Abstract
We have recently shown that penta-1,2,3,4,6-O-galloyl-beta-D-glucose (PGG), a naturally occurring hydrolyzable gallotannin, inhibited the in vivo growth of human androgen-independent p53-mutant DU145 prostate cancer (PCa) xenograft in athymic nude mice without adverse effect on their body weight. We have also shown that PGG induced caspase-mediated apoptosis in the DU145 cells and the androgen-dependent human p53-wild-type LNCaP cells. Here, we investigated the cell cycle effects of PGG in these and other PCa cells. Our data show that treatment with subapoptotic doses of PGG induced S-arrest, whereas higher doses of PGG induced not only S-arrest but also G1 arrest. We show, for the first time, that irrespective of the p53 functional status of the PCa cell lines, PGG exerted a rapid (within 2 h) and potent inhibition (inhibitory concentration by 50% ∼6 μM) of 5-bromo-2′-deoxyuridine incorporation into S phase cells. In isolated nuclei, PGG inhibited DNA replicative synthesis with superior efficacy than a known DNA polymerase alpha inhibitor, aphidocolin. In addition to the S-arrest action, we have found a close association of downregulation of cyclin D1 with G1 arrest induced by PGG. Overexpressing this G1 cyclin abolished G1 arrest, but hastened the S-arrest induction by PGG. Together, our data indicate that PGG induced PCa S-arrest probably through DNA replicative blockage and induced G1 arrest via cyclin D1 downregulation to contribute to anticancer activity. Our data raise the hypothesis that PGG may be a novel inhibitor of DNA polymerases.
Introduction
Polyphenolic compounds are among the various chemopreventive agents that have shown promise for inhibiting prostate cancer (PCa) in preclinical models (1–3). Penta-1,2,3,4,6-O-galloyl-beta-D-glucose (PGG) (Figure 1A) is a naturally occurring gallotannin polyphenolic compound in oriental herbs such as Galla Rhois, the gallnut of Rhus chinensis Mill and the root of peony Paeonia suffruticosa Andrews. We have recently shown that PGG suppressed the in vivo growth of human DU145 PCa xenografts in nude mice at a daily dose of 20 mg/kg without adverse effect on body weight (4), supporting its in vivo anticancer efficacy and potential usefulness as a PCa chemopreventive and therapeutic agent.
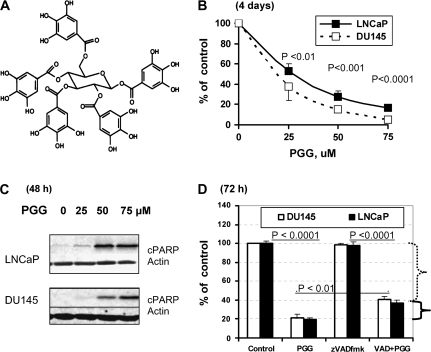
Fig. 1.
Effect of a general caspase inhibitor zVADfmk on the growth inhibitory action of PGG in DU145 and LNCaP cells. (A) Chemical structure of PGG. (B) Overall inhibitory effects on LNCaP and DU145 cell number after 4 days of daily treatment. Expressed as % of control cell set as 100 (mean ± SEM, n = 3 wells of 6-well plates). P values indicate statistical difference from controls. (C) Detection of cleaved poly (ADP-ribose) polymerase in LNCaP and DU145 cells as an indicator of caspase-mediated apoptosis after 48 h of exposure to increasing doses of PGG. (D) Effect of zVADfmk on the growth inhibitory action of PGG (75 μM) in LNCaP and DU145 cells treated in the presence or absence of 40 μM zVADfmk for 3 days without medium change. Expressed as % of control cell set as 100 (mean ± SEM, n = 3 wells of 12-well plates). Difference between/among key groups are indicated by P values.
In the same publication, we investigated the cell death signaling mechanisms induced by PGG in human PCa cells of different p53 functional status (4). We observed caspase-mediated apoptosis in the androgen-dependent human LNCaP cells that express wild-type p53 and in the androgen-independent p53-mutant DU145 cells. In LNCaP cells, caspase-mediated apoptosis induction by PGG was associated with and mediated in major part by activation of p53 as established through small interference RNA knockdown and dominant-negative mutant approaches. Intracellular reactive oxygen species production by PGG was found to be crucial for these molecular and cellular actions. In DU145 cells, which harbor constitutively active signal transducer and activator of transcription 3 (STAT3), caspase-mediated apoptosis induction by PGG was associated with an inhibition of STAT3 Tyr705 phosphorylation and the downregulation of STAT3 transcriptional targets Bcl-XL and Mcl-1. Overexpression of Bcl-XL or knockdown of its binding partner Bak attenuated apoptosis induction. Our published data therefore support the possible involvement of the p53 tumor suppressor activation and inhibition of STAT3 oncogenic signaling in the anticancer effect of PGG.
