Abstract
The matrix metalloproteinase (MMP) family degrade extracellular matrix and mediate pathways including apoptosis, angiogenesis and immunity. We studied the association between four MMP polymorphisms within three MMP genes and esophageal adenocarcinoma (EA) risk and prognosis. A total of 313 EA cases and 455 age and gender frequency-matched controls were genotyped for MMP1 1G/2G, MMP3 6A/5A, MMP12 −82A/G and MMP12 1082A/G. The association between individual MMP polymorphisms and EA risk was evaluated using regression models and adjusted for age, gender, adult body mass index and smoking status. Haplotype analysis was performed to investigate the combined effect of all four linked MMP polymorphisms and EA risk. The MMP1 and MMP3 polymorphisms were associated with increased EA risk: MMP1 1G/2G and 2G/2G had adjusted odds ratios of 1.46 [95% confidence interval 1.0–2.1; P = 0.04] and adjusted odds ratio 1.83 (1.2–2.8; P = 0.005), respectively, whereas MMP3 6A/5A had adjusted odds ratio 1.40 (95% confidence interval 1.0–2.1; P = 0.09) and MMP3 5A/5A had 1.61 (95% confidence interval 1.0–2.5; P = 0.03). Two MMP haplotypes [MMP1–MMP3–MMP12 (−82) 2G-5A-A (adjusted odds ratio 1.36, 95% confidence interval 1.0–1.8; P = 0.03) and 2G-5A-G (adjusted odds ratio 1.70, 95% confidence interval 1.1–2.6; P = 0.01)] were also associated with increased EA risk. The relationship between BE cases with the same set of controls was similar. No association was identified between the MMP polymorphisms and overall survival or progression free survival of patients with EA. MMP1, MMP3 and possibly MMP12 −82A/G polymorphisms and their haplotypes are associated with increased EA risk.
Introduction
Esophageal adenocarcinoma (EA) is a rare malignancy, but the incidence is rising rapidly. This is particularly evident among white men, in whom the reported average yearly increase in incidence exceeds 20% in some countries (1). Barrett's esophagus is a precursor to EA (2). In addition, smoking, obesity and gastroesophageal reflux disease (GERD) incur additional risk (3,4). However, since only a minority of patients diagnosed with Barrett's esophagus will develop EA, other factors, such as genetics, may play additional roles in disease pathogenesis (5).
The matrix metalloproteinase (MMP) family has diverse substrates. Functionally, they are best characterized for degrading the extracellular matrix and basement membrane, promoting invasion and metastastic spread of transformed cells (6). However, members of the MMP family are also mediators of apoptosis, angiogenesis, cell adhesion, cell signaling and immune response to malignancy (6). In esophageal cancer, MMP expression has been correlated with poor outcome (7,8), and in Barrett's esophagus, MMP expression has been shown to be upregulated (9,10), indicating this may be an important event in the progression of Barrett's metaplasia to EA.
Functional polymorphisms within MMP1, MMP3 and MMP12 are associated with an increased risk of developing a range of malignancies. MMP1 has a common deletion/insertion polymorphism within the gene promoter (MMP1 1G/2G, rs1799750). Insertion of a guanine (MMP1 2G) upregulates MMP1 transcription (11) and is associated with increased risk of lung (12), colorectal (13) and nasopharyngeal cancer (14). Similarly, the MMP3 5A genotype of MMP3 6A/5A (rs3025058) has greater gene expression relative to the 6A genotype (15) and is linked with lung cancer risk (16), but may also moderate the risk of the MMP1 1G/2G genotype. MMP12 has two putatively functional single-nucleotide polymorphisms. The variant allele of MMP12 −82A/G (rs2276109) increases activator protein 1 binding and MMP12 expression (17). A second single-nucleotide polymorphism, MMP12 1082A/G (rs652438), results in an asparagine to serine substitution at the coding region for the hemopexin domain, which is cleaved leaving the catalytic domain of MMP12. The functional significance of MMP12 1082A/G is unclear, but has been correlated with outcome in breast cancer (18). However, the impact of MMP1, MMP3 and MMP12 polymorphisms on EA risk has not been evaluated previously.
