Glanzmann’s thrombasthenia is a bleeding disorder caused by mutations of the ITGA2B or ITGB3 genes. This paper describes two Italian families with moderate thrombocytopenia with large platelets, defective platelet function and moderate/severe mucocutaneous bleeding, transmitted as an autosomal dominant trait and associated with a novel mutation in the ITGB3 gene, encoding the integrin β chain β3. This report adds to previous studies of mutant alleles which illustrate the importance of signaling via this integrin for platelet function as well as for platelet formation.
Keywords: glycoprotein IIb/IIIa, megakaryocytopoiesis, outside-in signaling, thrombo-cytopenia
Abstract
Background
Defects of integrin αIIbβ3 are typical of Glanzmann’s thrombasthenia, an inherited autosomal recessive bleeding disorder characterized by the failure of platelets to aggregate in response to all physiological agonists, but with no abnormalities in the number or size of platelets. Although large heterogeneity has been described for Glanzmann’s thrombasthenia, no family has so far been described as having an autosomal dominant form of this disease.
Design and Methods
We describe two Italian families with moderate thrombocytopenia with large platelets, defective platelet function and moderate/severe mucocutaneous bleeding, transmitted as an autosomal dominant trait and associated with a novel integrin β3-gene (ITGB3) mutation.
Results
The characteristics of our families are moderate macrothrombocytopenia and defective platelet function associated with a mild reduction of surface αIb β3, impaired platelet aggregation to physiological agonists but not to ristocetin, normal clot retraction, reduced fibrinogen binding and expression of activated αIIbβ3 upon stimulation, normal platelet adhesion to immobilized fibrinogen but reduced platelet spreading and tyrosine phosphorylation, indicating defective αIIbβ3-mediated outside-in signaling. Molecular analysis revealed a novel mutation of ITGB3 that determines an in-frame deletion producing the loss of amino acids 647–686 of the βTD ectodomain of integrin β3. Haplotype analysis indicated that the two families inherited the mutation from a common ancestral chromosome.
Conclusions
This novel autosomal dominant macrothrombocytopenia associated with platelet dysfunction raises interesting questions about the role of integrin β3, and its βTD domain, in platelet formation and function.
Introduction
Glanzmann’s thrombasthenia (GT) is a bleeding disorder caused by mutations of the ITGA2B or ITGB3 genes encoding for integrins αIIb and β3, respectively. Platelets do not aggregate in response to any physiological agonist, but they do not show abnormalities in number or size1. GT is an autosomal recessive disease and is, therefore, phenotypically expressed in homozygotes or compound heterozygotes, given that 50% of normal αIIbβ3 is sufficient to guarantee unimpaired platelet function.1,2 However, one GT-like patient with a partial deficiency of αIIbβ3, a life-long bleeding history and alterations in platelet size was previously reported.3,4 More recently, a missense mutation affecting only one allele of ITGB3 was described in a family with macrothrombocytopenia.5 Thus, depending on the site and the extent of the αIIbβ3 mutation, defective platelet function or impaired thrombocytopoiesis may occur. Indeed, a role for αIIbβ3 in platelet release from megakaryocytes has been shown.6
We report here two unrelated Italian families with an autosomal dominant form of macrothrombocytopenia showing impaired platelet function associated with a previously undescribed ITGB3 mutation and a partial integrin β3 defect.
Patients
The proband, a 35-year old woman from southern Italy, had life-long moderate/severe bleeding, mainly mucocutaneous. She and some members of her family have been studied on several occasions over a 4-year period; on each occasion one or more controls, members of the research and hospital staff, were simultaneously studied. No abnormalities in blood clotting were detected but her template bleeding time7 was more than 20 min (normal 5.8±1.0). Electronic platelet counts (Genius S, Seac, Italy) were 26–79×109/L (by optical microscopy: 69–98×109/L); mean platelet volume was 14.2 fL (normal: 10.7±0.9 fL), with 84% normal-sized (controls: 97±1.5%), 14% large (controls: 2.4±1.4%) and 2% giant (controls: 0.2±0.4%) platelets on peripheral blood smears.8
Bone marrow showed mild megakaryocytic hyperplasia with all stages of maturation. Clinical and laboratory investigations of 36 members of the proband’s family identified 17 subjects with thrombocytopenia transmitted as an autosomal dominant trait (Online Supplementary Figure S1).
