Abstract
Introduction and Objective
A great deal of effort has been focused on developing new treatment protocols to reduce tissue injury to improve the safety of shock wave lithotripsy. This has led to the discovery that pretreatment of the kidney with a series of low-energy shock waves (SWs) will substantially reduce the hemorrhagic lesion that normally results from a standard clinical dose of high-energy SWs. Because renal blood flow is reduced following low- or high-energy SWL, and may therefore contribute to this effect, this study was designed to test the hypothesis that the pretreatment protocol induces renal vasoconstriction sooner than the standard protocol for SW delivery.
Methods
Female farm pigs (6-weeks old) were anesthetized with isoflurane and the lower pole of the right kidney treated with SWs using the HM3 lithotripter. Pulsed Doppler sonography was used to measure resistive index (RI) in blood vessels as a reflection of resistance/impedance to blood flow. RI was recorded from a single intralobar artery located in the targeted pole of the kidney, and measurements taken from pigs given sham SW treatment (Group 1; no SWs, n = 4), a standard clinical dose of high-energy SWs (Group 2; 2000 SWs, 24 kV, 120 SWs/min, n = 7), low-energy SW pretreatment followed by high-energy SWL (Group 3; 500 SWs, 12 kV, 120 SWs/min + 2000 SWs, 24 kV, 120 SWs/min, n = 8) and low-energy SW pretreatment alone (Group 4; 500 SWs, 12 kV, 120 SWs/min, n = 6).
Results
Baseline RI (~ 0.61) was similar for all groups. Pigs receiving sham SW treatment (Group 1) had no significant change in RI. A standard clinical dose of high-energy SWs (Group 2) did not significantly alter RI during treatment, but did increase RI at 45-min into the post-SWL period. Low-energy SWs did not alter RI in Group 3 pigs, but subsequent treatment with a standard clinical dose of high-energy SWs resulted in a significantly earlier (at 1000 SWs) and greater (two-fold) rise in RI than that observed in Group 2 pigs. This rise in RI during the low/high-energy SWL treatment protocol was not due to a delayed vasoconstrictor response of pretreatment, as low-energy SW treatment alone (Group 4) did not increase RI until 65 min into the post-SWL period.
Conclusions
The pretreatment protocol induces renal vasoconstriction during the period of SW application whereas the standard protocol shows vasoconstriction occurring only during the post-SWL period. Thus the earlier and greater rise in RI during the pretreatment protocol may be causally associated with a reduction in tissue injury.
Keywords: Resistive index (RI), shock waves, extracorporeal shock wave lithotripsy
INTRODUCTION
Shock wave lithotripsy (SWL) is an effective means of removing uncomplicated stone burdens of 2 centimeters or less located in the upper urinary tract [1], and is the most preferred therapy by urologists [2] and patients [3,4] alike. Despite such wide acceptance, however, both human and animal studies have documented a consistent and predictable pattern of acute tissue injury and impaired renal hemodynamics after SWL [5]. The tissue injury primarily occurs in the microvascular system of the renal medulla and spreads into the cortex toward the capsule where a subcapsular hematoma may form. The injury is first noted in small veins and capillaries to small to medium sized arteries as lacerations of their endothelial/smooth muscle layers resulting in intraparenchymal bleeding [5].
The acute injury from SWL may have long-term adverse effects that include the onset of hypertension [6,7], diabetes [7] and brushite stone disease [8,9], all of which have the potential of greatly altering a patient’s quality of life. These long-term complications have raised questions of the present safety of SWL and have directed our research group to develop and study in animal models new therapeutic protocols for the safer administration of SWL for all stone patients. One of these treatment strategies has been termed the “protection” protocol, where the kidney is initially treated with low-energy shock waves (SWs) prior to administration of the standard clinical dose of SWs [10]. This reduces, possibly prevents, the tissue lesion that normally develops after the administration of a standard clinical dose of high-energy SWs to a porcine kidney in vivo [10].
Renal vasoconstriction is nearly always observed after SWL and is a feature that is independent of the strength of the administered SWs [11], Therefore, we have hypothesized that the low-energy SWs of the protection protocol induce renal vasoconstriction prior to the administration of the standard full-strength SWs, thereby minimizing hemorrhage and, accordingly, the size of the resulting lesion. As a first step in testing this hypothesis, we have measured and assessed the renal resistive index (RI), which provides the means for noninvasive monitoring of renal vascular resistance/impedance to blood flow [12]. We report here the results of experiments in which renal RI was measured in real time before, during and after standard SWL and SWL applied via the protection protocol.