We postulated that the MMP1 2G, MMP3 5A, MMP12 −82G and MMP12 1082G alleles associated with increased gene expression or transcription are associated with elevated EA risk and may have prognostic significance in EA patients.
Materials and methods
Study population
Details of the case and control population have been described previously (19,20). In summary, incident cases of histologically confirmed EA were recruited from two affiliated hospitals within the same catchment area: Massachussetts General Hospital, Boston, MA, between 1999 and 2005 and Dana-Farber Cancer Institute between 2003 and 2005. The control population was recruited in the same period. Of the control population, 47% were healthy friends and 53% were non-blood relatives of cardiothoracic patients, who mostly were being seen for lung lesions/cancers or cardiac conditions; none of patients attached to the friends/spouses had esophageal cancer (21). To be eligible, controls had to be healthy with no prior history of cancer, GERD or Barrett's esophagus. Controls were age and gender frequency matched to cases. After enrollment, cases and controls completed a questionnaire, and a sample of whole blood was drawn for genotyping. In an exploratory subgroup analysis, the association between MMP genotype and risk of Barrett's esophagus was evaluated. Cases of Barrett's esophagus were enrolled from the same institutions between 1999 and 2005. The study was approved by the Human Subjects Committee of Massachussetts General Hospital, Dana-Farber Cancer Institute and Harvard School of Public Health (Boston, MA) and the Research Ethics Board of Princess Margaret Hospital (Toronto, Ontario, Canada).
Epidemiologic and clinical questionnaire
Clinical and demographic data were collected from cases and controls in the form of a questionnaire performed in person by a trained interviewer. The demographic data included: weight as young adult (defined as weight in their third decade of life); adult height; gender; ethnicity; smoking and alcohol history (smoking and alcohol history taken at a time point 1 year prior to diagnosis or interview) and a history of GERD symptoms (up to 1 year prior to diagnosis/questionnaire). Alcohol intake was dichotomized by drinking more than two drinks per year for any year of their life (i.e. considered non-drinker otherwise). Young adult body mass indices (BMIs) were calculated based on weight as a young adult and adult height in part because the obesity risk had a latency period of >20 to 30 years. We also collected data on BMI at study entry, but these data were non-representative since most cases had lost weight as a symptom of their disease. Weight data were not collected at other points in a person's lifetime.
Genotyping
Whole blood for genotyping was drawn at the time of enrollment. DNA was extracted using the Puregene DNA isolation Kit (Gentra Systems, Minneaplois, MN), and four MMP polymorphisms MMP1 1G/2G, MMP3 6A/5A, MMP12 −82A/G and MMP12 1082A/G were genotyped using a commercial TaqMan assay in a 384-well ABI 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Primers and probe sequences are available on request and were obtained from Assays-by-Demand (Applied Biosystems Inc, Foster City CA).
Quality control
Quality control measures for data collection and genotyping were incorporated from the time of study initiation. Trained personnel around the time of entry into the study administered questionnaires. Questionnaire data were entered into an electronic database, with 10% double entered. Missing questionnaire data or data requiring clarification were sought by a follow-up telephone interview.
Blood for genotyping was stored in individual tubes, at −76°C, in an alarmed, locked freezer until time of use. All genotyping was performed blinded to clinical information and two investigators checked all results. Genotyping was repeated in a random 15% of samples and in all cases of equivocal, failed or discrepant results. Failed, equivocal or discrepant genotyping was repeated two times before excluding the case from the analysis. A third investigator arbitrated any remaining discrepant results. Genotyping data were double entered onto a computerized spreadsheet and compared with any discrepancies crosschecked using raw data results.
