Summary
The amino acids glutamate and gamma-aminobutyric acid (GABA) have primarily been characterized as the most prevalent excitatory and inhibitory, respectively, neurotransmitters in the vertebrate central nervous system. However, the role of these signaling molecules extends far beyond the synapse. GABA, glutamate, and their complement of receptors are essential signaling molecules that regulate developmental processes in both embryonic and young adult mammals. In this review, we describe the current knowledge on the role of GABA and glutamate in development, focusing on the perinatal cerebellum. We will then present novel data suggesting that GABA depolarizes granule cell precursors via GABAA receptors, which leads to calcium increases in these cells. Finally, we will consider the role of GABA and glutamate signaling on cell proliferation and perhaps neural cancers. From our review of the literature and these data, we hypothesize that GABAA receptors and metabotropic glutamate receptors may be a novel target for the pharmacological regulation of the cerebellar tumors, medulloblastomas.
Keywords: GABA, GABA receptor, cerebellum, external granule cell layer, migration, glutamate, medulloblastoma
GABA AND GLUTAMATE SIGNALING: A HISTORICAL PERSPECTIVE
γ-aminobutyric acid (GABA) and glutamate are structurally similar amino acids. They are related metabolically in that GABA is synthesized from glutamate by the enzyme glutamic acid decarboxylase (1). Yet, their strikingly opposite effects on the mature brain were realized almost concurrently with their discovery as neurotransmitters (2; 3). In the vertebrate brain, L-glutamate is the primary excitatory neurotransmitter and GABA is the primary inhibitory neurotransmitter. This striking complementation becomes more complicated and less apparent in immature networks. Perhaps because of their striking effects as synaptic neurotransmitters, the non-synaptic, developmental effects of GABA and glutamate were not initially considered. However, in the years since developmental biologist Daniel McMahon proposed his hypothesis of neurotransmitters as developmental signals (4), a wealth of evidence has been collected in its support. GABA and glutamate signaling have been implicated in embryonic and postnatal cell proliferation, migration, and differentiation (reviewed in (5–8)). In keeping with the “pleasing asceticism” hypothesized by McMahon, neurotransmitters have also been shown to play a critical role in postnatal neurogenesis as well (reviewed in (8–12)). However, it is unknown whether both transmitters, and their complement of receptors, exist in all postnatal neurogenic niches. Additionally, many of the cellular mechanisms underlying signaling in these regions have yet to be elucidated.
Compared to other organs, the brain was recognized to have a critical requirement of glutamate for efficient metabolism (13). Brain slices demonstrated the surprising capacity to actively accumulate it, even against the large concentration gradient created by high endogenous levels (14). Though it was debated whether such an ubiquitous amino acid could function as a discrete signaling molecule, the evidence for glutamate as an excitatory neurotransmitter was overwhelming, as it had an excitatory effect on all types of neurons to which it was applied (2; 15; 16).
In 1950, Eugene Roberts and Jorge Awapara independently discovered that there were large amounts of GABA in the mammalian central nervous system (approximately 1 mg GABA per gram of brain tissue) and, furthermore, that GABA was virtually undetectable in other tissues (1; 17). This dramatic enrichment of GABA in the brain hinted at a specialized role. Concurrently, Florey and McLennan had collected physiology data indicating that an unknown substance (factor I) had inhibitory effects on mammalian neurons (18). Shortly thereafter, factor I was identified as GABA (19). However, because of its seeming ubiquity in the brain and well as its then-unconventional inhibitory action, the idea of GABA as a neurotransmitter was heavily debated for several years before its acceptance (15; 20–24).
The potential problem of a ubiquitous compound being unable to carry a discrete signal seems to be solved by the diversity of molecules with which GABA and glutamate are able to interact. As they are potent signaling molecules, GABA and glutamate concentrations in the extracellular space are tightly regulated by a variety of transporters and exchangers. Many GABA and glutamate transporters use the sodium gradient to actively transport these neurotransmitters out of the extracellular space (25; 26).
GABA and glutamate each exert their effects on cells though activation of two main types of receptors: ionotropic receptors, which are ligand-gated, ion-permeable transmembrane pores, and metabotropic receptors, which are coupled to ion channels by G-protein intracellular signaling cascades. GABA ionotropic receptors are pentameric chloride channels of which there are two types: the predominant GABAA receptor (GABAAR) (27) and the more recently identified GABAC receptor (GABACR) (28). GABACRs are highly expressed in the retina (29), yet compelling evidence for their functional expression in postnatal neurogenic niches has yet to be shown. Metabotropic GABA receptors, GABAB receptors (GABABRs), are G-protein coupled receptors that activate cationic conductances, usually potassium channels, via the intracellular G-protein signaling cascade (30–32).