A limited number of cell culture studies have examined the cell cycle effects of PGG. PGG was shown to induce G1 cell cycle arrest and apoptosis of human HL-60 leukemia cells (5) and human Jurkat T cells (6). Against solid tumor cells, PGG was shown to induce G1 cell cycle arrest in human MCF-7 breast cancer cells (7,8). However, in PCa cells, we documented not only G1 arrest, but also S-arrest (4). The mechanisms of these cell cycle effects in PCa cells have not been investigated.
In this communication, we report, for the first time, a rapid and potent inhibitory activity of PGG toward DNA replicative synthesis as a probably primary mechanism for S-arrest and a downregulation of cyclin D1 for G1 arrest.
Materials and methods
Chemicals and reagents
PGG was isolated from the gallnut of R.chinensis Mill (9). The purity was ∼98%. The general caspase inhibitor (zVADfmk) was purchased from MP-Biomedicals (Aurora, OH). Antibodies including anti-cyclin D1, anti-cyclin dependent kinase (CDK)-2, anti-CDK4, anti-CDK6 and anti-cyclin E were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Additional antibodies, include anti-P21/CIP1 and monoclonal anti-P27/KIP1, were from NeoMarker, Fremont, CA.
Cell culture and treatments
LNCaP and DU145 cell lines were obtained from the American Type Culture Collection, Manassas, VA. LNCaP cells were grown in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) without antibiotics. DU145 cells were grown in eagle's minimum essential medium supplemented with 10% FBS without antibiotics. At 24–48 h after plating, when cells were 50–60% confluence, the medium was changed before starting the treatment with PGG or the other agents. To standardize all PGG/drug exposure conditions, cells were bathed in culture medium at a volume to surface area ratio of 0.2 ml/cm2 (e.g. 15 ml for a T75 flask and 5 ml for a T25 flask). For the experiments in which caspase inhibitor was used, the inhibitor and PGG were given to the cells at the same time. Dimethyl sulfoxide (DMSO, 2 μl/ml or less) was added as a vehicle solvent to the control culture that did not receive the inhibitor. This concentration of DMSO did not cause any adverse morphologic response.
Crystal violet staining
For the evaluation of the effect of caspase inhibitor on overall inhibitory effect of PGG on cell number, the cells were treated with PGG/zVADfmk for 3 days. After treatment, the culture medium was removed and the cells were fixed in 1% glutaraldehyde solution in phosphate-buffered saline (PBS) for 15 min. The fixed cells were stained with 0.02% aqueous solution of crystal violet for 30 min. After washing with PBS, the stained cells were solublized with 70% ethanol. The absorbance at 570 nm with the reference filter 405 nm was evaluated using a microplate reader (Beckman Coulter, Fullerton, CA).
Preparation of nuclei
Nuclei were prepared essentially as described previously (10). DU145 and LNCaP cells (3–4 T75 flasks) at 50% confluence (log phase growth) were trypsinized, pooled and washed once with ice-cold PBS, resuspended in lysis buffer (50 mM Tris–HCl, pH 7.5, 20 mM KCl, 5 mM MgCl2, 0.5 M sucrose and 0.1% Triton X-100) and then incubated on ice for 5 min. After centrifugation (1500g, 5 min, 4°C), pelleted nuclei were washed three times with PBS and resuspended in 0.1 ml of PBS containing 5% DMSO for assay.
In vitro DNA synthesis reaction
In vitro DNA synthesis reaction was performed on isolated nuclei as described with some modification (11). Twenty microliter of nuclei was incubated in a final volume of 50 μl for each sample in 40 mM K–N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.8, 7 mM MgCl2, 3 mM adenosine triphosphate, 0.1 mM each of guanosine triphosphate, cytidine triphosphate, uridine 5′-triphosphate, deoxyadenosine triphosphate, 2′-deoxyguanosine 5′-triphosphate and deoxycytidine triphosphate, 0.25 μM 5-bromo-2′-deoxyuridine 5′-triphosphate (BrdUTP), 0.5 mM dithiothreitol, 40 mM creatine phosphate and 5 μg of phosphocreatine kinase. PGG was added to the reaction mix in 10 μl DMSO of the following concentration: 0 (control); 60 and 150 μM to give rise to final concentration of 10 and 25 μM. The reaction mix was incubated for 2 h at 37°C and stopped by diluting with 450 μl of PBS and nuclei was pelleted (8 min, 2000g) and then resuspended in 100 μl of PBS/0.5% Tween 20/1% bovine serum albumin. Fluorescein isothiocyanate-conjugated 5-bromo-2′-deoxyuridine antibody was used as in the whole-cell protocol for BrdU incorporation detection. As a positive control, we compared PGG with aphidocolin (30 and 50 μM), which is a known DNA polymerase alpha inhibitor [inhibitory concentration 50 (IC50) ∼20 μM, (10,11)].