Design and Methods
Platelet morphology
Blood films stained with May-Grünwald Giemsa were examined using an immersion oil objective with a Laborux 12 microscope (Leitz, Germany). For electron microscopy, washed platelets were prepared from blood collected in EDTA, and fixed for 4 h at 4°C, using cacodylate buffer containing 4% wt/vol of glutaraldehyde. The samples were then washed, and kept in cacodylate buffer for 4 h; the buffer was then replaced with 1% osmic acid and the samples were pelleted by centrifugation at 10,000xg for 30 sec. Ultrathin sections of the platelet pellets were stained with uranyl acetate and lead citrate and observed with a Philips Electron Optics EM208 transmission electron microscope at 80 kv.9
Platelet function
Platelet aggregation induced by collagen (1.2-4 μg/mL), adenosine diphosphate (ADP 2–4 μM), epinephrine (10–100 μM), U46619 (1–2 μM), arachidonic acid (0.6-4 mM), A23187 (4–40 μM), PMA (1–50 nM) and PAF (0.1–10 μM), was studied with a four-channel aggregometer (APACT4, Helena Bioscences, UK), using either platelet rich-plasma or washed platelets.10
Clot retraction was assessed using non-anticoagulated whole blood added to pre-warmed glass tubes (45x12 mm) and placed in a water bath at 37°C for 24 h; tubes were then inspected for clot retraction and results expressed as percentage of the original volume.11
The PFA-100® (Dade Behring, Marburg, Germany) was employed using both collagen/ADP (C/ADP) and collagen/epinephrine (C/EPI) cartridges in citrate whole blood samples; normal ranges were established using samples from 40 healthy volunteers.12
Platelet adhesion to a collagen-coated surface (collagen from equine tendon, 30 μg/cm2) under flow conditions was studied in a parallel plate perfusion chamber, at a wall shear rate of 3000s−1.13
Flow cytometry
The expression of platelet membrane glycoproteins (GP) was investigated by flow cytometry with the following monoclonal antibodies: P2 and SZ22 for integrin αIIb (CD41); SAP, SZ21 and AP3 for integrin β3 (CD61); A2A9/6 and AP2 for integrin αIIbβ3; SZ2 for GPIbα (CD42b); SZ1 for GPIX (CD42a); FA6-152 for GPIV (CD36); ALB6 for CD9; AMF7 for αvβ3 (CD51, αV-chain); 5.6E for PECAM-1 (CD31), and 11E4B-7-6 for glycophorin A. An isotypic antibody was used as a negative control. All antibodies were fluorescein isothiocyanate (FITC)-conjugated, except for AP2 and AP3 which were unconjugated and their binding was determined using a secondary FITC-conjugated anti-mouse IgG. Mean fluorescence intensity and the percentage of positive cells were measured and normalized to those of GPIbα, in order to take into account the large volume of platelets.14 The activation of platelets, induced either by ADP (10 μM) or thrombin receptor activating peptide (TRAP-6, 20 μM) in whole blood, was assessed by measuring the expression of P-selectin on their surface.15
Moreover, the binding of PAC-1 (recognizing activated αIIbβ3) or of a FITC-conjugated monoclonal antibody anti-fibrinogen, was studied.16
All samples were analyzed in an EPICS XL-MCL flow cytometer (Coulter Corporation, Miami, FL, USA), equipped with an argon laser operating at 488 nm.
Western blotting
Washed platelets from the proband and control or chinese hamster ovary cells expressing αIIbβ3 (kindly provided by Prof. H. Deckmyn, University of Kortrijk, Belgium) were lysed and 20 μg of proteins for each sample were analyzed by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) (8% gel) for 4 h and 30 min at 20 mA. Separated proteins were transferred onto a nitrocellulose membrane at 100 V for 90 min. Membranes were saturated with 5% fat-free dried milk in T-TBS and incubated overnight with an anti-integrin β3 mouse monoclonal antibody (Calbiochem, USA) and then incubated with horseradish peroxidase-conjugated anti-mouse IgG for 2 h at room temperature. Immunoreactive bands were detected using peroxidase-conjugated IgG antibody and chemiluminescence detection.
Adhesion and spreading assays
Washed platelets resuspended in Tyrode’s buffer (200×109/L) were incubated for 30 or 60 min on 100 μg/mL fibrinogen-coated dishes under static conditions, as previously described.17
The protein content of adherent platelets was determined with the Bradford assay, and results expressed as a percentage of the total added platelets. Platelet lysates were used for the detection of tyrosine phosphorylation by immunoblotting. Aliquots from total platelets or adherent platelets, containing the same amount of proteins, were added to an equal volume of SDS-sample buffer, heated at 95°C for 5 min and then subjected to SDS-PAGE on 7.5% acrylamide gels. Proteins were transferred to nitrocellulose and tested with an antibody against phosphotyrosine. After extensive washing, blots were revealed by chemiluminescence with an ECL kit and the bands corresponding to pp125FAK were quantified by using Quantiscan software (Biosoft, Cambridge, UK) and expressed as arbitrary units.
