Abstract
Synergistic effects of dysregulation of the WNT/CTNNB1 and phosphatidylinositol 3-kinase (PI3K)/AKT pathways are thought to be important for the development and progression of many forms of cancer, including the granulosa cell tumor of the ovary. Sustained WNT/CTNNB1 signaling in Sertoli cells causes testicular degeneration and the formation of foci of poorly differentiated stromal cells in the seminiferous tubules in mice. To test if concomitant dysregulation of the WNT/CTNNB1 and PI3K/AKT pathways could synergize to cause testicular cancer, Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice that express a dominant, stable CTNNB1 mutant and lack the expression of phosphatase and tensin homolog (PTEN) in their Sertoli cells were generated. These mice developed aggressive testicular cancer with 100% penetrance by 5 weeks of age, and 44% of animals developed pulmonary metastases by 4 months, whereas Ptentm1Hwu/tm1Hwu;Amhr2tm3(cre)Bhr/+ controls were phenotypically normal. Surprisingly, the tumors could not be classified as Sertoli cell tumors, but rather bore histologic and ultrastructural characteristics of granulosa cell tumors of the testis (GCTT). Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumors did not express CYP17, CYP19, germ cell nuclear antigen, estrogen receptor 1 or progesterone receptor, but expressed the early granulosa cell markers WNT4 and FOXL2, confirming the diagnosis of GCTT. Immunohistochemical analyses of Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT demonstrated a tumor marker profile similar to that reported in human GCTT. Immunoblotting analyses revealed high levels of phosphorylation of AKT and the PI3K/AKT signaling effector FOXO1A in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT, suggesting the involvement of FOXO1A in the mechanism of GCTT development. Together, these data provide the first insights into the molecular etiology of GCTT and the first animal model for the study of GCTT biology.
Introduction
Testicular cancer is the most common cause of cancer in men aged between 15 and 44 in developed countries, representing 13.4% of new cancer cases (1). Most research in this field has focused on germ cell tumors, as these comprise the majority of testicular cancer (2). Comparatively, little attention has been paid to the sex cord/stromal tumors, although they are thought to represent 5% of all testicular neoplasms (3). Among the latter group, granulosa cell tumors of the testis (GCTT) are rare neoplasms. Nonetheless, case reports have shown that GCTT have a malignant potential to form distant metastases leading to a very short overall survival (reviewed in ref. 4). At the present time, treatment options for GCTT are not well established, and very little is known about its origin or of its molecular etiology (4).
Dysregulated WNT signaling is a hallmark of many forms of cancer (reviewed in refs 5–8). Recently, it has been shown that the sustained activation of the WNT/CTNNB1 pathway in the granulosa cells of a genetically engineered mouse model (Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+) leads to ovarian granulosa cell tumor (GCT) development (9). Interestingly, Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ ovaries develop multiple follicle-sized premalignant lesions around the time of puberty, and GCT develop in a stochastic manner from these premalignant lesions only after the age of 6 months (9). In male Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice, sustained activation of the WNT/CTNNB1 pathway occurs in Sertoli cells (10). This leads to degeneration of the seminiferous tubules, the progressive loss of all germ cells and sterility via a mechanism that is not well understood (10). Although testicular tumor development was not observed in male Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals, multilayered foci of poorly differentiated somatic cells accumulated in many seminiferous tubules, in a manner reminiscent of the premalignant lesions seen in the ovaries (10). It has been proposed that the cells that formed the multilayered foci could represent primitive gonadal somatic cells uncommitted to the Sertoli cell lineage (10).
Another intracellular signaling pathway that is dysregulated in many forms of cancer is the PI3K/AKT pathway. Most notably, the tumor suppressor phosphatase and tensin homolog (PTEN) acts as a negative regulator of the PI3K/AKT pathway and is frequently mutated and inactivated in many malignancies (11,12). Loss of PTEN leads to increased AKT kinase activity, which modulates the activities of multiple downstream effectors via phosphorylation (13). One downstream target of the PI3K/AKT pathway is the protein kinase glycogen synthase kinase 3β (GSK3β) (14). GSK3β also plays a crucial role in the WNT/CTNNB1 pathway as part of a multicomponent complex that is responsible for the phosphorylation and subsequent ubiquitination and proteosomal degradation of CTNNB1 (15). AKT can therefore promote WNT/CTNNB1 signaling by inactivating GSK3β and thereby causing the hypophosphorylation, stabilization and accumulation of CTNNB1, which translocates to the cell nucleus and modulates the transcriptional activity of specific target genes, including cyclin D2 (16). Several studies have suggested that this type of cross talk between the PI3K/AKT- and WNT/CTNNB1-signaling pathways is involved in the development and progression of several forms of cancer including mammary gland, prostate, liver and skin (14,17–19). We have recently reported that the addition of a granulosa cell-targeted deletion of Pten to the aforementioned Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ genetic background resulted in a much more aggressive GCT phenotype, characterized by 100% penetrance, perinatal onset and the ability to disseminate locally and distally (20). The mechanism of synergy between the PI3K/AKT and WNT/CTNNB1 pathways in the latter model remains to be elucidated, but is thought not to involve AKT regulation of CTNNB1 via GSK3β (20).
The objective of this study was to investigate the possible involvement and synergy of the PI3K/AKT and WNT/CTNNB1 pathways in the formation of sex cord tumors of the testis. Transgenic Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice were generated to test if derepression of PI3K/AKT signaling could trigger cancer development in the multilayered cell foci observed in the seminiferous tubules of Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals. Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice developed aggressive, metastatic testicular tumors that were diagnosed as GCTT based on histopathologic and molecular marker analyses. This study therefore demonstrates a synergy between the PI3K/AKT and WNT/CTNNB1 pathways in the pathogenesis of GCTT and provides the first model system for the study of GCTT biology.
