Allogeneic stem cell transplantation is being considered as a potentially curative treatment for patients with chronic lymphocytic leukemia. The findings of this study suggest that relapsed patients can achieve molecular remission after reduced intensity conditioning and allogeneic stem cell transplantation.
Keywords: chronic lymphocytic leukemia, minimal residual disease, allogeneic stem cell transplantation
Abstract
Background
The graft-versus-leukemia effect is able to induce clinical responses in patients with chronic lymphocytic leukemia treated with a reduced intensity conditioning regimen, followed by allogeneic stem cell transplantation. We investigated whether molecular remissions could be attained after reduced intensity conditioning and allogeneic stem cell transplantation in patients with relapsed chronic lymphocytic leukemia and whether the assessment of minimal residual disease might be used to predict the clinical outcome.
Design and Methods
Minimal residual disease was monitored by polymerase chain reaction using the immunoglobulin heavy-chain gene rearrangement as a molecular marker in 29 relapsed patients who achieved complete remission following reduced intensity conditioning and allogeneic stem cell transplantation. A nested-polymerase chain reaction with patient-specific primers derived from complementarity determining regions (CDR2 and CDR3) was carried out in all the patients. Real-time polymerase chain reaction was performed in patients whose nested reaction gave positive or mixed results.
Results
Three patterns of minimal residual disease were observed: negative (31%), mixed (24%), and always positive (45%). The cumulative incidence of relapse according to the minimal residual disease status at 6 and 12 months after transplantation was significantly different between polymerase chain reaction-negative and -positive patients (p=0.031 and p=0.04, respectively). Two-year disease-free survival was 93% and 46% for polymerase chain reaction-negative and -positive patients at 6 months after transplantation, respectively (p=0.012). Similarly, 2-year disease-free survival was 100% and 57% for polymerase chain reaction-negative and -positive patients at 12 months, respectively (p=0.037). No clinical or biological factors were predictive of the achievement of polymerase chain reaction negativity after allogeneic stem cell transplantation. Graft-versus-host disease was more frequent in patients who did not relapse (p=0.04). Quantitative monitoring of minimal residual disease was able to identify polymerase chain reaction-positive patients with a higher risk of relapse.
Conclusions
These findings demonstrate that relapsed patients can achieve molecular remission after reduced intensity conditioning and allogeneic stem cell transplantation and suggest a minimal residual disease-driven intervention that might be useful to prevent overt hematologic relapse.
Introduction
Patients with relapsed chronic lymphocytic leukemia (CLL) are candidates for intensive treatments to attain remission and possibly prevent disease recurrence. Autologous stem cell transplantation (SCT) may improve patients’ outcome, although a continuous pattern of relapse has been observed.1–3 At present, allogeneic SCT is considered a potentially curative treatment since survival curves of allografted patients have been observed to plateau.4–6 The main advantage provided by allografting may be the immune-mediated graft-versus-leukemia effect.7,8 In the last decade an increasing number of patients have been referred for an allogeneic SCT with reduced intensity conditioning (RIC), which has been shown to be feasible in patients over the age of 55 years, in heavily pre-treated subjects and even in those affected by other medical comorbidities.9–13
The availability of a molecular tumor marker, based on the immunoglobulin heavy-chain gene (IgH) rearrangement, allows post-transplant monitoring of minimal residual disease (MRD) in patients in complete remission. After autologous SCT, MRD negativity has been correlated with a better disease-free survival.14–16 In the allogeneic SCT setting, persistent MRD positivity without relapse has been described by some authors and has sometimes been used to modulate the post-transplant immunosuppressive therapy in order to stimulate anti-tumor activity.14–17
In the present study, we performed a prospective molecular monitoring of patients with relapsed and refractory CLL treated with RIC allogeneic SCT. Three main issues were addressed: i) the rate of molecular remissions; ii) the clinical relevance of MRD results; iii) the clinical and biological factors, if any, that can be correlated with MRD status.