For platelet spreading, washed platelets (40×109/L) were layered onto human fibrinogen-coated (100 μg/mL) glass coverslips.17 Adherent platelets were fixed, permeabilized and stained with FITC-conjugated phalloidin. Slides were then analyzed using a fluorescence microscope (Zeiss Axioplan fluorescence microscope) with a 100x objective lens, controlled by a Spot-2 cooled camera (Diagnostic Instruments) and digital images were acquired. Ten different fields were analyzed for each specimen. For scanning electron microscopy analysis, adherent platelets were fixed with 2.5% (v/v) glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.3, containing 2% sucrose. Fixed platelets were washed, and then processed for dehydration and gold-labeling, and scanning electron microscopy images were captured using a Philips XL30 scanning electron microscope.18
For all the assays the proband’s samples were compared with those of ten normal subjects.
Genome-wide search and mutational screening
The genome-wide search was performed using ABI PRISM Linkage Mapping Set v2.5 (Applied Biosystems, Foster City, CA, USA). Pairwise linkage analysis and multipoint analysis based on an autosomal dominant transmission with complete penetrance, were performed using the LINKAGE19 and the Simwalk2 packages,20 respectively. Data handling was performed using Mega2.21
The coding exons, together with the donor and acceptor splicing sites of ITGB3 and ITGA2B (GenBank accession numbers NM_000212.2 and NM_000419.3), were amplified and sequenced. Genomic DNA of family members and of 150 controls was tested by denaturating high performance liquid chromatography (Models 3500A and 3500 HT; Transgenomic, Omaha, NE, USA) or restriction enzyme analysis, as the mutation identified creates a restriction enzyme site for PvuII.
Total RNA of one affected family member and three healthy controls was extracted from buffy coats using Trizol.22 The first strand cDNA was synthesized (SuperScriptTM II, Invitrogen Corporation, Paisley, UK) and amplified using oligonucleotides ITGB3/Ex12F (5’-GAATGTGTGGAGTGTAAGAAG-3’) and ITGB3/15R (5’-TGACATTCTCCCAACCTACC-3’) before sequence analysis.
Results
On light microscopy platelets were large but did not show any other visible alterations. No leukocyte inclusions were observed.
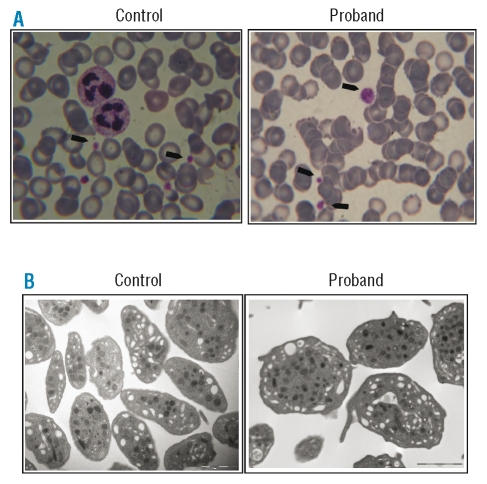
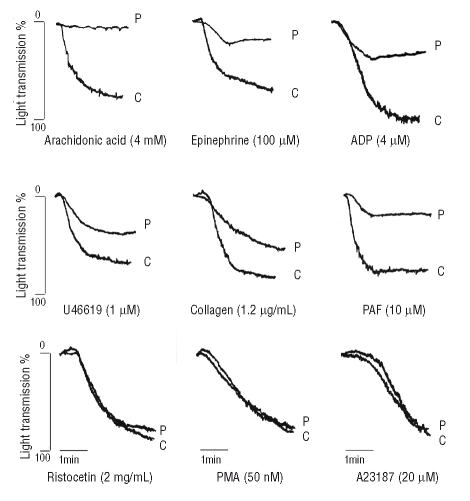
On transmission electron microscopy platelets were larger than normal (average diameter: 3.5±0.5 μm vs. 2.6±0.7 μm of controls, n=6, p<0.005) and revealed an increased number of δ and α granules/platelet, compatible with the larger platelet size (δ granules/platelet: 1.56±0.6 vs. 1.13±0.9, p<0.05; α granules/platelet: 10.9±5 vs. 6.1±2.7, p<0.005) (Figure 1). In the proband, platelet aggregation induced by ADP and epinephrine was severely impaired while that induced by collagen was reduced. Response to ristocetin, PMA or A23187 was normal; arachidonic acid-induced aggregation was absent (Figure 2). Spontaneous aggregation was always absent. The PFA-100® C/EPI closure time was unmeasurable (>300 sec vs. normal 135.3±27.5 sec) and the C/ADP closure time was markedly prolonged (213 sec vs. normal 89.9±20.8 sec).
Figure 1.
Platelet morphology as determined by optical microscopy and transmission electron microscopy. (A) Light microscopy (under oil immersion, 1000X) of a blood smear of control and proband’s platelets: platelets are indicated by arrowheads. (B) Trasmission electron microscopy of control and proband’s platelets (original magnification 13000X).
