Abstract
During development, elaborate patterns of cell differentiation and movement must occur in the correct locations and at the proper times. Developmental timing has been studied less than spatial pattern formation and the mechanisms integrating the two are poorly understood. Border cell migration in the Drosophila ovary occurs specifically at stage 9. Timing of the migration is regulated by the steroid hormone ecdysone, whereas spatial patterning of the migratory population requires localized activity of the JAK/STAT pathway. Ecdysone signaling is patterned spatially as well as temporally, although the mechanisms are not well understood. In stage 9 egg chambers, ecdysone signaling is highest in anterior follicle cells including the border cells. We identify the gene abrupt as a repressor of ecdysone signaling and border cell migration. Abrupt protein is normally lost from border cell nuclei during stage 9, in response to JAK/STAT activity. This contributes to the spatial pattern of the ecdysone response. Abrupt attenuates ecdysone signaling via a direct interaction with the bHLH domain of the P160 ecdysone receptor coactivator Taiman (Tai). Together these findings provide a molecular mechanism by which spatial and temporal cues are integrated.
INTRODUCTION
During development, elaborate patterns of cell differentiation and movement unfold in the correct locations and at the proper times due to programmed changes in patterns of gene expression. Precise spatial patterns of gene expression commonly develop in response to graded morphogens, which cause distinct patterns of gene expression at different concentrations and thereby pattern cell fates1-3.
Although developmental timing has been studied less than spatial patterning, it also depends on differential gene expression, and three general mechanisms have been recognized4. One mechanism, originally defined in C. elegans, is the regulation of transitions between larval stages by microRNAs5-7. A second mechanism is the regulation of larval transitions and metamorphosis in insects by hormone pulses8. Similarly, steroid hormones control puberty in mammals9, 10. Larval molts, metamorphosis and puberty are all global developmental transitions that involve the entire organism. More local developmental timing, such as the sequential production of ganglion mother cells and neurons from neuroblasts in the developing Drosophila nervous system employs cascades of transcription factors acting in series with no known input from microRNAs or hormones1. A significant remaining challenge is to elucidate the mechanisms responsible for integrating spatial and temporal patterning and to understand how global timing factors relate to local networks4.
One example of a specific cell behavior for which both spatial and temporal control mechanisms have been defined is migration of the border cells in the Drosophila ovary, which occurs specifically at stage 911-13. Border cells are a group of 6-8 cells that originate from the follicle cell epithelium. Border cells migrate in between nurse cells and reach the anterior border of the oocyte by stage 10 (Fig. 1a-c). Timing of the migration is regulated by the steroid hormone ecdysone14. Ecdysone synthesis rises during stage 9 and peaks at stage 1015. Inhibition of ecdysone synthesis or widespread loss of ecdysone receptor (EcR) function results in arrest of egg chamber development at stage 816-18, whereas loss of EcR function specifically in border cells leads to border cell migration defects in otherwise normal egg chambers14.
Figure 1. Spatial patterns of ecdysone signaling in stage 8-9 egg chambers.
a-c) Confocal micrographs of egg chambers of the indicated stages triple labeled with DAPI (blue) to stain all nuclei, GFP (green) to show the slbo-GAL4 expression pattern and anti-beta-galactosidase antibodies (red) to show the pattern of expression of the ecdysone reporter EcRE-lacZ. 15 Nurse cells (nc) and one oocyte (o) are surrounded by follicle cells to form a egg chamber. Arrows indicate border cells. Filled arrowheads indicate posterior follicle cells and open arrowheads indicate nurse-cell-associated follicle cells. d-f) the same micrographs as those shown in a-c showing the anti-beta-galactosidase staining only. g) Surface view to show nurse cell associated follicle cell expression of EcRE-lacZ in wild-type. h-i) Effect of dominant-negative EcR (EcRDN) on EcRE-lacZ expression in stage 10 egg chambers. h) Expression of EcRDN with slbo-GAL4 and mCD8-GFP (green) specifically reduced border cell (arrow) but not nurse cell associated follicle cell (open arrowheads) expression of beta-galactosidase (red). i) Same egg chamber as h, showing red channel only. j-l) Expression of a second ecdysone reporter (hs-GAL4-USP;UAS-mCD8-GFP) in stages 8-9 egg chambers. Scale bars represent 50 μm.
Spatial patterning of the migratory border cell population requires localized STAT activity19. The morphogen Unpaired is secreted by two follicle cells at each end of the egg chamber and activates STAT in a graded manner20. Loss of function of any component of the JAK/STAT pathway impairs border cell specification and migration19,21. Negative feedback regulation by the STAT target gene Apontic converts the graded STAT response into “on” (migratory) and “off” (non-migratory) states22.
Ecdysone signaling is patterned spatially as well as temporally in embryos23 and ovaries24, although the mechanisms are unclear. Understanding these mechanisms is important for understanding cell-type specific responses to global signals. Here we report that in stage 9 egg chambers, ecdysone signaling is highest in anterior follicle cells including the border cells. We identify the gene abrupt as a repressor of ecdysone signaling and border cell migration. Abrupt protein is widely expressed, however it is normally lost from border cell nuclei during stage 9, in response to STAT activity. We show that Abrupt attenuates ecdysone signaling via a direct interaction with the bHLH domain of the P160 EcR coactivator Tai. A form of Tai lacking the bHLH domain is hyperactive and renders the cells insensitive to Abrupt-mediated repression. Ecdysone signaling feeds back to further down-regulate Abrupt protein expression. Together these findings show that Abrupt represents a node of integration for steroid hormone and JAK/STAT signals.
RESULTS
Spatial pattern of the ecdysone response
To evaluate the pattern of ecdysone signaling, we examined the patterns of three different reporters. The first reporter is a transgene containing seven copies of an EcR responsive element (EcRE) upstream of a minimal promoter and the E. coli lacZ gene. Although present in every cell, it should only be expressed in those cells exposed to ecdysone and competent to respond to it23. We detected little or no expression of EcRE-lacZ prior to stage 9 in wild-type ovaries (Fig. 1a, d). Throughout stage 9, expression was detected in anterior follicle cells, including migrating border cells and nurse cell-associated follicle cells (Fig. 1b-g). EcRE-lacZ expression was reduced in border cells expressing a dominant-negative form of EcR (EcRDN) using slbo-GAL4, which drives expression specifically in border cells (Fig. 1h, i). Their migration was also strongly inhibited, consistent with earlier findings25. A similar pattern was observed for two other reporters, hs-GAL4-USP (Figure 1j-l) and hs-GAL4-EcR (not shown)23, 26, in which the ligand-binding domain of Ultraspiracle (USP) or EcR is fused to GAL4 rendering it hormone-sensitive. These findings were consistent with an earlier study that showed anterior follicle cell expression of these reporters at later stages24, and raise the question as to how this spatial pattern arises.