Design and Methods
Patients’ characteristics
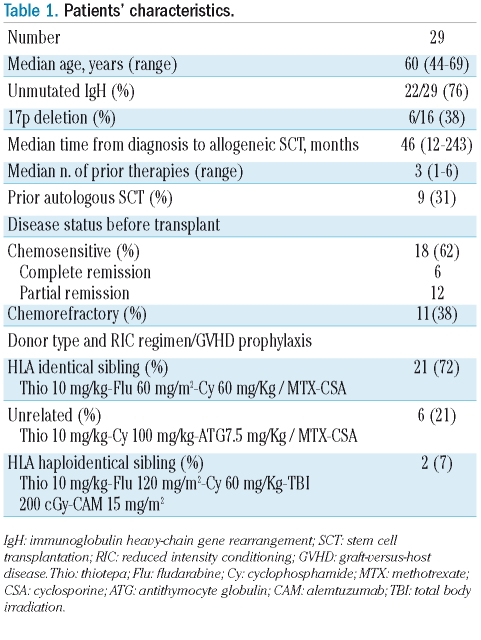
Twenty-nine patients with relapsed CLL who achieved complete remission after RIC allogeneic SCT were monitored for MRD by a polymerase-chain-reaction (PCR) assay. The patients’ characteristics and the RIC regimens are described in Tables 1 and 2. Patients were enrolled in three RIC allogeneic SCT Italian multi-center protocols because they were primary refractory to fludarabine-based regimens or had relapsed after two or more lines of chemotherapy or after an autologous SCT. The conditioning regimens consisted of thiotepa-cyclophosphamide-fludarabine for recipients of transplants from HLA identical siblings, thiotepa-cyclophos-phamide- antithymocyte globulin for unrelated allogeneic SCT (n=6) and thiothepa-cyclophosphamide-fludarabine-alemtuzumab and 2 Gy total body irradiation for haploidentical transplants (n=2).13,18,19 Graft-versus-host disease (GVHD) prophylaxis was carried out with a short-course of methotrexate and cyclosporine A in the case of HLA-matched donors. Cyclosporine A was tapered from day +100 in sibling transplants and from day +180 in unrelated transplants. Early withdrawal of immunosuppression followed by donor lymphocyte infusions was allowed for persistent, progressive or relapsed disease in patients without GVHD. The protocols did not include a pre-established plan of donor lymphocyte infusions or withdrawal of immunosuppression based on the MRD results. GVHD was graded according to consensus criteria.20,21 Chimerism was assessed on peripheral blood as previously described.22
Table 1.
Patients’ characteristics.
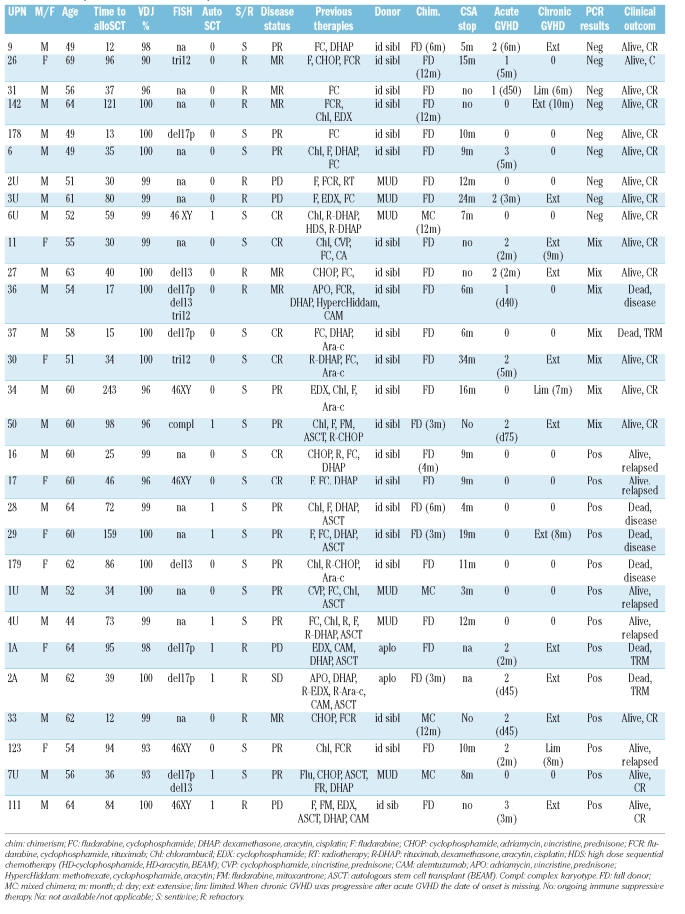
Table 2.
Pre-transplant characteristics of the patients’ and their outcome.
Molecular analyses were performed during the clinical follow-up and patients gave their written informed consent.