The eight types of metabotropic glutamate receptors (mGluRs, mGlu1-8 receptors) are divided into three groups (I–III) based on the intracellular signaling pathway they activate (33). Ionotropic glutamate receptors can be divided into three groups: N-methyl-D-aspartic acid (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors, each named for a selective agonist (34; 35). Variations in subunit composition modulate the biophysical properties of the receptors; however, broadly the three groups are ligand-gated, non-selective cation channels, permeable to sodium, potassium, and, in some cases, calcium ions (36). The main distinguishing factor between the groups is the voltage sensitivity of NMDA receptors (NMDARs) (37; 38) the other ionotropic glutamate receptors, AMPA and kainate receptors, and has interesting implications for the integration of developmental signals.
GABA AND GLUTAMATE SIGNALING IN THE PERINATAL CEREBELLUM
The cerebellum distinguishes itself by having its most prolific period of neurogenesis occur after birth in both rodents and humans (39; 40). However, in contrast with the subventricular zone (SVZ) and the the subgranular zone of the dentate gyrus (SGZ) neurogenesis does not persist into adulthood. The neurons generated during this period are fated to become glutamatergic interneurons called cerebellar granule cells. These neurons outnumber all other neuronal cell types in the central nervous system combined. Beginning at embryonic day 10 in mouse, cerebellar granule cell precursors (GCPs) migrate from the rhombic lip at the midbrain/hindbrain junction to the cerebellar anlage. There, postnatally, they create a prolific region called the external germinal layer (EGL) and undergo a rapid clonal expansion, vastly increasing their numbers (Figure 1). Within this layer, cerebellum GCPs migrate tangentially and then turn 90° and migrate across the molecular layer (ML) of the cerebellum, using Bergmann glial radial fibers as a scaffold (41–43).
Figure 1. Diagram summarizing the developmental stages of GCPs in neonates.
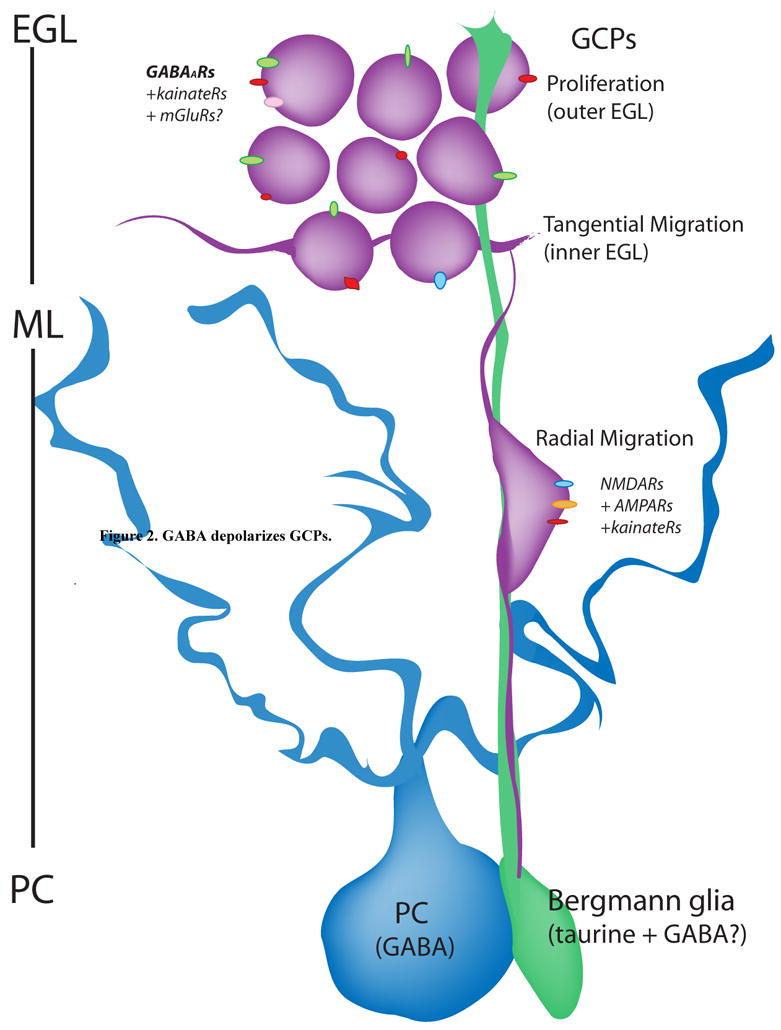
GCPs undergo clonal expansion (proliferation) in the outer part of the EGL. Once they exit the cell cycle, they move to the inner part of the EGL where they tangentially migrate and extend their processes. They then turn 90° angle, grab onto a Bergmann glial process and radially migrate throughout the molecular layer (ML) to the Purkinje cell layer (PC). In the outer EGL and other layers, GCPs express functional kainate receptors (kainateRs). It is unclear whether they express mGluRs. We show in this review article that GCPs also express functional GABAARs. In the inner EGL, they start expressing NMDA and AMPA receptors (NMDAR and AMPARs, respectively).
