Summary
Over one third of all known proteolytic enzymes are serine proteases. Among these, the trypsins underwent the most predominant genetic expansion yielding the enzymes responsible for digestion, blood coagulation, fibrinolysis, development, fertilization, apoptosis, and immunity. The success of this expansion resides in a highly efficient fold that couples catalysis and regulatory interactions. Added complexity comes from the recent observation of a significant conformational plasticity of the trypsin fold. A new paradigm emerges where two forms of the protease, E* and E, are in allosteric equilibrium and determine biological activity and specificity.
Keywords: serine proteases, enzyme catalysis, thrombin, allostery
PREAMBLE
A typical genome contains 2–4% of genes encoding for proteolytic enzymes (1). Among these, serine proteases emerged during evolution as the most abundant and functionally diverse group (2, 3). Because abundance is a measure of success in evolutionary terms, the molecular mechanisms that ensure catalysis and regulation in these enzymes deserve attention. Much has been learned on how serine proteases catalyze the hydrolysis of a peptide bond and excellent reviews have covered this subject (4, 5). Surprisingly, however, the rules encoding specificity in a given protease fold remain incompletely understood (6). New developments from the structural investigation of a number of serine proteases have recently challenged old paradigms. Allostery, originally conceived to explain the properties of multisubunit enzymes (7), emerges as a key embodiment of the serine protease fold especially evident in the trypsins. This new dimension changes our understanding of serine protease function, regulation, and specificity.
GENERAL PROPERTIES OF SERINE PROTEASES
Barrett and coworkers have devised a classification scheme based on statistically significant similarities in sequence and structure of all known proteolytic enzymes and term this database MEROPS (8). The classification system divides proteases into clans based on catalytic mechanism and families on the basis of common ancestry. Over one third of all known proteolytic enzymes are serine proteases grouped into 13 clans and 40 families. The family name stems from the nucleophilic Ser in the enzyme active site, which attacks the carbonyl moiety of the substrate peptide bond to form an acyl-enzyme intermediate (4). Nucleophilicity of the catalytic Ser is typically dependent on a catalytic triad of Asp, His, and Ser residues, commonly referred to as the charge relay system (9). At least four distinct protein folds as illustrated by trypsin, subtilisin, prolyl oligopeptidase, and ClpP peptidase utilize the Asp-His-Ser catalytic triad in identical configuration to catalyze hydrolysis of peptide bonds (2). This is testimony to the success of the triad as a catalytic machinery in four distinct evolutionary pathways. Many serine proteases employ a simpler dyad mechanism where Lys or His is paired with the catalytic Ser. Other serine proteases mediate catalysis via novel triads of residues, such as a pair of His residues combined with the nucleophilic Ser. A summary of catalytic units in all serine protease families is given in Table 1.
Table 1.
General properties of serine proteases
| Clan | Families | Representative member | Catalytic residues | Primary specificity |
|---|---|---|---|---|
| PA | 12 | Trypsin | His, Asp, Ser | A, E, F, G, K, Q, R, W, Y |
| SB | 2 | Subtilisin | Asp, His, Ser | F, W, Y |
| SC | 2 | Prolyl oligopeptidase | Ser, Asp, His | G, P |
| SE | 6 | D-A, D-A carboxypeptidase | Ser, Lys | D-A |
| SF | 3 | LexA peptidase | Ser, Lys/His | A |
| SH | 2 | Cytomegalovirus assemblin | His, Ser, His | A |
| SJ | 1 | Lon peptidase | Ser, Lys | K, L, M, R, S |
| SK | 2 | Clp peptidase | Ser, His, Asp | A |
| SP | 3 | Nucleoporin | His, Ser | F |
| SQ | 1 | Aminopeptidase DmpA | Ser | A, G, K, R |
| SR | 1 | Lactoferrin | Lys, Ser | K, R |
| SS | 1 | L,D-carboxypeptidase | Ser, Glu, His | K |
| ST | 5 | Rhomboid | His, Ser | D, E |
Multiple members of each clan may give rise to a wide variety of primary specificities, as documented by the PA, SJ, and SQ clans.
Serine proteases are widely distributed in nature and found in all kingdoms of cellular life as well as many viral genomes. However, significant differences exist in the distribution of each clan across species. For example, clan PA proteases are highly represented in eukaryotes, but rare constituents of prokaryotic and plant genomes. Vertebrates boast an array of clan PA proteases responsible for a variety of extracellular processes. SB and SC clans are most represented in other organisms. Serine proteases are usually endoproteases and catalyze bond hydrolysis in the middle of a polypeptide chain. However, several families of exoproteases have been described that remove one or more amino acids from the termini of target polypeptide chains. Within the metazoan lineage, a select subset of protease families underwent significant gene duplication and divergence (3). In fact, four protease families by themselves account for over 40% of all proteolytic enzymes in humans. These are the ubiq-uitin-specific proteases responsible for regulated intracellular protein turnover (10), the adamalysins controlling growth factors and integrin function (11), prolyl oligopeptidases (12), and the trypsin-like serine proteases (2), which are also the largest group of homologous proteases in the human genome. Of 699 proteases in man, 178 are serine proteases and 138 of them belong to the S1 protease family. Abundance of S1 proteases suggests the protein fold presents a selective advantage relative to other proteases. The trypsin fold of the S1 protease family (Fig. 1) nestles catalytic efficiency, substrate selectivity, and multiple levels of regulation in a scaffold that is readily associated with other auxiliary protein modules. Because of the scopes of this review, we will limit our discussion to PA proteases and the trypsins in particular. Properties of the members of other clans listed in Table 1 are discussed in detail elsewhere (2).