Molecular analysis
The IgH rearrangement was identified on diagnostic bone marrow or peripheral blood samples using 500 ng of genomic DNA extracted after mononuclear cell separation on a Ficoll-Hypaque density gradient. PCR amplification was performed using four sets of consensus sense primers derived from the IgH leader or framework 1 (FR1) regions and an antisense primer derived from the joining region (JH) at the 3′ end.23 Direct sequencing of amplified DNA was performed using automated sequencing. The IgH sequence was then compared with the human germline sequences using the Immunogenetics (IMGT) database (http://imgt.cines.fr, IMGT, European Bioinformatics Institute, Montepellier, France), the IGBLast search (http://www.ncbi.nlm.nih.gov/igblast/, National Cancer for Biotechnology Information, Bethesda, MD, USA), and VBASE directory of DNAPLOT (http://vbase.mrc-cpe.cam.ac.uk, Centre for Protein Engineering, Cambridge, United Kingdom). IgH rearrangements showing 98% or more homology to the closest matching germline sequence were assigned as unmutated.24
After their allogeneic transplant, patients were monitored from the time when complete remission was documented according to the NCI criteria.25 Serial post-transplant bone marrow samples were analyzed by nested-PCR, as previously described.23 Briefly, the first PCR amplification was carried out with the consensus primer derived from the tumor VH family and the anti-sense primer derived from the 3′ end of the JH region. The second reaction was performed using patient-specific primers constructed within the complementarity determining regions CDR2 and CDR3. The PCR conditions were established according to the melting temperature of the primers. Five hundred nanograms of DNA were used in the first PCR reaction, and 1 μL of the PCR product was used for the second PCR.
Real-time PCR was performed using an ABI Prism 7700 Sequence Detection System® (Applied Bio-systems, Foster City, CA, USA). Five hundred nanograms of DNA were amplified in 25 μL using 300 nm of the individual patient-specific primers, 200 nm of TaqMan probe and TaqMan Universal Mastermix (Applied Byosistems, Foster City, CA, USA). A FR3-derived consensus probe and the patient-specific primers designed for the nested-PCR were used.26 Reaction conditions were established based on the patients’ primers and probes. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was chosen as the reference standard. The relative quantification of MRD was calculated as follows: ΔΔCT= (CTIgH –CTGAPDH)follow-up – (CTIgH -CTGAPDH)pre-transplant.27 A mean CT value was obtained by running experiments in triplicate. In both qualitative and quantitative PCR reactions: (i) a pre-transplant PCR-positive sample was used as a positive control to assess the sensitivity; (ii) all the samples were run in triplicate; (iii) a pool of DNA from five healthy donors was used as a negative control in order to assess the specificity; and (iv) a no-template control sample was included to exclude any possible contamination. To avoid false-negative results, all the MRD-negative samples were tested by amplifying the sequence of p53 exon 5 or exon 6.
Sensitivity tests were performed randomly in 20% (n=6) of our patients, as part of the quality tests routinely performed in the laboratory for MRD assessment. We choose patients based on the availability of pre-transplant samples in which the tumor cells approach 100% of the cell content. Serial dilutions of CLL cells into a pool of normal blood samples from five healthy donors are performed in order to have samples with a tumor content ranging from 100 to 10−7. Five hundred nanograms of DNA of the samples are then used in real-time PCR and nested-PCR carried out as described above. A slope of the standard curves between −3.0 and −3.9 and a correlation coefficient greater than 0.95 are considered acceptable.28 The sensitivity ranges between 10−4 and 10−5 for real-time PCR, according to published guidelines,29 and 10−6–10−7 for nested-PCR (Online Supplementary Figure S1).
Statistical analysis
Molecular remission was defined as persistent PCR negativity on serial bone marrow samples collected after allogeneic SCT. Death from any cause and relapse were considered as events for the analysis of disease-free survival. Transplant-related mortality was defined as death not related to progression of the malignant disease. The cumulative incidence of relapse was calculated using the cumulative incidence method, considering relapse as an event and death without relapse as a competing event. Two landmark time points were set at 6 and 12 months after transplantation.30–32 The cumulative incidence of relapse of PCR-negative versus PCR-positive patients at 6 or 12 months was compared by Gray’s test.33 Disease-free survival rates were calculated by the Kaplan-Meier method and compared with the log-rank test. Patients who died or were censored before the time of the landmark evaluation were excluded from the analysis. Patients were analyzed and compared from the landmark time to the last follow-up according to MRD status at the landmark time regardless of any subsequent changes in MRD status. Contingency tables were used to compare proportions between groups.
Results
Polymerase chain reaction results
In 29 out of 30 evaluated patients we were able to identify the IgH-derived molecular marker.