Materials and methods
Animals and sample collection
Genetically modified animals were derived by selective breeding of the previously described Ptentm1Hwu, Ctnnb1tm1Mmt and Amhr2tm3(cre)Bhr parental strains (21–23). Genotyping analyses for the Amhr2tm3(cre)Bhr and Ctnnb1tm1Mmt alleles were performed by polymerase chain reaction (PCR) on DNA obtained from tail biopsies as described previously (23,24). Mice bearing the Ptentm1Hwu allele were obtained from The Jackson Laboratory (Bar Harbor, ME), and DNA samples from tail biopsies, testicular tumor samples or isolated testes were analyzed for Pten genotype by PCR as directed by the animal provider, except the following oligonucleotide primers were used: Pten-S 5′-GATACTAGTAAGATAAAAACCAGTAGT-3′, Pten-AS 5′-GTCACCCAGGCCTCTTGTCAAGT-3′ and Pten-P3 5′-GCTTGATATCGAATTCCTGCAGC-3′. These primers produce PCR products of ∼400 bp for the wild-type Pten allele, 572 bp for the floxed allele and 290 bp for the Cre-recombined allele. Blood samples were collected by cardiac puncture under anesthesia prior to euthanasia. Testosterone and estradiol immunoassays were done on serum samples at the Ligand Assay and Analysis Core within the Center for Research in Reproduction at the University of Virginia. Intra-assay coefficients of variability were <6% for the testosterone assay and <19% for the estradiol assay. Testes for light microscopy histopathologic analysis were fixed in Bouin's fixative for 24 h, rinsed and dehydrated in alcohol and subsequently embedded in paraffin, sectioned and stained with hematoxylin and eosin. Testes samples for electron microscopy were fixed by cardiac perfusion with 2.5% glutaraldehyde buffered in sodium cacodylate (0.1 M) containing 0.05% calcium chloride (pH 7.4) and processed as described previously (25). All procedures were approved by the Institutional Animal Care and Use Committee and conformed to the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Immunohistochemical and Immunofluorescence analyses
Immunohistochemistry was done on Bouin's-fixed, paraffin-embedded, 7 μm tissue sections using VectaStain Elite avidin–biotin complex method kits (Vector Laboratories, Burlingame, CA) as directed by the manufacturer. Sections were probed with primary antibodies against germ cell nuclear antigen 1, smooth muscle actin (ACTA2) (BioGenex, San Ramon, CA, #MU128-UC), cytokeratin (KRT) (Thermo Fisher Scientific, Neomarkers, Fremont, CA, #MS-343-P1), aromatase (CYP19A1) (Novus Biologicals, Littleton, CO, #APoooo1PU-N) and estrogen receptor alpha or progesterone receptor (Santa Cruz biotechnology, Santa Cruz, CA, #sc-543 and #sc-539, respectively) and staining was done using the 3,3′-diaminobenzidine peroxidase substrate kit (Vector Laboratories). For immunofluorescence experiments, testes were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA) and stored at −80°C before the preparation of 3 μm sections, fixed for 30 min in phosphate-buffered saline-buffered 4% paraformaldehyde at room temperature and overnight in phosphate-buffered saline-buffered 1% paraformaldehyde at 4°C. Sections were labeled or double labeled with primary antibodies against CTNNB1 (BD Transduction Laboratories, Mississauga, Ontario, #610153), anti-Müllerian hormone (AMH), cytochrome P450, family 17, subfamily a, polypeptide 1 (CYP17A1), Wilms tumor 1 (Santa Cruz Biotechnology, #sc-6886, sc-46081 and sc-846, respectively), PTEN (Cell Signaling, Danvers, MA, #9559) or vimentin (BD Transduction Laboratories, #5550513) in phosphate-buffered saline/0.3% Triton X-100 overnight at 4°C and subsequently probed with secondary Alexa Fluor 594- or 488-conjugated goat anti-rabbit, rabbit anti-goat, rabbit anti-mouse or goat anti-rat IgG antibodies (Molecular probes, Eugene, OR) for 1 h at room temperature. Slides were mounted using VectaShield with 4′,6-diamidino-2-phenylindole (Vector Laboratories).
Immunoblot analysis
Protein extracts from Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ ovaries and testes, Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testes and Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT were prepared using T-Per solution (Pierce, Rockford, IL) as directed by the manufacturer. The recovered supernatant was stored at −80°C until electrophoretic analyses were performed. Protein concentrations were determined by the Bradford method (Bio-Rad protein assay, Bio-Rad Laboratories, Hercules, CA). Samples (50 μg) were resolved by one-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis (12% acrylamide) under reducing conditions and electrophoretically transferred to a polyvinylidene difluoride membrane (GE Amersham, Piscataway, NJ). The membrane was probed with primary antibody against V-Akt murine thymoma viral oncogene homolog 1 (AKT1), phospho-AKT1 (S473), Forkhead box O1A (FOXO1A), phospho-FOXO1A (S256), FKBP12–rapamycin complex-associated protein 1 (FRAP1), phospho-FRAP1 (S2448) (Cell Signaling, #9272, #4051, #9462, #9461, #2972 and #2976, respectively), Forkhead transcription factor FOXL2 (Sigma, St Louis, MO, #F0805) or beta-actin (ACTB) (Santa Cruz Biotechnology, sc-47778) diluted in tris-buffered saline with 0.1% tween 20 containing 5% bovine serum albumin (Jackson ImmunoResearch Laboratories, West Grove, PA) or 5% dry milk. Following incubation with horseradish peroxidase-conjugated secondary goat anti-rabbit or rabbit anti-mouse antibody (GE Amersham, Pittsburgh, PA, #NA934VS and #NA931VS, respectively), the protein bands were visualized by chemiluminescence using the ECL Plus Western Blotting Detection Reagents (GE Amersham) and High-Performance Chemiluminescence film (GE Amersham).
