Platelet receptors are at the forefront of recent research and major advances have been made in understanding their molecular functions and their downstream signaling pathways. This review addresses the steps in the thrombus formation process in the arterial circulation, emphasizing our current knowledge on the role of platelet receptors and signaling.
Keywords: platelet, receptors, thrombus formation
Abstract
Hemostasis and pathological thrombus formation are dynamic processes that require a co-ordinated series of events involving platelet membrane receptors, bidirectional intracellular signals, and release of platelet proteins and inflammatory substances. This review aims to summarize current knowledge in the key steps in the dynamics of thrombus formation, with special emphasis on the crucial participation of platelet receptors and signaling in this process. Initial tethering and firm adhesion of platelets to the exposed subendothelium is mediated by glycoprotein (GP) Ib/IX/V complex and collagen receptors, GP VI and α2β1 integrin, in the platelet surface, and by VWF and fibrillar collagen in the vascular site. Interactions between these elements are largely influenced by flow and trigger signaling events that reinforce adhesion and promote platelet activation. Thereafter, soluble agonists, ADP, thrombin, TxA2, produced/released at the site of vascular injury act in autocrine and paracrine mode to amplify platelet activation and to recruit circulating platelets to the developing thrombus. Specific interactions of these agonists with their G-protein coupled receptors generate inside-out signaling leading to conformational activation of integrins, in particular αIIbβ3, increasing their ligand affinity. Binding of αIIbβ3 to its ligands, mainly fibrinogen, supports processes such as clot retraction and platelet aggregation. Stabilization of thrombi is supported by the late wave of signaling events promoted by close contact between aggregated platelets. The best known contact-dependent signaling is outside-in signaling through αIb β3, but new ones are being clarified such as those mediated by interaction of Eph receptors with ephrins, or by Sema 4D and Gas-6 binding to their receptors. Finally, newly identified mechanisms appear to control thrombus growth, including back-shifting of activated integrins and actuation of compensatory molecules such as ESAM or PECAM-1. The expanding knowledge of thrombotic disease is expected to translate into the development of new drugs to help management and prevention of thrombosis.
Introduction
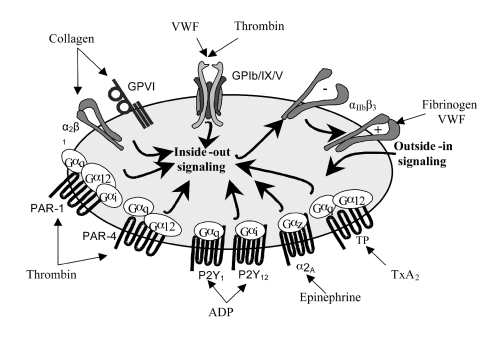
Platelet receptors are at the forefront of recent research and major advances have been made in understanding their molecular functions and their downstream signaling pathways. Studies with animal models, including pharmacological inhibition and knocking out of nearly all known receptors, adhesion molecules, and many signaling molecules have helped to reveal new mechanisms for how the thrombotic and hemorrhagic propensity of platelets is controlled in health and disease. A wide variety of mobile transmembrane receptors covers the platelet membrane, including many integrins (αIIbβ3, α2β1, α5β1, α6β1, αVβ3), leucine-rich repeated (LRR) receptors (Glycoprotein [GP] Ib/IX/V, Toll-like receptors), G-protein coupled seven transmembrane receptors (GPCR) (PAR-1 and PAR-4 thrombin receptors, P2Y1 and P2Y12 ADP receptors, TPα and TPβ TxA2 receptors), proteins belonging to the immunoglobulin superfamily (GP VI, FcγRIIA), C-type lectin receptors (P-selectin), tyrosine kinase receptors (thrombopoietin receptor, Gas-6, ephrins and Eph kinases) and a miscellaneous of other types (CD63, CD36, P-selectin ligand 1, TNF receptor type, etc). Many of these receptors are shared by other cell types, but some are only expressed on platelets. Nowadays, it is well established that the major platelet receptors have a prominent role in the hemostatic function of platelets, allowing specific interactions and functional responses of vascular adhesive proteins and of soluble platelet agonists (Figure 1). In addition, it is increasingly recognized that a range of receptors are involved in other less well-understood platelet functions such as inflammation, tumor growth and metastasis, or immunological host defense.1 Internally, platelets contain a cytoskeleton, a dense tubular system, few mitochondrias, glycogen granules, dense (δ) and α storage granules and peroxisomes. The α-granules retain relevant proteins for the hemostatic function of platelets, such as von Willebrand factor (VWF), fibrinogen, P-selectin, PECAM-1, CD40 ligand (CD154), platelet factor-4, β-thromboglobulin, thrombospondin, platelet derived growth factor (PDGF), FV, as well as a back-up of GP IIb/IIIa (αIIbβ3). δ granules, on the other hand, are rich in nucleotides (ADP and ATP), serotonin, histamine, pyrophosphate, and calcium. Upon activation, granule contents are stepwise released to further promote platelet adhesion and activation.2
Figure 1.