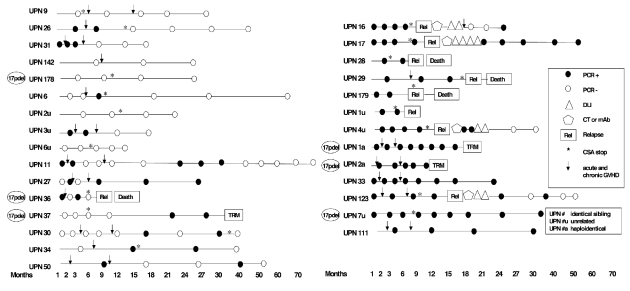
A total of 148 samples from these 29 patients were then analyzed for MRD, with the median time between samples being 3 months (range, 1–30) (Figure 1). The overall median molecular follow-up of patients alive and in complete remission was 26 months (range, 12–76) and the median clinical follow-up was 40 months (range, 12–85). At the last molecular follow-up nine patients (31%) were PCR-negative: five of them (17%) had always been PCR-negative, whereas the other four (14%) experienced delayed clearance of MRD within the first year after transplant. All these patients were alive in complete remission with a median follow-up of 40 months (range, 15–85). Seven patients (24%) showed a mixed pattern of alternating PCR positivity and negativity: one patient died of secondary acute myeloid leukemia, another patient had a nodal relapse (Richter’s syndrome) whereas the others are alive and in complete remission. The median follow-up of this group of patients was 46 months (range, 38–77). Thirteen patients (45%) were always PCR-positive: eight of them (28%) relapsed after a median of 9 months (range, 3–26). Two of the PCR-positive patients (UPN 1a, 2a) died of extensive chronic GVHD after a haploidentical transplant for 17p deletion refractory CLL at 18 and 12 months respectively, whereas the other three patients are alive and in complete remission at a median follow-up of 49 months (range, 33–57).
Figure 1.
Minimal residual disease monitoring by allele-specific oligonucleotide-nested-polymerase chain reaction. PCR: polymerase chain reaction; TRM: transplant-related mortality; CT: chemotherapy; mAb: monoclonal antibody; DLI: donor lymphocyte infusion; CSA: cyclosporine; GVHD: graft-versus-host disease.
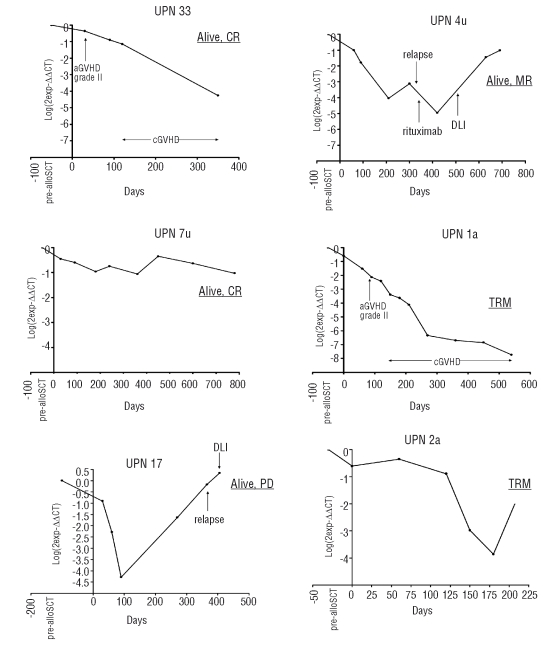
Real-time PCR was carried out in six out of the 13 PCR-positive patients (Figure 2). In the remaining PCR-positive patients quantitative monitoring was not performed because of the small amount of DNA available. In four patients (UPN 33, 1a, 2a, 7u) who did not relapse, a decreasing (n=3) or stable (n=1) tumor load was detected. The former were affected by extensive chronic GVHD. In two more patients (UPN 17, 4u) the tumor load increased on day +270 and +300 and both patients relapsed without experiencing any GVHD. In three out of the seven patients with a mixed PCR pattern (UPN 11, UPN 27, UPN 37) we performed real-time PCR, which gave negative results since we did not observe any amplification in the follow-up samples. To confirm the positivity by nested-PCR we sequenced the PCR products which were 100% matched with the DNA sequence of the diagnostic sample.
Figure 2.
Minimal residual disease monitoring by real-time polymerase chain reaction. MR: molecular remission; TRM: transplant-related mortality; CR: complete remission; PD: progressive disease; aGVHD: acute graft-versus-host disease; cGVHD: chronic graft-versus-host disease; DLI: donor lymphocyte infusion.
Four patients, who relapsed after allogeneic SCT, were treated with chemo-immunotherapy followed by donor lymphocyte infusions (DLI). UPN 16 developed extensive chronic GVHD and attained a mixed PCR status after chemotherapy and DLI: he is alive and in complete remission 6 years after his transplant. UPN 17 received chemo-immunotherapy and DLI followed by interferon-α: she achieved complete remission after interferon-α, but never developed GVHD and relapsed 31 months later. UPN 123 showed a PCR-mixed status lasting 34 months after rituximab and DLI followed by limited GVHD. After having relapsed on day + 330, UPN 4u received chemo-immunotherapy and DLI. He did not develop GVHD and achieved molecular remission after DLI.