GCPs express the full complement of io notropic glutamate receptors (see Figure 1). Within the EGL, GCPs express calcium-permeable kainate receptors and begin to express AMPA receptors in the ML (44; 45). In the EGL, GCPs also begin to express NMDARs, which are tonically activated by ambient glutamate (46). However, the sources of glutamate leading to tonic receptor activation remain unknown. GCPs in the EGL do not express mGlu1 (47), mGlu2/3 (48), mGlu6, mGlu7 and mGlu8 receptors (for references see (49; 50)). It is unclear whether GCPs express mGluR4, but mGluR4 are expressed presynaptically on parallel fibers (51) and mGluR4 mRNA is in granule cells (52; 53). mGluR5 is expressed in immature granule cells in vitro (54) but not in adult granule cells (49). GABAergic signaling in the cerebellar EGL is enigmatic. While in situ hybridization and immunohistochemical studies have shown that GCPs express GABAARs subunits, indicating they have the potential to assemble receptors (55), functional GABAARs in GCPs has not be demonstrated. Here we report that GCPs express functional GABAARs in the EGL.
NOVEL DATA: GCPS IN THE EGL EXPRESS FUNCTIONAL GABAARS
GABA depolarizes granule cell precursors in the EGL
To determine whether GCPs express functional GABAARs in the EGL, we obtained gramicidin-perforated patch clamp recordings from GCPs in acutely dissected parasagittal cerebellar slices from postnatal (P) 8 mice at near-physiological temperature (32–35°C). Experiments were performed in CD1 mice (Charles River, USA) in accordance with the Yale Animal Careand Use Committee guidelines. Gramicidin is an antibiotic that perforates the cell membrane, creating cation-selective pores, without disturbing the chloride gradient (56; 57). Considering that the chloride gradient across the cell membrane is the main force underlying the reversal potential of GABAA responses, we can determine whether GABAA is depolarizing or hyperpolarizing. Recorded cells were identified as GCPs based on their biophysical and morphological properties. GCPs have a small cell body (~8 μm, Fig. 2D), a high input resistance (>1.5 GΩ), no synaptic currents, and no voltage-gated sodium currents (data not shown). GCPs had a mean input resistance of 3.5 ± 0.7 GΩ (n=6), which resemble that of SVZ neuroblasts (58; 59). Pressure application of GABA (100 μM, 2 s) induced inward currents in all recorded GCPs in the EGL held at a membrane potential of −60 mV (n=6/6, Fig. 2A). Using a ramp protocol at the peak of the response, the reversal potential of GABAARs was found to be −30.9 ± 1.5 mV (n=6). Pressure application of GABA (100 μM, 2 s) during current clamp recordings depolarized GCPs located in the EGL by 20–30 mV (n=4, Figure 2C).
Figure 2. GABA depolarizes GCPs.
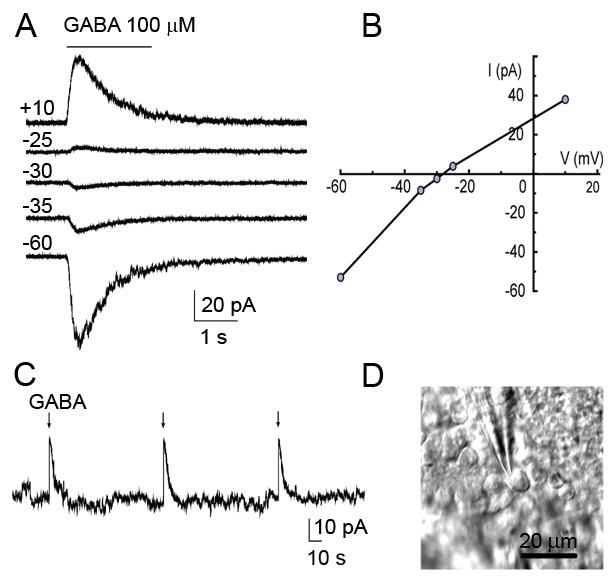
(A) GABA-induced currents recorded at different holding potentials ranging from −60 to +10 mV (marked to the left of the perforated patch-clamp records). (B) Current-voltage curve of GABA-induced currents for the traces shown in (B). (C) A representative current clamp record of a GCP depolarized by exogenous GABA application (100 μM, 2 s at arrow) in the EGL. Gramicidin perforated patch clamp was used. (D) DIC photograph of a patched cerebellar GCP in the EGL.
Based on the Nernst equation (see equation below) and knowing that GABAARs are also permeable to HCO3 with a HCO3−:Cl− permeability ratio of 0.2 (60), we can calculate the internal chloride concentration in GCPs. Using the following equation:
with [Cl−]e of 134.1 mM, [HCO3−]e of 24 mM, and assuming [HCO3−]i of 16 mM (61), the internal chloride concentration in GCPs is 39.4 ± 2.1 mM (n=6). This value is comparable to the mean internal chloride concentration calculated for SVZ neuroblasts (62).