METHODS
Anesthesia was induced in female farm pigs (6-weeks old, body wt ~ 15 kg) with an intramuscular injection of xylazine (15 to 20 mg/kg) and ketamine (2 mg/kg), and maintained with the inhalation of isoflurane (1 to 3%) and oxygen (100%). Catheters were placed in an ear vein for the intravenous infusion of saline to maintain hydration, a femoral artery for mean arterial blood pressure (MAP) measurements, and both ureters to collect urine from both kidneys and to permit the injection of X-ray contrast agent into the treated kidney for fluoroscopic visualization of the urinary collecting system and targeting of the SWs as previously described [11]. The lower pole of the right kidney was identified and then targeted for SWs delivered by an unmodified HM3 electrohydraulic lithotripter (Dornier GmbH, Germany). The pigs remained in the lithotripter’s heated water bath for the duration of the experimental protocol.
Each pig was assigned to one of four treatment groups: sham SW treatment (Group 1; no SWs, n = 4), a standard clinical dose of high-energy SWs (Group 2; 2000 SWs, 24 kV, 120 SWs/min, n = 7), the protection protocol [10] consisting of low-energy SW pretreatment followed by a 3-min pause and then the standard dose of SWs (Group 3; 500 SWs, 12 kV, 120 SWs/min + 2000 SWs, 24 kV, 120 SWs/min, n = 8) or low-energy SW pretreatment alone (Group 4; 500 SWs, 12 kV, 120 SWs/min, n = 6). The SW treatment was briefly stopped after every 500 SWs to verify targeting. Electrodes were replaced following 500 low-energy SWs and after 1000 high-energy SWs.
An experienced sonographer (M.P.) used color and pulsed Doppler ultrasound (Phillips C5-2 ultrasound transducer attached to a Phillips iU22 ultrasound machine) to identify an intralobar artery in the lower pole of the right kidney and measure RI [RI = (peak systolic velocity – peak diastolic velocity)/peak systolic velocity]. The ultrasound transducer was attached to a flexible positioning arm (CIVCO Medical Solutions, USA) that allowed serial RI measurements from the same intralobar artery before, during (at 500 SW intervals) and up to one hour after (at 5-min intervals) SWL. RI measurements were taken only during pauses in SW treatment (see above) because SWs often interfered with the ultrasound waveform.
Baseline RI was defined as the mean of three RI measurements taken over a 5- min interval that did not differ by more than 0.02 RI units from each other, and were taken immediately before high-energy SW delivery in Group 2, and before low-energy SW delivery in Groups 3 and 4. The timing of baseline and subsequent RI measurements in Group 1 (sham SW-treated animals) was similar to the latter two groups. Digital screen captures and cine loops were saved for each measurement and the imaging display was recorded to videotape over the duration of the entire experiment. These data formats were subsequently viewed to ensure accuracy of the calculated RI value by checking that peak systolic and diastolic blood velocities were accurately identified and that representative waveforms had been chosen for analysis.
STATISTICS
Descriptive statistics are presented as mean ± standard error for each of the four groups. One-way analysis of variance (ANOVA) was used to compare mean RI for the four groups at baseline. Two sets of mixed effect models were used to analyze repeated RI measures over time, where time was included as a categorical independent variable to accommodate nonlinear trends. The first set of models examined repeated RI values in each of the four groups over time, with time as the independent variable. The second set of models was used to examine whether the four groups differed in changes in RI measures over time by including group, time and the interaction between group and time as independent variables. If significant overall time effects were detected in the first set of models, or if significant interactions were evident between group and time in the second set of models, we conducted post-hoc analyses using linear contrasts of the fixed effects in the models to compare post-baseline RI values at each time point to baseline measures, or to identify the time points where differences in changes of RI were different among the four groups. Significance levels were adjusted, as appropriate, according to the procedure proposed by Holm (also known as the step-down Bonferroni procedure) [13,14]. Overall type I error rate for each model was set to 0.05. All analyses were conducted using SAS v9 software [15].