Immunoblot analyses
The cell lysate was prepared in ice-cold lysis buffer as described previously (12). Immunoblot analyses were essentially as described (12), except that the signals were detected by enhanced chemofluorescence with a Storm 840 scanner (Molecular Dynamics, Sunnyvale, CA).
Results
Cell cycle arrests contributed significantly to the growth inhibitory effect of PGG in different PCa cell lines
We have earlier shown that PGG treatment inhibits the overall growth of LNCaP (P53 wild-type) and DU145 (mutant P53) cells in a concentration-dependent manner following a regimen of daily change of fresh medium (10% FBS) containing PGG for 4 days (4). The IC50 for PGG for these cell lines were ≤25 μM (Figure 1B). We have reported that caspase-mediated apoptosis was the major, almost exclusive, pathway for cell death in both DU145 and LNCaP cells when exposed to 50 μM or higher levels of PGG (Figure 1C) (4). To evaluate the extent of the contribution by cell death to overall growth inhibition, we inhibited the caspases with a general inhibitor zVADfmk at the previously verified effective concentration of 40 μM (4). As shown in Figure 1D for DU145 and LNCaP cells, the inhibition of caspases had only a minor effect of restoring the cell number (e.g. from 80% reduction by PGG alone to 60% reduction of cell number by zVADfmk plus PGG) of the PGG-exposed cells in a 3 day growth assay (without changing medium). Therefore, the data support cell cycle arrests as an important avenue for PGG to decrease overall cell number.
Cell cycle perturbations induced by PGG in PCa cells
To determine the nature of cell cycle arrests, we examined the cell cycle distribution patterns of LNCaP and DU145 cells after exposure to increasing concentrations of PGG for 24 h in serum rich medium (10% FBS) (Figure 2A). At 10 μM PGG, an increase of S phase cells was accompanied by a small or no decrease of G1 cells with a reduction of G2–M phase cells, indicating an S phase arrest at this low concentration of PGG. At PGG exposure concentrations ≥20 μM, an enrichment of G1 cells was evident in both cell lines with further suppression of G2 phase cells, whereas the S phase cells remained unchanged or slightly lower than untreated cells, probably due to fewer cells able to leave G1 phase to enter S phase.
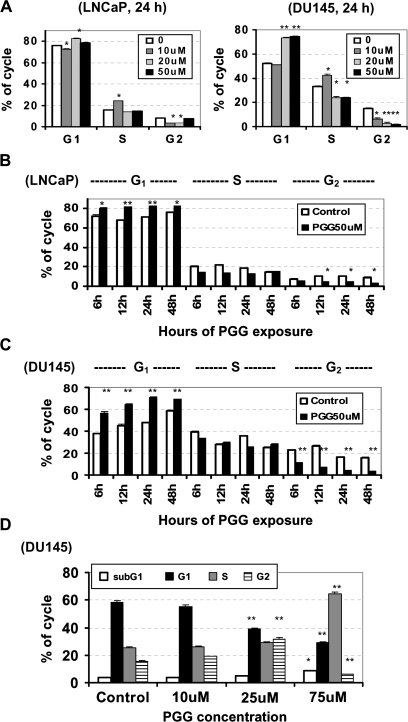
Fig. 2.
The effect of PGG on cell cycle distribution in LNCaP and DU145 PCa cells. (A) Propidium iodide flowcytometric analysis of the cell cycle distribution of LNCaP and DU145 cells exposed to increasing concentration of PGG for 24 h. Values are mean ± SEM, n = 3 T25 flasks. Difference from control *<0.05, **<0.01. (B and C) Propidium iodide flowcytometric analysis of the cell cycle distribution of LNCaP cells (B) and DU145 cells (C) exposed to 50 μM PGG for 6, 12, 24 and 48 h. Values are mean ± SEM, n = 3 T25 flasks. Difference from respective control *<0.05, **<0.01 (D) Propidium iodide flowcytometric analysis of subG1 (apoptotic) and the cell cycle distribution of DU145 cells exposed to increasing concentration of PGG for 24 h and then returned to drug-free medium for another 24 h. Values are mean ± SEM, n = 3 T25 flasks. Difference from respective control *<0.05, **<0.01.