GABAAR activation leads to calcium transients in GCPs
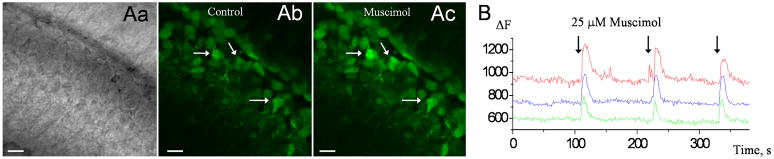
The depolarizing action of GABA on immature neurons has been shown to cause intracellular calcium transients in these cells (5; 10). Therefore, we next determined whether application of muscimol, a specific GABAAR agonist, increased intracellular Ca2+ levels in GCPs of the EGL. Parasagittal cerebellar slices were pressure-loaded with Oregon green BAPTA-1-AM (20 μM, 3–4 times each of 2 min). After a 20 min wait period, images were acquired every 1.1 s using an Olympus Fluoview confocal microscope (laser 488 nm). Pressure application of muscimol (25 μM, 5 s) induced reproducible, transient increases in intracellular Ca2+ levels in 93% of the fluo-4-loaded GCPs in the EGL (12–15 cells analyzed per slice, n=2 slices, Figure 3). These data support the patch clamp data in suggesting that GCPs in the EGL express functional GABAARs, whose activation increases intracellular Ca2+ presumably via cell depolarization and opening of voltage-gated Ca2+ channels as shown in SVZ neuroblasts (63).
Figure 3. GABAAR activation leads to calcium transients in GCPs.
(A) Confocal photographs of a live sagittal slice containing the EGL. (Aa) DIC image of the Oregon Green BAPTA-1 AM-loaded slice before and during a GABAAR agonist (muscimol, 25 μM, 5 s) application. Images were acquired every 1.1 s. Scale bar: 20 μm. (B) Representative calcium activity graphs as a function of time for the three cells whose ROI are shown in the (A).
POSSIBLE MECHANISMS FOR GABA’S DEPOLARIZATION AND SOURCES OF GABA
Finding that GABAA is depolarizing in GCPs add to its well know depolarizing function in embryonic and immature postnatal neurons via GABAARs (62; 64–66). The depolarizing action of GABA is thought to result from a higher intracellular chloride concentration in immature neurons than in mature neurons, created by expression of a different pattern of chloride transporters (67). Two families of transporters have been studied: the Na+–K+–2Cl− co-transporters (NKCCs) and the K+–Cl− co-transporters (KCCs). NKCCs are chloride importer and, therefore, typically raise [Cl−]i (68) while KCCs, in particular KCC2, whose expression is delayed during development, extrude chloride. In the cerebellum, expression of KCC2 in GCPs does not occur until cells reach the IGL (69). This delayed expression of KCC2 presumably explains the high intercellular chloride content and GABAA depolarizing effect.
The GABAAR activation in GCP depends on the existence of an endogenous source of GABA, or another GABAAR agonist, proximal to the cerebellar EGL. GCPs are fairly isolated in the EGL. Similar to the arrangement in the rostral migratory stream (RMS, a migratory pathway from the SVZ to the olfactory bulb), the GCPs are in intimate contact with their migratory scaffold, Bergmann glia (70). In general, GABA release from glia has not been reported. However, Bergmann glia have been shown to synthesize and release taurine (71; 72). Taurine is one of the most abundant free amino acids in the brain and has been shown to be an agonist for GABAARs (73–76). Therefore, Bergmann glia are a potential source for GABAAR activation in GCPs. However, it now remains to be examined whether GABAARs are tonically activated in GCPs under physiological conditions.
FUNCTIONS OF GABA AND GLUTAMATE SIGNALING ON THE PROLIFERATION OF GCPs
GABA and glutamate exert profound effects on cell development including on adult neurogenesis in the SVZ and SGZ. The functions of GABA and glutamate has been extensively reviewed by others (5; 7–10; 77–79). Here, we will essentially focus on their effects on cell proliferation in vivo or in organotypic slices to extrapolate on their hypothetical proliferative function in the neonatal cerebellum. GCPs undergo clonal expansion in the outer EGL before exiting the cell cycle and tangentially migrating in the inner EGL (80).
All types of glutamate receptors AMPA/kainate receptors, NMDARs and mGluRs have been shown to regulate cell proliferation with differential effects depending on the region studied and the age of the mice. In vivo acute administration of NMDAR and AMPA/kainate receptor blockers reduced the number of proliferative cells (marked by incorporation of bromodeoxyuridine, BrdU) in the striatal and cortical ventricular zone (VZ) of embryos (E), respectively (81; 82). These data suggested that activation of NMDAR and AMPA/kainate receptor by ambient glutamate promotes cell proliferation in the striatal and cortical VZ, respectively. However, chronic blockade of NMDARs in organotypic E17 slices increased the number of proliferative cells in the neocortical VZ, suggesting that NMDAR activation limits cell proliferation in this region (83). Blockade of NMDARs or AMPA/kainate receptors in vivo increased BrdU or thymidine-labelled cells in the SGZ of neonatal mice and adult gerbils (84; 85). Some of the discrepancies in the effects of receptor blockade on cell proliferation could be due to a decrease in neuronal activity impinging on progenitor cells and thus indirectly affecting their proliferation. mGlu5 receptors (group I) activation promotes cell proliferation in some embryonic neurogenic zones of the forebrain (86) as well as adult neurogenic zones (87). Chronic administration of a group II mGluR antagonist enhanced the number of BrdU-labelled cells in the SGZ (88). In the cerebellar EGL, proliferating GCPs do not express NMDARs and AMPA receptors, but they express kainate receptors (see section above). It is thus conceivable that kainate receptors control the rate of GCP proliferation or the exit from the cell cycle. Regarding mGluRs, treatment with a mGlu4 receptor enhancer reduces GCP proliferation (89). However, this effect may have been indirect, as it remains unknown whether GCPs express mGlu4 receptors. If mGlu5 receptors were present in GCPs, their activation may promote GCP proliferation as shown in the adult neurogenic zones.