RESULTS
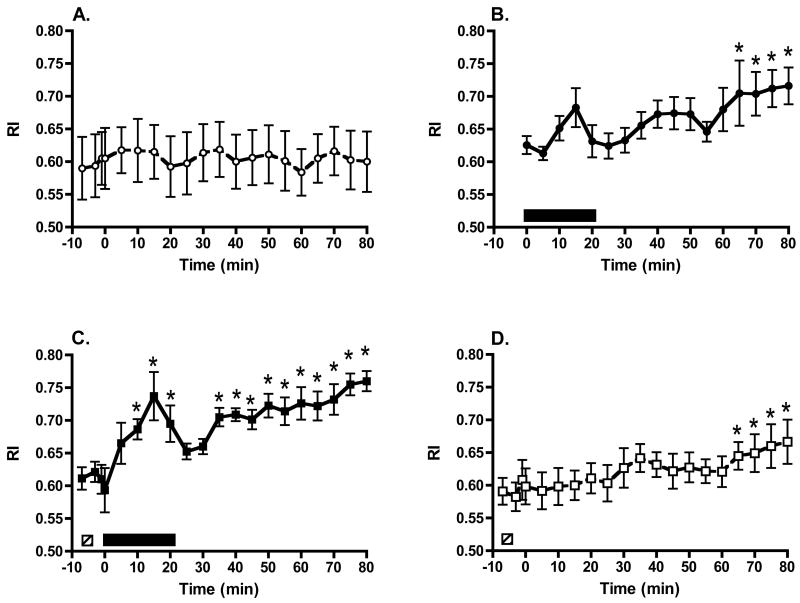
Baseline RI was similar for all groups (Group 1, 0.61 ± 0.05; Group 2, 0.63 ± 0.01; Group 3, 0.61 ± 0.02; Group 4, 0.59 ± 0.02; P = 0.72; see Fig 1A through Fig 1D, respectively). Pigs receiving sham SW treatment (Group 1, Fig. 1A) had no significant change in RI throughout the experimental observation period (P = 0.91 for overall time effect). The delivery of a standard clinical dose of 2000 high-energy SWs (Group 2, Fig. 1B) did not significantly change RI during the period of SW administration. The apparent trend for RI to rise at 1500 SWs (Fig. 1B) did not attain statistical significance (P = 0.16), but RI rose progressively after SWL, ultimately attaining values that were 14 ± 4% greater than baseline at the end of the experiment (P = 0.0041). Pigs treated with the protection protocol (Group 3, Fig. 1C) showed no significant change in RI during the delivery of 500 low-energy SWs, but subsequent delivery of high-energy SWs resulted in a significant rise in RI that remained elevated both during and following SWL treatment. During the high-energy SW phase of the protection protocol, RI increased 9 ± 4% at 500 SWs (P = 0.0687), 13 ± 4% at 1000 SWs (P = 0.0052), 21 ± 6% at 1500 SWs (P < 0.0001), and 14 ± 4% at 2000 SWs (P = 0.0017). Thereafter, there was a brief decline in RI followed by a progressive increase such that RI was 26 ± 1% higher than baseline at the end of the experiment (P < 0.0001). Treatment with only 500 low-energy SWs (Group 4, Fig. 1D) did not alter RI during and ~ 60 min following SW delivery, but RI did increase thereafter, as was the case with Group 2.
Figure 1.
Serial RI measurements from a single intralobar artery during sham SW treatment (panel A), a standard clinical dose of high-energy SWs (panel B), pretreatment with low-energy SWs followed by a 3-min pause and then the standard dose of SWs (panel C) and pretreatment with low-energy SWs alone (panel D). □ = treatment period with 500 low-energy SWs; ■ = treatment period with 2000 high-energy SWs. * = P < 0.05 from baseline (pre-SW) RI values.