Although the precise domain of ecdysone synthesis is not known, it is produced within the egg chamber8, 15, 27. Some enzymes in the biosynthetic pathway are expressed in germline cells and others are found predominantly in follicle cells17, 28-32, suggesting that the lipophilic intermediates diffuse from one cell type to the other. Therefore, spatially localized ecdysone synthesis seems unlikely. Another possibility is that either the receptor is expressed in a spatially restricted pattern. There are three isoforms of EcR: EcRA, EcRB1, and EcRB2. Antibodies specific for EcRA label all cells of the egg chamber equally at all stages14, 16 (Fig. 2a). Similarly USP, the heterodimeric partner of EcR, is uniformly distributed (Fig. 2b). The B1 isoform of EcR was more highly expressed in follicle cells than germline cells and showed a 4-fold enrichment in anterior follicle cells at early stage 9 (Fig. 2c, e). This enrichment was less apparent by mid stage 9 (Fig. 2d) and was undetectable by stage 10 (not shown). There is no specific antibody against EcRB2. The P160 EcR co-activator Tai is enriched in follicle cells relative to the germline14 but is uniform within that population.
Figure 2. Normal and ectopic expression of ecdysone receptor isoforms.
a-d) Immunofluorescence staining for the indicated ecdysone receptor isoforms. Follicle cells (open arrowheads), border cells (arrows) and nurse cells (open arrows) are indicated. e) Quantification of the indicated ecdysone receptor subunits. The ratio of immunofluorescence to DAPI staining was measured for 123 border cells and 123 posterior follicle cells at early stage 9. The ratio of border cell (bc) to follicle cell (fc) signal is shown. The error bars represent the standard deviation (n=5-7). f-i) Effects of over-expression of EcRA on EcRE-lacZ expression (red) in clones of follicle cells (green) generated using FLP-OUT GAL4 (see methods for details). f, g) Border cells and h, i) nurse cell associated follicle cells over-expressing EcRA exhibit reduced EcRE-lacZ expression (arrows) compared to neighboring wild-type cells (open arrowheads). j, k) stage 10 egg chamber from a slbo-GAL4; UAS-GFP; EcRE-lacZ female. l, m) Down-regulation of EcRA expression via RNAi. slbo-GAL4; UAS-GFP; UAS-EcRA-dsRNA results in elevated EcRE-lacZ, which is particularly evident in posterior follicle cells (arrow) which normally express little or no detectable β-Galactosidase (arrow in k). n, o) Over-expression of EcRB1 using slbo-GAL4 elevates EcRE-lacZ in posterior follicle cells (compare to k). While all cells that show ectopic EcRE-lacZ also express some GFP, we consistently observed higher levels of EcRE-lacZ in cells expressing low levels of GFP. Scale bars = 50 μm.
To explore the functions of the EcR isoforms, we used the FLP-OUT technique to over-express each one in the presence of the EcRE-lacZ reporter. In anterior follicle cells, including border cells, EcRA over-expression caused a reduction in EcRE-lacZ expression relative to neighboring wild-type cells (Fig. 2f-i). Consistent with this result, expression of an EcRA-specific RNAi construct using slbo-GAL4 increased EcRE-lacZ in the slbo expression domain (Fig. 2j-m). Similarly, over-expression of EcRA in the wing imaginal disk reduces ecdysone target gene expression33. In contrast, over-expression of EcRB1 (Fig. 2n, o) or B2 (not shown) increased EcRE-lacZ expression. These findings suggest that the relative expression of different EcR isoforms could affect the magnitude of the ecdysone response.
Identification of Abrupt as a repressor of ecdysone signaling
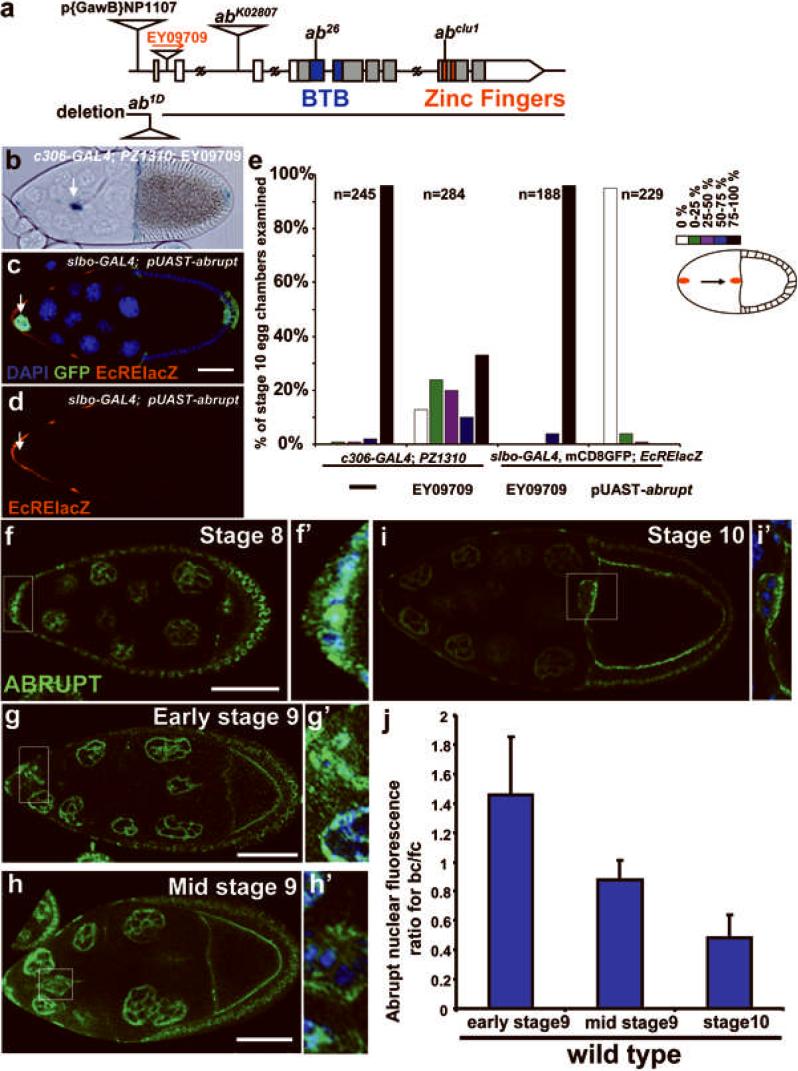
The elevated ratio of EcRB to EcRA in anterior follicle cells compared to posterior cells may contribute to the pattern of the ecdyone response. However, the enrichment of EcRB1 was transient and therefore did not seem to account fully for the EcRE-lacZ expression pattern. Therefore we postulated that, in addition, there might be a repressor of ecdysone signaling that is differentially down-regulated in anterior follicle cells. When over-expressed in border cells, such a factor should inhibit migration. Therefore we over-expressed random genes in border cells by crossing the c306-GAL4 line, which drives expression to high levels in anterior and posterior follicle cells (Fig. S1), to 1,942 EP and EY lines from the Bloomington stock center. Out of 20 lines that caused border cell migration defects, two also reduced EcRE-lacZ expression. The strongest effect was due to an EY insertion (EY09709) into the locus known as abrupt, which encodes a BTB domain and zinc finger protein (Fig. 3a-e). When crossed to c306-GAL4, EY09709 led to incomplete migration in 70% of stage 10 egg chambers (Fig. 3e). Over-expression of Abrupt using a UAS-abrupt transgene and slbo-GAL4 caused nearly complete inhibition of border cell migration (Fig. 3e). These findings suggested that Abrupt could be a repressor of ecdysone signaling.