Statistical analysis
Chi-squared tests were utilized to determine deviation from Hardy–Weinberg equilibrium (HWE) in cases and controls separately. Case and control demographic data were compared using Pearson's chi-square test and the Wilcoxon rank tests, where appropriate. Unconditional logistic regression models were used to compare genotype and EA risk, adjusting for age, gender and any significant clinical covariates that remained in stepwise models, using P < 0.10 as the cutpoint. These significant clinical covariates were smoking status (never, former and current smoker) and young adult BMI, while other variables such as alcohol and smoking pack-years were removed during the stepwise regression. Sensitivity testing was performed for race, given that >96% of all cases were Caucasians. Haplotype analysis was performed to assess the combined effects of four MMP polymorphisms together. Haplotype frequencies were estimated using SAS macro HAPPY; haplotypes with frequencies ≥5% were included in logistic regression analyses. SAS version 9.1 (Cary, NC) or greater was used for all analyses. The association between genotype and overall survival (OS) and progression free survival for each individual MMP polymorphism was investigated using the methods of Kaplan–Meier and log-rank. Cox proportional hazard ratios were adjusted for gender, age, performance status and disease stage.
Results
Case and control characteristics and genotype
The participation rate for eligible cases and controls were both >85%. For cases, the disease stage distribution was as follows: stage I, 22 (7%); stage IIA, 69 (22%); stage IIB, 55 (18%); stage III, 79 (26%); stage IVA, 27 (9%); stage IVB, 57 (18%) and unknown 4 (1%). Table I summarizes the characteristics of EA cases (n = 313) and matched controls (n = 455). GERD, defined as the presence of reflux or heartburn symptoms at least once a month for at least 6 months period, was reported in 49% of cases. None of the controls described any GERD symptoms. A prior history of smoking and higher young adult BMI was more common among cases compared with controls (P = 0.0003 and P = 0.0004, respectively). We found no effect of race in any of our analyses because of the overwhelming proportion of Caucasians in our sample: excluding Caucasians or adjusting them in the model resulted in minimal changes in the adjusted odds ratios for each of the main effects at the third significant digit only. For example, the adjusted odds ratio for MMP1 2G allele using an additive model was 1.34 (1.1–1.7) in all-comers, 1.33 (1.1–1.7) when restricted to Caucasians and 1.34 (1.1–1.7) when adjusting for race (Caucasians versus non-Caucasians); results for other main effects were similarly unchanged by race.
Table I.
Demographic characteristics and MMP polymorphism frequencies for cases (n = 313) and controls (n = 455)
| Characteristic | Cases | Controls | P-value |
| Gendera | |||
| Female | 11% | 13% | 0.40 |
| Male | 89% | 87% | |
| Age years (range)b | 64 (21–91) | 64 (19–96) | 0.07 |
| Ethnicitya | |||
| Caucasian | 98% | 98% | 0.65 |
| Other | 2% | 2% | |
| Smoking statusac | |||
| Non-smoker | 20% | 32% | 0.0003 |
| Ex-smoker | 55% | 51% | |
| Current smoker | 25% | 17% | |
| Median pack-years of smoking (range) in ever smokersb | 34 (0.2–212) | 30 (0.1–218) | 0.42 |
| Median young adultd BMI (Kg/m2) (range)b | 23 (15–37) | 22 (14–36) | 0.0004 |
| Alcohol intakea | |||
| Never | 11% | 18% | 0.01 |
| Ever | 89% | 82% | |
| MMP1 1G/2Gac | |||
| 1G/1G | 25% | 33% | 0.02 |
| 1G/2G | 49% | 46% | |
| 2G/2G | 26% | 20% | |
| MMP3 6A/5Aa | |||
| 6A/6A | 19% | 26% | 0.07 |
| 6A/5A | 54% | 51% | |
| 5A/5A | 27% | 23% | |
| MMP12 −82A/Gac | |||
| A/A | 77% | 82% | 0.07 |
| A/G | 22% | 17% | |
| G/G | 2% | 1% | |
| MMP12 1082A/Gac | |||
| A/A | 88% | 89% | 0.63 |
| A/G | 12% | 10% | |
| G/G | 1% | 1% |
Cases and controls were compared using Pearson's Chi-squared tests.
Wilcoxon rank test.
Does not add up to 100 due to rounding.
In third decade of life (e.g. twenties).