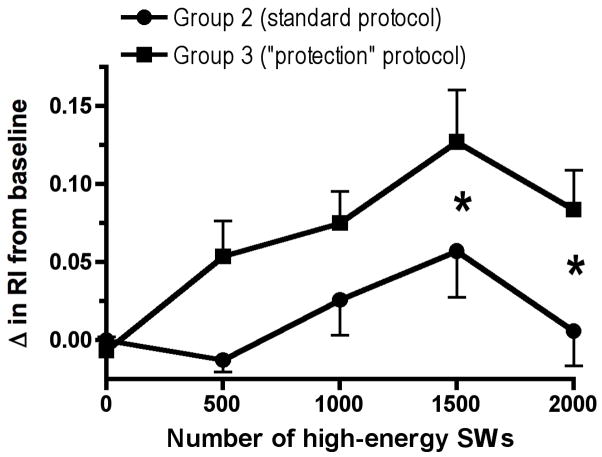
Fig. 2 shows the change in RI from their respective baseline values for Groups 2 and 3 only during the 20 min period of high-energy SW delivery. The rise in RI after the application of 1500 and 2000 high-energy SWs was significantly greater in the protected animals than in pigs exposed only to the standard SW protocol (P = 0.0470 and P = 0.0224, respectively).
Figure 2.
The change in RI from baseline values for Group 2 (standard clinical SW dose) and Group 3 (pretreatment/standard clinical SW dose) animals during the period of high-energy SW delivery. * = P < 0.05 between the two groups following the application of 1500 and 2000 high-energy SWs.
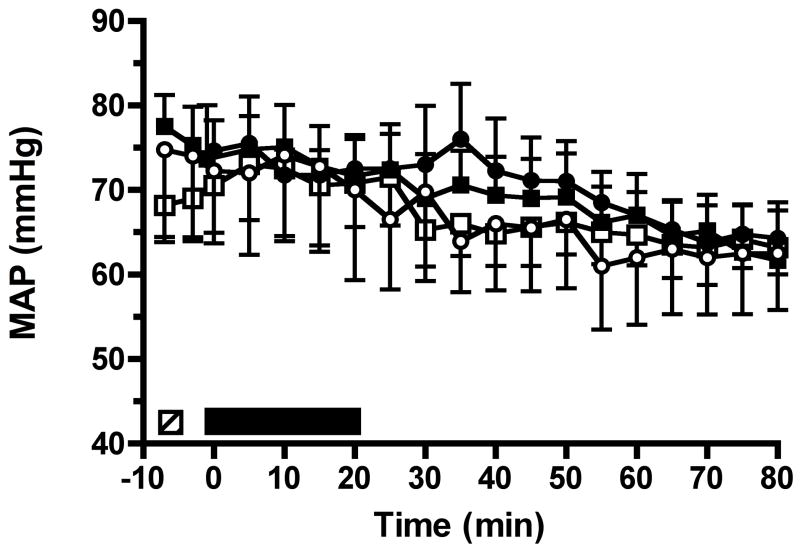
Baseline MAP was similar in all groups (Group 1, 74.8 ± 10.9 mmHg; Group 2, 74.6 ± 3.6 mmHg; Group 3, 77.5 ± 3.7 mmHg; Group 4, 68.2 ± 3.8 mmHg; P = 0.28). Linear trend models demonstrated that blood pressure did not differ between the four groups (P = 0.24) and that MAP significantly decreased (P < 0.0001) over time in all groups, with an estimated decrease of 0.13 mmHg/min (Fig. 3). Mixed-effect models examined whether there was an association between MAP and RI. Both linear trend and discrete time point models demonstrated that the decrease in MAP was associated with the increase in RI over time in all groups (P < 0.0001). However, there was no difference in the association of MAP with RI among the four groups, i.e., the progressive fall in MAP did not account for the differences in RI that was observed between the standard protocol (Group 2) and protection protocol (Group 3) following the delivery of 1500 and 2000 high-energy SWs.
Figure 3.
Measurements of mean arterial blood pressure (MAP) measured during the four experimental protocols. Group symbols as in figure 1.
DISCUSSION
The results demonstrate that the pretreatment protocol induced renal vasoconstriction during the latter half of the SW application, whereas the standard protocol showed measurable vasoconstriction only during the post-SWL period. The detection of vasoconstriction during the latter half of the SW application period, a time when tissue injury doubtless is occurring [16], allows us to suggest that this vasoconstrictive event correlates with the reduced injury reported for the low-energy pretreatment protocol. The data support the hypothesis that renal vasoconstriction may protect the kidney from the tissue damaging effects of high-energy SWL.