Figure 2.
Representative platelet aggregation tracings, in response to different agonists in platelet-rich plasma from the proband (P) and controls (C). Agonist concentrations are indicated in the figure.
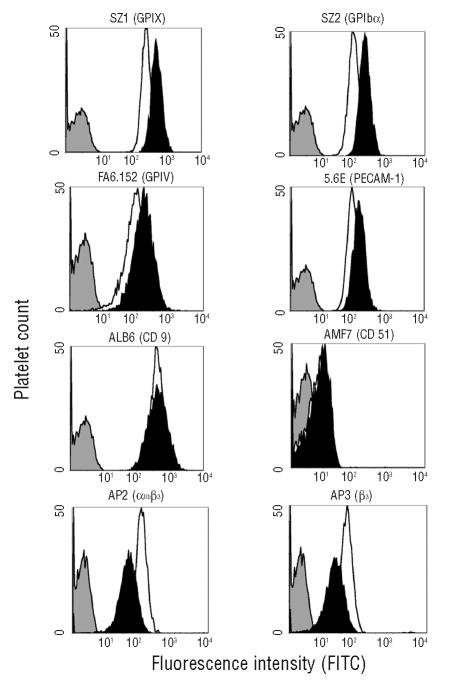
Platelet adhesion to collagen at high shear rate was impaired (4±0.9% vs. 22±2.6% of surface covered by platelets). Clot retraction was normal (77.5% vs. normal of 71.4±5.4%), unlike that of a simultaneously studied case of GT (0%). All surface glycoproteins were normal, or moderately increased, except for αIIbβ3 which was reduced with all anti αIIbβ3-clones tested (Figure 3), even when values were corrected for platelet volume14 (Online Supplementary Table S1). Upon stimulation with TRAP-6 (20 μM), surface expression of αIIbβ3, normalized for GPIbα14, increased in the patient’s platelets, although it did not reach normal values, indicating a normal internal pool of αIIbβ3 (data not shown).
Figure 3.
Flow cytometry of surface glycoproteins on platelets from the proband (black histograms) and controls (white histograms). The expression of CD9, αvβ3, GPIb/IX/V, PECAM-1 or GPIV on the platelet surface was normal, or slightly increased. In contrast, expression of GPIIIa (integrin β3) and the complex GPIIb/IIIa (αIIbβ3) was reduced. The gray curve is a non-specific signal.
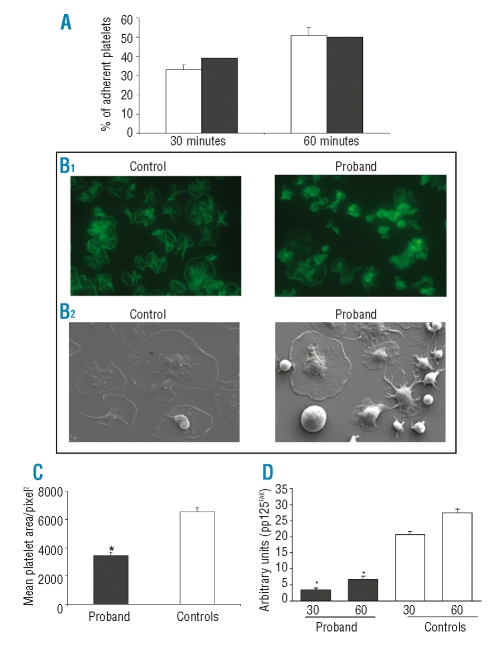
Fibrinogen- and PAC-1-binding to resting platelets was slightly enhanced, however possibly due to increased platelet size, while it was significantly impaired (approximately reduced by 50%) upon stimulation with ADP 10 μM or TRAP-6 20 μM (data not shown). P-selectin expression on the platelet surface after stimulation with TRAP-6 20 μM was normal, as it was on the platelets from a simultaneously studied GT patient.16 The proband’s platelets adhered to a fibrinogen-coated surface under static conditions with the same efficiency as that of control platelets (39% at 30 min. vs. 33±2.5% and 50% at 60 min. vs. 50.8±4%) (Figure 4A), while spreading on fibrinogen was severely impaired (Figure 4B). In particular, while most of the platelets from controls spread on fibrinogen, almost 50% of the proband’s platelets remained round and failed to spread following attachment (Figure 4C). Moreover, while the adhesion of control platelets was followed by a time-dependent tyrosine phosphorylation, in patient’s platelets this was strongly reduced (30 min: 3.52 vs. 20.6±1 arbitrary units; 60 min: 6.7 vs. 27.4±1.3 arbitrary units) (Figure 4D).
Figure 4.