Clinical impact of polymerase chain reaction results
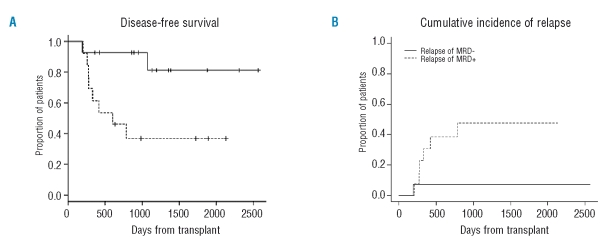
The relapse rate was significantly higher in PCR-positive patients than in PCR-negative patients and patients with a mixed PCR pattern (p=0.003). The cumulative incidence of relapse according to the MRD status at 6 months after allogeneic SCT was significantly different between PCR-negative and PCR-positive patients (p=0.031) (Figure 3B). The 2-year disease-free survival was 93% and 46% for PCR-negative and PCR-positive patients at 6 months after transplantation, respectively (p=0.012) (Figure 3A). A second landmark analysis was carried out at 12 months: the cumulative incidence of relapse was 0% and 28% (p=0.040) and 2-year disease-free survival was 100% and 57% for PCR-negative and PCR-positive patients, respectively (p=0.037).
Figure 3.
Clinical impact of polymerase chain reaction results. (A) Disease-free survival from 6 months after RIC allogeneic SCT of PCR-negative and PCR-positive patients based on MRD status at that time point (p=0.012). (B) Cumulative incidence of relapse of PCR-negative and PCR-positive patients based on MRD status at 6 months after RIC allogeneic SCT (p=0.031).
Univariate analysis
In univariate analysis, chemorefractory status before transplantation (p=0.702), the number of previous lines of therapy (≤2 versus >2, p=0.451) and donor type (HLA identical sibling versus matched unrelated/mismatched donors, p=0.225) were not significantly correlated with the achievement of molecular remission. Since most of the patients had an unmutated IgH, any difference in MRD status between patients with mutated and unmutated IgH could not be detected. Three of six patients carrying a 17p deletion achieved a PCR-negative or mixed status (UPN 178, 36, 37), and one of them relapsed with Richter’s syndrome. Of the other patients with 17p deletion, UPN 7u is alive, in PCR-positive complete remission at 57 months after allogeneic SCT, whereas UPN 1a and 2a died of GVHD in PCR-positive complete remission. We did not observe any significant correlation between the achievement of a rapid PCR-negativity and disease burden, assessed by peripheral lymphocytosis, bone marrow lymphocyte infiltration greater than 50% or the presence of bulky lymph nodes (> 5 cm) and splenomegaly at the time of transplantation. In particular, UPN 178 and 2U had splenomegaly in association with a bone marrow infiltrate greater than 50% and peripheral lymphocytosis, respectively.
As regard chimerism status, 69% of the patients who were PCR-negative or had a mixed PCR pattern always showed full donor reconstitution after their transplant compared to 46% of PCR-positive patients (p=0.274).
Seventy-five percent of PCR-negative/mixed patients experienced GVHD compared to 46% of PCR-positive patients (p=0.142). In PCR-negative patients with a delayed clearance of MRD (UPN 26, 31, 6, 3u), the onset of GVHD preceded the achievement of molecular remission. In the PCR-positive group, GVHD occurred in 25% of patients who relapsed and in 80% of those who did not. Overall, the crude incidence of grade 2–4 acute and chronic GVHD was 22% and 70% in relapsed and not relapsed patients, respectively (p=0.040).
Discussion
Molecular monitoring of MRD has been used in CLL patients undergoing autologous or allogeneic transplantation with rather different results. After autologous SCT, it has been shown that PCR status correlates with disease-free survival: PCR-positive patients and PCR-negative patients becoming positive inevitably relapse.14–16 On the other hand, after conventional allogeneic SCT a delayed clearance of MRD has been observed, suggesting a role for the postulated graft-versus-leukemia effect.14–16 Moreover, quantitative PCR monitoring revealed three different kinetics of the tumor clone: stable and decreasing MRD levels were associated with persistent complete remission, whereas increasing MRD levels predicted relapse.16,17 Recently, data from a series of 30 patients treated in the setting of a prospective RIC allogeneic SCT protocol were reported by the German CLL Study group.34 It was confirmed that post-transplant immune therapy can induce molecular responses through the graft-versus-leukemia effect, although some patients may be or become resistant to such an effect due to unknown mechanisms of immune escape. Along with this study our series of PCR-monitored CLL patients is the largest published so far concerning molecular outcomes following RIC allogeneic SCT. The results support the existence of different molecular disease patterns and show that about one third of the patients can achieve stable molecular remission. The lower rate of molecular remission compared to those in other studies may be explained by three observations: (i) the sensitivity of a nested-PCR with patient-specific primers can be higher than that of real-time PCR or one-step PCR; (ii) the assessment of MRD was performed on bone marrow rather than peripheral blood samples: although recent guidelines for MRD detection have demonstrated the correlation between bone marrow and peripheral blood data, the authors did not exclude that results could change in different settings;35 and (iii) in our group of patients the rate of first or second remissions as well as the number of matched unrelated donors were lower (41% vs. 59% and 21% vs. 57%, respectively), whereas more patients were chemorefractory at the time of transplantation than in the series studied by the German group (38% vs. 23%).