Reverse transcription–PCR and semiquantitative reverse transcription–PCR
Reverse transcription (RT)–PCR to detect expression of wild-type Ctnnb1 transcripts, transcripts derived from the Cre-recombined Ctnnb1tm1Mmt allele and Cre transcripts in testes, testicular tumor samples, ovaries and lungs was done using the Superscript one-step RT–PCR kit (Invitrogen, Burlington, Ontario) on 1 μg RNA samples purified with the RNeasy mini kit (Qiagen, Mississauga, Ontario). Oligonucleotide primers used for Ctnnb1 were ‘Ctnnb1-GF2’ (5′-GGTAGGTGAAGCTCAGCGCAGAGC-3′) and ‘Ctnnb1-AS5’ (5′-ACGTGTGGCAAGTTCCGCGTCATCC-3′). For Cre, primers were ‘Cre-S’ (5′-CTCTGGTGTAGCTGATGATC-3′) and ‘Cre-AS’ (5′-TAATCGCCATCTTCCAGCAG-3′). Cycling conditions were 94°C for 1 min followed by 30 cycles of 94°C for 30 s, 55°C for 1 min and 72°C for 1 min. Semiquantitative RT–PCR to evaluate expression of Wingless-type MMTV integration site family member 4 (Wnt4) and ribosomal protein L19 (Rpl19, control gene) was performed on 1 μg RNA samples purified with the RNeasy mini kit (Qiagen) from Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT and lung, Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testes and Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ testes, ovaries and lung. Oligonucleotides primers used for Wnt4 were Wnt4-S 5′-AGCTGTCATCGGTGGGCAGCAT-3′ and Wnt4-AS 5′-ACTGTCCGGTCACAGCCACACT-3′, and primers for Rpl19 were Rpl19-S 5′-CTGAAGGTCAAAGGGAATGTG-3′ and Rpl19-AS 5′-GGACACAGTCTTGATGATCTC-3′. Cycling conditions were 94°C for 1 min followed by 25 cycles (Rpl19) or 35 cycles (Wnt4) of 94°C for 30 s, 55°C for 1 min and 72°C for 1 min. Preliminary experiments done for Wnt4 and Rpl19 ensured that the cycle numbers selected fell within the linear range of PCR amplification (data not shown). PCR products were separated by electrophoresis on 1.8% tris acetate ethylenediaminetetraacetic acid-agarose gels containing ethidium bromide and photographed under ultraviolet light.
Statistical methods
Effects of genotype on estradiol and testosterone levels were analyzed by unpaired, two-tailed T-tests. P values <0.05 were considered statistically significant. Analyses were done using Prism 4.0a software (GraphPad Software, San Diego, CA).
Results
Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice develop testicular degeneration and metastatic testicular tumors
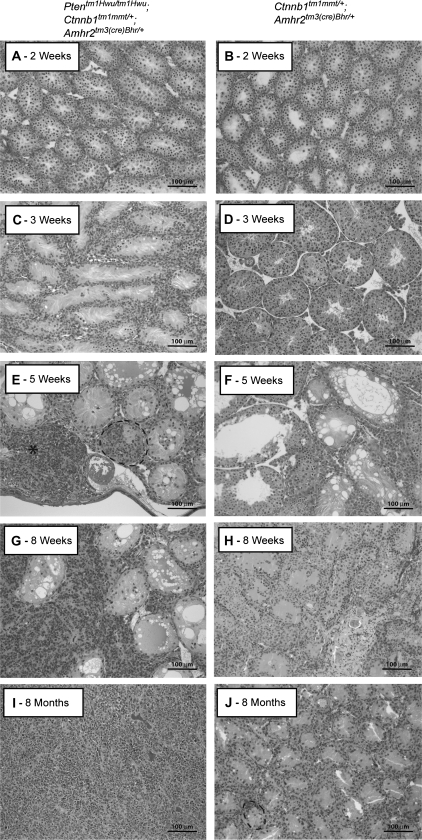
To determine if the PI3/AKT and WNT/CTNNB1 pathways could interact to trigger the formation of neoplasms from the multilayered cell foci observed in the seminiferous tubules of Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals, a mouse model (Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+) was designed to obtain concurrent constitutive activation of both pathways in Sertoli cells. Male Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice were born in the expected Mendelian and male:female ratios and had morphologically normal gonads at birth (data not shown). However, histopathologic evaluation of Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testes during post-natal development revealed clear signs of seminiferous tubule degeneration by 3 weeks of age, as evidenced by the loss of germ cells associated with presence of large clear vacuoles in Sertoli cells (Figure 1C). Atrophy of the seminiferous epithelium and associated germ cell loss then progressed rapidly, leading to the progressive decrease of seminiferous tubule diameter and the disappearance of the tubular lumen within several weeks (Figure 1E and G). These observations were reminiscent of what had been previously observed in Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals (ref. 10 and Figure 1F, H and J), except the onset of the degenerative process occurred earlier in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals (i.e. 3 versus 5 weeks) and progressed more rapidly.
Fig. 1.
Formation of sex cord tumors in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice. (A, C, E, G and I) Photomicrographs demonstrating progressive tumor formation and degeneration of the seminiferous tubules in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice, compared with (B, D, F, H and J) Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice at 2 weeks to 8 months of age. Multilayered, focal accumulations of cells in some seminiferous tubules are circumscribed with black dotted lines. A microtumor is indicated with an asterisk. Original magnification, ×200.