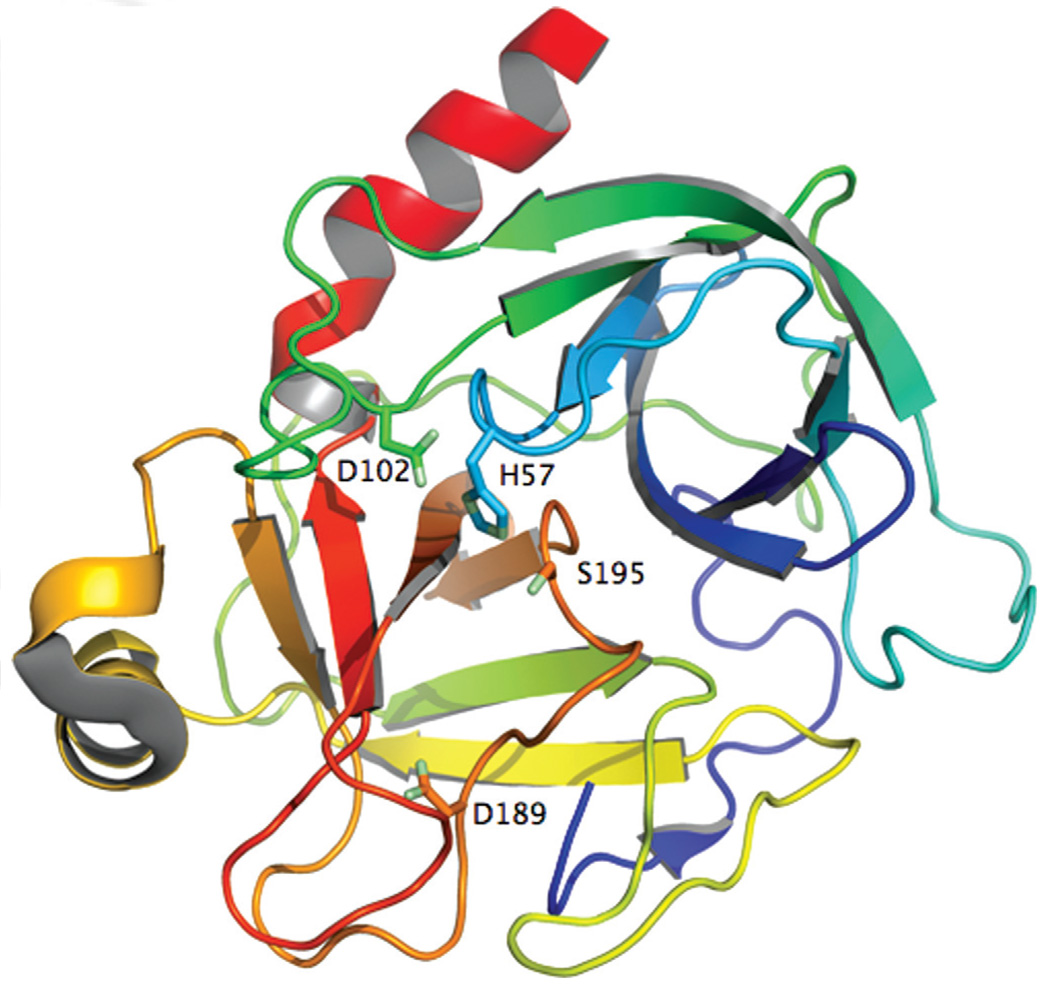
Figure 1.
Ribbon representation of trypsin (2PTN) (25), the prototypic example of serine protease from family S1 in clan PA (Table 1), colored according to the spectrum from the N-terminus (violet) to the C-terminus (red). The catalytic triad (sticks) is hosted at the interface of two similar β-barrels. D189 (sticks) occupies the bottom of the primary specificity pocket and confers specificity toward Arg or Lys side chain at the P1 position of substrate.