Our MRD monitoring identified a group of patients with a mixed PCR pattern who had a similar outcome to that of the PCR-negative patients. We hypothesized that these patients have a very low tumor burden that may not always be detected by nested-PCR. The persistence of a very low level of MRD could also explain the negative results found by real-time PCR that, at least in our hands, is usually one-log less sensitive. To exclude the occurrence of false positive results, PCR products of patients with a mixed PCR pattern were randomly sequenced showing a 100% match with the DNA sequence of the positive control. The demonstration of persistent low tumor burden in patients in long-term complete remission could support the role of the graft-versus-leukemia effect. In the clinical setting of prospective MRD monitoring, we suggest assigning the term PCR-mixed at the time when a PCR-positive result is obtained in previously PCR-negative patients. Although real-time PCR was negative in our tested patients, in these cases quantitative MRD monitoring might be useful to exclude a rapid increase in tumor genomes.
Interestingly, we sought to identify some factors, such as donor type and previous treatment, which could predict the probability of achieving molecular remission, but no significant associations were found. Besides the limitation of the sample size, this result may suggest that the graft-versus-leukemia effect provided by allogeneic SCT may overcome the poor clinical prognostic factors. Similarly, other authors have already shown the advantage of an allogeneic SCT in patients with mutated as well as unmutated IgH.6,17 As regards the presence of the 17p deletion, which is usually correlated with a poor outcome, the fact that two patients (UPN 178, UPN 37) achieved negative and mixed PCR results lasting 30 months is encouraging, as is the observation of an ongoing complete remission at 3 years of follow-up in a PCR-positive patient (UNP 7u). Moreover, since our patients had unfavorable prognostic features the relatively long disease control further supports the hypothesis that both complete and molecular remissions are sustained by mechanisms different from the cytotoxicity provided by the conditioning regimen.
Donor-derived immune cells are potentially capable of mediating an anti-tumor effect either specifically or as a part of an alloreactive phenomenon. Thus, the graft-versus-leukemia effect may be revealed by the occurrence of GVHD, as demonstrated by the significantly lower incidence of GVHD in relapsed patients and by the occurrence of GVHD prior to achievement of molecular remission in those displaying a delayed clearance of MRD. The correlation between GVHD and response has already been reported by other authors in the context of MRD and in clinically detectable disease.34,36,37
It is of note that in our series the MRD status at 6 months was able to predict relapse, differently from the report by Moreno et al. after conventional allogeneic SCT.16 The difference was maintained even for the 12-month landmark analysis, as reported by the German group in the setting of RIC allogeneic SCT.34 Although survival analysis based on MRD results must be considered with caution,30–32 disease-free survival rates from 6 and 12 months after RIC allogeneic SCT were better for PCR-negative patients, based on PCR data at that time point. Our data support the notion that rather early PCR monitoring is able to predict the long-term outcome. In particular, since the withdrawal of immunosuppression in our patients usually started from day +100 and +180 in recipients of related and unrelated transplants, respectively, the time point at 6 months could be particularly useful for driving clinical decisions towards DLI administration in HLA-identical sibling transplants or faster withdrawal of immunosuppression in the unrelated setting, in the case of MRD positivity. Based on our results and those of others, we can conclude that assessments at 6 and 12 months are informative and should, therefore, be included in the post-transplant monitoring of patients.
In summary, the evidence of three patterns of molecular disease and their significant correlation with disease-free survival could lead to MRD-oriented interventions: (i) PCR-negative patients are at a very low risk of disease recurrence and they can have a slow decrease of their immunosuppressive therapy, thus lowering their risk of GVHD; (ii) patients with a mixed PCR pattern had an outcome similar to that of PCR-negative patients, but closer PCR monitoring is necessary because of the persistence of a small clone, probably controlled by the immune system, and quantitative monitoring may be advisable when positive results occur; we can speculate that altogether, PCR-negative patients and patients with a mixed PCR pattern represent most of the 50–60% of progression-free survivors reported in many phase II RIC allogeneic SCT trials in CLL;9–13 and (iii) PCR-positive patients are at high risk of overt relapse and withdrawal of their immunosuppressive therapy followed by therapeutic intervention may be encouraged. Quantitative PCR monitoring is strongly indicated in this setting to assess the timing and type of intervention. The fact that some PCR-positive patients do not relapse or can be rescued after DLI further supports the role of the graft-versus-leukemia effect and the development of pre-emptive approaches based on molecular results.
In conclusion, our data indicate that molecular monitoring should be incorporated into clinical studies employing allogeneic SCT in CLL patients in order to further confirm these results and to generate robust MRD-based guidelines for post-transplant management of the patients.