To better define the kinetics of cell cycle arrests, we analyzed cell cycle distribution after 50 μM PGG treatment for different durations. We observed a rapid enrichment of G1 cells at the expense of G2 cells in LNCaP (Figure 2B) and DU145 (Figure 2C) cell lines from 6 to 48 h exposure. During the exposure period, S phase cells held steady or slightly decreased.
To determine whether the action of PGG on cell cycle arrests was transient or sustained, we exposed DU145 cells to increasing concentrations (10, 25 and 75 μM) of PGG for 24 h and then provided drug-free medium for another 24 h. Cell cycle distribution patterns suggested that the PGG-exposed cells were still detained in S (75 μM) or G2–M phases (25 μM) even after 24 h of PGG removal, whereas those exposed to the lowest tested concentration of 10 μM resumed cycling (Figure 2D). These data indicated that PGG inhibited PCa cell proliferation by arresting cells not only in S phase but also in G1 phase, depending on the levels of PGG.
PGG caused a rapid inhibition of DNA replicative synthesis
To probe the nature and possible mechanisms of S phase arrest by PGG, we used BrdU pulse labeling for 30 min before cell harvest to label S phase cells that were actively engaged in DNA replicative synthesis in DU145 and LNCaP cells. As shown in Figure 3A for DU145 cells, there was almost a complete shutdown of DNA synthesis in the PGG-treated cells throughout the S phase after 24 h treatment at 50 μM. Additional experiments showed that the PGG action was very rapid, producing a complete blockade of BrdU incorporation within 2.5 h of exposure to 20 μM PGG, while the S phase cell percentage estimated by propidium iodide staining intensity remained unchanged (Figure 3B). The other noteworthy feature was the high potency for PGG to inhibit BrdU incorporation into S phase cells: >95% inhibition by 10 μM PGG and 40% inhibition by 5 μM PGG for an exposure of 6 h (Figure 3C). The rapid inhibitory effect of PGG on BrdU incorporation was also found in LNCaP cells (Data not shown).
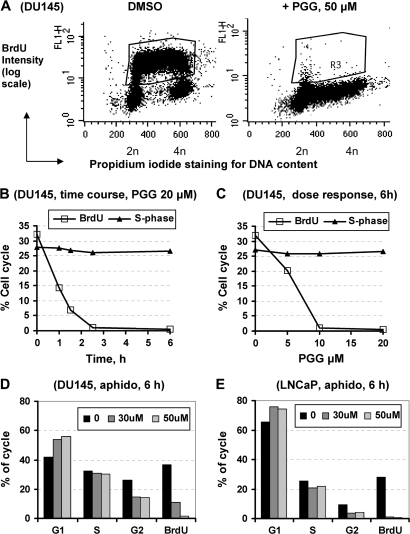
Fig. 3.
BrdU incorporation detection by immunofluorescence in (A) control DU145 cells and cells treated with PGG for 24 h. R3 region encompasses S phase cells that were active in DNA replicative synthesis. (B) Acute time course of PGG (20 μM)-induced inhibition of BrdU incorporation (R3 region) in DU145 cells. (C) Dose-dependent inhibition of BrdU incorporation (R3 region) by PGG exposure for 6 h in DU145 cells. The % of S-phase cells based on DNA content determined by propidium iodide remained unaffected. (D and E) Effects of aphidocolin, a known inhibitor of DNA polymerase alpha, on cell cycle distribution and BrdU incorporation in DU145 cells (D) and LNCaP cells (E). Similar patterns were observed for 12 and 24 h. Each bar represents the average of two T25 flasks, the deviation within 2%.
Because the uniform suppression by PGG of BrdU incorporation throughout S phase (Figure 3A) suggested a probable inhibition of the major replicative DNA polymerases, we compared the cell cycle arrest actions and effect on BrdU incorporation of PGG with a known DNA polymerase alpha inhibitor aphidocolin (10,11) in both DU145 (Figure 3D) and LNCaP cells (Figure 3E) at 6 h of treatment. In both cell types, aphidocolin increased G1 cells and depleted G2–M cells without causing a significant change of S phase cells. In the DU145 cells, PGG was ∼4–5 times more potent than aphidocolin (i.e. 10 μM PGG ≈50 μM aphidocolin) on suppressing BrdU incorporation (Figure 3C versus Figure 3D). Considering the speed and potency of PGG to inhibit BrdU incorporation and the diverse pathogenetic backgrounds of the PCa cell lines, we speculate that a rapid and direct inhibition of DNA replicative synthesis by PGG might be the common underpinning of S-arrest.