Major platelet receptor-ligand interactions.
The primary function of platelets is to stop blood loss after tissue trauma and exposure of the subendothelial matrix.2,3 However, the frontier between physiological hemostasis and pathological thrombosis is very narrow, and it is increasingly recognized that platelets are at least partially liable for the pathological development of atherothrombosis, the leading cause of death in the developed world.4 The contribution of platelets to thrombus growth is highly dependent on its location within the blood circulation. Venous thrombosis is to a large extent due to changes in the composition of the blood favoring coagulation, while in the arterial circulation, the high flow rate precludes accumulation of activated coagulation factors thus limiting fibrin formation, with platelets however playing a crucial role in the establishment of these thrombi. Nevertheless, the classical nature of arterial and venous thrombosis has been recently challenged, and recent evidence suggests a potential link between these disorders.5
The participation of platelets in hemostasis and thrombosis has been extensively studied in the last decades. Traditional research in this area, with techniques common to the study of biochemical processes, cell and molecular biology, has provided a valuable insight into the elements and signals regulating platelet adhesion, activation and aggregation. Emergent high-speed imaging techniques with arrays of fluorescent and chemiluminescent probes now allow the study of hemostasis and thrombus formation in live animals, and activation signaling events in live cells. These new technologies will help to unravel the complex role of platelets in physiological and pathological thrombus formation, and to the design of effective and specific antithrombotic drugs.6
This review addresses the steps in the thrombus formation process in the arterial circulation, emphasizing our current knowledge on the role of platelet receptors and signaling.
Dynamics of platelet plug formation
Formation of platelet plugs at sites of vascular damage requires a co-ordinated, both in time and place, series of events leading to: i) platelet arrest onto the exposed subendothelium creating a monolayer of activated cells (initiation phase); ii) recruitment and activation of additional platelets through the local release of major platelet agonists (extension phase); iii) stabilization of the platelet plug preventing premature disaggregation until wound healing occurs (stabilization phase). Currently, the existing static model of thrombus formation has been visualized as a dynamic model of thrombus build-up and stabilization in which continuous signaling is needed to stabilize thrombi and prevent their dissolution.7
Additionally, negative regulation of platelets is essential to prevent uncontrolled thrombosis, and therefore the balance between inhibitory and activatory signaling in platelets regulates the balance between platelet inhibition and activation, which is important to ensure that the thrombus is restricted to the initial site of injury.