GABAAR activation has essentially been shown to limit cell proliferation in embryonic and adult neurogenic zones (63; 65; 90; 91). The function of GABABRs on cell proliferation is less clear. In the neonatal cerebellum, it is conceivable that GABA acting at GABAARs contributes to cell cycle exit of GCPs, but this needs to be thoroughly examined.
FUNCTIONS OF GABA AND GLUTAMATE SIGNALING IN DISEASES OF THE GCPs
The balance between proliferation and programmed withdrawal from the cell cycle is critical to the differentiation of GCPs. Persistent proliferation may lead to the presence of undifferentiated cells as seen in tumors. If GABAergic and glutamatergic signaling play a role in the regulation of physiological neural proliferation, it follows that aberrations in GABA and glutamate signaling pathways may lead to neuro-pathophysiology or alternatively could be used to control cell proliferation in diseased tissue. Tumorigenesis is one such neuro-pathophysiology. Medulloblastomas are cerebellar tumors that are among the most common tumors in children (20–30% of all pediatric brain tumors in 5–10 years old children). They are highly malignant and are associated with substantial mortality (only 50% of patients survive 5 years after diagnosis). Moreover, irradiation results in severe neurological impairment. New approaches to the diagnosis and treatment of medulloblastoma are necessary and will likely come from a deeper understanding of the cellular and molecular basis of cerebellar development, and the biology of this tumor. The ontogeny of this tumor remains controversial since its original description in 1925 (92). These tumors have been postulated to arise from two sources: the neuroepithelial cells at the surface of the 4th ventricle (i.e. the first germinal zone) (93) and the GCPs of the EGL generating the so-called classic and desmoplastic medulloblastoma (94; 95), respectively. A recent study raised the possibility that white-matter cerebellar stem cells constitute an alternative cell of origin for medulloblastoma (96).
Desmoplastic medullablastoma cells have been shown to share electrophysiological properties with GCPs, displaying a variety of potassium and calcium currents, and most notably, currents in response to GABA application (97). Of the mGluRs, activation of mGluR4 in vivo inhibits the growth of medulloblastoma cell lines grafted in mice (98). In addition medulloblastoma tissue expresses high levels of mGluR4s. mGluR4 ligands may thus be good candidates to control medulloblastoma growth. The expression of ionotropic glutamate receptors by medulloblastoma is less clear. Whereas some molecular studies have shown that several medulloblastoma cell lines express the protein subunits of these receptors, whether they are assembled into functional receptors is unclear (99). In addition, as we have shown here that GCPs express functional GABAARs, GABAAR ligands may provide additional therapeutic targets assuming that they either directly or indirectly through mGluR regulation alter GCP proliferation and medulloblastoma growth.
Evidence from the postnatal forebrain and more recent evidence from embryonic stem cells shows GABA’s influence on cell proliferation to be largely inhibitory (91). While no broad generalizations can be made about the pathophysiology of cancer cells, several lines of evidence have supported the idea that GABA and activation of GABAARs may regulate proliferation in some cancers (for review see (100)). Further, recent studies suggest that GABABRs may also play a prominent role. Bacoflen, an agonist of metabotropic GABABRs has recently been shown to inhibit human hepatocellular carcinoma cell growth (101) and activation of GABABRs, with GABA, decreased proliferation in an immortalized pulmonary adenocarcinoma line (102). However, in certain cases, stimulation with GABA induces matrix metalloproteinases that serve to enhance cancer’s invasive potential (103; 104). Therefore, despite their long, established history as neurotransmitters, we still have much to learn about the potential of GABA and glutamate as regulators of proliferation, both in physiological and pathological contexts.
Acknowledgments
This work was supported by grants from the National Institute of Health (NS048256 and DC007681, A.B.) and the National Science Foundation Graduate Research Fellowship (K.A.D.)