CLAN PA PROTEASES
Clan PA proteases bearing the trypsin fold are the largest family of serine proteases and perhaps the best studied group of enzymes. Digestive enzymes such as trypsin and chymotrypsin cleave polypeptide chains at positively charged (Arg/Lys) or large hydrophobic (Phe/Trp/Tyr) residues, respectively. Most clan PA proteases have trypsin-like substrate specificity and prefer Arg or Lys side chains at the P1 position of substrate (13). A number of key biological processes rely on clan PA proteases. Chief among them are blood coagulation and the immune response, which involve cascades of sequential zymogen activation. In both systems, the protease domain is combined with one or more apple, CUB, EGF, fibronectin, kringle, sushi, and von Willebrand factor domains. These domains are present on the N-terminus as an extension of the propeptide segment of the protease and typically remain attached to the protease domain through a covalent disulphide bond.
Family S1 of clan PA is partitioned into two subfamilies, S1A and S1B, that are phylogenetically distinct but share a common two β-barrel architecture. The S1B proteases are found in all cellular life and are responsible for intracellular protein turnover. In contrast, the S1A proteases are the trypsins that mediate a variety of extracellular processes. S1A proteases have a limited distribution in plants, prokaryotes, and the archaea. In humans, they are phylogenetically grouped into six functional categories: digestion, coagulation and immunity, tryptase, matriptase, kallikrein, and granzymes. A variety of enzymes are involved in the breakdown of proteins in the digestive system (14). Trypsins, chymotrypsins, and elastases are endoproteases that breakdown polypeptides into shorter chains. Further digestion is mediated by a number of exoproteases (15, 16). Tryptases are major components in the secretory granules of mast cells that are unique among clan PA proteases because of their homotetrameric structure (17). Matriptases are membrane bound S1 proteases bearing primary substrate selectivity similar to trypsin (18). Physiological substrates of this subfamily of proteases are largely unknown, but high gene expression levels for matriptases are associated with a variety of cancers (19, 20). Similar association with cancer has led to great interest in kallikreins (21), a large family better known for its role in regulation of blood pressure through the kinin system (22). Granzymes are mediators of directed apoptosis by natural killer cells and cytotoxic T cells that play key roles in the defense against viral infection (23). These enzymes are unique in their primary specificity for acidic residues.
THE CATALYTIC MECHANISM
Activation of many trypsin-like serine proteases requires proteolytic processing of an inactive zymogen precursor (24). Cleavage of the proprotein precursor occurs at the identical position in all known members of the family, that is, between residues 15 and 16 (chymotrypsinogen numbering). The nascent N-terminus induces conformational change in the enzyme through formation of an ion-pair with the highly conserved D194 that organizes both the oxyanion hole and substrate-binding site (25, 26). Alternatively, proteases like tissue-type plasminogen activator carry Lys at position 156 that engages D194 with an ion-pair that confers a catalytically competent fold without proteolytic cleavage at residue 15 (27).
Nearly all clan PA proteases utilize the canonical catalytic triad and hydrolyze the peptide bond via two tetrahedral intermediates (28). Initially, the hydroxyl O atom of the catalytic S195 attacks the carbonyl of the substrate peptide bond utilizing H57 as a general base. The oxyanion tetrahedral intermediate is stabilized by the backbone N atoms of G193 and S195, which together generate a positively charged pocket within the active site known as the oxyanion hole. H-bonding interactions in the oxyanion hole contribute 1.5–3.0 kcal/mol to ground and transition state stabilization (29). Collapse of the tetrahedral intermediate generates the acyl-enzyme intermediate and stabilization of the newly created N-terminus is mediated by H57. A water molecule displaces the free polypeptide fragment and attacks the acylenzyme intermediate. The oxyanion hole stabilizes the second tetrahedral intermediate of the pathway and collapse of this intermediate generates a new C-terminus in the substrate (4).
PRIMARY SPECIFICITY
The trypsin fold is remarkable for the even distribution of catalytic residues across the entire polypeptide sequence (Fig. 1). Two six-stranded β-barrels come together asymmetrically to host at their interface the residues of the catalytic triad. H57 and D102 belong to the N-terminal β-barrel with S195 and the oxyanion hole (S195 and G193) hosted by the C-terminal β-barrel. Eight surface exposed loops in the protein decorate the entrance to the active site. Within the same fold, trypsins prefer Arg/Lys side chains at the P1 position of substrate (13) but chymotrypsins prefer Phe/Tyr/Trp residues (2). The molecular origin of this difference resides in part in the architecture of the primary specificity pocket, shaped as a cavity about 10 Å deep and connected to the active site, at the bottom of which trypsins carry D189 and chymotrypsins carry S189. The simplicity of this arrangement belies the difficulty of swapping specificity between trypsin and chymotrypsin. Pioneering studies have shown that conversion of trypsin into chymotrypsin requires the expected D189S replacement, but also extensive additional replacements at loops not directly in contact with substrate (30). Despite these replacements, however, the conversion is never complete and enzyme activity in the mutant constructs remains poor. Furthermore, the same strategy fails in the reverse conversion of chymotrypsin into trypsin (31) or the conversion of trypsin into elastase (32). Recent mutagenesis studies have shown that the simple S189D replacement in chymotrypsin is sufficient to confer trypsin-like specificity when access to the primary specificity pocket is enhanced with the A226G substitution (33), a result that echoes the impressive recent successes in the redesign of primary specificity in the different protein scaffold of OmpT (34). Yet, the S189D/A226G mutant of chymotrypsin remains a poor protease, 5,000-fold slower than trypsin at cleaving substrates with Lys at the P1 position. Apparently, the structure- function link required to understand the molecular basis of serine protease specificity is missing a key element. The considerable conformational plasticity of the trypsin fold may hold important clues.