Initiation phase
Thrombus formation in response to tissue trauma initiates with platelet interactions with the extracellular matrix components exposed to blood, particularly VWF, collagen, fibronectin, thrombospondin, and laminin. The rheological conditions largely influence these adhesive interactions. Thus, while at low shear rate, such as that in veins and larger arteries, platelet adhesion to the vessel wall primarily involves binding to fibrilar collagen, fibronectin and laminin, under conditions of elevated shear stress, such as those encountered in the microvasculature or in stenotic arteries, platelet tethering to the damaged subendothelium is critically dependent on their interaction with subendothelial bound VWF.8,9 Several collagens are present in the vessel wall, of which collagens I and III are considered the most important in supporting platelet adhesion to the damaged vasculature. Type I, III, and VI collagen filaments have affinity for VWF and the two molecules are associated in the extracellular matrix. While soluble VWF does not bind to platelets to prevent aggregation in the normal circulation, immobilized VWF onto collagen is highly reactive toward flowing platelets. This may be because immobilized VWF assumes an extended shape under the effect of shear allowing its A1 domain to interact with platelets.10,11 Perfusion assays simulating in vivo flow conditions have revealed that platelet adhesion to VWF is a dynamic process in which initial platelet tethering is characterized by transient interactions mediated through GP Ibα. This deceleration then allows platelets to form new bonds with slower intrinsic binding kinetics (collagen to platelet collagen receptors, VWF to integrin αIIbβ3) and to become activated.8,9
It has long been known that the GP Ib/IX/V complex is the major platelet receptor mediating interaction with VWF. This complex consists of leucine-rich repeat glycoproteins: GP Ibα (130 kDa) and GP Ibβ that are disulfide-linked and non-covalently associated with GP IX (22kDa) and GP V as a 2:4:2:1 complex.12,13 In humans, lack or dysfunction of this receptor is associated with the Bernard Soulier syndrome (BSS), a congenital bleeding disorder characterized by macrothrombocytopenia and inability of these platelets to aggregate in response to the antibiotic ristocetin. In addition to VWF, the GP Ib/IX/V complex also binds to other adhesive proteins (collagen, thrombospondin-1), α-thrombin and coagulation factors (kininogen, FXI, FXII). It also plays a substantial role in platelet interaction with activated endothelial cells and with leukocytes, through the binding of P-selectin and Mac-1 (αMβ2), respectively. The N-terminal globular domain of GP Ibα (residues 1-282) contains the non-identical but overlapping binding sites for all these ligands. In vivo, experiments with VWF knockout mice and mice expressing modified GP Ibα have demonstrated that GP Ibα is absolutely essential for arterial thrombus formation, while absence of VWF can be somehow overcome by the binding of the receptor with alternative ligands.14,15 Fab fragments of the monoclonal antibody 6B4, that blocks the GP Ibα-binding site for VWF, exhibit a powerful antithrombotic effect in baboons without significant prolongation of the skin bleeding time.16 This antibody was recently humanized and may provide a promising therapeutic antithrombotic alternative.17
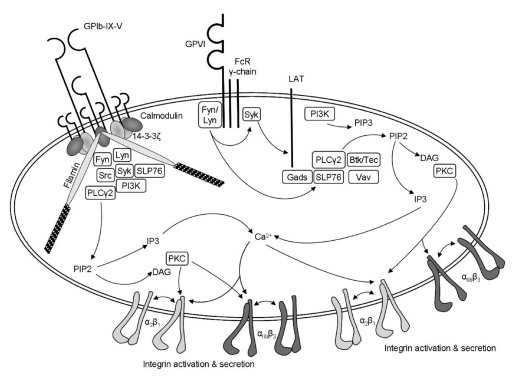
The model of how the VWF-GP Ibα interaction onto the vessel wall signals and contributes to subsequent platelet activation has still not been completely defined. The cytoplasmic region of GP Ibα is associated with filamin (also referred to as actin binding protein), calmodulin, and 14-3-3ζ, thus providing potential links to relevant signaling proteins such as phosphatidylinositol 3-kinase (PI-3K), focal adhesion kinase (FAK), Src-related tyrosine kinases, GTPase-activating protein and tyrosine phosphatases (PTP1b and SHPTP10).18,19 In addition, recent evidence shows topographical association of the GP Ib/IX/V complex with other important proteins in platelet signaling such as GP VI, FcR γ-chain, α2β1, FcγRIIA, most likely within specialized membrane microdomains known as lipid rafts,20 supporting a cross-linking mechanism involved in GP Ibα signaling. Thus, despite the fact that GP Ib/IX/V lacks built-in tyrosine kinase activity, is not directly coupled to G-proteins, nor contains phosphorylatable tyrosine residues to directly link signaling molecules, it profits from all these signaling mechanisms by associating with other platelet receptors. The engagement of GP Ibα by immobilized VWF elicits typical activation signals such as transient cytoplasmic Ca2+ elevations, protein phosphorylation (PLCγ2, ERK-1/2, Syk), TxA2 synthesis, ADP release and ultimately activation of αIIbβ318,19 (Figure 2). The picture of how all this occurs remains to be fully clarified.
Figure 2.