Figure 3. Abrupt represses ecdysone signaling in Drosophila egg chambers.
a) Genomic organization of the abrupt (ab) locus and domain structure of Abrupt protein. b) Stage 10 egg chamber showing border cell migration defect caused by Abrupt over-expression using c306-GAL4. PZ1310 is a slbo enhancer trap line used to mark border cells (arrow) and centripetal follicle cells. c, d) Stage 10 egg chamber of the genotype slbo-GAL4, mCD8GFP/+; UAS-ab/EcRE-lacZ stained with anti-beta-galactosidase antibodies (red) and DAPI (blue). GFP indicates the slbo-GAL4 expression domain. EcRE-lacZ staining was reduced in border cells (arrow). e) Quantification of border cell migration in the indicated genotypes. The migration path was divided into five sections as shown in the schematic drawing on the right side. The indicated number (n) of stage 10 egg chambers were evaluated for the extent of border cell migration. White bars represent the percentage of egg chambers in which border cells failed to detach from the anterior of the egg chamber. Black bars represent the percentage of egg chambers in which border cells migrated all the way to the oocyte. Other colors represent intermediate phenotypes. f-i) Anti-Abrupt antibody staining (green). f'-i') Higher magnification of the boxed regions shown in (f-i) including DAPI (blue) to mark all nuclei. Scale bars = 50 μm. j) The fluorescence intensity of anti-Abrupt antibody staining was measure for 42 border cells (bc) and 44 oocyte-associated follicle cells (fc) to quantify the change in Abrupt concentration over time. Error bars indicate the standard deviation.
An antibody against Abrupt showed widespread nuclear staining of germline and somatic cells (Fig. 3f-i). Interestingly, the nuclear Abrupt protein accumulation decreased specifically in border cells throughout stage 9. To quantify the effect, we measured the ratio of Abrupt/DAPI fluorescence intensity (Fig. 3j). Prior to migration, presumptive border cells expressed a level of nuclear Abrupt protein equivalent to that of other follicle cells (Figs. 3f' and 3j). As border cells migrated, this protein level decreased (Fig. 3g', h'. and j) until it was undetectable (Figs. 3i' and j). The nuclear Abrupt staining was specific because it was lost from follicle cell clones of the null allele (Fig. S2 h-j). Such clones were infrequent and were only detected in early stage egg chambers, suggesting that abrupt loss of function was cell lethal. In addition to nuclei, the Abrupt antibody stained the apical surfaces of follicle cells, the oocyte cortex, and ring canals. In the border cells, cortical staining was evident, which did not decrease during stage 9 as the nuclear staining did. It is unclear what the function is of the cortical protein, or if it is specific.
If Abrupt normally contributes to the spatial pattern of ecdysone signaling then its loss should cause elevated or ectopic EcRE-lacZ expression. Since loss of abrupt was cell lethal in mosaic clones, we examined egg chambers from females that were transheterozygous for combinations of hypomorphic abrupt alleles34-36(Fig. 3a, S2 a-e). Ectopic EcRE-lacZ expression in posterior follicle cells occurred in more than 50% of egg chambers from abclu1/abk02807, abk02807/abP[GawB]NP1107 and ab26/abP[GawB]NP1107 (Fig. S2 a-g). Therefore, both loss- and gain-of-function experiments indicated that Abrupt was a repressor of ecdysone signaling.
Interactions between Abrupt and Tai in vitro and in vivo
The effects of Abrupt were precisely opposite of those caused by the EcR co-activator Tai, suggesting that Abrupt might exert its effect on ecdysone signaling by antagonizing Tai. To test for an interaction between Tai and Abrupt we carried out co-immunoprecipitation (co-IP). Lysates from S2 cells expressing Abrupt alone or Abrupt and full-length Tai [Tai(FL)] were incubated with either control IgG or with anti-Tai antibody. Immunoprecipitates were then subjected to SDS-PAGE and Western blotting with the anti-Abrupt antibody. Abrupt protein co-precipitated with Tai(FL) (Fig. 4a).
Figure 4. Tai and Abrupt proteins interact.
a) Co-immunoprecipitation (IP) between full length Tai[Tai(FL)] and Abrupt. Extracts from S2 cells expressing Abrupt together with Tai(FL) or a form of Tai lacking the bHLH domain [Tai(ΔB)] were incubated with non-specific rabbit IgG antibodies (rab IgG) or with anti-Tai antibodies. Immunoprecipitates were subjected to Western blotting (WB) and probed with anti-Abrupt (αAB) antibody. b) Arrangement of domains in Tai. c) Extracts from S2 cells expressing a FLAG-tagged bHLH domain from Tai and Abrupt were IPed with or without anti-FLAG antibody and Western blotted with αAB. d) GST pulldown showing interaction between the BTB domain of Abrupt and the bHLH domain of Tai.
Like other P160 coactivators, Tai possesses N-terminal basic-helix-loop-helix (bHLH) and PAS domains, LXXLL motifs, and glutamine-rich transactivation domains (Fig. 4b). The LXXLL domain in mammalian proteins37 and in Tai14 mediates ligand-dependent binding to hormone receptors. However the functions of the bHLH and PAS domains have been enigmatic. Intriguingly, Tai lacking the bHLH domain [Tai(ΔB)] failed to interact with Abrupt (Fig. 4a). Consistent with this observation, FLAG-tagged bHLH domain alone did co-IP with Abrupt (Fig. 4c).
To determine which domain within Abrupt was responsible for the interaction with Tai, we carried out GST pull-down assays. GST fused to the bHLH domain pulled down 35S-labeled BTB domain but not the Zn2+ finger domain (Fig. 4d). In contrast, the GST-fused LXXLL domain did not interact (Fig. 4d). Taken together these results indicate that the BTB domain of Abrupt binds directly to the bHLH domain of Tai.