THE E* FORM
Recent kinetic studies on thrombin (35), the key serine protease of the blood coagulation cascade, vouch for an unexpected plasticity of the trypsin fold. Thrombin exists in three forms at equilibrium under physiological conditions. The Na+-free form E and the Na+-bound form E:Na+ are the low and high activity conformations of the enzyme, with E:Na+ being responsible for the procoagulant, prothrombotic, and signaling functions. A third form, E*, is in equilibrium with E and is basically inactive toward substrate and unable to bind Na+ (36). The structural differences between E and E:Na+ are subtle (37), almost disappointingly small considered the significant functional differences observed between the two forms in solution. However, enzymologists have long recognized that subtle short-scale rearrangements within the active site of an enzyme may lead to profound energetic changes in the ground and transition states (38, 39). On the other hand, E* differs dramatically from E and E:Na+ in its collapse of the 215–217 β-strand into the active site, a disruption of the oxyanion hole due to a flip of the peptide bond between E192 and G193 from a type II to a type I β-turn, and other changes around D189 F2 and the Na+ site (40) (Fig. 2). Importantly, the ion-pair between I16 and D194 remains intact, suggesting that E* is not equivalent to the zymogen form of thrombin. Existing structures of the zymogen forms of trypsin (26), chymotrypsin (41), and chymase (42) all feature a broken I16-D194 ion-pair and do not document a collapse of the 215–217 β-strand. Stopped-flow experiments demonstrate that the E*–E conversion takes place on a time scale <10 msec (36), as opposed to the zymogen-protease conversion that evolves over a much longer (100–1,000 msec) time scale (43, 44).
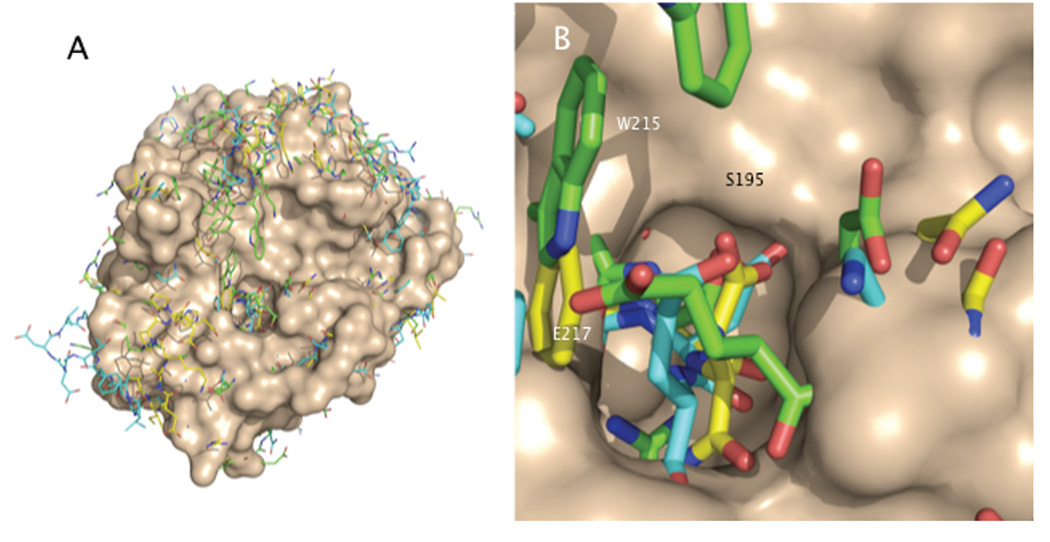
Figure 2.
A: The E* form shows a collapse of the 215–217 β-strand into the active site. The surface (wheat) refers to trypsin oriented as in Fig. 1. Sticks refer to the superimposed structures of thrombin (3BEI, green) (45), αI-tryptase (1LTO, cyan) (46), and prostasin (3DFJ, yellow) (47). B: Details of the collapse that completely occludes access to the active site. Black labels refer to trypsin. White labels indicate the positions of W215 and E217 in the E* form of thrombin.