A major obstacle to understanding how vasoconstriction protects the kidney from SWL-induced injury is the paucity of information on how SWs damage tissue. We know that the SWL-induced renal injury is largely hemorrhagic, i.e. that the SWs rupture blood vessels, and the resulting hemorrhagic lesion comprises approximately 5.2% of the functional renal volume (FRV) in the juvenile pig [10]. However, the role of factors such as SW-induced shear waves and/or cavitation activity (bubble expansion and/or SWs generated by bubble collapse) in the initiation and progression of the hemorrhagic lesion is not known. Nevertheless, we speculate on several mechanisms, acting alone or in combination, which could potentially explain how renal vasoconstriction protects tissue from the damaging effects of high-energy SWL and reduces mean hemorrhagic lesion size to less than 0.4% FRV [10].
Mechanism 1: A constricted blood vessel would arguably be stiffer due to contraction of its smooth muscle cells resulting in reduced strain on the vessel wall due to shear and/or as a bubble expanded, and reduced violence during bubble collapse. Accordingly, the likelihood of blood vessels being ruptured would be reduced. Mechanism 2: A localized decrease in blood flow through renal tissue might be associated with reduced O2 and elevated CO2 levels in the affected tissue [17]. Because some investigators have suggested that CO2 can reduce cavitation activity [18–20], a rising renal CO2 level during vasoconstriction could potentially suppress cavitation activity and the damage that it causes. Mechanism 3: Renal vasoconstriction should reduce bleeding from a ruptured vessel and thereby diminish the amount of blood that accumulates in the nearby parenchyma. Since a smaller pool of intraparenchymal blood would be expected to support fewer cavitation nuclei, less cavitation activity, and subsequently less tissue injury to surrounding structures would be expected. However, we should keep in mind that with the protection protocol only few damaged vessels are seen in the cortex and medulla [10]. This would imply a protective mechanism that limits the rupture of vessels rather than bleeding. Also, the mechanism(s) of protection must be capable of influencing the entire kidney as pretreatment of a lower renal pole with SWs protects the upper pole of the same kidney from injury when a clinical dose of high-energy SWs is applied to that pole [10].
While the cause of the protection is unknown, factors that could potentially trigger the response include the number of pretreatment SWs, the starting energy (voltage) of the pretreatment SWs, and the time interval between the SW applications. We have shown that a similar degree of protection occurs when the number of low-energy SWs administered in the pretreatment period was reduced in a series of experiments from 2000 to 100, indicating that the threshold number of SWs required to initiate the protective response must be 100 or less [10]. In addition, the protection was comparable whether the initial 100 pretreatment SWs were begun at strengths of 12 kV, 18 kV or 24 kV [21]. This finding, together with our original observation that pretreatment of one pole of the kidney with either 2000 SWs at 12 kV or 24 kV will protect the opposite pole of the same kidney from the damaging actions of 2000 high-energy SWs [10], suggests that the onestep voltage ramping in the protection protocol per se is not solely responsible for limiting lesion size. Consequently, the time interval between the pretreatment and clinical doses of SWs emerges as the only other obvious factor that could contribute to the protection phenomena. This is illustrated by the fact that pretreatment with 100 high-energy SWs followed by a 3-min pause and then the clinical dose of 2000 high-energy SWs produced a lesion that was at least 10-fold smaller than that observed with a clinical dose of 2000 high-energy SWs alone [21]. Therefore, there appears to be a critical interaction between the initial non-damaging SW stimulus and the delay interval that permits the development of the protective response, presumably by allowing sufficient time for the sensitization of the renal vasculature and/or vasoconstrictor system(s) (e.g. neural, paracrine, autocrine) such that a robust renal vasoconstriction is apparent when high-energy SWs are subsequently applied. Renal vasoconstriction, through the suppression of SW forces that rupture blood vessels, and the reduction in intraparenchymal hemorrhage, may then protect the kidney from SWL-induced tissue injury.
The RI data also gives us some new insight into the timing of the renal vasoconstriction to a standard clinical dose of SWs. Previous studies focused on the impact of SWL on renal blood flow only after treatment and showed that renal vasoconstriction was detectable 0.5 to 4 hours after low- or high-energy SWL [11,22–28]. The factors that may reduce renal blood flow under these circumstances are not known, but we have always assumed that renal vasoconstriction was initiated at some point during SWL. The results obtained with the standard high-energy SWL protocol (Group 2) and low-energy SW pretreatment alone (Group 4) suggest that renal vasoconstriction is not readily apparent — as assessed by RI methodology — during lithotripsy, but instead becomes most intense after SW treatment.