A ( ) Adhesion to immobilized human fibrinogen. Washed platelets were allowed to attach to wells coated with 100 μg/mL fibrinogen for 30 and 60 min. After washing out non-adherent platelets, the protein content of adherent platelets was determined with the Bradford assay, and results were expressed as the percentage of total added platelets. The percentages of adherent platelets were similar in the proband (black columns) and in controls (white columns). (B) Spreading and protein phosphor ylation upon adhesion on immobilized fibrinogen. Washed platelets were layered onto fibrinogen-coated plates for 30 min. After washing away unbound platelets, adherent platelets were examined by fluorescence microscopy (B1) or by scanning electron microscopy (B2) and digital images (x100) were acquired. (C) Platelet spreading expressed as mean platelet area/pixel2. The proband’s platelets show an approximately 50% reduction of spreading (n=10, *p<0.05 vs. controls). (D) Protein phosphorylation. Densitometric analysis of pp125FAK, showing a significant reduction in the proband’s samples as compared to normal controls (*p<0.05 vs. controls). For all the assays the proband’s samples were compared to those of five normal subjects.
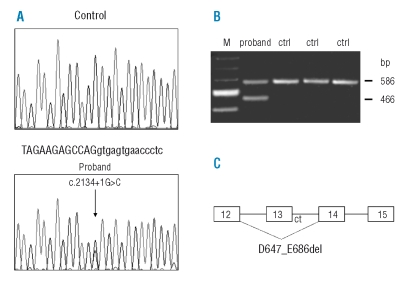
A genome-wide search mapped the involved gene to chromosome 17q in a candidate region of approximately 18 Mb between microsatellites D17S798 and D17S1319 (Online Supplementary Figure S1). Marker D17S950 gave a maximum multi-point location score of 8.4. Among possible candidates, ITGB3 and ITGA2B were analyzed in two affected individuals and a heterozygous G>C transversion (c.2134+1G>C) in the donor splice site of intron 13 of ITGB3 was identified (Figure 5). Mutational analysis in 36 family members showed that only the affected subjects were heterozygous for the mutation while this was absent in 150 unrelated control subjects. The effect of the splice site mutation was determined by reverse transcriptase-polymerase chain reaction (PCR) and sequencing analysis showing an in-frame deletion of 120 bp of exon 13, with loss of 40 residues of the extracellular βTD subdomain of integrin β3, from aspartic acid 647 to glutamic acid 686 of the mature polypeptide (p.D647_E686del) (Figure 5).
Figure 5.
Identification of the c.2134+1G>C mutation and its effect on the splicing of the ITGB3 gene. (A) Sequencing analysis of exon 13 and its flanking region from a control and from the proband, showing the nucleotide substitution G>C in the first position of intron 13 (lower case); (B) Reverse transcriptase-PCR of RNA extracted from peripheral blood of three controls (ctrl) and of patient IV-12 (proband) (Online Supplementary Figure S1): the proband presents an additional fragment of 486 bp corresponding to an in frame deletion of 120 bp of exon 13, as detected by sequence analysis; M, marker 100 bp (Biolabs); (C) Schematic representation of the alternative splicing of the ITGB3 gene due to the c.2134+1G>C mutation leading to a deletion of 40 amino acids (p.D647_E686del).
The 120 bp deletion was confirmed in platelet β3 mRNA by reverse transcriptase-PCR of purified platelet cDNA23 using primers flanking the deletion (data not shown).
Moreover, the expression level of normal β3 mRNA was quantified by real-time PCR using primers overlapping the deleted region: expression of the normal allele was reduced by approximately 50% in the proband (Online Supplementary Figure S2).
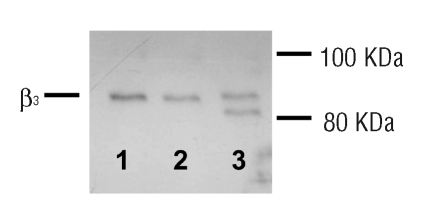
The mutated β3 was expressed in the proband’s platelets. Western blotting of control platelet lysate (Figure 6) showed a 90 kDa band, consistent with wild type β3, while platelet lysate from the proband showed normal β3 and a low molecular weight band product of mutated β3, of an expected molecular weight of approximately 85 kDa (Figure 6).
Figure 6.
Western blot of integrin β3. Control platelet lysate (lane 1) showed a band at the same level of the band of extract of chinese hamster ovary cells expressing normal αIIbβ3 (lane 2). The proband’s platelet lysate showed two distinct bands (lane 3): a band corresponding to normal integrin β3 (~90 kDa) and a lower band of the molecular weight expected for the mutated protein (~85 kDa).