E* is not a peculiarity of thrombin. Some of the most striking features of the E* conformation, like the collapse of the 215–217 β-strand into the active site and disruption of the oxyanion hole, have been observed in other structures of serine proteases in the free form (Fig. 2). In the inactive form of αI-tryp-tase, D216 collapses into the oxyanion hole (46). A collapsed 215–217 segment is observed in the structures of the high-temperature-requirement-like protease (48), complement factor D (49), granzyme K (50), hepatocyte growth factor activator (51), prostate kallikrein (52), and prostasin (47). A disrupted oxyanion hole is observed in complement factor B (53) and the arterivirus protease nsp4 (54). In all these cases the I16-D194 ion-pair remains intact, and therefore the conversion of E* into the active form E of the protease cannot involve the canonical zymogen-protease conversion or a cofactor-induced zymogen activation as observed for the prothrombin-staphylocoagulase interaction (55). The conformational transition of E* into E must follow other routes, as demonstrated recently for thrombin in unprecedented detail (45). Binding of ligands to E* away from the active site region organizes a network of H-bonds that induces a long-range rearrangement of the 215–217 β-strand and a flip of the oxyanion hole back into the active incarnations of the E form (45).
ALLOSTERY AND SERINE PROTEASES
All these recent observations support E* as an inactive form of the protease in allosteric equilibrium with the active form E, regardless of the ability of the protease to bind Na+ and further convert E into E:Na+. In other words, serine proteases are allosteric enzymes whose activity can be modulated by affecting the E*–E equilibrium. Allostery is often associated with largescale conformational transitions in multimeric proteins like hemoglobin (56), aspartate transcarbamylase (57), the nicotinic receptor (58), or GroEL (59). However, the ability of monomeric enzymes to express complex kinetic behavior like hysteresis (60), cooperativity (61), and allosteric regulation (62) has long been recognized. The molecular plasticity documented in monomeric proteins like serine proteases is further testimony to the importance of allostery as an intrinsic property of all dynamic proteins closely intertwined with catalysis (63). This plasticity undoubtedly dictates many aspects of protease function, regulation, and even substrate specificity (35, 64, 65).
What is the physiological advantage of the E*–E equilibrium? For each protease within its biological niche activity can be fine tuned by properly setting the E*–E equilibrium. In the case of complement factors, kallikreins, tryptase, and some coagulation factors activity must be kept to a minimun until binding of a trigger factor ensues. Stabilization of E* may afford a “resting” state of the protease waiting for action, a strategy implemented by other enzymes (66–68). The role of E* can be exploited in engineering studies aimed at stabilizing a protease in the inactive form until a cofactor unravels its full catalytic power. In fact, thrombin has been redesigned to display activity only in the presence of thrombomodulin and protein C along the anticoagulant pathway (69, 70). Notably, the structures of two anticoagulant variants have been solved (71, 72) and show collapse of the 215–217 β-strand and disruption of the oxyanion hole as seen in E* (40). These are exciting new developments that challenge old paradigms about the structural rigidity of the trypsin fold and the molecular basis of specificity. Future work on the properties of E* in serine proteases promises to be ground-breaking.
ACKNOWLEDGEMENTS
This work was supported by NIH research grants HL49413, HL58141, and HL73813.
REFERENCES
- 1.Puente XS, Sanchez LM, Gutierrez-Fernandez A, Velasco G, Lopez-Otin C. A genomic view of the complexity of mammalian proteolytic systems. Biochem. Soc. Trans. 2005;33:331–334. doi: 10.1042/BST0330331. [DOI] [PubMed] [Google Scholar]
- 2.Page MJ, Di Cera E. Serine peptidases: classification, structure and function. Cell Mol. Life Sci. 2008;65:1220–1236. doi: 10.1007/s00018-008-7565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page MJ, Di Cera E. Evolution of peptidase diversity. J. Biol. Chem. 2008;283:30010–30014. doi: 10.1074/jbc.M804650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedstrom L. Serine protease mechanism and specificity. Chem. Rev. 2002;102:4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- 5.Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Cera E. Engineering protease specificity made simple, but not simpler. Nat. Chem. Biol. 2008;4:270–271. doi: 10.1038/nchembio0508-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 8.Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2008;36:D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blow DM, Birktoft JJ, Hartley BS. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969;221:337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- 10.Ciechanover A, Iwai K. The ubiquitin system: from basic mechanisms to the patient bed. IUBMB Life. 2004;56:193–201. doi: 10.1080/1521654042000223616. [DOI] [PubMed] [Google Scholar]