References
- 1.Roberts E, Frankel S. gamma-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. [PubMed] [Google Scholar]
- 2.Curtis DR, Watkins JC. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- 3.Krnjevic K, Phillis JW. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon D. Chemical messengers in development: a hypothesis. Science. 1974;185:1012–1021. doi: 10.1126/science.185.4156.1012. [DOI] [PubMed] [Google Scholar]
- 5.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 6.Barker JL, Behar T, Li YX, Liu QY, Ma W, Maric D, Maric I, Schaffner AE, Serafini R, Smith SV, Somogyi R, Vautrin JY, Wen XL, Xian H. GABAergic cells and signals in CNS development. Perspect Dev Neurobiol. 1998;5:305–322. [PubMed] [Google Scholar]
- 7.Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 8.Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Bordey A. Adult Neurogenesis: Basic Concepts of Signaling. Cell Cycle. 2006;5:722–728. doi: 10.4161/cc.5.7.2614. [DOI] [PubMed] [Google Scholar]
- 10.Bordey A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res Brain Res Rev. 2007;53:124–134. doi: 10.1016/j.brainresrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol (Lond) 2008;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Weil-Malherbe H. Studies on brain metabolism: The metabolism of glutamic acid in brain. Biochem J. 1936;30:665–676. doi: 10.1042/bj0300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern JR, Eggleston LV, Hems R, Krebs HA. Accumulation of glutamic acid in isolated brain tissue. Biochem J. 1949;44:410–418. [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis DR, Phillis JW, Watkins JC. Chemical excitation of spinal neurones. Nature. 1959;183:611–612. doi: 10.1038/183611a0. [DOI] [PubMed] [Google Scholar]
- 16.Curtis DR, Phillis JW, Watkins JC. The depression of spinal neurones by gamma-amino-n-butyric acid and beta-alanine. J Physiol. 1959;146:185–203. doi: 10.1113/jphysiol.1959.sp006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awapara J, Landua AJ, Fuerst R, Seale B. Free gamma-aminobutyric acid in brain. J Biol Chem. 1950;187:35–39. [PubMed] [Google Scholar]
- 18.Florey E, McLennan H. Effects of an inhibitory factor (factor I) from brain on central synaptic transmission. J Physiol. 1955;130:446–455. doi: 10.1113/jphysiol.1955.sp005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basemore AW, Elliot KA, Florey E. Isolation of factor I. J Neurochem. 1957;1:334–339. doi: 10.1111/j.1471-4159.1957.tb12090.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuffler SW, Edwards C. Mechanism of gamma aminobutyric acid (GABA) action and its relation to synaptic inhibition. J Neurophysiol. 1958;21:589–610. doi: 10.1152/jn.1958.21.6.589. [DOI] [PubMed] [Google Scholar]
- 21.Krnjevic K, Randic M, Straughan DW. Cortical inhibition. Nature. 1964;201:1294–1296. doi: 10.1038/2011294a0. [DOI] [PubMed] [Google Scholar]
- 22.Krnjevic K, Schwartz S. Is gamma-aminobutyric acid an inhibitory transmitter? Nature. 1966;211:1372–1374. doi: 10.1038/2111372a0. [DOI] [PubMed] [Google Scholar]
- 23.Florey E. GABA: history and perspectives. Can J Physiol Pharmacol. 1991;69:1049–1056. doi: 10.1139/y91-156. [DOI] [PubMed] [Google Scholar]
- 24.Roberts E. GABA in the nervous system: the view at 50 years. Philadelphia: Lippincott Williams and Wilkins; 2000. Adventures with GABA: Fifty years on; pp. 1–24. [Google Scholar]
- 25.Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 26.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 27.Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 28.Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- 29.Feigenspan A, Gustincich S, Raviola E. Pharmacology of GABA(A) receptors of retinal dopaminergic neurons. J Neurophysiol. 2000;84:1697–1707. doi: 10.1152/jn.2000.84.4.1697. [DOI] [PubMed] [Google Scholar]
- 30.Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 31.Hill DR, Bowery NG. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981;290:149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- 32.Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 33.Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist. 2002;8:551–561. doi: 10.1177/1073858402238514. [DOI] [PubMed] [Google Scholar]
- 34.Jahr CE, Stevens CF. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987;325:522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- 35.Cull-Candy SG, Usowicz MM. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 37.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 38.Ascher P, Bregestovski P, Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 40.Altman J, Bayer SA. Development of the cerebellar system: In Relation to its Evolution, Structure and Functions. Boca Raton: CRC; 1997. [Google Scholar]
- 41.Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development. 1991;113:755–765. doi: 10.1242/dev.113.3.755. [DOI] [PubMed] [Google Scholar]
- 42.Kuhar SG, Feng L, Vidan S, Ross ME, Hatten ME, Heintz N. Changing patterns of gene expression define four stages of cerebellar granule neuron differentiation. Development. 1993;117:97–104. doi: 10.1242/dev.117.1.97. [DOI] [PubMed] [Google Scholar]