PGG inhibited cell-free DNA replicative synthesis in isolated nuclei
Since a number of steps could be affected by PGG in the BrdU incorporation assay, such as the uptake of BrdU into the cells and its metabolism to BrdUTP substrate for DNA polymerases, we next determined the impact of PGG on DNA replicative synthesis in isolated nuclei under the assay condition that provided deoxyribonucleotide (deoxynucleoside 5′-triphosphate) substrates (11), bypassing the needs for cellular uptake and deoxyribonucleotide metabolism.
As shown in Figure 4, PGG incubation with DU145 isolated nuclei for 2 h dose dependently (Figure 4c and d) decreased BrdUTP incorporation (R3 region) from the DMSO control (Figure 4b), with IC50 ∼10–15 μM. The potency of PGG was approximately two times higher than that of aphidocolin (Figure 4e and f). These data therefore support the hypothesis that a direct inhibition of DNA replicative synthesis in S phase cells by PGG, possibly targeting DNA polymerase alpha, could be a primary cause of PGG-induced S-arrest.
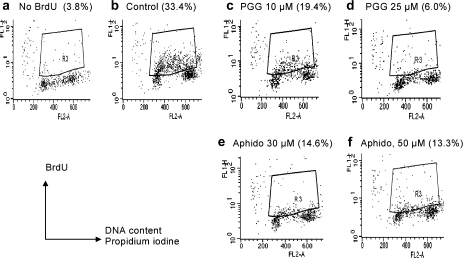
Fig. 4.
The direct effect of PGG or aphidocolin on BrdUTP incorporation into S phase nuclei (in vitro DNA synthesis) of isolated nuclei preparations from DU145 cells. Nuclei were treated with PGG (c and d) or aphidocolin (e and f) for 2 h in the test tubes in the presence of BrdUTP to label those active in replicative synthesis. BrdU detection was carried out by immunofluoresence flowcytometry as for whole cells (Figure 3). LNCaP nuclei showed the same responses.
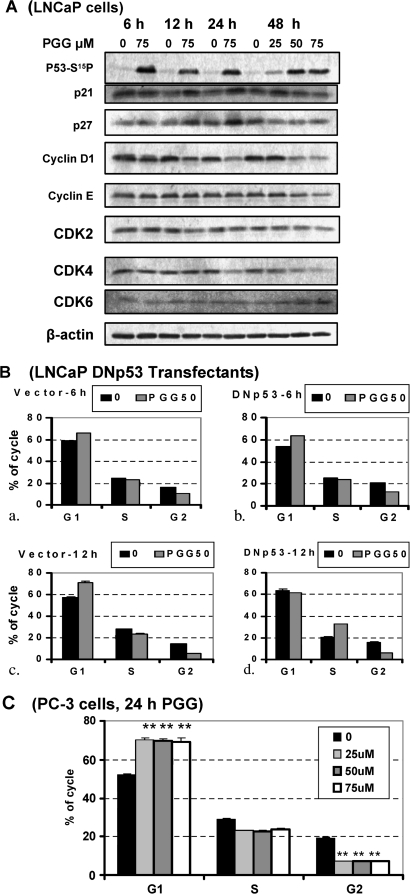
P53-P21Cip1 axis contributed to PGG-induced G1 arrest, but was not obligatory
To define possible molecular mediators for the cell cycle arrests, we used western blot to profile the effects of PGG on the expression level of key cell cycle regulators in time course and dose-response experiments. Since we have reported the rapid activation by PGG treatment of P53 in LNCaP cells (4) and that P53-P21Cip1 axis is best known for mediating G1 arrest by genotoxic stress (13), we therefore focused on their expression changes. We observed an increased expression of CDK inhibitor protein P21Cip1 between 12–24 h of PGG treatment in LNCaP cells, temporally behind p53 phosphorylative activation (Figure 5A) as we reported before (4). Another CDK inhibitor protein P27Kip1 followed a similar pattern of change. Interestingly, we observed a reduction of cyclin D1 (early G1 cyclin) as early as 6 h, persisting throughout 48 h and in a PGG concentration-dependent manner (Figure 5A). In LNCaP cells, no significant change of cyclin E (late G1 and S cyclin) was observed until 48 h. CDK4 expression was decreased by 24 h. There was no decrease of CDK6 for the first 24 h and an increase by 48 h (both CDK4 and CDK6 activated by cyclin D1). CDK2 (activatable by cyclin E) expression was not changed.