- 11.Gomis-Ruth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Mol. Biotechnol. 2003;24:157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 12.Rea D, Fulop V. Structure-function properties of prolyl oligopeptidase family enzymes. Cell Biochem. Biophys. 2006;44:349–365. doi: 10.1385/CBB:44:3:349. [DOI] [PubMed] [Google Scholar]
- 13.Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 14.Rothman SS. The digestive enzymes of the pancreas: a mixture of inconstant proportions. Annu. Rev. Physiol. 1977;39:373–389. doi: 10.1146/annurev.ph.39.030177.002105. [DOI] [PubMed] [Google Scholar]
- 15.Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig. Dis. Sci. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 16.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 17.Pereira PJ, Bergner A, Macedo-Ribeiro S, Huber R, Matschiner G, Fritz H, Sommerhoff CP, Bode W. Human β-tryptase is a ring-like tetramer with active sites facing a central pore. Nature. 1998;392:306–311. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- 18.Lin CY, Anders J, Johnson M, Sang QA, Dickson RB. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J. Biol. Chem. 1999;274:18231–18236. doi: 10.1074/jbc.274.26.18231. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay AJ, Reid JC, Velasco G, Quigley JP, Hooper JD. The type II transmembrane serine protease matriptase-2—identification, structural features, enzymology, expression pattern and potential roles. Front. Biosci. 2008;13:569–579. doi: 10.2741/2702. [DOI] [PubMed] [Google Scholar]
- 20.Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, Antalis TM. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- 21.Diamandis EP, Yousef GM, Luo LY, Magklara A, Obiezu CV. The new human kallikrein gene family: implications in carcinogenesis. Trends Endocrinol. Metab. 2000;11:54–60. doi: 10.1016/s1043-2760(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 22.Proud D, Kaplan AP. Kinin formation: mechanisms and role in inflammatory disorders. Annu. Rev. Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- 23.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 24.Neurath H, Dixon GH. Structure and activation of trypsinogen and chymotrypsinogen. Fed. Proc. 1957;16:791–801. [PubMed] [Google Scholar]
- 25.Fehlhammer H, Bode W. The refined crystal structure of bovine β-trypsin at 1.8 A resolution. I. Crystallization, data collection and application of patterson search technique. J. Mol. Biol. 1975;98:683–692. doi: 10.1016/s0022-2836(75)80004-0. [DOI] [PubMed] [Google Scholar]
- 26.Fehlhammer H, Bode W, Huber R. Crystal structure of bovine trypsinogen at 1–8 A resolution. II. Crystallographic refinement, refined crystal structure and comparison with bovine trypsin. J. Mol. Biol. 1977;111:415–438. doi: 10.1016/s0022-2836(77)80062-4. [DOI] [PubMed] [Google Scholar]
- 27.Renatus M, Engh RA, Stubbs MT, Huber R, Fischer S, Kohnert U, Bode W. Lysine 156 promotes the anomalous pro-enzyme activity of tPA: X-ray crystal structure of single-chain human tPA. EMBO J. 1997;16:4797–4805. doi: 10.1093/emboj/16.16.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartley BS, Kilby BA. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem J. 1954;56:288–297. doi: 10.1042/bj0560288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bobofchak KM, Pineda AO, Mathews FS, Di Cera E. Energetic and structural consequences of perturbing Gly-193 in the oxyanion hole of serine proteases. J. Biol. Chem. 2005;280:25644–25650. doi: 10.1074/jbc.M503499200. [DOI] [PubMed] [Google Scholar]
- 30.Hedstrom L, Szilagyi L, Rutter WJ. Converting trypsin to chymotrypsin: the role of surface loops. Science. 1992;255:1249–1253. doi: 10.1126/science.1546324. [DOI] [PubMed] [Google Scholar]
- 31.Venekei I, Szilagyi L, Graf L, Rutter WJ. Attempts to convert chymotrypsin to trypsin. FEBS Lett. 1996;383:143–147. [PubMed] [Google Scholar]
- 32.Hung SH, Hedstrom L. Converting trypsin to elastase: substitution of the S1 site and adjacent loops reconstitutes esterase specificity but not amidase activity. Protein Eng. 1998;11:669–673. doi: 10.1093/protein/11.8.669. [DOI] [PubMed] [Google Scholar]