- 43.Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 44.Smith TC, Wang LY, Howe JR. Distinct kainate receptor phenotypes in immature and mature mouse cerebellar granule cells. J Physiol. 1999;517(Pt 1):51–58. doi: 10.1111/j.1469-7793.1999.0051z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith TC, Wang LY, Howe JR. Heterogeneous conductance levels of native AMPA receptors. J Neurosci. 2000;20:2073–2085. doi: 10.1523/JNEUROSCI.20-06-02073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi DJ, Slater NT. The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology. 1993;32:1239–1248. doi: 10.1016/0028-3908(93)90018-x. [DOI] [PubMed] [Google Scholar]
- 47.Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- 48.Meguro R, Ohishi H, Hoshino K, Hicks TP, Norita M. Metabotropic glutamate receptor 2/3 immunoreactivity in the developing rat cerebellar cortex. J Comp Neurol. 1999;410:243–255. doi: 10.1002/(sici)1096-9861(19990726)410:2<243::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 49.Knopfel T, Grandes P. Metabotropic glutamate receptors in the cerebellum with a focus on their function in Purkinje cells. Cerebellum. 2002;1:19–26. doi: 10.1007/BF02941886. [DOI] [PubMed] [Google Scholar]
- 50.Petralia RS, Wang YX, Singh S, Wu C, Shi L, Wei J, Wenthold RJ. A monoclonal antibody shows discrete cellular and subcellular localizations of mGluR1 alpha metabotropic glutamate receptors. J Chem Neuroanat. 1997;13:77–93. doi: 10.1016/s0891-0618(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 51.Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- 52.Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- 53.Makoff A, Lelchuk R, Oxer M, Harrington K, Emson P. Molecular characterization and localization of human metabotropic glutamate receptor type 4. Brain Res Mol Brain Res. 1996;37:239–248. doi: 10.1016/0169-328x(95)00321-i. [DOI] [PubMed] [Google Scholar]
- 54.Copani A, Casabona G, Bruno V, Caruso A, Condorelli DF, Messina A, Di GGV, Pin JP, Kuhn R, Knopfel T, Nicoletti F. The metabotropic glutamate receptor mGlu5 controls the onset of developmental apoptosis in cultured cerebellar neurons. Eur J Neurosci. 1998;10:2173–2184. doi: 10.1046/j.1460-9568.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- 55.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- 57.Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- 58.Bolteus AJ, Bordey A. GABA Release and Uptake Regulate Neuronal Precursor Migration in the Postnatal Subventricular Zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang DD, Krueger DD, Bordey A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J Neurophysiol. 2003;90:2291–2302. doi: 10.1152/jn.01116.2002. [DOI] [PubMed] [Google Scholar]
- 60.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 62.Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol (Lond) 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, Rigo JM. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- 65.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 66.Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 68.Fukuda A, Muramatsu K, Okabe A, Shimano Y, Hida H, Fujimoto I, Nishino H. Changes in intracellular Ca2+ induced by GABAA receptor activation and reduction in Cl− gradient in neonatal rat neocortex. J Neurophysiol. 1998;79:439–446. doi: 10.1152/jn.1998.79.1.439. [DOI] [PubMed] [Google Scholar]
- 69.Takayama C, Inoue Y. Developmental localization of potassium chloride co-transporter 2 in granule cells of the early postnatal mouse cerebellum with special reference to the synapse formation. Neuroscience. 2006;143:757–767. doi: 10.1016/j.neuroscience.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 70.Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–120. [PubMed] [Google Scholar]
- 71.Almarghini K, Remy A, Tappaz M. Immunocytochemistry of the taurine biosynthesis enzyme, cysteine sulfinate decarboxylase, in the cerebellum: evidence for a glial localization. Neuroscience. 1991;43:111–119. doi: 10.1016/0306-4522(91)90421-j. [DOI] [PubMed] [Google Scholar]
- 72.Barakat L, Wang D, Bordey A. Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J Physiol. 2002;541:753–767. doi: 10.1113/jphysiol.2001.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Prog Neurobiol. 1989;32:471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 74.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 75.Horikoshi T, Asanuma A, Yanagisawa K, Anzai K, Goto S. Taurine and beta-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Res. 1988;464:97–105. doi: 10.1016/0169-328x(88)90002-2. [DOI] [PubMed] [Google Scholar]
- 76.Puopolo M, Kratskin I, Belluzzi O. Direct inhibitory effect of taurine on relay neurones of the rat olfactory bulb in vitro. Neuroreport. 1998;9:2319–2323. doi: 10.1097/00001756-199807130-00031. [DOI] [PubMed] [Google Scholar]
- 77.Ben Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 78.Nacher J, McEwen BS. The role of N-methyl-D-asparate receptors in neurogenesis. Hippocampus. 2006;16:267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- 79.Schlett K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem. 2006;6:949–960. doi: 10.2174/156802606777323665. [DOI] [PubMed] [Google Scholar]