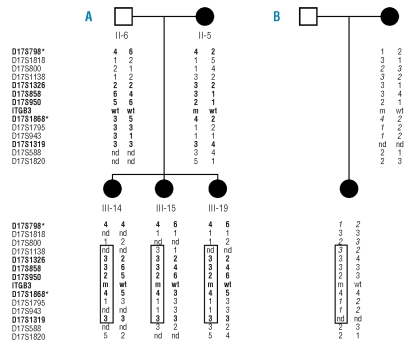
In order to define whether other patients with thrombocytopenia carried the c.2134+1G>C mutation, we selected from our database of patients with inherited forms of thrombocytopenia of unknown origin six patients with platelet surface expression of αIIbβ3 lower than 50% of control:24 in one of them we found the same mutation. This was a 34-year old woman from southern Italy, with moderate/severe bleeding since birth, electronic platelet counts of 48–93×109/L (by optical microscopy: 52–95×109/L), mean platelet volume 14.5fL, and 78% of normal-sized, 21% large and 1% giant platelets on blood smears.8 This patient’s 9-year old daughter had similar clinical and laboratory features. Platelet membrane glycoproteins and platelet aggregation showed the same alterations as the proband of the first family. Although no consanguinity was reported, microsatellites within the candidate region revealed that the affected members from the two families shared the same haplotype (Figure 7), indicating that the mutation occurred as a single event on an ancestral chromosome and that other cases with the same mutation are likely to be found in the Italian population.
Figure 7.
Haplotype construction using different microsatellites in two families carrying the c.2134+1G>C mutation (m) of the ITGB3 gene. (A) Branch of the pedigree used for linkage analysis; alleles of microsatellites indicated in bold are the same as those reported in Online Supplementary Figure S1. (B) Mother and daughter of the second family; alleles in italics are not informative. The largest haplotype potentially shared by affected individuals is boxed. Asterisks indicate microsatellites of the ABI PRISM Linkage Mapping set 2.5 (see Materials and Methods). nd, allele not determined.
Discussion
The macrothrombocytopenia with platelet dysfunction we report here represents a novel clinical entity because it does not meet the criteria for the diagnosis of any of the known forms.8,24–26
The p.D647_E686del mutation we found produces the loss of 40 amino acids of a region of integrin β3 located in the carboxyterminal tail of the ectodomain called βTD.27 Little is known about the function of this domain. Although random mutagenesis of βTD has produced mainly gain-of-function mutants, with a permanently activated αIIbβ3,28 the loss of amino acids 657–692, which partially overlaps the deletion in our families, was shown to prevent intracellular αIIbβ3 complex formation.29 We have shown the concomitant presence of both the normal and a mutated β3 protein in platelet lysates of the proband; thus, a loss-of-function hypothesis, with a dominant negative effect resulting from the p.D647_E686del mutation, can be made. The β3 mutation in our family leads to impaired αIIbβ3-mediated outside-in signaling. The patient’s platelets adhere to fibrinogen but fail to spread on it and tyrosine phosphorylation is impaired too, suggesting that the signal transduction pathways activated by the αIIbβ3 ligation and clustering are altered in our patient.30
In addition to platelet dysfunction our patient also had macrothrombocytopenia. It has been reported that the interaction of fibrinogen with αIIbβ3 is capable of stimulating proplatelet development in megakaryocytes and that the integrin is necessary for proplatelet formation.6
Recently, an ITGB3 mutation associated with autosomal dominant macrothrombocytopenia was described, and the β3 dysfunction was shown to cause defective megakaryopoiesis,5 in accordance with a role of αIIbβ3 in platelet formation and release.6 Differently from our cases, however, this mutation resulted in a significant constitutive, partial activation of αIIbβ3 and was not associated with bleeding. Moreover, unlike GT patients, our families manifest additional features, such as macrothrombocytopenia, defective response to arachidonic acid but a normal clot retraction. It has previously been reported that some variant αIIbβ3 molecules retain clot retraction capacity, even if aggregation is prevented.31
We cannot exclude that the platelet defects in our families are associated with an unidentified mutant allele in linkage disequilibrium with c.2134+1G>C; however, all candidate genes of known autosomal dominant thrombocytopenias were normal in our families (data not shown), and it is unlikely that two independent mutational events causing the same disease occurred in two different families and in the same relatively small region.
In conclusion, we have reported here a novel autosomal dominant macrothrombocytopenia with platelet dysfunction associated with a previously undescribed ITGB3 mutation. Although the full comprehension of the pathogenic mechanisms caused by p.D647_E686del requires further investigations, this novel hereditary thrombocytopenia raises interesting questions about the β3 domains involved in thrombocytopoiesis and outside-in signaling.
Supplementary Material
Acknowledgments
we thank the proband for her continued collaboration and helpfulness, Dr. D. Bartoli for practical advice about fluorescence microscopy and Prof. M. Torti for critical reading of the manuscript.