Fig. 5.
Role of P53-p21 axis for G1 and S-arrests by PGG. (A) Effect of PGG on expression of phospho-Ser15-p53, P21Cip1 and cyclin D1 and selected cell cycle proteins in LNCaP cells detected by western blot. (B) Comparison of PGG-induced cell cycle arresting effects in dominant-negative (DN) p53 transfectant (P151S) (panels b and d) versus vector-transfectant LNCaP cells (panels a and c). Each value represents the average of two T25 flasks, with deviation range indicated. (C) Propidium iodide flowcytometric analysis of the cell cycle distribution of p53-null PC-3 cells exposed to increasing concentration of PGG for 24 h. Values are mean ± SEM, n = 3 T25 flasks. Difference from control, **<0.01.
Since P21Cip1 is a transcriptional target of p53 (13), we suspected that the activation of p53-p21Cip1 axis by PGG treatment in the LNCaP cells might contribute to the G1 arrest. We tested this by comparing the cell cycle responses of LNCaP cells stably expressing a dominant-negative mutant p53 P151S (4) with vector-transfectant cells (Figure 5B). In the DNp53-cells G1 arrest was observed with PGG treatment at 6 and 8 h (patterns shown for 6 h, Figure 5B, b versus a). By 12 h of exposure to 50 μM PGG, when p21Cip1 level would be increased in the p53-wild-type LNCaP parent cells (Figure 5A), the DNp53-transfectant cells abolished G1 arrest (Figure 5B, d versus c). Instead, more cells arrested in the S phase in comparison with the vector control cells (Figure 5B). These data suggest that the rapid onset of G1 arrest (6–8 h) and the S-arrest were independent of p53 activity, but if p53 was present to activate p21Cip1 transcription, they might contribute to further sustaining G1 arrest. In further support of the non-obligatory need for p53 for G1 and S-arrests by PGG, PGG was capable of inducing sustained G1 arrest and S-arrests in the p53-null PC-3 cells (Figure 5C).
Overexpression of cyclin D1 hastened S-arrest by PGG
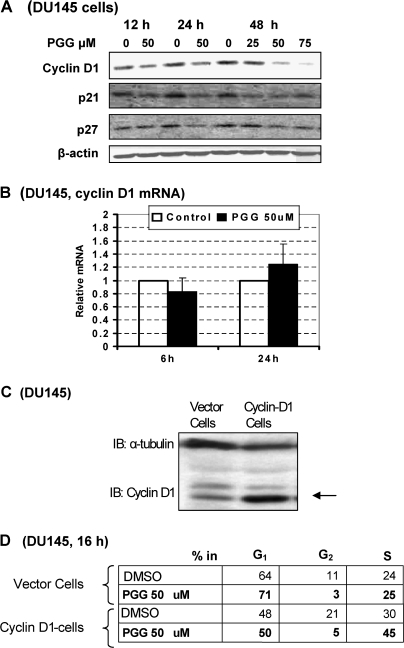
In p53-mutant DU145 cells, PGG treatment did not increase the protein abundance of p21Cip1 or p27Kip1 (Figure 6A), in contrast with LNCaP cells in which PGG induced both proteins after 12 h (Figure 5A). Nevertheless, PGG treatment decreased the expression of cyclin D1 in DU145 cells (Figure 6A). The messenger RNA level for cyclin D1 was not affected by PGG in DU145 cells at either 6 or 24 h of treatment (Figure 6B). The consistent decrease of cyclin D1 in different cell lines after PGG treatment and the inconsistent effects on P21Cip1 and P27Kip1 among the PCa cells of diverse p53 functional status led us to hypothesize that cyclin D1 downregulation was involved in achieving G1 arrest independent of, or in addition to, S-arrest.
Fig. 6.
Effect of PGG on cyclin D1 and P21Cip1 and P27Kip1 expression in DU145 cells and impact of ectopic expression of cyclin D1 on PGG-induced G1 and S-arrest in DU145 cells. (A) Western blot analyses of cyclin D1 and P21Cip1 and P27Kip1 expression. (B) Lack of effect of PGG on cyclin D1 steady state messenger RNA (mRNA) level detected by real-time reverse transcription–polymerase chain reaction. (C) Western blot verification of stable forced overexpression of cyclin D1 in DU145 cells. (D) Cell cycle analyses after 16 h treatment with/without PGG (50 μM) of the vector-transfected cells and cyclin D1-transfected cells. Each value represents the average of two T25 flasks.