Since Abrupt is a repressor of ecdysone signaling and Tai is an activator, their interaction should be antagonistic. Therefore Tai(ΔB), which cannot interact with Abrupt should enhance ecdysone signaling in vivo, compared to Tai(FL). To test this hypothesis we generated transgenic flies expressing GAL4-inducible Tai(FL) or Tai(ΔB) and expressed them in egg chambers using slbo-GAL4 (Fig. 5). We expressed these constructs, as well as several other truncated proteins (Fig. 5a), in wild-type border cells to test for dominant effects (Fig. S3 and S4) and in homozygous tai mutant border cells to test for rescue (Fig. S5). We included EcRE-lacZ in the genetic background so that we could monitor the level of ecdysone signaling.
Figure 5. Effects of Tai constructs on ecdysone dependent transcription in vivo.
a) Schematic diagram showing the domains found in full length and truncated Tai proteins. The bHLH (B), PAS (P), LXXLL and transactivation domain containing polyglutamine repeats (QQQ) are shown. In some cases, an exogenous nuclear localization sequence (NLS) and/or FLAG tag or GFP tag were included as indicated. Ab shows the epitope which was used to generate Tai antibody.
b-i) Immunofluorescence micrographs of stage 10 egg chambers expressing the indicated transgenes induced with slbo-GAL4. (b, d, f and h) The merged images display with DAPI (blue), GFP and the anti-beta-galactosidase staining (red). (c, e, g and i) The same micrographs as those in (b, d, f and h) show the anti-beta-galactosidase staining only. j-k) Beta-galactosidase activity was measured from purified slbo-GAL4 expressing follicle cells53 of the indicated genotypes. Values are reported as a fold-change relative to wild-type. Error bars represent the standard deviation (n=3-5). Scale bars = 50 μm.
Over-expression of Tai(FL) using slbo-GAL4 led to a pronounced increase in EcRE-lacZ expression in posterior follicle cells (Fig. 5b, c) but did not lead to precocious activation of EcRE-lacZ and did not affect border cell migration, even when two copies of Tai(FL) were expressed (not shown). Expression of the LXXLL domain fused to GFP impaired border cell migration and reduced expression of EcRE-lacZ (Fig. 5d, e), consistent with a dominant-negative effect, which was expected since this domain can bind to the receptor in a hormone-dependent manner but cannot activate transcription14. In contrast, expression of Tai(ΔB) caused a dramatic increase in EcRE-lacZ expression in the slbo-GAL4 expression domain (Fig. 5f, g). This construct caused the strongest dominant inhibition of border cell migration of any of those tested. In addition, Tai(ΔB), unlike any other construct, led to precocious EcRE-lacZ activity at the very onset of slbo-GAL4 expression in stage 8 (Fig. 5h, i).
To quantify these effects, we purified the slbo-GAL4-expressing cells and measured their beta-galactosidase activity level. Relative to the control, over-expression of full length Tai(FL) increased the beta-gal activity by a factor of 12, whereas Tai(ΔB) caused a 30-fold increase (Fig. 5j). In contrast Tai(LXXLLGFP) decreased beta-gal activity to 40% of the control, similar to the effect of a dominant-negative EcR (Fig. 5k). This analysis established that, in vivo, the bHLH domain acts as an inhibitory domain, which attenuates the ecdysone response. In the absence of this domain, ecdysone signaling is both precocious and hyperactive.
We also tested the ability of the truncated Tai proteins to rescue tai mutant border cells using MARCM (Mosaic Analysis with a Repressive Cell Marker) analysis38. In the absence of a rescuing transgene, tai61G1 mutant border cells exhibit a fully penetrant border cell migration defect (Fig. 6a). Tai(FL) rescued this phenotype to wild-type (Fig. 6b) whereas Tai(ΔB) provided partial rescue (Fig. 6c). Tai(LXXLL) (Fig. 6d), Tai(bHLH), Tai(PAS) and Tai(BP) (Fig. S5) failed to rescue. Deletion of the PAS domain or the C-terminus did not impair rescue, suggesting that these domains are not essential in border cells (Fig. S5a, b). We conclude that the bHLH domain, which binds to Abrupt, is an essential negative regulatory domain in vivo.
Figure 6. Rescue of tai mutant border cells by full length and truncated Tai proteins.
Homozygous tai mutant clones are labeled with GFP using the MARCM technique (see methods for details), either in the absence (a) or presence (b-d) of expression of the indicated Tai transgenes. In each case, the indicated number (n) of egg chambers was analyzed in which all border cells were GFP-positive and thus homozygous tai-/-. Histograms were constructed as described for Figure 3e. White bars represent the percentage of egg chambers in which border cells failed to detach from the anterior of the egg chamber. Black bars represent the percentage of egg chambers in which border cells migrated all the way to the oocyte. Other colors represent intermediate phenotypes as shown in the schematic. e-j) Effect of Abrupt (AB) overexpression in the presence of Tai(FL) (e-g) or Tai(ΔB) (h-j). The red channel shows anti-beta-galactorsidase expression from EcRE-lacZ. The purple channel shows Tai (g) or Abrupt (j) antibody staining. Scale bars = 50 μm.
If Abrupt inhibits the ecdysone response by interacting with the bHLH domain of Tai, then cells expressing Tai(ΔB), which cannot interact with Abrupt, should be insensitive to Abrupt-mediated repression. To test this hypothesis, we co-expressed Abrupt with either Tai(FL) or Tai(ΔB). Abrupt inhibited EcRE-lacZ expression in the presence of Tai(FL) (Fig. 6e-g) but had no effect on Tai(ΔB) (Fig. 6h-j), demonstrating that the bHLH domain of Tai was required for Abrupt-mediated repression of the ecdysone response in vivo.
