplasmic localization of CCAAT/enhancer-binding protein delta in osteoblasts. J. Biol. Chem. 2001;276:15354–15361. doi: 10.1074/jbc.M009973200. [DOI] [PubMed] [Google Scholar]
- 52.Umayahara Y, et al. Protein kinase C-dependent, CCAAT/enhancer-binding protein beta-mediated expression of insulin-like growth factor I gene. J. Biol. Chem. 2002;277:15261–15270. doi: 10.1074/jbc.M110827200. [DOI] [PubMed] [Google Scholar]
- 53.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 55.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 56.Friedman JM, et al. Molecular mapping of the mouse ob mutation. Genomics. 1991;11:1054–1062. doi: 10.1016/0888-7543(91)90032-a. [DOI] [PubMed] [Google Scholar]
- 57.Chehab FF, et al. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 58.Ahima RS, et al. Leptin accelerates the onset of puberty in normal female mice. J. Clin. Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein S, et al. Adipose tissue leptin production and plasma leptin kinetics in humans. Diabetes. 1996;45:984–987. doi: 10.2337/diab.45.7.984. [DOI] [PubMed] [Google Scholar]
- 60.Mason MM, et al. Regulation of leptin promoter function by Sp1, C/EBP, and a novel factor. Endocrinology. 1998;139:1013–1022. doi: 10.1210/endo.139.3.5792. [DOI] [PubMed] [Google Scholar]
- 61.Hwang CS, et al. Transcriptional activation of the mouse obese (ob) gene by CCAAT/enhancer binding protein alpha. Proc. Natl Acad. Sci. USA. 1996;93:873–877. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruhl CE, et al. Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am. J. Clin. Nutr. 2001;74:295–301. doi: 10.1093/ajcn/74.3.295. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz MW, et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 64.Baskin DG, et al. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 65.Bruning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 66.Lu M, et al. Pancreatic beta-cell-specific repression of insulin gene transcription by CCAAT/enhancer-binding protein beta. Inhibitory interactions with basic helix-loop-helix transcription factor E47. J. Biol. Chem. 1997;272:28349–28359. doi: 10.1074/jbc.272.45.28349. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence MC, et al. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J. Biol. Chem. 2005;280:26751–26759. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- 68.Sebastian T, et al. Stop and go: anti-proliferative and mitogenic functions of the transcription factor C/EBPbeta. Cell Cycle. 2006;5:953–957. doi: 10.4161/cc.5.9.2733. [DOI] [PubMed] [Google Scholar]
- 69.Zhu S, et al. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc. Natl Acad. Sci. USA. 2002;99:207–212. doi: 10.1073/pnas.012437299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sterneck E, et al. Conditional ablation of C/EBP beta demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene. 2006;25:1272–1276. doi: 10.1038/sj.onc.1209144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, et al. A gene expression signature for relapse of primary wilms tumors. Cancer Res. 2005;65:2592–2601. doi: 10.1158/0008-5472.CAN-04-1532. [DOI] [PubMed] [Google Scholar]
- 72.Piva R, et al. Functional validation of the anaplastic lymphoma kinase signature identifies CEBPB and BCL2A1 as critical target genes. J. Clin. Invest. 2006;116:3171–3182. doi: 10.1172/JCI29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moschos SJ, et al. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 74.Yee D. Are the insulin-like growth factors relevant to cancer? Growth Horm. IGF Res. 2001;11:339–345. doi: 10.1054/ghir.2001.0256. [DOI] [PubMed] [Google Scholar]
- 75.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 76.Berrigan D, et al. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 77.Mai V, et al. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res. 2003;63:1752–1755. [PubMed] [Google Scholar]
- 78.Ishikawa M, et al. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 79.Garofalo C, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin. Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 80.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 81.Wu KK, et al. Transcriptional control of COX-2 via C/EBP{beta} Arterioscler. Thromb. Vasc. Biol. 2005;25:679–685. doi: 10.1161/01.ATV.0000157899.35660.61. [DOI] [PubMed] [Google Scholar]
- 82.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]