To test this idea, we made a stable transfectant of DU145 cells with forced overexpression of cyclin D1 by 5- to 10-fold (wild-type expression plasmid kindly provided by Prof Joshua D.Liao, Hormel Institute) (Figure 6C). Compared with vector-transfectant cells, the D1-overexpressing cells were relieved of PGG-induced G1 arrest, but did not overcome the S-arrest (Figure 6D). In fact, by relieving G1 arrest, the D1-overexpressing cells entered into PGG-induced S-arrest more rapidly as seen by 16 h of PGG treatment (Figure 6D). The data therefore support our notion of separate mechanisms for S versus G1 arrests by PGG.
Discussion
PCa is the most commonly diagnosed cancer that affects one in six American men in their lifetime (14). Notable features of PCa that make chemoprevention a rational and cost effective approach include its high prevalence and long latency between premalignant lesions and clinically evident cancers (15). Because of its high incidence, even a modest reduction in risk can translate into a great number of men spared of PCa in the USA. The long latency provides a wide window of opportunities to prevent and intervene the cancer development process. PGG possesses multiple targeting actions to potentially inhibit prostate carcinogenesis (4). The current work further adds to the mechanistic understanding of how PGG induces PCa cell cycle arrests in S and G1 phases to contribute to the rationale for its merit for PCa chemoprevention.
The most novel aspect of our finding is the rapid and potent inhibitory action of PGG on DNA replicative synthesis as the most probable cause of S phase arrest effect, which has been overlooked by other researchers who reported cell cycle studies with PGG (6–8). We have shown by regular flowcytometry that treatment with subapoptotic doses of PGG induced S-arrest in LNCaP and DU145 cells (Figure 2A). With the help of BrdU incorporation assay and flowcytometry, we showed that irrespective of the p53 functional status and genetic background of the PCa cell lines, PGG exerts a rapid (within 2 h) and potent inhibition (IC50 ∼6 μM) of BrdU incorporation into S phase cells (Figure 3). Using isolated nuclei and BrdUTP as substrate, we showed that PGG inhibited DNA replicative synthesis (Figure 4) with a superior efficacy than the known DNA polymerase alpha inhibitor, aphidocolin. This cell-free assay bypassed a number of cellular processes that could complicate the interpretation of the BrdU incorporation data in whole cells. Furthermore, we observed that aphidocolin essentially replicated the cell cycle arrest patterns and BrdU inhibition in DU145 and LNCaP cells (Figure 3D and E) (data not shown for 12 and 24 h). These data therefore support the hypothesis that PGG may be a novel inhibitor of DNA polymerases, such as DNA polymerase alpha, in PCa cells leading to their replicative blockage. As to the question of whether this action is specific to PCa or applicable to cancer cells of other organ sites, our unpublished data show that several breast cancer cell lines varying in malignancy states all responded in the same manner as the PCa cells, supporting a generalizable effect. Additional work is needed to elucidate which DNA replicative enzymes are targeted by PGG and the detailed inhibition mechanisms.
Although P53 was involved in mediating the caspase-mediated apoptosis events induced by PGG in the LNCaP cells as we reported before (4) and that it contributed to sustaining G1 arrest in LNCaP cells (Figure 5B) probably in conjunction with p21Cip1 induction (Figure 5A), both p53 and p21Cip1 were probably not obligatory for G1 arrest or S-arrest by PGG since we have shown a full competence of p53-null PC-3 cells to display these cell cycle responses (Figure 5C) and the diminution of P21Cip1 abundance in p53-mutant DU145 cells (Figure 6A) while G1 and S-arrests were induced by PGG (Figure 2). Because p53 mutation and inactivation contribute to carcinogenesis and progression in many cancers including PCa (16,17), the potent S and G1 arrest actions of PGG against PCa cells of diverse P53 functional status support its chemopreventive as well as therapeutic application toward a wide spectrum of cancer etiology.