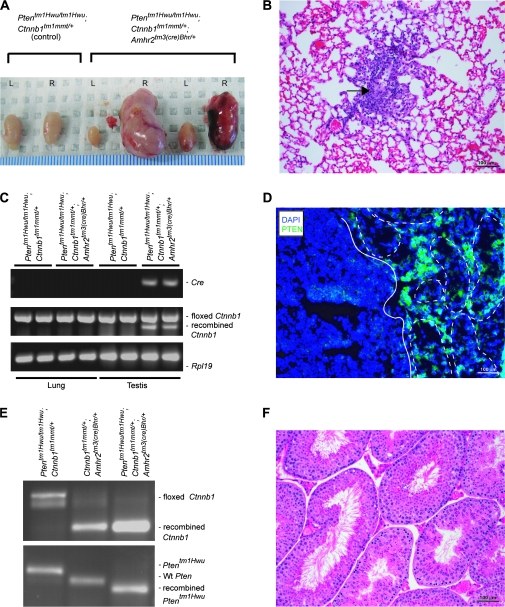
In addition to the atrophy of the tubules, a cell population accumulated in multilayered foci in many seminiferous tubules in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice in a manner similar to what was observed in Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice (ref. 10 and Figure 1E and J). However, unlike the foci that occurred in Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testes, focal cells proliferated rapidly in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals, eventually escaping the confines of the tubules and forming microtumors that were observable in all animals at 5 weeks of age (Figure 1E and G). Invasion and replacement of testicular tissue by proliferating tumor cells (Figure 1G and I) and subsequent tumor growth occurred at a somewhat variable rate. By 3 months of age, ∼70% of animals had grossly evident unilateral testicular tumors, and 30% had bilateral tumors, typically with one tumor being much larger than the other (Figure 2A). By 6 months of age, bilateral tumors were observed in all animals. Although some animals were euthanized for ethical reasons following the development of large tumors, Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice generally remained healthy despite their testicular tumors, and mortality attributable to the tumors was not observed even in mice kept up to the age of 1 year. Methodical histopathologic analyses of tissues from 4-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice revealed the presence of small tumors in the lungs of four of nine animals examined (Figure 2B). These appeared to grow slowly, as larger tumors were not observed in the lungs of older animals. Although the morphological similarity of the lung tumor cells to the testicular tumor cells suggested that the lung tumors were metastases, an additional experiment was conducted to rule out that they were primary lung tumors caused by ectopic expression of Cre. The expression of Cre and transcripts derived from the Cre-recombined Ctnnb1tm1Mmt allele was evaluated in 3-week-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testes and lungs (Figure 2C). Neither Cre nor recombined Ctnnb1tm1Mmt messenger RNA was detected in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ lungs, supporting the conclusion that the tumors observed in the lungs were metastases of the testicular cancer. Predictably, loss of PTEN protein expression, recombination of the floxed Pten alleles and abundant messenger RNA encoding dominant, stable CTNNB1 were observed in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumor samples (Figure 2D and E), associating tumorigenesis with both sustained CTNNB1 signaling and the loss of Pten. Neither degenerative nor abnormal proliferative processes were observed in Ptentm1Hwu/tm1Hwu;Amhr2tm3(cre)Bhr/+ testes at any age (Figure 2F), and these animals were fertile. This result suggests that Pten may not be required for Sertoli cell function in mice and that its loss may be insufficient by itself to induce sex cord/stromal tumor formation.
Fig. 2.
Testicular tumors in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testis. (A) Photograph of testes from 3-month-old Ptentm1Hwu/tm1Hwu; Ctnnb1tm1Mmt/+ (control, left) and Ptentm1Hwu/tm1Hwu; Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice showing unilateral (center) or bilateral (right) tumors. Ruler shown at bottom is in millimeters. (B) Lung metastasis (arrow) in a Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse (original magnification, ×200). (C) RT–PCR analysis of Cre and Ctnnb1 expression in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ lungs compared with Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testis (positive control) and lungs and testis from Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ mice (negative controls). Cre expression and transcripts derived from the Ctnnb1tm1Mmt allele following Cre-mediated recombination were never detected in the lungs of Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals. Rpl19 was used as a housekeeping control gene. (D) Immunofluorescence analyses of PTEN expression (green) in Ptentm1Hwu/tm1Hwu; Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testis. Seminiferous tubules are shown circumscribed with white dotted lines and the tumor is delimited with a white line. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) (original magnification, ×200). (E) RT–PCR analysis of Ctnnb1 expression and PCR analysis of Cre-mediated recombination of the Ptentm1Hwu allele in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT compared with Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testis and Ptentm1Hwu/tm1Hwu; Ctnnb1tm1Mmt/+ testis. The shorter RT–PCR product for Ctnnb1 is produced from transcripts derived from the Ctnnb1tm1Mmt allele following Cre-mediated recombination. PCR products for Pten of ∼572 bp for the floxed allele (Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+), 400 bp for the wild-type Pten allele (Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+) and 290 bp for the Cre-recombined allele (Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+) were detected. (F) Photomicrographs of a testis from a 1-year-old Ptentm1Hwu/tm1Hwu; Amhr2tm3(cre)Bhr/+ mouse (original magnification, ×200).
Testicular tumors in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice are GCTT
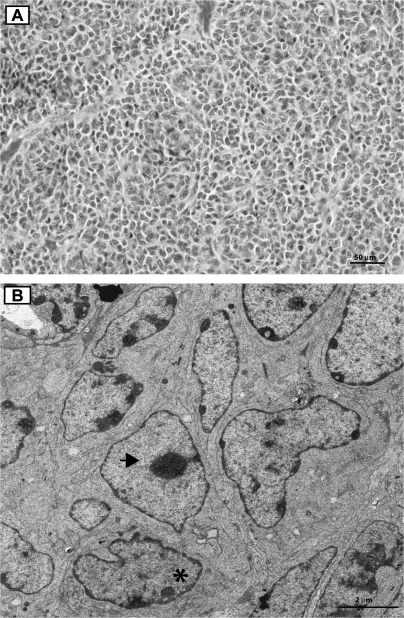
As the Amhr2tm3(cre)Bhr allele directs Cre expression and genetic recombination of the Ctnnb1tm1Mmt allele exclusively in the Sertoli cells of Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice (10), a Sertoli cell tumor phenotype was predicted in the Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ model. However, histopathologic analysis of Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumors revealed morphological characteristics inconsistent with Sertoli cell tumors (26). Notably, tumor cells were not organized into tubular structures, but rather tended to form dense, follicle-like nests (Figure 3A). Furthermore, Ptentm1Hwu/tm1Hwu,Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumor cells were round to pleomorphic with a scant, eosinophilic cytoplasm, unlike tumorous Sertoli cells that are generally elongated with an abundant, vacuolar cytoplasm (26). However, all observed morphological characteristics were consistent with those described for the GCTT (26). Electron microscopy analyses revealed the tumor cells to be polygonal in shape and to measure ∼4 to 6 μm in diameter (Figure 3B). The tumor cells were in close apposition to one another, and several tight junctions and cytoplasmic projections were observed multifocally. The cytoplasm featured small round mitochondria, few parallel arrays of rough endoplasmic reticulum and rare lysosomes and lipid droplets. The nuclei varied in shape and form, measuring between 2 and 4 μm in diameter and were euchromatic with a peripheral margination of nuclear chromatin; they were often deeply indented and occasionally featured prominent nucleoli (Figure 3B). All aforementioned ultrastructural characteristics were consistent with those described in GCTT in humans and mice (27,28).