Supplementary Material
Footnotes
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
LF: designed the study, performed the experiments, analyzed the results and wrote the paper; CC: performed and reviewed all the experiments, contributed to the interpretation of the results, and reviewed the paper; AD: collected clinical data and samples, contributed to the interpretation of the results, and reviewed the paper; AV: performed the experiments and created the figures; AR: performed the experiments and contributed to the interpretation of the results; FS: performed the statistical analysis, contributed to the interpretation of the results, and reviewed the paper; FP, FN, FB, and AO: collected clinical data and samples, and reviewed the paper; PC: designed the study, contributed to the interpretation of the results and reviewed the paper.
The authors reported no potential conflicts of interest.
Funding: this work was supported by the Associazione Italiana Ricerca sul Cancro (AIRC) and Fondazione Michelangelo.
References
- 1.Pavletic ZS, Bierman PJ, Vose JM, Bishop MR, Wu CD, Pierson JL, et al. High incidence of relapse after autologous stem-cell transplantation for B-cell chronic lymphocytic leukemia or small lymphocytic lymphoma. Ann Oncol. 1998;9:1023–6. doi: 10.1023/A:1008474526373. [DOI] [PubMed] [Google Scholar]
- 2.Dreger P, Stilgenbauer S, Benner A, Ritgen M, Kröber A, Kneba M, et al. The prognostic impact of autologous stem cell transplantation in patients with chronic lymphocytic leukemia: a risk-matched analysis based on the VH gene mutational status. Blood. 2004;103:2850–8. doi: 10.1182/blood-2003-05-1549. [DOI] [PubMed] [Google Scholar]
- 3.Gribben JG, Zahrieh D, Stephans K, Bartlett-Pandite L, Alyea EP, Fisher DC, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–96. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavletic ZS, Arrowsmith ER, Bierman PJ, Goodman SA, Vose JM, Tarantolo SR, et al. Outcome of allogeneic stem cell transplantation for B cell chronic lymphocytic leukemia. Bone Marrow Transplant. 2000;25:717–22. doi: 10.1038/sj.bmt.1702237. [DOI] [PubMed] [Google Scholar]
- 5.Doney KC, Chauncey T, Appelbaum FR. Allogeneic related donor hematopoietic stem cell transplantation for treatment of chronic lymphocytic leukemia. Bone Marrow Transplant. 2002;29:817–23. doi: 10.1038/sj.bmt.1703548. [DOI] [PubMed] [Google Scholar]
- 6.Moreno C, Villamor N, Colomer D, Esteve J, Martino R, Nomdedéu J, et al. Allogeneic stem-cell transplantation may overcome the adverse prognosis of unmutated VH gene in patients with chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3433–8. doi: 10.1200/JCO.2005.04.531. [DOI] [PubMed] [Google Scholar]
- 7.Mehta J, Powles R, Singhal S, Iveson T, Treleaven J, Catovsky D. Clinical and hematologic response of chronic lymphocytic and prolymphocytic leukemia persisting after allogeneic bone marrow transplantation with the onset of acute graft-versus-host disease: possible role of graft-versus-leukemia. Bone Marrow Transplant. 1996;17:371–5. [PubMed] [Google Scholar]
- 8.Rondón G, Giralt S, Huh Y, Khouri I, Andersson B, Andreeff M, et al. Graft-versus-leukemia effect after allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant. 1996;18:669–72. [PubMed] [Google Scholar]
- 9.Schetelig J, Thiede C, Bornhauser M, Schwerdtfeger R, Kiehl M, Beyer J, et al. Evidence of a graft-versus-leukemia effect in chronic lymphocytic leukemia after reduced-intensity conditioning and allogeneic stem-cell transplantation: the Cooperative German Transplant Study Group. J Clin Oncol. 2003;21:2747–53. doi: 10.1200/JCO.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Khouri IF, Lee MS, Saliba RM, Andersson B, Anderlini P, Couriel D, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: impact of rituximab on immunomodulation and survival. Exp Hematol. 2004;32:28–35. doi: 10.1016/j.exphem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Sorror ML, Maris MB, Sandmaier BM, Storer BE, Stuart MJ, Hegenbart U, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3819–29. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 12.Delgado J, Thomson K, Russell N, Ewing J, Stewart W, Cook G, et al. Results of alemtuzumab-based reduced-intensity allogeneic transplantation for chronic lymphocytic leukemia: a British Society of Blood and Marrow Transplantation Study. Blood. 2006;107:1724–30. doi: 10.1182/blood-2005-08-3372. [DOI] [PubMed] [Google Scholar]
- 13.Corradini P, Dodero A, Farina L, Fanin R, Patriarca F, Miceli R, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21:2316–23. doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 14.Esteve J, Villamor N, Colomer D, Cervantes F, Campo E, Carreras E, et al. Stem cell transplantation for chronic lymphocytic leukemia: different outcome after autologous and allogeneic transplantation and correlation with minimal residual disease status. Leukemia. 2001;15:445–51. doi: 10.1038/sj.leu.2402036. [DOI] [PubMed] [Google Scholar]