Platelet signaling through GP VI and GP Ib/IX/V complex. Adapted with permission.21
Initiation of platelet adhesion to the injured vessel wall also requires platelet interaction with exposed collagen. Indeed, collagen and VWF form a functional unit for thrombus formation in flowing blood, with VWF contributing to the initial capturing of platelets on the vessel surface and collagen allowing the establishment of stable bonds for firm adhesion and triggering platelet activation. The mechanism by which the collagen structure influences platelet adhesion and activation is still poorly understood and, although platelets can adhere to monomeric collagen, the more complex structure of fibrillar collagen is required for optimal platelet activation by this adhesive protein.22 Two receptors have been demonstrated in the platelet surface that bind directly to collagen, the GP VI immunoglobulin superfamily member and the integrin α2β1.
GP VI (62kDa) is a platelet–specific low-affinity collagen receptor of high potency in terms of initiating signal generation.23 It has two extracellular immunoglobulin domains, a mucin-like core, a short peptide linker sequence, a transmembrane domain and a short cytoplasmic tail that binds Fyn and Lyn Src kinases. GP VI is also constitutively complexed with FcR γ-chain dimer, which bears an immunoreceptor tyrosine-based activation motif (ITAM) acting as the signal-transducing subunit of the receptor. When the GP VI receptor is cross-linked by binding to collagen or by GP VI specific ligands such as convulxin or alborhagin, the constitutively bound Src kinases phosphorylate the ITAM sequence in the FcR γ-chain allowing the assembly and activation of Syk and initiating activation of a downstream signaling pathway that has many similarities with that employed by immune receptors. Central to this signaling cascade is the formation of the signalosome, composed of various adapter and effector proteins (LAT, SLP-76, Gads), which associates to and activates PLCγ2, thus leading to liberation of 1, 2 diacylglycerol and inositol 1,4,5-triphosphate and to promotion of full platelet activation24 (Figure 2). Mice that lack either GP VI or the FcR γ-chain have significantly impaired platelet response to collagen and reduced thrombus formation in the ferric chloride injury model.25,26 Additionally, the Fab fragment of a novel monoclonal antihuman GP VI antibody, OM4, inhibits thrombosis in vivo in a model of thrombosis in rats without the prolongation of bleeding time that is seen with anti-GP IIb/IIIa antibodies.27 Further studies are needed to investigate whether OM4 and the other anti-GPVI antibodies can be developed for future clinical use.
The α2β1 integrin, commonly referred to as GP Ia/IIa, VLA-2 or CD49b/CD29, also plays a role in the adhesion of platelets to collagen and for subsequent optimal activation. The expression level of α2β1, as that of GP VI, is controlled by silent polymorphisms and correlates with the in vitro rapidity in platelet adhesion and responsiveness to collagen.28,29 The involvement of α2β1 in hemostasis had been anticipated by the mild bleeding tendency and impaired platelet responses to collagen displayed by subjects with heritable reduced expression of this integrin.30 Mice deficient in α2 or β1 have normal bleeding times, and display minor defects on platelet adhesion and aggregation to collagen.31,32 Studies of experimental in vivo arterial thrombosis in these mice have led to conflicting results showing both unaltered formation of thrombi32 and mildly delayed, reduced and unstable thrombi.33,34 Like other integrins, conformational activation of α2β1 increases its affinity for collagen, and seems to require inside-out signaling events that might be driven by engagement of GP VI with collagen23 and/or by activation of αIIbβ3.35 Recent findings indicate that the mechanism of activation of α2β1 is similar to that of other integrins and involve unclasping of the corresponding transmembane domain upon interaction of the α1 cytoplasmic tail with talin and kindlin-3.36 Moreover, collagen binding to α2β1 also triggers outside-in signaling resembling that induced by GP VI,23 that reinforces platelet activation. It is noteworthy that combined deficiency of α2β1 and GP VI in mice causes complete