Fig. 3.
Tumors in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice have histologic characteristics of GCTT. (A) Section of a tumor from a 3-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse showing tumor cell organization into follicle-like nests. Original magnification, ×400. (B) Electron microscopy photomicrograph of a tumor from a 3-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse showing indented nuclei with marginated chromatin (asterisk) and a nucleus with a large nucleolus (black arrow). Original magnification, ×2550.
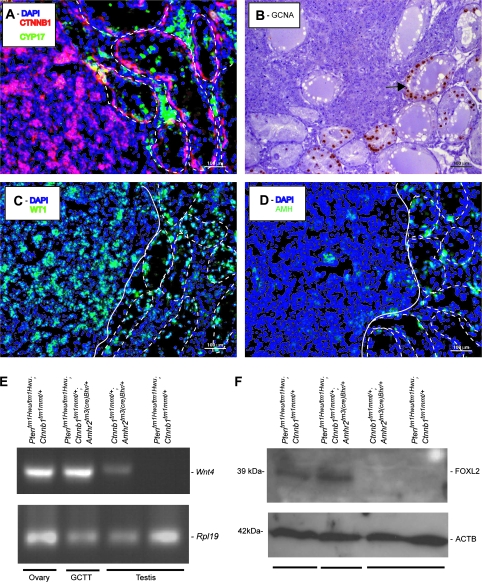
To substantiate the histopathologic diagnosis of GCTT, the expressions of a series of gonadal cell molecular markers were examined. As previously observed in the multilayered cell foci that accumulate in the seminiferous tubules of Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice (10), Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumors expressed neither CYP17 nor germ cell nuclear antigen (Figure 4A and B), indicating that they were neither of Leydig nor of germ cell origin. Rather, the tumor cells were strongly positive for Wilms tumor 1, a marker for the somatic lineage that gives rise to both granulosa and Sertoli cells (Figure 4C). AMH, a marker of both immature Sertoli cells and granulosa cells (29,30), was expressed at low levels, although interspersed foci of higher expression were usually present (Figure 4D). Molecular markers associated with later stages of granulosa cell differentiation, including progesterone receptor, estrogen receptor 1 (data not shown) and CYP19A1 (Figure 5C), were undetectable in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumors, suggesting a relatively undifferentiated granulosa cell phenotype. Consistent with this, 4-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice bearing testicular tumors did not have increased serum levels of testosterone or estradiol relative to age-matched Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ controls (T: 43.03 ± 8.933 versus 46.82 ± 13.26 ng/dl; E2: 25.48 ± 2.852 versus 30.32 ± 3.576 pg/ml, mean ± SEM, n = four to five animals per genotype, P > 0.05), indicating that the tumor cells had not differentiated sufficiently to support steroidogenesis. We therefore examined the expression of two early markers of commitment to the granulosa cell fate. Wnt4 is upregulated in ovarian somatic cells at the time of sex determination (31) and is expressed in granulosa cells at variable levels throughout folliculogenesis (32). By RT–PCR, we detected Wnt4 messenger RNA in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumors at levels comparable with control adult ovaries, but not in control testes (Figure 4E). Interestingly, low levels of Wnt4 expression were also detected in Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testes, suggesting that the sustained activation of the CTNNB1 signal causes testicular somatic cells to commit to the granulosa cell fate (Figure 4E).
Fig. 4.
GCTT in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice express granulosa cell markers. (A, C and D) Immunofluorescence analyses of GCTT in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice. (A) CYP17 (green), CTNNB1 (red), (C) Wilms tumor 1 (WT1) (green), (D) AMH (green). Seminiferous tubules in immunofluorescence photomicrographs are shown circumscribed with white dotted lines and the tumor is delimited with a white line. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (B) Immunohistochemical analysis of germ cell nuclear antigen (GCNA) expression in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT. Arrow indicates a seminiferous tubule in which germ cell nuclear antigen-positive germ cells are present, in contrast with the germ cell nuclear antigen-negative tumor cells (upper left). Original magnification, ×200. (E) Semiquantitative RT–PCR analysis of Wnt4 expression in GCTT from a 3-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse (lane 2) compared with Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ ovary (positive control, lane 1), Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testis (lane 3) and Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ testis (control, lane 4). Rpl19 was used as a housekeeping control gene. (F) Western blot analysis of FOXL2 expression in GCTT from a 3-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse (lane 2) compared with Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ ovary (positive control, lane 1), Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testis (lane 3) and Ptentm1Hwu/tm1Hwu; Ctnnb1tm1Mmt/+ testis (control, lane 4). ACTB was used as a housekeeping control gene.
Fig. 5.
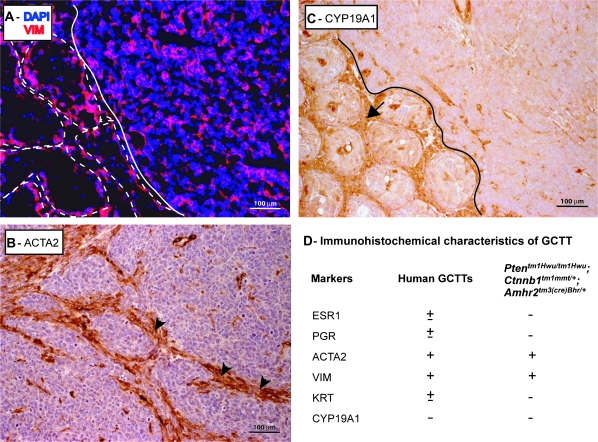
Immunohistochemical analyses of GCTT in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice. (A) Immunofluorescence analysis of vimentin (VIM) expression (red) in GCTT from a 3-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse. Seminiferous tubules are shown circumscribed with white dotted lines and the tumor is delimited with a white line. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) (original magnification, ×200). (B and C) Immunohistochemical analysis of (B) ACTA2 and (C) CYPl9A1 in GCTT from a 3-month-old Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse. Arrowheads indicate expression of ACTA2 localized mainly to cells forming the connective tissue of the tumor. Arrow indicates CYP19A1 expression in Leydig cells, in contrast with the GCTT (upper right, delimited with a black line). Original magnification, ×200. (D) Table comparing the reported immunohistochemical marker expression profile of human GCTT to GCTT from Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice. ‘+’ indicates expressed in all GCTT, ‘±’indicates variable expression, ‘−’ indicates never expressed in GCTT.