Frequency of MMP1, MMP3 and MMP12 polymorphisms
Genotyping was unsuccessful or indeterminate for MMP3 5A/6A in seven controls and three cases, for MMP12 −82A/G in six controls and two cases and for MMP12 1082A/G in one case. The frequency of the homozygous wild-type, heterozygous and homozygous variant genotypes for each of the four MMP polymorphisms evaluated in the cases and controls are summarized in Table I. Cases were in HWE. In the control cohort, MMP1 1G/2G, MMP3 6A/5A and MMP12 −82A/G were in HWE (P > 0.10 for each comparison). MMP12 1082A/G was just barely out of HWE (P = 0.04); thus, haplotype analyses excluded this last polymorphism. There was a higher frequency of MMP1 1G/2G and MMP1 2G/2G genotypes among cases (P = 0.02).
Association between individual MMP polymorphisms and EA risk
After adjustment for age, gender, smoking status and young adult BMI, the heterozygous and homozygous variant genotypes of MMP1 1G/2G and MMP3 6A/5A were individually associated with an increased EA risk (Table II). Using an alternative approach that assesses the effect of each copy of the variant allele (a dose-response or additive model), the variant alleles of both MMP1 and MMP3 polymorphisms, along with the G allele of MMP12 −82A/G were each individually associated with increased EA risk (Table II). No association was identified between MMP12 1082A/G and EA risk.
Table II.
Association between MMP polymorphisms and risk of esophageal adenocarcinoma
| Genotype | Odds ratio (95% confidence interval); P-value |
|||
| Model that considers homozygous and heterozygous variants independently |
Model that evaluates dose effect of variant allele (additive model) |
|||
| Crude | Adjusteda | Crude | Adjusteda | |
| MMP1 | ||||
| 1G/1G | 1.00 | 1.00 | ||
| 1G/2G | 1.44 (1.0–2.0); 0.04 | 1.46 (1.0–2.1); 0.04 | ||
| 2G/2G | 1.76 (1.2–2.6); 0.006 | 1.83(1.2–2.8); 0.005 | ||
| 1G allele | 1.00 | 1.00 | ||
| 2G allele | 1.33 (1.1–1.6); 0.005 | 1.34 (1.1–1.7); 0.004 | ||
| MMP3 | ||||
| 6A/6A | 1.00 | 1.00 | ||
| 6A/5A | 1.45 (1.0–2.1); 0.05 | 1.40 (1.0–2.1); 0.09 | ||
| 5A/5A | 1.62 (1.1–2.5); 0.03 | 1.61 (1.0–2.5); 0.03 | ||
| 6A allele | 1.00 | 1.00 | ||
| 5A allele | 1.27 (1.0–1.6); 0.03 | 1.27 (1.0–1.6); 0.04 | ||
| MMP12 −82A/G | ||||
| A/A | 1.00 | 1.00 | ||
| A/G | 1.37 (1.0–2.0); 0.09 | 1.37 (0.9–2.0); 0.10 | ||
| G/G | 3.11 (0.8–12.6); 0.11 | 2.89 (0.7–12.0); 0.14 | ||
| A allele | 1.00 | 1.00 | ||
| G allele | 1.45 (1.0–2.0); 0.03 | 1.44 (1.0–2.0); 0.04 | ||
| MMP12 1082A/G | ||||
| A/A | 1.00 | 1.00 | ||
| A/G | 1.18 (0.7–1.9); 0.47 | 1.27 (0.8–2.0); 0.33 | ||
| G/G | 0.59 (0.1–3.1); 0.53 | 0.64 (0.1–3.4); 0.60 | ||
| A allele | 1.00 | 1.00 | ||
| G allele | 1.06 (0.7–1.6); 0.78 | 1.12 (0.7–1.7); 0.58 | ||
Adjusted for age, gender, smoking status (current smokers, ex-smokers and pack-years) and young adult BMI.