inhibition of thrombus formation as compared with the partial defect in formation of thrombi associated with isolated deficiency of either collagen receptor.37 Thus, it is widely recognized that α2β1 and GP VI act synergistically for optimal platelet adhesion and activation by collagen, and that their relative contribution to the dynamics of thrombus formation depends on the nature of the vascular lesion, flow conditions, and other still unknown factors. Even though deficiency of α2β1 and GP VI in humans does not seem to be associated with major hemostatic defects,30,38 targeting α2β1, and the collagen itself, is being considered as a potential antithrombotic strategy.39
Extension phase
After deposition of a platelet monolayer over the exposed VWF and collagen, the next step required for thrombus formation is the recruitment of additional platelets from the flowing blood, which upon activation acquire the ability to stick to each other in a process commonly referred to as platelet aggregation. This is made possible by the local accumulation of soluble agonists that are secreted/produced by adherent-activated platelets, including ADP, TxA2, epinephrine and thrombin. The final step is activation of αIIbβ3, causing a conformational change that enables it to bind fibrinogen and VWF, allowing stable bridges between platelets. The great number of αIIbβ3 copies on the platelet surface, (40,000–80,000), allows the assembly of large aggregates at the site of vascular injury. Activation of αIIbβ3 integrin requires agonist-driven activation events in recruited platelets, referred to as inside-out signaling, including the sequential activation of one or more PLC isoforms yielding a rise in cytosolic Ca2+, activation of PKC and PI-3K, reorganization of the platelet cytoskeleton and activation of cytoskeletal proteins such as talin. The activated talin can bind to the cytoplasmic domain of the β3 subunit causing dissociation of the cytoplasmic tails and transmembrane domains of αIIb and β3, and promoting integrin oligomerization and fibrinogen binding.40 A novel integrin activation pathway, which links inside-out signals to αIIbβ3 affinity modulation via a Rap-1–RIAM–talin signaling complex was recently identified.41 Moreover, the adhesion plaque protein kindlin-3, has been shown to directly bind to regions of β-integrin tails distinct from those of talin and trigger integrin activation in mice platelets.42 The relevance of these two elements for platelet integrin activation has been revealed in patients suffering from the rare leukocyte adhesion deficiency (LAD) syndrome, associated with severe defects in leukocyte and platelet integrin activation causing a Glanzmann-type bleeding disorder. Such disease has been shown to be caused by an aberrant Rap-1 activity43 or kindlin-3 protein expression.44
GPCRs are important seven-transmembrane spanning signaling molecules that play crucial roles in the extension of the platelet plug by most soluble platelet agonists.45,46 GPCRs can activate associated heterotrimeric guanine nucleotide–binding proteins (G proteins), which in turn act on various effectors (adenylyl cyclase, PLC, PI-3K, p115-RhoGEF). In an orchestrated manner, in platelets, agonists acting through GPCRs: i) stimulate PLCβ isoforms via Gqα, causing an increase in cytosolic Ca2+ and activation of PKC; ii) reorganize the actin cytoskeleton via G12α and G13α promoting the microtubular ring change and the filopodia and lamellopodia formation that drive platelet shape change; iii) suppress cAMP synthesis via Giα family members by inhibiting adenylyl cyclase, which is particularly relevant when intracellular cAMP levels are high due to the action of endothelial cell-derived prostaglandin I2 (PGI2) and nitric oxide (NO). There is evidence that Giα-associated Gβγ subunits also activate other signaling pathways such as PI-3Kψ, Src kinases and the Rap1B Ras family protein, which is an important contributor to pathways converging on the activation of αIIbβ3.46 Studies on mice deficient in specific G-proteins have helped to establish the role of these proteins in platelet activation and thrombus formation.46
Platelet agonists with a prominent role in the process of extension of the platelet plug are ADP, TxA2, thrombin and epinephrine.