The transcription factor FoxL2 is expressed in the pregranulosa cells of newborn mice, and follicle development in FoxL2-null mice is arrested at a very early stage (33). As for Wnt4, we detected FOXL2 protein in the testicular tumors by immunoblotting at levels that were comparable with those expressed in control ovaries (Figure 4F). No FOXL2 expression was detected in control testes, as had been previously reported (34) (Figure 4F). Together, these results indicate that Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testicular tumor cells have morphological and molecular characteristics consistent with a poorly differentiated granulosa cell phenotype.
Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT express immunohistochemical markers characteristic of the human disease
To compare the tumors seen in the Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals with human adult GCTT, a series of immunohistochemical markers were evaluated and compared with the reported human GCTT marker profile (4). Vimentin and ACTA2, which are expressed in all human GCTT (4), were also detected in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT (Figure 5A and B), although ACTA2 expression localized mainly to the tumor connective tissue rather than the malignant granulosa cells (Figure 5B). Other markers that are not expressed or that are expressed at a variable frequency in human GCTT (4) were not detected in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT (Figure 5C and D).
AKT and FOXO1A phosphorylation in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT
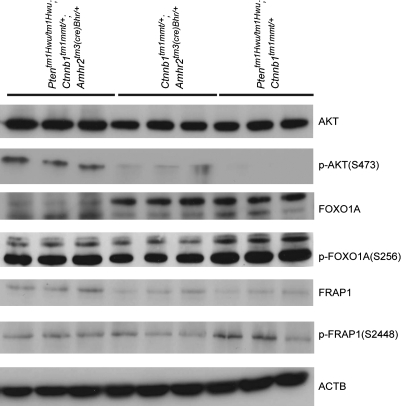
To study the signaling mechanisms underlying GCTT development in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ animals, the expression of various PI3K/AKT pathway effectors was examined. Loss of PTEN activity normally relieves inhibition of PI3K activity, resulting in phosphorylation and activation of AKT (13). As expected, the loss of PTEN expression in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT (Figure 2E) was associated with AKT phosphorylation that was virtually undetectable by immunoblotting in testis samples from Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ or Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ control mice (Figure 6). The phosphorylation status of the AKT targets FOXO1A and FRAP1 (also known as mammalian target of rapamycin) was then examined, as both are believed to function as PI3K/AKT signaling effectors in ovarian granulosa cells (reviewed in ref. 35). FRAP1 phosphorylation was readily detectable in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+; Amhr2tm3(cre)Bhr/+ GCTT, but at levels comparable with control testes samples, suggesting that FRAP1 may not be a major downstream target of AKT in this model (Figure 6). Phosphorylation of FOXO1A was also observed in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT, and levels of phospho-FOXO1A were comparable with those detected in control testes (Figure 6). However, levels of total FOXO1A protein in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT were drastically lower than those observed in testes (Figure 6), and therefore, the relative level of FOXO1A phosphorylation was proportionately much higher in the tumor samples. Taken together, our results suggest that loss of PTEN expression in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT increases PI3K/AKT pathway activity and that the resultant signal is transduced via FOXO1A but not FRAP1.
Fig. 6.
FOXO1A, but not FRAP1, is a target of PI3K/Akt signaling in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT. Western blot analysis of AKT, p-AKT(S473), FOXO1A, p-FOXO1A(S256), FRAP1 and p-FRAP1(S2448) expression in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT (lanes 1–3), Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testis (lanes 4–6) and Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+ (control) testis (lanes 7–9). ACTB was used as a housekeeping control gene.
Discussion
The GCTT are a rare form of cancer for which nothing is known of its molecular etiology. Yet, the elucidation of the mechanisms underlying GCTT development not only provides insight into the biology of testicular sex cord/stromal tumors but may also provide important information regarding the process of sex determination. Sertoli and granulosa cells are believed to originate from the same multipotent progenitor cell type during embryogenesis, and the commitment of the progenitor cells to either the Sertoli or granulosa cell fate is a defining element of sex determination (reviewed in ref. 36). Tumor cells originating from the testis that bear morphological and gene expression characteristics of granulosa cells must therefore be derived either from persistent progenitor cells with a defect in cell fate determination or, perhaps less likely, from Sertoli cells that have been provoked to ‘dedifferentiate’ to the point of acquiring a granulosa cell-like phenotype.
Ample evidence now exists that WNT signaling is a critical regulator of sex determination. Notably, dysregulation of Wnt4 results in anomalies of gonadal and reproductive tract development, and XX Wnt4-null mice have a partial female-to-male sex reversal phenotype (reviewed in ref. 37). R-spondin1 (Rspo1) is a gene that has been identified as a potential regulator of the canonical WNT-signaling pathway and is able to activate CTNNB1 signaling (38). As for Wnt4, loss of Rspo1 in mice results in partial sex reversal (39,40) and human XY patients with chromosomal duplications including both WNT4 and RSPO1 show complete male-to-female sex reversal (41). In addition, the stabilization of CTNNB1 during gonadal development leads to male-to-female sex reversal (42). Consistent with the aforementioned studies, the present report demonstrates that post-natal constitutive stabilization of CTNNB1 results in the commitment of stromal cells to the granulosa cell fate in both Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ testes and in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT. Our findings therefore provide further evidence that WNT signaling induces female sex determination and that its sustained activation can direct the granulosa cell fate even in genetically male cells. Our results also indicate that a genetic lesion that provokes the inappropriate commitment of testicular somatic cells to the granulosa cell fate is a requisite preliminary step in the development of GCTT. We propose that the multilayered foci of somatic cells that accumulate in the seminiferous tubules of Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice could represent primitive granulosa cells and that these foci are premalignant lesions from which GCTT can develop following additional genetic mutations. Consistent with this, we demonstrate herein for the first time that sustained PI3K/AKT-signaling pathway activation can cause the premalignant state to progress to metastatic GCTT. The Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mouse therefore represents the first relevant experimental model for the study of GCTT and provides significant novel insight into its cellular and molecular pathogenesis.