- 80.Komuro H, Yacubova E, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–540. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luk KC, Kennedy TE, Sadikot AF. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci. 2003;23:2239–2250. doi: 10.1523/JNEUROSCI.23-06-02239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luk KC, Sadikot AF. Glutamate and regulation of proliferation in the developing mammalian telencephalon. Dev Neurosci. 2004;26:218–228. doi: 10.1159/000082139. [DOI] [PubMed] [Google Scholar]
- 83.Hirasawa T, Wada H, Kohsaka S, Uchino S. Inhibition of NMDA receptors induces delayed neuronal maturation and sustained proliferation of progenitor cells during neocortical development. J Neurosci Res. 2003;74:676–687. doi: 10.1002/jnr.10795. [DOI] [PubMed] [Google Scholar]
- 84.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bernabeu R, Sharp FR. NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab. 2000;20:1669–1680. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Gandhi R, Luk KC, Rymar VV, Sadikot AF. Group I mGluR5 metabotropic glutamate receptors regulate proliferation of neuronal progenitors in specific forebrain developmental domains. J Neurochem. 2008;104:155–172. doi: 10.1111/j.1471-4159.2007.04955.x. [DOI] [PubMed] [Google Scholar]
- 87.Di Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, Biagioni F, Iacovelli L, Canudas AM, Parati E, De Maria R, Nicoletti F. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimizu T, Chaki S. Increased cell proliferation in the adult mouse hippocampus following chronic administration of group II metabotropic glutamate receptor antagonist, MGS0039. Biochem Biophys Res Commun. 2004;315:493–496. doi: 10.1016/j.bbrc.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 89.Canudas AM, Giorgi-Gerevini V, Iacovelli L, Nano G, D’Onofrio M, Arcella A, Giangaspero F, Busceti C, Ricci-Vitiani L, Battaglia G, Nicoletti F, Melchiorri D. PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors. J Neurosci. 2004;24:10343–10352. doi: 10.1523/JNEUROSCI.3229-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, Wilbertz J, Arenas E, Koltzenburg M, Charnay P, El Manira A, Ibanez CF, Ernfors P. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 92.Bailey P, Cushing H. Medulloblastoma cerebelli: Common type midcerebellar glioma of childhood. Arch Neurol Psych. 1925;14:192–223. [Google Scholar]
- 93.Yachnis AT, Rorke LB, Trojanowski JQ. Cerebellar dysplasias in humans: development and possible relationship to glial and primitive neuroectodermal tumors of the cerebellar vermis. J Neuropathol Exp Neurol. 1994;53:61–71. doi: 10.1097/00005072-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 94.Vandenberg SR, Herman MM, Rubinstein LJ. Embryonal central neuroepithelial tumors: current concepts and future challenges. Cancer Metastasis Rev. 1987;5:343–365. doi: 10.1007/BF00055377. [DOI] [PubMed] [Google Scholar]
- 95.Provias JP, Becker LE. Cellular and molecular pathology of medulloblastoma. J Neurooncol. 1996;29:35–43. doi: 10.1007/BF00165516. [DOI] [PubMed] [Google Scholar]
- 96.Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Codina C, Kraft R, Pietsch T, Prinz M, Steinhauser C, Cervos-Navarro J, Patt S. Voltage- and gamma-aminobutyric acid-activated membrane currents in the human medulloblastoma cell line MHH-MED-3. Neurosci Lett. 2000;287:53–56. doi: 10.1016/s0304-3940(00)01134-4. [DOI] [PubMed] [Google Scholar]
- 98.Iacovelli L, Arcella A, Battaglia G, Pazzaglia S, Aronica E, Spinsanti P, Caruso A, De Smaele E, Saran A, Gulino A, D’Onofrio M, Giangaspero F, Nicoletti F. Pharmacological activation of mGlu4 metabotropic glutamate receptors inhibits the growth of medulloblastomas. J Neurosci. 2006;26:8388–8397. doi: 10.1523/JNEUROSCI.2285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshioka A, Ikegaki N, Williams M, Pleasure D. Expression of N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor genes in neuroblastoma, medulloblastoma, and other cells lines. J Neurosci Res. 1996;46:164–178. doi: 10.1002/(SICI)1097-4547(19961015)46:2<164::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 100.Watanabe M, Maemura K, Oki K, Shiraishi N, Shibayama Y, Katsu K. Gamma-aminobutyric acid (GABA) and cell proliferation: focus on cancer cells. Histol Histopathol. 2006;21:1135–1141. doi: 10.14670/HH-21.1135. [DOI] [PubMed] [Google Scholar]
- 101.Wang T, Huang W, Chen F. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci. 2008;82:536–541. doi: 10.1016/j.lfs.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 102.Schuller HM, Al Wadei HA, Majidi M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis. 2008;29:1979–1985. doi: 10.1093/carcin/bgn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Azuma H, Inamoto T, Sakamoto T, Kiyama S, Ubai T, Shinohara Y, Maemura K, Tsuji M, Segawa N, Masuda H, Takahara K, Katsuoka Y, Watanabe M. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. 2003;63:8090–8096. [PubMed] [Google Scholar]
- 104.Inamoto T, Azuma H, Sakamoto T, Kiyama S, Ubai T, Kotake Y, Watanabe M, Katsuoka Y. Invasive ability of human renal cell carcinoma cell line Caki-2 is accelerated by gamma-aminobutyric acid, via sustained activation of ERK1/2 inducible matrix metalloproteinases. Cancer Invest. 2007;25:574–583. doi: 10.1080/07357900701522471. [DOI] [PubMed] [Google Scholar]