Precocious border cell migration induced by early ecdysone and JAK/STAT signaling
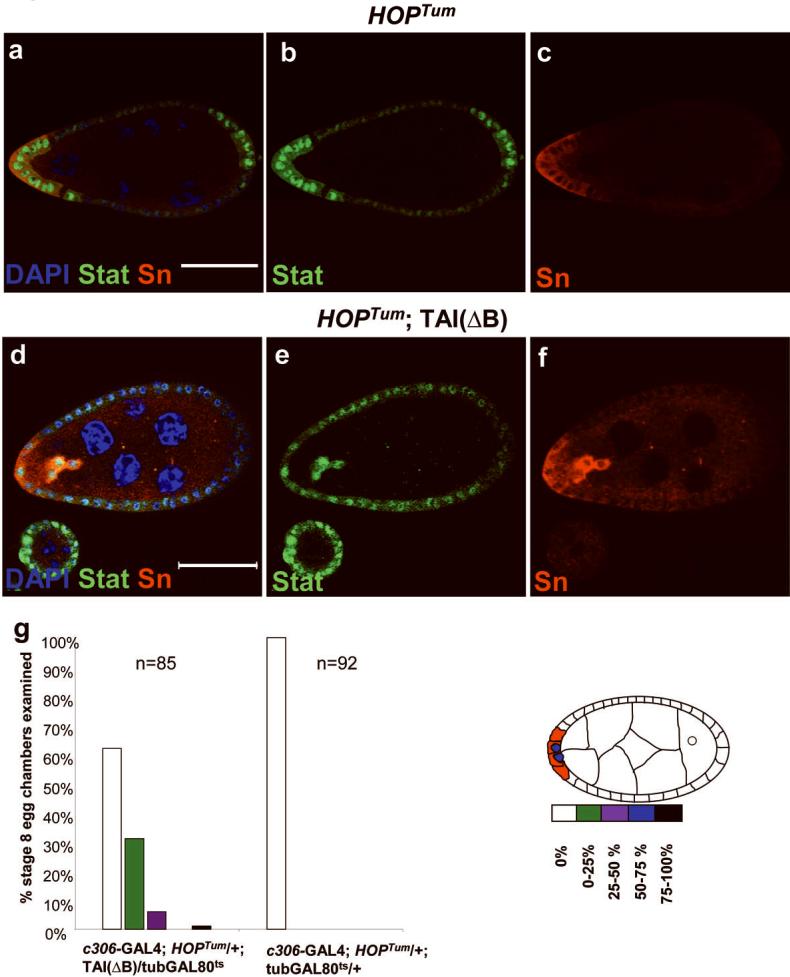
Since ecdysone signaling is thought to be a timing factor whereas JAK/STAT activity specifies the migratory population, precocious activation of both pathways should be sufficient to cause early migration. We took advantage of Tai(ΔB) to activate ecdysone signaling early. Precocious activation of JAK/STAT was accomplished by expressing a constitutively active form of JAK known as HOPTUM39. While HOPTUM resulted in early activation of specific border cell markers including Singed (Fig. 7a-c), it never caused early migration. Similarly, Tai(ΔB) resulted in early activation of EcRE-lacZ but not precocious migration (Fig. 5i). However, when co-expressed, HOPTUM and Tai(ΔB) caused migration 12 hours earlier than normal in 38% of egg chambers examined (Fig. 7d-g). Therefore the coincident activation of ecdysone and JAK/STAT can determine the timing of border cell migration.
Figure 7. Precocious border cell migration induced by co-expression of Tai(ΔB) and activated JAK.
a-c) Expression of a constitutively active form of the Drosophila JAK (HOPTum) using c306-GAL4, which expresses in anterior and posterior follicle cells (see supplementary information Figure S1), resulted in nuclear translocation of STAT (green) and precocious expression of the border cell marker singed (SN, red), but no migration. d-f) Co-expression of Tai(ΔB) with hopTum resulted in early detachment and migration of border cells. The effect is quantified in g. Scale bars = 50 μm.
Abrupt levels regulated by both JAK/STAT and ecdysone signaling
The specific down-regulation of nuclear Abrupt protein levels in border cells, which receive the highest levels of STAT signaling, led us to test whether endogenous STAT signaling affects Abrupt. We examined the effect stat loss-of-function, taking advantage of a temperature-sensitive allele because stat null mutant cells do not differentiate as border cells. At the permissive temperature, egg chambers from statts/stat3391 females were indistinguishable from wild-type: border cells migrated normally and nuclear abrupt levels were very low at stage 10 (Fig. 8a). After 4-6 hours at the non-permissive temperature, about 40% of stage 10 border cells showed incomplete migration, consistent with earlier findings21, and we found a strong correlation between the degree of migration defect, which reflects the degree of impairment of STAT function, and the level of Abrupt protein. Border cells that failed to leave the anterior end due to reduced stat function exhibited levels of Abrupt protein 1.4 fold higher than non-migratory follicle cells (Fig. 8b, c, j). Clusters that migrated partially exhibited lower Abrupt protein levels, presumably because residual STAT function promoted Abrupt down-regulation and migration (not shown). This result demonstrates that JAK/STAT signaling reduces the concentration of the repressor Abrupt. Abrupt then antagonizes the co-activator Tai, thereby enhancing ecdysone signaling. Therefore Abrupt serves as a point of integration for the ecdysone and JAK/STAT pathways (Fig. 8l).
Figure 8. Relationship between Jak/Stat, EcR, and Abrupt.
Confocal fluorescence micrographs of stage 10 egg chambers stained with anti-Abrupt (green) and in some cases with DAPI (blue). a-c) Stage 10 egg chambers from statts/stat3391 females kept at the permissive (a) or non-permissive temperature (b, c). Red staining in b is anti-Armadillo (Arm). d-i) High magnification views of stage 10 border cell clusters expressing EcRDN (EcR-F645A) (d, e), RacN17 (f, g) or PVRDN and EGFRDN (h, i). Panels c, e, g, and i show Abrupt staining only. Insets in a and c show high magnification of Abrupt staining in border cells. Transgenes were expressed in border cells by slbo-GAL4. j) Quantification of the fluorescence intensity of anti-Abrupt staining was measured for 57 border cells (bc) and 59 oocyte-associated follicle cells (fc) in the indicated genotypes. Error bars indicate the standard deviation (n=3-5). k) Quantification of border cell migration in the indicated genotypes. The diagram was constructed as Fig 3e. 1) Summary of regulatory relationships between Abrupt and the JAK/STAT and ecdysone pathways.
Ecdysone signaling also affected nuclear accumulation of Abrupt. Nuclear Abrupt was elevated in border cells expressing EcR-DN (Fig. 8d, e) or in tai mutant border cells (data not shown) compared to wild-type. This increase was specific because we did not observe it in cells over-expressing RacN17 or dominant negative guidance receptors (PVRDN and EGFRDN), even though these treatments inhibited migration (Fig. 8f-i). Therefore Abrupt protein levels responded to both STAT and ecdysone, further supporting the conclusion that Abrupt represents a point of integration for spatial and temporal signals in the control of border cell migration.
This model predicts that one function of ecdysone signaling is to reduce the concentration of Abrupt in border cells. To determine the functional significance of this effect, we tested for a genetic interaction. Specifically, we predicted that reducing the gene dosage of Abrupt might rescue reduced ecdysone signaling. To test this prediction, we used slbo-GAL4 to express EcRDN in the presence or absence of the abrupt null allele ab1D. Whereas EcRDN caused incomplete border cell migration in 60% of stage 10 egg chambers at 29°C, reducing the abrupt gene dosage by half reduced this effect to 34% (Fig. 8k). We did not observe a similar rescue of the statts allele, presumably because there are many additional stat targets that are required for border cell migration including known genes such as slbo. These results provided functional evidence in support of the model shown in Fig. 8l.