Although acute injury to the renal microvasculature and a reduction in renal blood flow are hallmarks of SWL [5], there has been limited clinical and basic research on whether such effects may have long-term consequences. Some clinical data suggest a link between SWL-induced renal damage and new-onset hypertension, primarily in older patients [6,27], and recent studies show a correlation between multiple lithotripsies and the transition from calcium oxalate to calcium phosphate (brushite) stone disease [8,9]. In both cases, the initial sites of acute injury in the renal cortex and medulla would result in a loss of microvessels and nephron segments that lead to tissue scarring. The loss of parenchymal tissue would be expected to have a pathological outcome at some point when enough functional tissue has been lost, especially in patients with other risk factors. Tissue damage that is specific to medullary collecting duct cells would likely result in a loss of urinary pH regulation by these cells, which in turn would alkalinize the urine and favor apatite crystal retention and the production of brushite stones, which are resistant to SWL [9]. In addition, the findings of a nineteen-year follow up study of SWL-treated patients suggest that they have an increased risk of developing diabetes as well as hypertension [7]. Therefore, an intuitive benefit of SWL treatment using the protection protocol is not only the significant reduction in acute renal injury, but also that it will likely lessen, or even prevent, the induction of these potential long-term complications.
Further studies are needed to confirm that renal vasoconstriction is indeed the cause of the protection, and that this protocol indeed reduces tissue injury with the newer-generation single-head lithotripters. The mechanisms that initiate renal vasoconstriction and how it may lead to reduced tissue injury require investigation. We need to understand how to manipulate lithotripter SW delivery settings to invoke the protective response and tailor the SW delivery protocols to the type of lithotripter, and perhaps even the type of patient. These are some of the directions of study that will advance our knowledge of the protection response, which will have significant translational implications, as they will aid in optimizing lithotripsy protection protocol settings, and thus improve the safety of SWL.
Acknowledgments
This work was supported by funds from U.S. Public Health Service grants PO1-DK43881 and RO1-DK67133.
References
- 1.Lingeman JE, Matlaga B, Evan AP. Surgical management of urinary lithiasis. In: Walsh PC, Retik AB, Vaughan ED, et al., editors. Campbell’s urology. Philadelphia: WB Saunders Company; 2006. pp. 1431–507. [Google Scholar]
- 2.Gerber GS. Trends in Endourologic Practice: Management of lower-pole caliceal stones. J Endourol. 2003;17:501–3. doi: 10.1089/089277903769013676. [DOI] [PubMed] [Google Scholar]
- 3.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2005;173:S69–S73. doi: 10.1016/j.juro.2008.03.140. [DOI] [PubMed] [Google Scholar]
- 4.Karlsen SJ, Renkel J, Tahir AR, et al. Extracorporeal shockwave lithotripsy versus ureterscopy for 5- to 10-mm stones in the proximal ureter: Prospective effectiveness patient-preference trial. J Endourol. 2007;21:28–33. doi: 10.1089/end.2006.0153. [DOI] [PubMed] [Google Scholar]
- 5.Evan AP, Willis LR. Extracorporeal shock wave lithotripsy: Complications. In: Smith AD, Badlani GH, Baggley DH, et al., editors. Smith’s textbook on endourology. Hamilton, Ontario, Canada, BC: Decker Inc; 2007. pp. 353–65. [Google Scholar]
- 6.Janetschek G, Frauscher F, Knapp R, et al. New onset hypertension after extracorporeal shock wave lithotripsy: Age related incidence and prediction by intrarenal resistive index. J Urol. 1997;158:346–51. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 7.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742–47. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 8.Parks JH, Worcester EM, Coe FL, et al. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–85. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 9.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–91. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 10.Willis LR, Evan AP, Connors BA, et al. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol. 2006;17:663–73. doi: 10.1681/ASN.2005060634. [DOI] [PubMed] [Google Scholar]