In addition to causing GCTT development, concurrent activation of PI3K/AKT and WNT/CTNNB1 signaling in Sertoli cells also greatly accelerated the testicular degeneration phenotype described previously in Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice (10). The mechanisms underlying this apparent synergy between the pathways remain to be elucidated and may differ between the testicular degeneration and GCTT development phenotypes. Our study of the signal transduction mechanisms downstream of PI3K/AKT revealed a high level of phosphorylation of the AKT target FOXO1A. In addition, FOXO1A protein levels were very low compared with control testicular tissue, suggesting that a decrease in FOXO1A expression may also be involved in GCTT pathogenesis in this model. It has been previously reported that sustained activation of PI3K/AKT signaling can downregulate FOXO1A expression by promoting its proteosomal degradation (43), and therefore, both increased FOXO1A phosphorylation and its decreased expression are consistent with the notion that it transduces the PI3K/AKT signal in Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT. FOXO1A is thought to induce apoptosis in several cell types including cancer cells (44) and to induce G1 arrest by upregulating cyclin kinase inhibitor p27KIP1 (45) and downregulating cyclin D1 and D2 (46). AKT negatively regulates FOXO1A activity via phosphorylation, which results in either its sequestration in the cytoplasm (47) or its degradation by the proteasome machinery (44). It therefore seems reasonable to propose that loss and phosphorylation of FOXO1A in the Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTT model could be a major mechanism by which the premalignant cells acquire an increased rate of proliferation and escape apoptosis and thereby progress to a malignant tumor cell phenotype. We chose to limit our analyses to FOXO1A and FRAP1, as these are the only effectors presently known to transduce physiological PI3K/AKT signals in ovarian granulosa cells (reviewed in ref. 35). However, a large number of AKT substrates have been identified in various cell types that may also play critical roles in tumor development, including Bc12 antagonist of cell death, caspase-9 and mouse double minute 2 homolog (48). Further studies will be required to determine if these or other PI3K/AKT signaling effectors are involved in GCTT development in the Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ model.
We have recently reported that female Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice develop ovarian GCTs that metastasize to the lung (20). While this suggests that the targeted genetic lesions cause the development of the same disease in both males and females, Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ GCTs and GCTTs differ in several important ways. Notably, tumor development is initiated perinatally in females, and the tumors grow much more quickly and are fatal before 9 weeks of age (20). Likewise, the pulmonary metastases are much more frequent and grow more aggressively in female Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice (20). Taken together, these findings indicate that, despite being caused by identical genetic lesions, the GCTs that developed in females were of a more malignant phenotype. This sexual dimorphism may be explained by the fact that the Amhr2tm3(cre)Bhr allele drives Cre expression in the gonadal somatic cells at distinct stages of development in each sex. Indeed, it was shown previously that the Amhr2tm3(cre)Bhr allele can direct Cre activity in embryonic ovaries, whereas activity in the testes can only be weakly detected starting at 7 day post partum, with a large increase at 2 weeks of age (49). Differences in granulosa cell malignancy between male and female Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+; Amhr2tm3(cre)Bhr/+ mice may therefore be the consequence of differences in gonadal stromal cell differentiation at the time of Cre-mediated recombination of the Ctnnb1tm1Mmt allele. Other factors that may influence the sexually dimorphic behavior of the tumors could stem from differences between the ovary and testis. For instance, the structural and hormonal microenvironment of the testis may be less favorable than the ovary to support tumor cell growth, although this does not explain why tumor cells in lung metastases grew more aggressively in female mice than in males, whose lungs presumably provide similar environments for the establishment and growth of metastases. Genetic and/or epigenetic differences between male and female gonadal stromal cells may also create differences in gene expression that could influence the behavior of tumor cells. Importantly, aging Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ females develop GCT (9), whereas testicular tumors were never observed in males of this genotype, despite the presence of premalignant lesions in the gonads of both sexes (10). This suggests that premalignant granulosa cells have a lesser inherent susceptibility to develop into tumors in males, in addition to their aforementioned propensity for developing a less aggressive form of the disease. These observations may help explain the rarity of GCTT in all species in which they have been described (4,50).
In summary, this study reports for the first time that dysregulated WNT/CTNNB1 and PI3K/AKT signaling interact in a synergistic manner to cause GCTT. Ptentm1Hwu/tm1Hwu;Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice represent a model for GCTT that closely resembles the human disease in terms of morphology and marker expression and that will serve to provide the first insights into the pathobiology of this poorly understood disease.