Footnotes
Authorship and Disclosures
PG contributed patients to the study, designed and supervised the research and wrote the paper; EF, SG, TC, AMM, and GG collected patients’ material, carried out experimental studies and analyzed data; LC performed RT-PCR and real-time PCR of platelet mRNA; CLB contributed patients to the study; PN carried out experimental studies; FDB, AS, PD’A and AD’E performed sequencing and molecular analysis and analyzed data; CLB and AS revised the final manuscript.
The authors reported no potential conflicts of interest.
The online version of this article contains a supplementary appendix.
Funding: this work was supported in part by grants to PG from the Fondazione Cassa di Risparmio di Perugia (Project n. 2007.0130.020) and to CLB and AS from the Italian Ministry of Health (Italy-USA Program –“Rare Diseases”).
References
- 1.Nurden AT. Glanzmann thrombasthenia. Orphanet J Rare Dis. 2006;1:1–8. doi: 10.1186/1750-1172-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Andrea G, Colaizzo D, Vecchione G, Grandone E, Di Minno G, Margaglione M. Glanzmann’s thrombasthenia: identification of 19 new mutations in 30 patients. Thromb Haemost. 2002;87:1034–42. [PubMed] [Google Scholar]
- 3.Hardisty R, Pidard D, Cox A, Nokes T, Legrand C, Bouillot C, et al. A defect of platelet aggregation associated with an abnormal distribution of glycoprotein IIb-IIIa complexes within the platelet: the cause of a lifelong bleeding disorder. Blood. 1992;80:696–708. [PubMed] [Google Scholar]
- 4.Peyruchaud O, Nurden AT, Milet S, Macchi L, Pannochia A, Bray PF, et al. R to Q amino acid substitution in the GFFKR sequence of the cytoplasmic domain of the integrin IIb subunit in a patient with a Glanzmann’s thrombasthenia-like syndrome. Blood. 1998;92:4178–87. [PubMed] [Google Scholar]
- 5.Ghevaert C, Salsmann A, Watkins NA, Schaffner-Reckinger E, Rankin A, Garner SF, et al. A non-synonymous SNP in the ITGB3 gene disrupts the conserved membrane-proximal cytoplasmic salt bridge in the αIIbβ3 integrin and co-segregates dominantly with abnormal proplatelet formation and macrothrombocytopenia. Blood. 2007;111:3407–14. doi: 10.1182/blood-2007-09-112615. [DOI] [PubMed] [Google Scholar]
- 6.Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin αIIbβ3. Blood. 2006;108:1509–14. doi: 10.1182/blood-2005-11-011957. [DOI] [PubMed] [Google Scholar]
- 7.Gresele P, Arnout J, Deckmyn H, Huybrechts E, Pieters G, Vermylen J. Role of proaggregatory and antiaggre-gatory prostaglandins in hemostasis. Studies with combined thromboxane synthase inhibition and thromboxane receptor antagonism. J Clin Invest. 1987;80:1435–45. doi: 10.1172/JCI113223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balduini CL, Cattaneo M, Fabris F, Gresele P, Iolascon A, Pulcinelli FM, et al. Inherited thrombocytopenias: a proposed diagnostic algorithm from the Italian Gruppo di Studio delle Piastrine. Haematologica. 2003;88:582–92. [PubMed] [Google Scholar]
- 9.Laffi G, Marra F, Gresele P, Romagnoli P, Palermo A, Bartolini O, et al. Evidence for a storage pool defect in platelets from cirrhotic patients with defective aggregation. Gastroenterology. 1992;103:641–6. doi: 10.1016/0016-5085(92)90859-w. [DOI] [PubMed] [Google Scholar]
- 10.Vezza R, Roberti R, Nenci GG, Gresele P. Prostaglandin E2 potentiates platelet aggregation by priming protein kinase C. Blood. 1993;82:2704–13. [PubMed] [Google Scholar]
- 11.Rooney MM, Farrell DH, van Hemel BM, de Groot PG, Lord ST. The contribution of the three hypothesized integrin-binding sites in fibrinogen to platelet-mediated clot retraction. Blood. 1998;92:2374–81. [PubMed] [Google Scholar]
- 12.Giannini S, Mezzasoma AM, Leone M, Gresele P. Laboratory diagnosis and monitoring of desmopressin treatment of von Willebrand’s disease by flow cytometry. Haematologica. 2007;92:1647–54. doi: 10.3324/haematol.11313. [DOI] [PubMed] [Google Scholar]
- 13.Momi S, Impagnatiello F, Guzzetta M, Caracchini R, Guglielmini G, Olivieri R, et al. NCX 6560, a nitric oxide-releasing derivative of atorvastatin, inhibits cholesterol biosynthesis and shows anti-inflammatory and anti-thrombotic properties. Eur J Pharma-col. 2007;570:115–24. doi: 10.1016/j.ejphar.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Savoia A, Balduini CL, Savino M, Noris P, Del Vecchio M, Perrotta S, et al. Autosomal dominant macrothrombocytopenia in Italy is most frequently a type of heterozygous Bernard-Soulier syndrome. Blood. 2001;97:1330–5. doi: 10.1182/blood.v97.5.1330. [DOI] [PubMed] [Google Scholar]