- 11.Connors BA, Evan AP, Willis LR, et al. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–18. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 12.Tublin ME, Bude RO, Platt JF. The resistive index in renal Doppler sonography: Where do we stand? AJR. 2003;180:885–92. doi: 10.2214/ajr.180.4.1800885. [DOI] [PubMed] [Google Scholar]
- 13.Holm S. A simple sequentially rejective Bonferroni test procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
- 14.Shaffer JP. Modified sequentially rejective multiple test procedures. J Am Statistical Assoc. 1986;81:826–31. [Google Scholar]
- 15.Littell RC, Milliken GA, Stroup WW, et al. SAS system for mixed models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- 16.Connors BA, Evan AP, Blomgren PM, et al. Reducing shock number dramatically decreases lesion size in a juvenile kidney model. J Endourol. 2006;20:607–11. doi: 10.1089/end.2006.20.607. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BA, Weil MH. Redefining ischemia due to circulatory failure as dual defects of oxygen deficits and of carbon dioxide excesses. Crit Care Med. 1991;19:1432–8. doi: 10.1097/00003246-199111000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T, Kuwabara M, Sato F, et al. Influence of dissolved gases on chemical and biological effects of ultrasound. Ultrasound Med Biol. 1986;12:151–5. doi: 10.1016/0301-5629(86)90020-7. [DOI] [PubMed] [Google Scholar]
- 19.Carstensen EL, Kelly P, Church CC, et al. Lysis of erythrocytes by exposure to CW ultrasound. Ultrasound Med Biol. 1993;19:147–65. doi: 10.1016/0301-5629(93)90007-b. [DOI] [PubMed] [Google Scholar]
- 20.Topaz M, Motiei M, Assia E, et al. Acoustic cavitation in phacoemulsification: chemical effects, modes of action and cavitation index. Ultrasound Med Biol. 2002;28:775–784. doi: 10.1016/s0301-5629(02)00514-8. [DOI] [PubMed] [Google Scholar]
- 21.Connors BA, Evan AP, Blomgren PM, et al. Effect of initial shock wave voltage on SWL-induced lesion size during step-wise voltage ramping. BJU Int. 2008 doi: 10.1111/j.1464-410X.2008.07922.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsen SJ, Smevik B, StenstrØm J, et al. Acute physiological changes in canine kidneys following exposure to extracorporeal shock waves. J Urol. 1990;143:1280–3. doi: 10.1016/s0022-5347(17)40255-2. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto T, Senu M, Sugimoto T, et al. Effects of high energy shock wave exposure on renal function during extracorporeal shock wave lithotripsy for kidney stones. Eur Urol. 1990;18:290–8. doi: 10.1159/000463931. [DOI] [PubMed] [Google Scholar]
- 24.Mostafavi MR, Chavez DR, Cannillo J, et al. Redistribution of renal blood flow after SWL evaluated by Gd-DTPA-enhanced magnetic resonance imaging. J Endourol. 1998;12:9–12. doi: 10.1089/end.1998.12.9. [DOI] [PubMed] [Google Scholar]
- 25.Nazaroglu H, Akay AF, Bükte Y, et al. Effects of extracorporeal shock-wave lithotripsy on intrarenal resistive index. Scand J Urol Nephrol. 2003;37:408–12. doi: 10.1080/00365590310006354. [DOI] [PubMed] [Google Scholar]
- 26.Mohseni MG, Khazaeli MH, Aghamir SMK, et al. Changes in intrarenal resistive index following electromagnetic extracorporeal shock wave lithotripsy. Urol J. 2007;4:217–20. [PubMed] [Google Scholar]
- 27.Mitterberger M, Pinggera GM, Neururer R, et al. Multimodal evaluation of renal perfusional changes due to extracorporeal shock wave lithotripsy. BJU Int. 2007;101:731–5. doi: 10.1111/j.1464-410X.2007.07281.x. [DOI] [PubMed] [Google Scholar]
- 28.Handa RK, Willis LR, Evan AP, et al. Effect of shock wave lithotripsy on renal hemodynamics. In: Evan AP, Lingeman JE, McAteer JA, et al., editors. Renal Stone Disease 2: Proceedings of the Second International Urolithiasis Research Symposium, American Institute of Physics; Melville, NY. 2008. In Press. [Google Scholar]