Exploratory subgroup analysis
To investigate the biologic significance MMP genotype further, an exploratory subgroup analysis was undertaken in a cohort of Barrett's disease cases (n = 99). The cohort of Barrett's disease cases were older (P = 0.02), had a higher BMI at 18 years (0.0008), and there were more females in the cases than controls (P = 0.0005). Table III summarizes the associations between MMP polymorphisms and risk of Barrett's disease. The variant allele of MMP1 1G/2G and MMP12 −82A/G in the additive model were associated with an increased Barrett's esophagus risk. No associations were identified for MMP3 6A/5A and MMP12 1082A/G.
Table III.
Association between MMP polymorphisms and risk of Barrett's esophagus
| Genotype | BE odds ratio (95% confidence interval); P-value |
|||
| Model that considers homozygous and heterozygous variants independently |
Model that evaluates dose effect of variant allele (additive model) |
|||
| Crude | Adjusteda | Crude | Adjusteda | |
| MMP1 | ||||
| 1G/1G | 1.00 | 1.00 | ||
| 1G/2G | 2.21 (1.26–3.86); 0.005 | 2.28 (1.27–4.11); 0.006 | ||
| 2G/2G | 1.91 (0.98–3.72); 0.06 | 2.21 (1.08–4.53); 0.03 | ||
| 1G allele | 1.00 | 1.00 | ||
| 2G allele | 1.38 (1.02–1.87); 0.04 | 1.50 (1.07–2.09); 0.02 | ||
| MMP3 | ||||
| 6A/6A | 1.00 | 1.00 | ||
| 6A/5A | 1.39 (0.79–2.43); 0.25 | 1.46 (0.81–2.64); 0.20 | ||
| 5A/5A | 1.33 (0.69–2.54); 0.39 | 1.37 (0.68–2.74); 0.38 | ||
| 6A allele | 1.00 | 1.00 | ||
| 5A allele | 1.15 (0.84–1.57); 0.41 | 1.34 (0.90–2.00); 0.14 | ||
| MMP12 −82A/G | ||||
| A/A | 1.00 | 1.00 | ||
| A/G | 1.53 (0.90–2.60); 0.12 | 1.69 (0.96–2.96); 0.06 | ||
| G/G | 5.06 (1.00–25.54); 0.05 | 4.47 (0.72–27.59); 0.11 | ||
| A allele | 1.00 | 1.00 | ||
| G allele | 1.66 (1.00–2.77); 0.05 | 1.79 (1.04–3.09); 0.04 | ||
| MMP12 1082A/G | ||||
| A/A | 1.00 | 1.00 | ||
| A/G | 1.12 (0.56–2.26); 0.75 | 1.26 (0.60–2.62); 0.54 | ||
| G/G | To few to analyze | To few to analyze | ||
| A allele | 1.00 | 1.00 | ||
| G allele | 1.01 (0.51–2.02); 0.98 | 1.16 (0.56–2.40); 0.69 | ||
Adjusted for age, gender, smoking status (current smokers, ex-smokers and pack-years) and young adult BMI
Haplotype analysis
Haplotype analysis was performed to investigate the effect of all four linked MMP polymorphisms combined and EA risk. Five haplotypes of MMP1 1G/2G–MMP3 6A/5A–MMP12−82A/G had a frequency of >5% and were included in the same model that evaluated probabilities for these haplotypes, using the most common haplotype as reference (Table IV). Of these, 2G-5A-A and 2G-5A-G were associated with increased risk of esophageal cancer compared with the wild-type haplotype after adjustment for age, gender, smoking status and young adult BMI (Table IV).
Table IV.
Association between MMP haplotypes and risk of esophageal adenocarcinoma
| Haplotypes are presented in this order: MMP1 1G/2G; MMP3 6A/5A; MMP12 −82A/G | Frequency (%) | Adjusted odds ratioa (95% confidence interval); P-value |
| 1G-6A-A | 36 | 1.00 (reference) |
| 1G-5A-A | 14 | 0.93 (0.6–1.3); 0.63 |
| 2G-5A-A | 22 | 1.37 (1.0–1.8); 0.03 |
| 2G-5A-G | 9 | 1.72 (1.1–2.6); 0.01 |
| 2G-6A-A | 12 | 0.89 (0.6–1.3); 0.56 |
Adjusted for age, gender, smoking status (current smokers, ex-smokers and pack-years) and young adult BMI. Only haplotypes with frequencies >5% were included. The analysis consisted of a single logistic regression model utilizing the probabilities of haplotype combinations.