- 33.Jelinek B, Antal J, Venekei I, Graf L. Ala226 to Gly and Ser189 to Asp mutations convert rat chymotrypsin B to a trypsin-like protease. Protein Eng. Des. Sel. 2004;17:127–131. doi: 10.1093/protein/gzh014. [DOI] [PubMed] [Google Scholar]
- 34.Varadarajan N, Rodriguez S, Hwang B-Y, Georgiu G, Iverson BI. Engineering a family of highly active and selective endo-peptidases with programmed substrate specificities. Nat. Chem. Biol. 2008;4:290–294. doi: 10.1038/nchembio.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Cera E. Thrombin. Mol. Aspects. Med. 2008;29:203–254. doi: 10.1016/j.mam.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bah A, Garvey LC, Ge J, Di Cera E. Rapid kinetics of Na+ binding to thrombin. J. Biol. Chem. 2006;281:40049–40056. doi: 10.1074/jbc.M608600200. [DOI] [PubMed] [Google Scholar]
- 37.Pineda AO, Carrell CJ, Bush LA, Prasad S, Caccia S, Chen ZW, Mathews FS, Di Cera E. Molecular dissection of Na+ binding to thrombin. J. Biol. Chem. 2004;279:31842–31853. doi: 10.1074/jbc.M401756200. [DOI] [PubMed] [Google Scholar]
- 38.Kirby AJ, Hollfelder F. Biochemistry: enzymes under the nanoscope. Nature. 2008;456:45–47. doi: 10.1038/456045a. [DOI] [PubMed] [Google Scholar]
- 39.Sigala PA, Kraut DA, Caaveiro JM, Pybus B, Ruben EA, Ringe D, Petsko GA, Herschlag D. Testing geometrical discrimination within an enzyme active site: constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole. J. Am. Chem. Soc. 2008;130:13696–13708. doi: 10.1021/ja803928m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pineda AO, Chen ZW, Bah A, Garvey LC, Mathews FS, Di Cera E. Crystal structure of thrombin in a self-inhibited conformation. J. Biol. Chem. 2006;281:32922–32928. doi: 10.1074/jbc.M605530200. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Bode W, Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A? resolution. J. Mol. Biol. 1985;185:595–624. doi: 10.1016/0022-2836(85)90074-9. [DOI] [PubMed] [Google Scholar]
- 42.Reiling KK, Krucinski J, Miercke LJ, Raymond WW, Caughey GH, Stroud RM. Structure of human pro-chymase: a model for the activating transition of granule-associated proteases. Biochemistry. 2003;42:2616–2624. doi: 10.1021/bi020594d. [DOI] [PubMed] [Google Scholar]
- 43.Fersht AR. Conformational equilibria in -and -chymotrypsin. The energetics and importance of the salt bridge. J. Mol. Biol. 1972;64:497–509. doi: 10.1016/0022-2836(72)90513-x. [DOI] [PubMed] [Google Scholar]
- 44.Fersht AR, Requena Y. Equilibrium and rate constants for the interconversion of two conformations of -chymotrypsin. The existence of a catalytically inactive conformation at neutral pH. J. Mol. Biol. 1971;60:279–290. doi: 10.1016/0022-2836(71)90294-4. [DOI] [PubMed] [Google Scholar]
- 45.Gandhi PS, Chen Z, Mathews FS, Di Cera E. Structural identification of the pathway of long-range communication in an allosteric enzyme. Proc. Natl. Acad. Sci. USA. 2008;105:1832–1837. doi: 10.1073/pnas.0710894105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohr KB, Selwood T, Marquardt U, Huber R, Schechter NM, Bode W, Than ME. X-ray structures of free and leupeptin-complexed human alphaI-tryptase mutants: indication for an alpha→beta-tryptase transition. J. Mol. Biol. 2006;357:195–209. doi: 10.1016/j.jmb.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 47.Rickert KW, Kelley P, Byrne NJ, Diehl RE, Hall DL, Montalvo AM, Reid JC, Shipman JM, Thomas BW, Munshi SK, Darke PL, Su HP. Structure of human prostasin, a target for the regulation of hypertension. J. Biol. Chem. 2008;283:34864–34872. doi: 10.1074/jbc.M805262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krojer T, Garrido-Franco M, Huber R, Ehrmann M, Clausen T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature. 2002;416:455–459. doi: 10.1038/416455a. [DOI] [PubMed] [Google Scholar]
- 49.Jing H, Babu YS, Moore D, Kilpatrick JM, Liu XY, Volanakis JE, Narayana SV. Structures of native and complexed complement factor D: implications of the atypical His57 conformation and self-inhibitory loop in the regulation of specific serine protease activity. J. Mol. Biol. 1998;282:1061–1081. doi: 10.1006/jmbi.1998.2089. [DOI] [PubMed] [Google Scholar]
- 50.Hink-Schauer C, Estebanez-Perpina E, Wilharm E, Fuentes-Prior P, Klinkert W, Bode W, Jenne DE. The 2.2-A crystal structure of human pro-granzyme K reveals a rigid zymogen with unusual features. J. Biol. Chem. 2002;277:50923–50933. doi: 10.1074/jbc.M207962200. [DOI] [PubMed] [Google Scholar]