DISCUSSION
Embryonic development unfolds as a series of changes in gene expression that are regulated in both space and time. The fundamental mechanisms of spatial patterning have been established40, 41. Temporal patterns of gene expression can be regulated globally by circulating hormones or locally by the sequential actions of transcription factors on one another. What remains to be elucidated are the mechanisms by which spatial and temporal patterns are integrated. Here we identify the gene Abrupt as playing such a part in border cells.
We propose the following model for the molecular integration of spatial and temporal control of border cell migration (Fig. 8l). Early in stage 9 the ecdysone titer begins to rise15. Although we do not know the precise pattern in which it is produced, it may be uniform. At this stage, EcRB1 expression is enriched in anterior follicle cells, leading to an enhanced ecdysone response in these cells. In response to ecdysone signaling, the levels of Abrupt protein begin to fall in anterior follicle cells, leading to a feedback amplification of the ecdysone response in those cells, further reduction in Abrupt protein levels and thus a gradually decreasing level of nuclear Abrupt throughout stage 9. Since the asymmetry in EcRB1 expression is transient, this feedback mechanism is necessary to maintain the spatially localized effect in the absence of the initiating event. Abrupt protein levels also decrease in response to JAK/STAT signaling, which is sustained and highest in border cells.
The gradual decrease in the concentration of Abrupt in border cell nuclei due to the combined action of ecdysone signaling and JAK/STAT leads to a gradual increase in ecdysone signaling throughout stage 9, producing a temporal gradient. The gradual nature of the effect may serve as a buffer against any excessively rapid increase in the ecdysone concentration that might occur. As we have shown in Tai(ΔB) overexpression, very high levels of ecdysone signaling are not compatible with border cell migration and may even serve as a stop signal since the highest level of ecdysone reporter expression occur at stage 10, which is the stage at which border cells stop migrating.
Two other BTB domain proteins that function in developmental timing are Chinmo (Chronologically inappropriate morphogenesis)2 and BrC (Broad Complex)42. These factors contribute to the temporal sequence of neuronal cell fates during postembryonic development. Early neuronal precursors express high levels of Chinmo, which subsequently decay by a post-transcriptional mechanism. Loss of Chinmo from early neuroblasts converts their progeny to later identities whereas over-expression of Chinmo has the opposite effect2. In the developing larval CNS, early born neurons express higher levels of Chinmo and later born cells express BrC in a largely complementary pattern42. Thus temporal regulation may be a general property of proteins containing both BTB and Zn2+ finger domains.
Could the three temporal control mechanisms that have been studied largely separately, actually represent a single unified mechanism? The work presented here demonstrates that Abrupt protein levels respond to ecdysone signaling and in turn affect the ecdysone response in the Drosophila ovary. Very recently, Abrupt has also been shown to be a direct target for the let-7 microRNA in larval muscle cells43, 44. Drosophila let-7 is homologous to one of the original miRNAs identified as a heterochronic gene in C. elegans45. Drosophila let-7 is widely expressed in ecdysone-responsive tissues including ovaries46 and like ecdysone, is required for metamorphosis and for female (not male) fertility. Drosophila let-7 expression may require EcR46, 47 or ecdysone and let-7 may function in parallel pathways to regulate developmental timing48. The border cells now represent a well-developed model in which spatial and temporal control can be examined at the single cell level so that precise molecular mechanisms can be unraveled. Further investigation of this model system could help determine whether hormone, microRNA and BTB domain transcription factors are all part of one unified developmental timing pathway or function in parallel.
METHODS
Drosophila strains and genetics
The following Drosophila strains were used in this study: w1118 was used for microinjection and wild type analysis, slbo-GAL449 and c306-GAL450 for expression of transgenes. pUAST-hopTum and pUAST-hop were obtained from Dr. Doug Harrison39. EcRE-lacZ, hs-GAL4-EcR, and hs-GAL4-USP strains were kindly provided by Dr. Thummel23. hsGAL4-EcR/pUAST-mCD8GFP or hsGAL4-USP/pUAST-mCD8GFP female flies were heat-shocked once at 37°C for one hour and then ovaries were dissected for antibody staining after overnight fattening with wet yeast at 25°C. ab26, FRT40A35 and abP[GawB]NP1107 were acquired from Dr. Fen-Biao Gao and KYOTO stock center respectively. abK02807, FRT40A and abclu1, FRT40A36 flies were obtained from Dr. Tadashi Uemura. The transgenic flies carrying Pp91, P{UAS-EcR.A}3a, P{UAS-EcR.B1}3b, P{UAS-EcR.B2}3a and P{UAS-EcR.B1-DeltaC655.W650A}TP1 were received from the Bloomington stock center. P[hsp70-flp]; AyGAL4, nls-GFP flies were used in FLP-OUT technique to induce ectopic expression clones. Female flies were heat shock at 37°C for one hour and then incubated at 25°C for one and half days before dissection.
In MARCM (mosaic analysis with a repressible cell marker)38 rescue experiments, female flies with the genotype, P[hsp70-flp], UAS-mCD8GFP/c306-GAL4; FRT40A, tubGal80/dp, tai61G1, FRT40A, were heat-shocked at 37°C for one hour, four times in one day, and then ovaries were dissected after 3.5-4.5 days at 25°C. Only egg chambers in which all border cells were homozygous tai-/- were analyzed.
For experiments in which both Tai (ΔB) and HOP(TUM) were expressed using c306-GAL4, the required genotype was lethal, so we added the temperature sensitive (ts) transcriptional repressor GAL80ts51 to inactivate GAL4 activity throughout development by growing flies at or below 20°C. These flies were shifted to 31°C, to inactivate GAL80 and allow GAL4 activation, for 12 hours. Then the ovaries were dissected, stained and examined.
DNA constructs and generation of transgenic flies
Tai domain deletion constructs were modified from the original full length pUAST-Tai14. LXXLL was removed from GH06208 by Nae I and Nhe I, and was subcloned into EcoRV site of pBS-nls-GFP to obtain pBS-nls-LXXLL-GFP. The fragment nls-LXXLL-GFP was cut out from pBS-nls-LXXLL-GFP by Xho I and Xba I sites and cloned into the same sites of pUAST to obtain pUAS-nls-LXXLL-GFP. To make the pUAST-Tai (ΔC), the pUAST-Tai was digested by NotI and XbaI then filled in the cohesive ends by DNA polymerase to delete C terminal amino acid 1516-2036. The bHLH domain was removed by digesting with ZraI and EcoRV to produce pUAST-Tai (ΔB). A similar approach was used to generate pUAST-Tai (ΔP) by deleting PAS domain with EcoRV and KpnI. The protein expression levels and subcellular localizations were evaluated using Tai specific antibodies14. For quantification of protein expression levels, immunofluorescence intensity was measured using Zeiss LSM 510-Meta software. The anti-Tai antibody staining intensity was normalized to the DAPI staining intensity and this number was averaged over at least six nuclei from each of three different egg chambers. The IF:DAPI ratio for each deletion construct was then normalized to the IF:DAPI ratio for the full-length Tai protein. The anti-Tai antibody was not effective in Western blotting.