- 15.Esteve J, Villamor N, Colomer D, Montserrat E. Different clinical value of minimal residual disease after autologous and allogeneic stem cell transplantation for chronic lymphocytic leukemia. Blood. 2002;99:1873–4. doi: 10.1182/blood.v99.5.1873. [DOI] [PubMed] [Google Scholar]
- 16.Moreno C, Villamor N, Colomer D, Esteve J, Giné E, Muntañola A, et al. Clinical significance of minimal residual disease, as assessed by different techniques, after stem cell transplantation for chronic lymphocytic leukemia. Blood. 2006;107:4563–9. doi: 10.1182/blood-2005-09-3634. [DOI] [PubMed] [Google Scholar]
- 17.Ritgen M, Stilgenbauer S, Von Neuhoff N, Humpe A, Brüggemann M, Pott C, et al. Graft-versus-leukemia activity may overcome therapeutic resistance of chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy chain gene status: implications of minimal residual disease measurement with quantitative PCR. Blood. 2004;104:2600–2. doi: 10.1182/blood-2003-12-4321. [DOI] [PubMed] [Google Scholar]
- 18.Lamparelli T, van Lint MT, Gualandi F, Raiola AM, Barbanti M, Sacchi N, et al. Alternative donor transplants for patients with advanced hematologic malignancies, conditioned with thiotepa, cyclophosphamide and antithymocyte globulin. Bone Marrow Transplant. 2000;26:1305–11. doi: 10.1038/sj.bmt.1702719. [DOI] [PubMed] [Google Scholar]
- 19.Corradini P, Raganato A, Carniti C, Carrabba M, Farina L, Montefusco V, et al. CD8-depleted donor lymphocyte infusions can boost immune reconstitution after haploidentical stem cell transplantation following reduced-intensity conditioning regimen. Blood. 2006;108:3138. [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 21.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–76. [PubMed] [Google Scholar]
- 22.Corradini P, Tarella C, Olivieri A, Gianni AM, Voena C, Zallio F, et al. Reduced-intensity conditioning followed by allografting of hematopoietic cells can produce clinical and molecular remissions in patients with poor-risk hematologic malignancies. Blood. 2002;99:75–82. doi: 10.1182/blood.v99.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Voena C, Ladetto M, Astolfi M, Provan D, Gribben JG, Boccadoro M, et al. A novel nested-PCR strategy for detection of rearranged immunoglobulin heavy chain genes in B-cell tumours. Leukemia. 1997;11:1793–8. doi: 10.1038/sj.leu.2400801. [DOI] [PubMed] [Google Scholar]
- 24.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Un-mutated IgVH genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–54. [PubMed] [Google Scholar]
- 25.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 26.Ladetto M, Donovan JW, Harig S, Trojan A, Poor C, Schlossnan R, et al. Real-time polymerase chain reaction of immunoglobulin rearrangements for quantitative evaluation of minimal residual disease in multiple myeloma. Biol Blood Marrow Transplant. 2000;6:241–53. doi: 10.1016/s1083-8791(00)70006-1. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 –ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17:1013–34. doi: 10.1038/sj.leu.2402922. [DOI] [PubMed] [Google Scholar]
- 29.van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–11. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
- 30.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26:3913–5. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 32.Anderson JR, Neuberg DS. Analysis of outcome by response flawed. J Clin Oncol. 2005;23:8122–3. doi: 10.1200/JCO.2005.03.0148. [DOI] [PubMed] [Google Scholar]
- 33.Gray B. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 34.Ritgen M, Böttcher S, Stilgenbauer S, Bunjes D, Schubert J, Cohen S, et al. Quantitative MRD monitoring identifies distinct GVL response patterns after allogeneic stem cell transplantation for chronic lymphocytic leukemia: results from the GCLLSG CLL3X trial. Leukemia. 2008;22:1377–86. doi: 10.1038/leu.2008.96. [DOI] [PubMed] [Google Scholar]
- 35.Rawstron AC, Villamor N, Ritgen M, Böttcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–64. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 36.Dreger P, Brand R, Hansz J, Milligan D, Corradini P, Finke J, et al. Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning. Leukemia. 2003;17:841–8. doi: 10.1038/sj.leu.2402905. [DOI] [PubMed] [Google Scholar]
- 37.Schetelig J, van Biezen A, Brand R, Caballero D, Martino R, Itala M, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094–100. doi: 10.1200/JCO.2008.16.2982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.