- 51.Shia S, Stamos J, Kirchhofer D, Fan B, Wu J, Corpuz RT, Santell L, Lazarus RA, Eigenbrot C. Conformational lability in serine protease active sites: structures of hepatocyte growth factor activator (HGFA) alone and with the inhibitory domain from HGFA inhibitor-1B. J. Mol. Biol. 2005;346:1335–1349. doi: 10.1016/j.jmb.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 52.Carvalho AL, Sanz L, Barettino D, Romero A, Calvete JJ, Romao MJ. Crystal structure of a prostate kallikrein isolated from stallion seminal plasma: a homologue of human PSA. J. Mol. Biol. 2002;322:325–337. doi: 10.1016/s0022-2836(02)00705-2. [DOI] [PubMed] [Google Scholar]
- 53.Ponnuraj K, Xu Y, Macon K, Moore D, Volanakis JE, Narayana SV. Structural analysis of engineered Bb fragment of complement factor B: insights into the activation mechanism of the alternative pathway C3-convertase. Mol. Cell. 2004;14:17–28. doi: 10.1016/s1097-2765(04)00160-1. [DOI] [PubMed] [Google Scholar]
- 54.Barrette-Ng IH, Ng KK, Mark BL, Van Aken D, Cherney MM, Garen C, Kolodenko Y, Gorbalenya AE, Snijder EJ, James MN. Structure of arterivirus nsp4. The smallest chymotrypsin-like proteinase with an alpha/beta C-terminal extension and alternate conformations of the oxyanion hole. J. Biol. Chem. 2002;277:39960–39966. doi: 10.1074/jbc.M206978200. [DOI] [PubMed] [Google Scholar]
- 55.Friedrich R, Panizzi P, Fuentes-Prior P, Richter K, Verhamme I, Anderson PJ, Kawabata S, Huber R, Bode W, Bock PE. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–539. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 56.Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 57.Kantrowicz ER, Lipscomb WM. Escherichia coli aspartate transcarbamylase: the molecular basis for a concerted allosteric transition. Trends Biochem Sci. 1990;15:53–59. doi: 10.1016/0968-0004(90)90176-c. [DOI] [PubMed] [Google Scholar]
- 58.Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2006;308:1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 59.Xu Z, Horwich AL, Sigler PB. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature. 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- 60.Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J. Biol. Chem. 1970;245:5788–5799. [PubMed] [Google Scholar]
- 61.Botts J, Morales M. Analytical description of the effects of modifiers and of multivalency upon the steady state catalyzed reaction rate. Trans. Faraday Soc. 1953;49:696–707. [Google Scholar]
- 62.Ainslie GR, Jr, Shill JP, Neet KE. Transients and cooperativity. A slow transition model for relating transients and cooperative kinetics of enzymes. J. Biol. Chem. 1972;247:7088–7096. [PubMed] [Google Scholar]
- 63.Goodey NM, Benkovic SJ. Allosteric regulation and catalysis emerge via a common route. Nat. Chem. Biol. 2008;4:474–482. doi: 10.1038/nchembio.98. [DOI] [PubMed] [Google Scholar]
- 64.Hedstrom L. Trypsin: a case study in the structural determinants of enzyme specificity. Biol. Chem. 1996;377:465–470. [PubMed] [Google Scholar]
- 65.Di Cera E. A structural perspective on enzymes activated by monovalent cations. J. Biol. Chem. 2006;281:1305–1308. doi: 10.1074/jbc.R500023200. [DOI] [PubMed] [Google Scholar]
- 66.Eisenmesser EZ, Bosco DA, Akke M, Kern D. Enzyme dynamics during catalysis. Science. 2002;295:1520–1523. doi: 10.1126/science.1066176. [DOI] [PubMed] [Google Scholar]
- 67.Lu HP, Xun L, Xie XS. Single-molecule enzymatic dynamics. Science. 1998;282:1877–1882. doi: 10.1126/science.282.5395.1877. [DOI] [PubMed] [Google Scholar]
- 68.Sytina OA, Heyes DJ, Hunter CN, Alexandre MT, van Stokkum IH, van Grondelle R, Groot ML. Conformational changes in an ultrafast light-driven enzyme determine catalytic activity. Nature. 2008;456:1001–1004. doi: 10.1038/nature07354. [DOI] [PubMed] [Google Scholar]
- 69.Cantwell AM, Di Cera E. Rational design of a potent anticoagulant thrombin. J. Biol. Chem. 2000;275:39827–39830. doi: 10.1074/jbc.C000751200. [DOI] [PubMed] [Google Scholar]
- 70.Gibbs CS, Coutre SE, Tsiang M, Li WX, Jain AK, Dunn KE, Law VS, Mao CT, Matsumura SY, Mejza SJ, Paborsky LR, Leung LLK. Conversion of thrombin into an anticoagulant by protein engineering. Nature. 1995;378:413–416. doi: 10.1038/378413a0. [DOI] [PubMed] [Google Scholar]
- 71.Carter WJ, Myles T, Gibbs CS, Leung LL, Huntington JA. Crystal structure of anticoagulant thrombin variant E217K provides insights into thrombin allostery. J. Biol. Chem. 2004;279:26387–26394. doi: 10.1074/jbc.M402364200. [DOI] [PubMed] [Google Scholar]
- 72.Pineda AO, Chen ZW, Caccia S, Cantwell AM, Savvides SN, Waksman G, Mathews FS, Di Cera E. The anticoagulant thrombin mutant W215A/E217A has a collapsed primary specificity pocket. J. Biol. Chem. 2004;279:39824–39828. doi: 10.1074/jbc.M407272200. [DOI] [PubMed] [Google Scholar]