The constructs pUAST-flag-nls-Tai(bHLH), pUAST-flag-nls-Tai(PAS) and pUAST-flag-nls-Tai(BP) were amplified by PCR and cloned into the pUAST-flag-nls vector52. For expression level and protein localization of each transgenes, the mouse anti-FLAG antibody (Sigma) was used. Two to four independent transformants were established for each construct to test the expression level and border cell phenotype. Quantification of protein expression level was carried out as described above and using Western blotting, with similar results.
MARCM rescue analysis was carried out as follows: males with a third chromosome insertion of the relevant transgene Sco CyO; Tai(X) were crossed with females with the genotype, P[hsp70-flp], UAS-mCD8GFP; FRT40A, tubGal80; MKRS/TM6B, Tb, Ser to get male flies with genotype, P[hsp70-flp], UAS-mCD8GFP; FRT40A, tubGal80; Tai(X)/TM6B,Tb, Ser. These males were then crossed with c306-GAL4; dp, tai61G1, FRT40A females to obtain female flies with the genotype P[hsp70-flp], UAS-mCD8GFP/c306-GAL4; FRT40A, tubGal80/dp, tai61G1, FRT40A; Tai(X)/+ for the rescue experiments.
Immunohistochemistry
Ovarioles were dissected in S2 cell medium (Sigma) containing 10% fetal bovine serum, fixed on ice in PBS with 4% formaldehyde for 30 minutes and then rinsed three times in PBS with 0.3% Triton X-100. The following primary antibodies from Developmental Studies Hybridoma Bank (DSHB) were used for immunostaining: mouse anti-Armadillo (N27A1, 1:25); rat anti-dCAD2 (1:10) and anti-Sn (7C, 1:25); mouse anti-beta-galactosidase (40-1a, 1:25). Rabbit anti-β-galactosidase (1:800, Cappel); mouse anti-FLAG (1:500, Sigma) and rabbit anti-GFP (1:2000, Molecular Probes) were performed as the same protocol described above for ovarioles staining. Tai rabbit polyclonal antibody specifically recognized the LXXLL domain was used to stain ovarioles as described14. A rabbit anti-STAT peptide antibody was used in 1: 500 dilution for ovariole staining. Rabbit anti-Abrupt antibody (1:300) was generous gift from Dr. Stephen T. Crews. Secondary antibodies that conjugated with Alexa-488, Alex-568 or Alexa-647 were used in 1:400 dilutions (Molecular Probes). The images were scanned by Zeiss LSM 510-Meta confocal microscope or on Zeiss Axioplan 2 microscope using the ApoTome system and AxioVision 4 software.
Border cell purification and β-galactosidase activity measurements
Virgin females were collected and kept with male flies at 18°C for 1-3 days, then flies were fattened and incubated at 29°C for 14 hours before the dissection. 30 pair of ovaries were digested with elastase (Sigma E0127) in dissociation buffer (Sigma C-1544). The GFP positive cells were sorted with anti-mouse CD8 antibody following by magnetic cell purification procedures as described previously53. The Galacto-Light Plus™ kit (Tropix) was used to detect β-galactosidase activity of purified follicle cells.
Immunoprecipitation
The following procedures were performed at 4°C. S2 cells were lysed in RIPA buffer54 with protease inhibitor cocktail (Roche) for 30 minutes with gentle rocking and were spun down at 8000 × g for 10 minutes. Cell extracts were incubated with the primary antibody for overnight before adding protein A (Roche) or FLAG-protein G (Sigma) beads for one hour incubation. Beads were washed four 4 times prior to the 10% SDS PAGE and Western blot analysis and which blots were probing with rabbit anti-Abrupt (1:3000) and HRP conjugated secondary antibody (1:10,000).
GST pull-down assays
35S-methionine labeled BTB and Zn finger domains were generated by the TNT in vitro translation system (Promega). The plasmids were constructed by PCR amplification using the following oligos: atgaccgaatccacacagc, ttcgtgtttcggattggcc for the pTNT-BTB and atgctttggagaacagca, ctatgtgttgtgcactccc for pTNT-Zn. Glutathione S-transferase (GST), GST-LXXLL14 and GST-bHLH (amino acid 1-375) were expressed in BL-21 cells (invitrogene). The GST pull-down assays were done as described by Dr. Tsai et al55.
Supplementary Material
Acknowledgements
This work was supported by NIH grants R01 GM46425 and R01 GM73164 to DJM.