- 15.Ciferri S, Emiliani C, Guglielmini G, Orlacchio A, Nenci GG, Gresele P. Platelets release their lysosomal content in vivo in humans upon activation. Thromb Haemost. 2000;83:157–64. [PubMed] [Google Scholar]
- 16.Giannini S, Mezzasoma A, Guglielmini G, Rossi R, Falcinelli E, Gresele P. A new case of acquired Glanzmann’s thrombasthenia: diagnostic value of flow cytometry. Cytometry Part B. 2008;74:194–9. doi: 10.1002/cyto.b.20396. [DOI] [PubMed] [Google Scholar]
- 17.Bernardi B, Guidetti GF, Campus F, Crittenden JR, Graybiel AM, Balduini C, et al. The small GTPase Rap 1b regulates the cross talk between platelet integrin α2β1 and integrin αIIbβ3. Blood. 2006;107:2728–35. doi: 10.1182/blood-2005-07-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau LM, Wee JL, Wright MD, Moseley GW, Hogarth PM, Ashman LK, et al. The tetraspanin superfamily member CD151 regulates outside-in integrin αIIbβ3 signaling and platelet function. Blood. 2004;104:2368–75. doi: 10.1182/blood-2003-12-4430. [DOI] [PubMed] [Google Scholar]
- 19.Lathrop GM, Lalouel JM, Julier C, Ott J. Strategies for multilocus analysis in humans. Proc Natl Acad Sci USA. 1984;81:3443–6. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobel E, Lange K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet. 1996;58:1323–37. [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay N, Almasy L, Schroeder M, Mulvihill WP, Weeks DE. Mega2: data-handling for facilitating genetic linkage and association analyses. Bioinformatics. 2005;21:2556–7. doi: 10.1093/bioinformatics/bti364. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynsky P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, et al. Escaping the nuclear confines: signal dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–91. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noris P, Pecci A, Di Bari F, Di Stazio MT, Di Pumpo M, Ceresa IF, et al. Application of a diagnostic algorithm for inherited thrombocytopenias to 46 consecutive patients. Haematologica. 2004;89:1219–25. [PubMed] [Google Scholar]
- 25.Balduini CL, Iolascon A, Savoia A. Inherited thrombocytopenias: from genes to therapy. Haematologica. 2002;87:860–80. [PubMed] [Google Scholar]
- 26.Van Geet C, Freson K, Devos R, Vermylen J. Hereditary thrombocytopenias. In: Gresele P, Page C, Fuster V, Vermylen J, editors. Platelets in thrombotic and non-thrombotic disorders. Cambridge, UK: Cambridge University Press; 2002. pp. 515–27. [Google Scholar]
- 27.Bennett JS. Structure and function of the platelet integrin αIIbβ3. J Clin Invest. 2005;115:3363–9. doi: 10.1172/JCI26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butta N, Arias-Salgado EG, González-Manchón C, Ferrer M, Larrucea S, Ayuso MS, Parrilla R. Disruption of the beta3 663–687 disulfide bridge confers constitutive activity to beta3 integrins. Blood. 2003;102:2491–7. doi: 10.1182/blood-2003-01-0213. [DOI] [PubMed] [Google Scholar]
- 29.Yatuv R, Rosenberg N, Zivelin A, Peretz H, Dardik R, Trakhtenbrot L, et al. Identification of a region in glycoprotein IIIa involved in subunit association with glycoprotein IIb: further lessons from Iraqi-Jewish Glanzmann thrombasthenia. Blood. 2001;98:1063–9. doi: 10.1182/blood.v98.4.1063. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Shattil S, Ambruso DR, Newman PJ. Truncation of the cytoplasmatic domain of β3 in a variant form of Glanzmann thrombasthenia abrogates signaling through the integrin αIIbβ3 complex. J Clin Invest. 1997;100:2393–403. doi: 10.1172/JCI119780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiyoi T, Tomiyama Y, Honda S, Tadokoro S, Arai M, Kashiwagi H, et al. A naturally occurring Tyr143His αIIb mutation abolishes αIIbβ3 function for soluble ligands but retains its ability for mediating cell adhesion and clot retraction: comparison with other mutations causing ligand-binding defects. Blood. 2003;101:3485–91. doi: 10.1182/blood-2002-07-2144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.