Multiple comparisons
After Bonferroni adjustment for the four individual and one haplotype main effects for these MMP genes, the MMP1 2G/2G genotype and the 2G-5A-G haplotype remained significantly associated with elevated EA risk.
Association between MMP genotype and esophageal adenocarcinoma OS
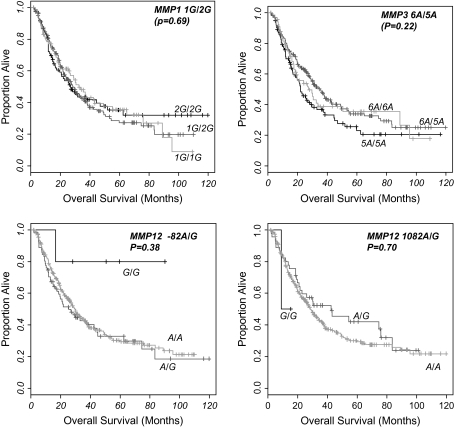
The median OS for a cohort of 292 patients with outcome data was 29 months with a median follow-up time of 22 months. No association between the individual MMP genotype and OS was identified (Figure 1). For MMP 1 1G/2G after adjustment for gender, performance status, age and disease stage, the OS-adjusted hazard ratio for heterozygous and homozygous variant genotype were 0.93 (0.64–1.35; P = 0.69), 0.89 (0.57–1.37; P = 0.59), and for MMP3 6A/5A adjusted hazard ratio OS was 0.84 (0.54–1.30; P = 0.44) and 1.18 (0.73–1.89; P = 0.50), respectively. Results for progression free survival were similar.
Fig. 1.
Kaplan–Meier curves of MMP 1G/2G, MMP3 6A/5A, MMP12 −82A/G and MMP12 1082A/G and esophageal adenocarcinoma OS. Log-rank P-values displayed.
Discussion
This is the first published study to evaluate the association between MMP polymorphisms and EA risk. Results of this study support the strongest association with the MMP1 2G allele conferring greater risk of EA, followed by the MMP3 5A and MMP12 −82 G alleles. These results are further confirmed in haplotype analyses. The MMP relationships with risk were still present after adjusting for age, gender and smoking status and young adult BMI. Despite associations with risk, MMP polymorphisms were not associated with disease outcome or prognosis.
Our primary results are consistent with both the reported functional change associated with these MMP polymorphisms (11,15,17) and case–control studies investigating the association between these polymorphisms and risk of malignancies of the aero-digestive tract (13,14,16,22,23). Zhu et al. (22) and Fang et al. (16) demonstrated that MMP1 2G/2G and MMP3 6A/5A, respectively, were associated with increased lung cancer risk. In the former study, the risk was greater in smokers, whereas the risk was restricted to smokers in the latter, suggesting a possible polymorphism–smoking interaction. In the present study, we found no evidence of such an interaction, though the modest sample size prevents us from making further conclusions. Similar primary risk associations with MMP polymorphisms have also been reported in head and neck (14,23) and colorectal cancer (13). However, results have not been consistent across all studies. In a large case–control study of 1752 cases of lung cancer and 1363 controls, no overall association was found between lung cancer risk and MMP1 1G/2G, except in male non-smokers (12). The authors concluded that the impact of MMP expression might be attenuated by the expression of tissue inhibitors of MMP, which are altered by smoking. Associations between MMP1, MMP3 and MMP12 polymorphisms and EA have not been reported previously.