As mentioned above, ADP is stored in δ-granules and secreted upon platelet activation. Red cells at sites of vascular injury also release ADP. This agonist causes a full range of activation events including intraplatelet Ca2+ elevation, TxA2 synthesis, protein phosphorylation, shape change, granule secretion, activation of αIIbβ3, and aggregation. All these events are mediated by interaction with two classes of purinergic GPCR, P2Y1 and P2Y12, which couple to Gqα and Giα, respectively.47 Recent studies in knockout mice for P2Y1 and P2Y12 have helped to define the probably different and complementary role of these two receptors in thrombus formation.48 P2Y1 deficient mice show minimal increase in bleeding time, no spontaneous hemorrhage, and some resistance to thromboembolic mortality upon injection of ADP, a mixture of collagen and epinephrine, or tissue factor. Platelet responses to ADP and collagen, but not cAMP synthesis, are impaired in these mice. At high concentration or in combination with serotonin, a mild activator of PLC, ADP causes aggregation of P2Y1−/− platelets. In contrast, in P2Y12−/− mice ADP does not induce aggregation nor inhibits cAMP synthesis, despite the maintenance of other P2Y1-mediated responses, such as shape change or PLC activation.46–48 The platelet responses to other agonists are also severely affected in P2Y12-deficient mice, which have a significantly prolonged bleeding time and are protected from arterial thrombosis in the FeCl3 model. Few patients have been identified with hemorrhagic diathesis associated to P2Y12 congenital defects, whereas so far there has been no report of patients with a P2Y1 deficiency.49 Together with results from in vitro studies using specific inhibitors, such data identify P2Y12 as the major receptor to amplify and sustain ADP-mediated platelet activation initiated via P2Y1. Indeed, P2Y12 is the target of thienopyridine drugs (ticlopidine, clopidogrel, prasugrel) widely used and with probed efficacy in the prevention of vascular events in patients with cardiovascular disease, specially those having stent insertion.39 Despite their likely categorized roles, co-activation of both P2Y1 and P2Y12 seems to be required for optimal ADP-induced aggregation and ADP-promoted thrombus growth. The observation that over-expressing the P2Y1 receptor results in full ADP-induced secretion and irreversible aggregation, shortened bleeding time and more susceptibility to ADP and collagen-induced thromboembolism and arterial thrombosis triggered by FeCl350 reinforces the physiological relevance of this receptor and the rationale for its consideration as target of new antithrombotic compounds.39,47 In addition to P2Y1 and P2Y12, human platelets express a third purinergic receptor P2X1, with a significant role in platelet function. P2X1 is an ATP-driven calcium channel unable to trigger platelet aggregation by itself, but under high shear conditions acts as a positive regulator of platelet responses to collagen and thus plays a significant role in thrombus formation. P2X1-deficient mice show a normal bleeding time but resistance to thromboembolism upon collagen-epinephrine injection, while mice overexpressing this receptor display increased systemic thrombosis. These results indicate that drugs targeting P2X1 might be effective antithrombotics specially at sites of severe stenosis where shear forces are high.47
Thromboxane A2 is a labile prostanoid synthesized by activated platelets through the sequential actions of cyclooxygenase (COX) and TxA2 synthase enzymes. TxA2 is a vasoconstrictor and a potent platelet agonist causing shape change, phosphoinositide hydrolysis, Ca2+ mobilization, protein phosphorylation, secretion, and aggregation.51
Once synthesized, it diffuses across the platelet membrane and activates other recruited platelets, thus favoring the growth of the platelet plug.
Overproduction of TxA2 has been implicated in the pathogenesis of thrombotic diseases, including myocardial infarction, unstable angina, pulmonary embolism and atherosclerosis.52 Indeed, as established by current clinical guidelines, blockade of TxA2 synthesis through COX inhibition by aspirin represents a valuable approach for managing high cardiovascular risk patients.53 However, the role of TxA2 in thrombus formation under pathological conditions of high shear remains unclear, with studies showing that aspirin has no inhibitory effect either on in vitro thrombus formation at high shear and disturbed laminar flow in a parallel-plate perfusion chamber device or on in vivo high-degree coronary artery stenosis in a dog animal model.54,55 This may be among the causes of clinical resistance or inefficacy of aspirin in patients with a high degree of arterial stenosis.53,56 The TxA2 receptor (TP) exists in two splice variants (TPα and TPβ) which differ only in their C-terminal cytoplasmic domains and that are encoded by a single gen located at 19p13.3. It is assumed that the ligand binding characteristics (mainly in the extracellular region of TPs) are identical for both splice variants. However, several reports have shown a different pattern of coupling to G protein effectors between TPα and TPβ.56,57 In human platelets, TPα is the only translated isoform, although mRNA for TPβ is also present.56 Biochemical studies have shown that TPs in platelets couple to Gqα and G13α activating pathways, but not to Giα. Loss of Gqα abolishes IP3 formation and Ca2+ mobilization, but not the G12/13α mediated activation of Rho GTPases and shape change, after activation with U46619, a TxA2 analogue. In contrast, platelets deficient in both Gqα and G13α do not respond to TxA2. Similarly, TP−/− mice are unable to aggregate in response to TxA2 analogs, display impaired responses to collagen, and show a prolonged bleeding time.48,58 Several homozygous and heterozygous patients suffering from lifelong mucosal bleeding due to genetic changes in TP or in other elements of the TxA2 signaling pathway have also been reported.49 All these data, in addition to the compelling evidence about the benefit of aspirin in the prevention/therapy of cardiovascular diseases, fully demonstrate the major contribution of TxA2 in platelet plug formation.