REFERENCES
- 1.Doe CQ. Chinmo and neuroblast temporal identity. Cell. 2006;127:254–256. doi: 10.1016/j.cell.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S, et al. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–422. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 3.Kornberg TB, Guha A. Understanding morphogen gradients: a problem of dispersion and containment. Curr Opin Genet Dev. 2007;17:264–271. doi: 10.1016/j.gde.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss EG. Heterochronic genes and the nature of developmental time. Curr Biol. 2007;17:R425–434. doi: 10.1016/j.cub.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Frasch M. A matter of timing: microRNA-controlled temporal identities in worms and flies. Genes Dev. 2008;22:1572–1576. doi: 10.1101/gad.1690608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karp X, Ambros V. Developmental biology. Encountering microRNAs in cell fate signaling. Science. 2005;310:1288–1289. doi: 10.1126/science.1121566. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 8.Riddiford LM. Hormones and Drosophila development. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. pp. 899–929. [Google Scholar]
- 9.Banerjee I, Clayton P. The genetic basis for the timing of human puberty. J Neuroendocrinol. 2007;19:831–838. doi: 10.1111/j.1365-2826.2007.01598.x. [DOI] [PubMed] [Google Scholar]
- 10.Carel JC, Leger J. Clinical practice. Precocious puberty. The New England journal of medicine. 2008;358:2366–2377. doi: 10.1056/NEJMcp0800459. [DOI] [PubMed] [Google Scholar]
- 11.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 12.Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 13.Rorth P. Initiating and guiding migration: lessons from border cells. Trends Cell Biol. 2002;12:325–331. doi: 10.1016/s0962-8924(02)02311-5. [DOI] [PubMed] [Google Scholar]
- 14.Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MB, Kelly TJ, Woods CW, Imberski RB. Ecdysteroid fluctuations in adult Drosophila melanogaster caused by elimination of pupal reserves and synthesis by early vitellogenic ovarian follicles. Insect Biochem. 1989;19:243–249. [Google Scholar]
- 16.Buszczak M, et al. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- 17.Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 2004;131:2715–2725. doi: 10.1242/dev.01143. [DOI] [PubMed] [Google Scholar]
- 18.Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics. 2000;154:1203–1211. doi: 10.1093/genetics/154.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver DL, Montell DJ. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell. 2001;107:831–841. doi: 10.1016/s0092-8674(01)00607-9. [DOI] [PubMed] [Google Scholar]
- 20.Xi R, McGregor JR, Harrison DA. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev Cell. 2003;4:167–177. doi: 10.1016/s1534-5807(02)00412-4. [DOI] [PubMed] [Google Scholar]
- 21.Silver DL, Geisbrecht ER, Montell DJ. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development. 2005;132:3483–3492. doi: 10.1242/dev.01910. [DOI] [PubMed] [Google Scholar]
- 22.Starz-Gaiano M, Melani M, Wang X, Meinhardt H, Montell DJ. Feedback inhibition of Jak/STAT signaling by apontic is required to limit an invasive cell population. Dev Cell. 2008;14:726–738. doi: 10.1016/j.devcel.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Kozlova T, Thummel CS. Essential roles for ecdysone signaling during Drosophila mid-embryonic development. Science. 2003;301:1911–1914. doi: 10.1126/science.1087419. [DOI] [PubMed] [Google Scholar]
- 24.Hackney JF, Pucci C, Naes E, Dobens L. Ras signaling modulates activity of the ecdysone receptor EcR during cell migration in the Drosophila ovary. Dev Dyn. 2007;236:1213–1226. doi: 10.1002/dvdy.21140. [DOI] [PubMed] [Google Scholar]
- 25.Cherbas L, Hu X, Zhimulev I, Belyaeva E, Cherbas P. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development. 2003;130:271–284. doi: 10.1242/dev.00205. [DOI] [PubMed] [Google Scholar]
- 26.Kozlova T, Thummel CS. Spatial patterns of ecdysteroid receptor activation during the onset of Drosophila metamorphosis. Development. 2002;129:1739–1750. doi: 10.1242/dev.129.7.1739. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein EC, Kelly TJ, Schwartz MB, Woods CW. in vitro synthesis and secretion of ecdysteroids by Drosophila melanogaster ovaries. The Journal of Experimental Zoology. 1982;223:305–308. [Google Scholar]
- 28.Chavez VM, et al. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development. 2000;127:4115–4126. doi: 10.1242/dev.127.19.4115. [DOI] [PubMed] [Google Scholar]
- 29.Freeman MR, Dobritsa A, Gaines P, Segraves WA, Carlson JR. The dare gene: steroid hormone production, olfactory behavior, and neural degeneration in Drosophila. Development. 1999;126:4591–4602. doi: 10.1242/dev.126.20.4591. [DOI] [PubMed] [Google Scholar]
- 30.Petryk A, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A. 2003;100:13773–13778. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren JT, et al. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99:11043–11048. doi: 10.1073/pnas.162375799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren JT, et al. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol. 2004;34:991–1010. doi: 10.1016/j.ibmb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Schubiger M, Tomita S, Sung C, Robinow S, Truman JW. Isoform specific control of gene activity in vivo by the Drosophila ecdysone receptor. Mech Dev. 2003;120:909–918. doi: 10.1016/s0925-4773(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 34.Hu S, Fambrough D, Atashi JR, Goodman CS, Crews ST. The Drosophila abrupt gene encodes a BTB-zinc finger regulatory protein that controls the specificity of neuromuscular connections. Genes Dev. 1995;9:2936–2948. doi: 10.1101/gad.9.23.2936. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Wang F, Menut L, Gao FB. BTB/POZ-zinc finger protein abrupt suppresses dendritic branching in a neuronal subtype-specific and dosage-dependent manner. Neuron. 2004;43:823–834. doi: 10.1016/j.neuron.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 36.Sugimura K, Satoh D, Estes P, Crews S, Uemura T. Development of morphological diversity of dendrites in Drosophila by the BTB-zinc finger protein abrupt. Neuron. 2004;43:809–822. doi: 10.1016/j.neuron.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, Tsai SY, Tsai MJ. SRC-3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol Sin. 2006;27:387–394. doi: 10.1111/j.1745-7254.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 39.Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. Embo J. 1995;14:2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- 41.Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007;128:245–256. doi: 10.1016/j.cell.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133:891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 43.Sokol NS, Xu P, Jan YN, Ambros V. Drosophila let-7 microRNA is required for remodeling of the neuromusculature during metamorphosis. Genes Dev. 2008;22:1591–1596. doi: 10.1101/gad.1671708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caygill EE, Johnston LA. Temporal Regulation of Metamorphic Processes in Drosophila by the let-7 and miR-125 Heterochronic MicroRNAs. Curr Biol. 2008;18:943–950. doi: 10.1016/j.cub.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 46.Sempere LF, Sokol NS, Dubrovsky EB, Berger EM, Ambros V. Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-Complex gene activity. Dev Biol. 2003;259:9–18. doi: 10.1016/s0012-1606(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 47.Sempere LF, Dubrovsky EB, Dubrovskaya VA, Berger EM, Ambros V. The expression of the let-7 small regulatory RNA is controlled by ecdysone during metamorphosis in Drosophila melanogaster. Dev Biol. 2002;244:170–179. doi: 10.1006/dbio.2002.0594. [DOI] [PubMed] [Google Scholar]
- 48.Bashirullah A, et al. Coordinate regulation of small temporal RNAs at the onset of Drosophila metamorphosis. Dev Biol. 2003;259:1–8. doi: 10.1016/s0012-1606(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 49.Rorth P, et al. Systematic gain-of-function genetics in Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- 50.Manseau L, et al. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev Dyn. 1997;209:310–322. doi: 10.1002/(SICI)1097-0177(199707)209:3<310::AID-AJA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 52.Yao JG, Sun YH. Eyg and Ey Pax proteins act by distinct transcriptional mechanisms in Drosophila development. Embo J. 2005;24:2602–2612. doi: 10.1038/sj.emboj.7600725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, et al. Analysis of cell migration using whole-genome expression profiling of migratory cells in the Drosophila ovary. Dev Cell. 2006;10:483–495. doi: 10.1016/j.devcel.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Geisbrecht ER, Montell DJ. A role for Drosophila IAP1-mediated caspase inhibition in Rac-dependent cell migration. Cell. 2004;118:111–125. doi: 10.1016/j.cell.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Tsai CC, Kao HY, Yao TP, McKeown M, Evans RM. SMRTER, a Drosophila nuclear receptor coregulator, reveals that EcR-mediated repression is critical for development. Mol Cell. 1999;4:175–186. doi: 10.1016/s1097-2765(00)80365-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.