EA is believed to arise from the chronic exposure of the distal esophagus to reflux of gastric contents, resulting in chronic inflammation, epithelial metaplasia and ultimately invasive adenocarcinoma. The molecular aberrations underlying this progression are incompletely understood; however, the small number of patients with Barrett's esophagus that develop EA is suggestive of either a genetic or a gene-environmental effect. Tissue invasion and metastases is one of the hallmarks of malignant transformation (24). In mouse models, overexpression of MMPs (including MMP1) is associated with increased susceptibility to tumor development (25). While polymorphisms within MMP genes may incur subtle changes of MMP expression, we postulate that in the setting of chronic inflammation, the combined effect of an at risk genotype within cells of the esophageal mucosa, extracellular matrix and inflammatory pathway may favor malignant transformation. Unfortunately, we did not have sufficient tumor samples to evaluate MMP protein expression to evaluate this further. In addition, smoking has been shown to increase MMP12 expression within macrophages, which may compound the risk for individuals with an already unfavorable genotype (26). Our preliminary results in Barrett's esophagus (BE) are consistent with a similar relationship with EA.
There are limitations to this study. Firstly, this was a hospital-based case–control study, though we did utilize healthy gender and age frequency-matched controls. Our control group was fairly representative of the Massachusetts general Caucasian population, except that there was a slightly greater proportion of non-smokers in our group (Massachusetts non-smoker rates for adults aged 45 years and older is 27 versus 32% in our sample). How this could skew the results is unknown, except that this group of controls may be slightly healthier than the regular population, which possibly is linked to the lack of GERD symptoms; we did attempt to adjust for the other lifestyle factors that may be important (obesity, alcohol use) as a result. Secondly, of the four MMP polymorphisms evaluated, MMP12 1082A/G, which yielded a negative result in our study, was not in HWE in the control population. We have utilized this control sample for other studies, and none of our other polymorphisms were out of HWE. The likely reason for falling out of HWE stems from the low percentage of homozygous variant individuals (1%); the addition or subtraction of a single person in this small group leads to a change in the HWE P-value from 0.006 to 0.17, demonstrating how rare alleles can generate unstable calculations of HWE (19,20,27). Thirdly, a candidate polymorphism approach was used, selecting polymorphisms based on evidence of putative functional change or association with increased risk of other malignancies. We chose a set of polymorphisms within the same haplotype structure, other MMP genes still require evaluation in future studies. Finally, we utilized non-GERD controls in part because this dataset was utilized to evaluate specific questions in our other esophageal case analyses (19,20,27). Analysis of the subset of non-GERD cases and controls (data not shown) actually found no relationships, suggesting that the main effect of MMP1, MMP3 and MMP12 are being driven by a gene–GERD interaction. A future study will need to explore this further.
In summary, we demonstrated an independent association between EA risk and the individual MMP1 1G/2G, MMP3 6A/5A and MMP12 −82A/G and no effect on outcome. Preliminary results in BE were similar to EA.
Funding
National Institutes of Health (R01 CA074386); Doris Duke Charitable Foundation; Alan B.Brown Chair in Molecular Genomics; Posluns Family; Princess Margaret Foundations; Canadian Institutes of Health Research; Flight Attendant Medical Research Institute young investigator award.
Acknowledgments
We acknowledge the assistance of Peggy Suen, Salvatore Mucci, Richard Rivera-Massa, David P.Miller, Andrea Shafer and Paul Wheatley-Price.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BMI
body mass index
- EA
esophageal adenocarcinoma
- GERD
gastroesophageal reflux disease
- HWE
Hardy–Weinberg equilibrium
- MMP
matrix metalloproteinase
- OS
overall survival
References
- 1.Bollschweiler E, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–555. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Souza RF, et al. Review article: a conceptual approach to understanding the molecular mechanisms of cancer development in Barrett's oesophagus. Aliment. Pharmacol. Ther. 2001;15:1087–1100. doi: 10.1046/j.1365-2036.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 4.Engel LS, et al. Population attributable risks of esophageal and gastric cancers. J. Natl Cancer Inst. 2003;95:1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AJ. Barrett's esophagus: does the incidence of adenocarcinoma matter? Am. J. Gastroenterol. 1997;92:193–194. [PubMed] [Google Scholar]