In addition to the potent and novel S-arrest action of PGG, we have established a critical role of cyclin D1 downregulation by PGG for the G1 arrest action. We found a close connection of downregulation of cyclin D1 by PGG with G1 arrest when the exposure concentration of PGG was in the proapoptotic range (Figures 5 and 6). By overexpressing this G1 cyclin that drives CDK4/6 and subsequently phosphorylative inactivation of the Rb protein, we established a causal mediator role of targeting cyclin D1 for G1 arrest by PGG in PCa cells (Figure 6). Additional work with real-time reverse transcription–polymerase chain reaction has shown that the cyclin D1 messenger RNA level was not decreased by PGG treatment either at 6 or 24 h (Figure 6B and data not shown). It is therefore very probably that cyclin D1 downregulation by PGG occurred at the protein level. Further detailed studies are in progress in synchronized cell models to address the mechanisms of cyclin D1 downregulation and its impacts on G1 phase CDKs.
In summary, we showed, for the first time, that PGG could act as a novel inhibitor of DNA replicative synthesis in PCa cells that most probably underlies the rapid and potent S-arrest observed in this study. We also established a causal role of cyclin D1 downregulation by PGG as a primary mechanism for G1 arrest. The current work provides novel insights into how PGG induces PCa cell cycle arrests irrespective of p53 functional status for PCa chemoprevention or treatment. Further studies are warranted to explore the detailed mechanisms of the cell cycle actions and their interrelationships with the diverse molecular targets and pathways.
Funding
Hormel Foundation; National Institutes of Health (CA136953); Korea Ministry of Education, Science and Technology, Medical Research Center grant (R13-2007-019-00000-0).
Acknowledgments
We thank Todd Schuster for performing flowcytometry and BrdU detection and Dr Lei Wang for technical help. We also thank Professor Joshua Liao for generously providing the cyclin D1 expression plasmid.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BrdU
5-bromo-2′-deoxyuridine
- BrdUTP
5-bromo-2′-deoxyuridine 5′-triphosphate
- CDK
cyclin-dependent kinase
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- IC
inhibitory concentration
- PCa
prostate cancer
- PBS
phosphate-buffered saline
- PGG
penta-1,2,3,4,6-O-galloyl-beta-D-glucose
- STAT3
signal transducer and activator of transcription 3
References
- 1.Gupta S, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl Acad. Sci. USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert JD, et al. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am. J. Clin. Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, et al. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr. Relat. Cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, et al. Penta-1,2,3,4,6-O-galloyl-beta-D-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008;7:2681–2691. doi: 10.1158/1535-7163.MCT-08-0456. [DOI] [PubMed] [Google Scholar]
- 5.Pan MH, et al. Induction of apoptosis by penta-O-galloyl-beta-D-glucose through activation of caspase-3 in human leukemia HL-60 cells. Eur. J. Pharmacol. 1999;381:171–183. doi: 10.1016/s0014-2999(99)00549-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen WJ, et al. Induction of G1 arrest and apoptosis in human jurkat T cells by pentagalloylglucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/WAF1, and Bax proteins. J. Biol. Chem. 2004;279:13496–13505. doi: 10.1074/jbc.M212390200. [DOI] [PubMed] [Google Scholar]
- 7.Chen WJ, et al. Induction of G1 phase arrest in MCF human breast cancer cells by pentagalloylglucose through the down-regulation of CDK4 and CDK2 activities and up-regulation of the CDK inhibitors p27(Kip) and p21(Cip) Biochem. Pharmacol. 2003;65:1777–1785. doi: 10.1016/s0006-2952(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 8.Hua KT, et al. Pentagalloylglucose inhibits estrogen receptor alpha by lysosome-dependent depletion and modulates ErbB/PI3K/Akt pathway in human breast cancer MCF-7 cells. Mol. Carcinog. 2006;45:551–560. doi: 10.1002/mc.20226. [DOI] [PubMed] [Google Scholar]
- 9.Huh JE, et al. Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis. 2005;26:1436–1445. doi: 10.1093/carcin/bgi097. [DOI] [PubMed] [Google Scholar]
- 10.Ikegami S, et al. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978;275:458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- 11.Borel F, et al. Prolonged arrest of mammalian cells at the G1/S boundary results in permanent S phase stasis. J. Cell Sci. 2002;115:2829–2838. doi: 10.1242/jcs.115.14.2829. [DOI] [PubMed] [Google Scholar]
- 12.Jiang C, et al. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61:3062–3070. [PubMed] [Google Scholar]
- 13.Dulic V, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 15.Klein EA. Chemoprevention of prostate cancer. Annu. Rev. Med. 2006;57:49–63. doi: 10.1146/annurev.med.57.121304.131435. [DOI] [PubMed] [Google Scholar]
- 16.Hollstein M, et al. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 17.Olivier M, et al. TP53 mutation spectra and load: a tool for generating hypotheses on the etiology of cancer. IARC Sci. Publ. 2004:247–270. [PubMed] [Google Scholar]