Funding
Discovery Grant from the National Sciences and Engineering Research Council (to D.B.); Canada Research Chair in Ovarian Molecular Biology and Functional Genomics (to D.B.); Canadian Institutes of Health Research (to D.B. and L.H.); a Post-doctoral Fellowship from the Fonds de la Recherche en Santé du Québec (to A.B.); National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction Research) (U54-HD28934); University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Acknowledgments
We thank Ms Céline Forget for technical assistance and mouse colony management, Dr George C.Enders (University of Kansas Medical Center, Kansas City) for providing the germ cell nuclear antigen antibody, Dr Dan Bernard (McGill University, Montreal) for providing the FOXL2 antibody, Dr Richard R.Behringer (University of Texas, Houston) for providing the Amhr2tm3(cre)Bhr/+ mice, Dr Makoto M.Taketo (Kyoto University, Kyoto) for providing the Ctnnb1tm1Mmt/tm1Mmt mice and Mrs Ye Lauren Liu for assistance with electron microscopy analyses.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AMH
anti-Müllerian hormone
- GCT
granulosa cell tumor
- GCTT
granulosa cell tumors of the testis
- GSK3β
glycogen synthase kinase 3β
- PCR
polymerase chain reaction
- PI3K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homolog
- RT
reverse transcription
References
- 1.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, et al. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 3.Dilworth JP, et al. Non-germ cell tumors of testis. Urology. 1991;37:399–417. doi: 10.1016/0090-4295(91)80100-l. [DOI] [PubMed] [Google Scholar]
- 4.Hammerich KH, et al. Malignant advanced granulosa cell tumor of the adult testis: case report and review of the literature. Hum. Pathol. 2008;39:701–709. doi: 10.1016/j.humpath.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Tycko B, et al. The Wnt/beta-catenin pathway in Wilms tumors and prostate cancers. Curr. Mol. Med. 2007;7:479–489. doi: 10.2174/156652407781387118. [DOI] [PubMed] [Google Scholar]
- 6.Segditsas S, et al. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;225:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 7.Van Scoyk M, et al. Wnt signaling pathway and lung disease. Transl. Res. 2008;151:175–180. doi: 10.1016/j.trsl.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Mohinta S, et al. Wnt pathway and breast cancer. Front. Biosci. 2007;12:4020–4033. doi: 10.2741/2368. [DOI] [PubMed] [Google Scholar]
- 9.Boerboom D, et al. Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 2005;65:9206–9215. doi: 10.1158/0008-5472.CAN-05-1024. [DOI] [PubMed] [Google Scholar]
- 10.Boyer A, et al. Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in sertoli cells. Biol. Reprod. 2008;79:475–485. doi: 10.1095/biolreprod.108.068627. [DOI] [PubMed] [Google Scholar]
- 11.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 12.Steck PA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 13.Stiles B, et al. Essential role of AKT-1/protein kinase B alpha in PTEN-controlled tumorigenesis. Mol. Cell. Biol. 2002;22:3842–3851. doi: 10.1128/MCB.22.11.3842-3851.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desbois-Mouthon C, et al. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20:252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- 15.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, et al. GSK3 beta mediates suppression of cyclin D2 expression by tumor suppressor PTEN. Oncogene. 2007;26:2471–2482. doi: 10.1038/sj.onc.1210033. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland DJ, et al. PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer. Oncogene. 2006;25:329–337. doi: 10.1038/sj.onc.1209020. [DOI] [PubMed] [Google Scholar]
- 18.Satyamoorthy K, et al. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer Res. 2001;61:7318–7324. [PubMed] [Google Scholar]
- 19.Wang Y, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 20.Laguë MN, et al. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29:2062–2072. doi: 10.1093/carcin/bgn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groszer M, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 22.Jamin SP, et al. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat. Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 23.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgez CJ, et al. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol. Endocrinol. 2004;18:953–967. doi: 10.1210/me.2003-0301. [DOI] [PubMed] [Google Scholar]
- 25.Grover A, et al. Structural and functional modifications of sertoli cells in the testis of adult follicle-stimulating hormone receptor knockout mice. Biol. Reprod. 2004;71:117–129. doi: 10.1095/biolreprod.103.027003. [DOI] [PubMed] [Google Scholar]
- 26.Mohr U. International Classification of Rodent Tumors. 1st edn. Springer–Verlag, Berlin, Germany, Heidelberg; 2001. p. p474. [Google Scholar]
- 27.Genton CY. Some observations on the fine structure of human granulosa cell tumors. Virchows Arch. A Pathol. Anat. Histol. 1980;387:353–369. doi: 10.1007/BF00454838. [DOI] [PubMed] [Google Scholar]
- 28.Demopoulos RI, et al. Fine structural evidence on the origin of gonadotropin-induced ovarian tumors in mice. Cancer Res. 1981;41:871–876. [PubMed] [Google Scholar]
- 29.Knight PG, et al. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 30.Münsterberg A, et al. Expression of the mouse anti-müllerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113:613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- 31.Brennan J, et al. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat. Rev. Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh M, et al. Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology. 2002;143:898–908. doi: 10.1210/endo.143.3.8684. [DOI] [PubMed] [Google Scholar]
- 33.Uda M, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- 34.Pisarska MD, et al. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology. 2004;145:3424–3433. doi: 10.1210/en.2003-1141. [DOI] [PubMed] [Google Scholar]
- 35.Hunzicker-Dunn M, et al. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell. Signal. 2006;18:1351–1359. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiNapoli L, et al. SRY and the standoff in sex determination. Mol. Endocrinol. 2008;22:1–9. doi: 10.1210/me.2007-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernard P, et al. Wnt4 action in gonadal development and sex determination. Int. J. Biochem. Cell Biol. 2007;39:31–43. doi: 10.1016/j.biocel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Kim KA, et al. R-spondin family members regulate the wnt pathway by a common mechanism. Mol. Biol. Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chassot AA, et al. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum. Mol. Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 40.Tomizuka K, et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum. Mol. Genet. 2008;17:1278–1279. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- 41.Jordan BK, et al. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am. J. Hum. Genet. 2001;68:1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maatouk DM, et al. Stabilization of {beta}-catenin in XY gonads causes male-to-female sex-reversal. Hum. Mol. Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki M, et al. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc. Natl Acad. Sci. USA. 2004;101:13613–13617. doi: 10.1073/pnas.0405454101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl Acad. Sci. USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura N, et al. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramaswamy S, et al. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 47.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 48.Kim D, et al. Akt: versatile mediator of cell survival and beyond. J. Biochem. Mol. Biol. 2002;35:106–115. doi: 10.5483/bmbrep.2002.35.1.106. [DOI] [PubMed] [Google Scholar]
- 49.Arango NA, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol. Reprod. Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 50.Bontempo RA, et al. Metastasising granulosa cell tumour of the testis: a case report in the dog. Vet. Res. Commun. 2005;29(suppl. 2):169–171. doi: 10.1007/s11259-005-0034-0. [DOI] [PubMed] [Google Scholar]