- 6.Egeblad M, et al. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 7.Murray GI, et al. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J. Pathol. 1998;185:256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Tanioka Y, et al. Matrix metalloproteinase-7 and matrix metalloproteinase-9 are associated with unfavourable prognosis in superficial oesophageal cancer. Br. J. Cancer. 2003;89:2116–2121. doi: 10.1038/sj.bjc.6601372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herszenyi L, et al. Alterations of glutathione S-transferase and matrix metalloproteinase-9 expressions are early events in esophageal carcinogenesis. World J. Gastroenterol. 2007;13:676–682. doi: 10.3748/wjg.v13.i5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmela MT, et al. Upregulation and differential expression of matrilysin (MMP-7) and metalloelastase (MMP-12) and their inhibitors TIMP-1 and TIMP-3 in Barrett's oesophageal adenocarcinoma. Br. J. Cancer. 2001;85:383–392. doi: 10.1054/bjoc.2001.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutter JL, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58:5321–5325. [PubMed] [Google Scholar]
- 12.Su L, et al. Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis. 2006;27:1024–1029. doi: 10.1093/carcin/bgi283. [DOI] [PubMed] [Google Scholar]
- 13.Hinoda Y, et al. Association of functional polymorphisms of matrix metalloproteinase (MMP)-1 and MMP-3 genes with colorectal cancer. Int. J. Cancer. 2002;102:526–529. doi: 10.1002/ijc.10750. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa R, et al. The 2G allele of promoter region of matrix metalloproteinase-1 as an essential pre-condition for the early onset of oral squamous cell carcinoma. BMC Cancer. 2007;7:187. doi: 10.1186/1471-2407-7-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye S, et al. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J. Biol. Chem. 1996;271:13055–13060. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- 16.Fang S, et al. Polymorphisms in the MMP1 and MMP3 promoter and non-small cell lung cancer in North China. Carcinogenesis. 2005;26:481–486. doi: 10.1093/carcin/bgh327. [DOI] [PubMed] [Google Scholar]
- 17.Jormsjo S, et al. Allele-specific regulation of matrix metalloproteinase-12 gene activity is associated with coronary artery luminal dimensions in diabetic patients with manifest coronary artery disease. Circ. Res. 2000;86:998–1003. doi: 10.1161/01.res.86.9.998. [DOI] [PubMed] [Google Scholar]
- 18.Shin A, et al. Genetic polymorphisms in the matrix metalloproteinase 12 gene (MMP12) and breast cancer risk and survival: the Shanghai Breast Cancer Study. Breast Cancer Res. 2005;7:R506–R512. doi: 10.1186/bcr1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, et al. XRCC1 and XPD polymorphisms and esophageal adenocarcinoma risk. Carcinogenesis. 2007;28:1254–1258. doi: 10.1093/carcin/bgm020. [DOI] [PubMed] [Google Scholar]
- 20.Lanuti M, et al. A functional epidermal growth factor (EGF) polymorphism, EGF serum levels, and esophageal adenocarcinoma risk and outcome. Clin. Cancer Res. 2008;14:3216–3222. doi: 10.1158/1078-0432.CCR-07-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DP, et al. Combinations of the variant genotypes of GSTP1, GSTM1, and p53 are associated with an increased lung cancer risk. Cancer Res. 2002;62:2819–2823. [PubMed] [Google Scholar]
- 22.Zhu Y, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances lung cancer susceptibility. Cancer Res. 2001;61:7825–7829. [PubMed] [Google Scholar]
- 23.Zinzindohoue F, et al. Single nucleotide polymorphisms in MMP1 and MMP3 gene promoters as risk factor in head and neck squamous cell carcinoma. Anticancer Res. 2004;24:2021–2026. [PubMed] [Google Scholar]
- 24.Hanahan D, et al. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Lochter A, et al. The significance of matrix metalloproteinases during early stages of tumor progression. Ann. N. Y. Acad. Sci. 1998;857:180–193. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- 26.Churg A, et al. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am. J. Respir. Crit. Care Med. 2003;167:1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- 27.Tse D, et al. Polymorphisms of the NER pathway genes, ERCC1 and XPD are associated with esophageal adenocarcinoma risk. Cancer Causes Control. 2008;19:1077–1083. doi: 10.1007/s10552-008-9171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]