It is well established that thrombin rapidly generated at sites of vascular injury plays a major role in promoting and stabilizing thrombi under all shear conditions.9,59 Generation of thrombin requires several surface-mediated reactions carried out by the tenase complex (FIXa in complex with FVIIa, which activate FX) and by the prothrombinase complex (FXa in complex with FVa, which activate FII). It has long been assumed that the activated platelet membrane, with phosphatidylserine translocated from the inner to the outer leaflet, mainly provides such a procoagulant surface. However, this dogma may need to be reconsidered according to recent evidence. For instance, mice deficient in the PAR-4 thrombin receptor, refractory to thrombin-induced platelet activation, do not develop a significant platelet thrombus but generate normal amounts of fibrin around the vessel injury.60 This invites the question as to whether the required procoagulant surfaces come from other sources such as endothelial cells or microparticles instead of platelets. In vivo, experiments also show that thrombin activity is distributed throughout the platelet thrombus and not only at the thrombus-blood interface, indicating that the arterial thrombi are porous and allow the flow of agonists within.6 Thrombin is perhaps the most effective platelet activator, and provokes a full range of responses (shape change, secretion, TxA2 generation, Ca2+ mobilization, protein phosphorylation and aggregation). It is capable of activating platelets at very low concentrations (0.1 nM) and no other platelet agonist seems to be as efficiently coupled to PLCβ activation. Within seconds, thrombin increases ten-fold the cytosolic level of Ca2+, triggering downstream events as activation of PLA2.46 The thrombin-induced platelet responses are mediated at least partially by the GP Ib/IX/V complex,12–19 and mainly by two protease activated receptors (PAR), namely PAR-1 and PAR-4 in humans and PAR-3 and PAR-4 in mice.61
The GP Ibα subunit of the GP Ib/IX/V complex contains a high affinity binding site for α-thrombin, accounting for as much as 90% of the total protease that can bind to platelets.62 This binding site is located within residues 268–287 at the N-terminal globular domain of GP Ibα and can bind two separate thrombin molecules by interacting with both exosite I and II of the protease.63,64 Binding of α-thrombin to GP Ibα induces platelet adhesion and spreading, secretion and aggregation,62,65 and thus, platelets from patients with the BSS which lack GP Ibα display impaired thrombin responsiveness.66 Antibodies that block the thrombin binding to GP Ibα attenuate platelet activation by thrombin.67 Blood banking storage of platelet concentrates causes proteolysis of GP Ibα, reduces the number of high affinity binding sites for thrombin, and impairs platelet responsiveness towards thrombin.68 These observations strongly support the view that GP Ibα participates in platelet activation by thrombin, but its exact role remains unclear. Since blockade of the thrombin- GP Ibα interaction with antibodies impairs the cleavage of PAR-1, it has been proposed that GP Ibα may serve as a co-factor that localizes thrombin to the platelet surface allowing its proteolytic action over PARs.61,63,69 Apart from GP Ibα, GP V may also be involved in this scenario, since it is a prominent α-thrombin substrate and GP V−/− mice display enhanced platelet sensitivity to low doses of thrombin leading to in vivo increased thrombogenesis and embolus formation.70,71 It has been suggested that proteolysis of GP V by α-thrombin reveals the ability of GP Ibα to act as a thrombin ligand and induce stimulatory responses.72
Despite recognizing that an intact GP Ib/IX/V complex may be required for optimal thrombin responsiveness, there is little doubt that PARs are sufficient to activate platelets and account for most, if not all, of the thrombin-induced signaling. Unlike other GPCRs, such as those for ADP or TxA2 which signal through standard receptor/ligand interactions, PAR-1 and PAR-4 are activated by a unique irreversible proteolytic cleavage within the first extracellular loop exposing a new N-terminus that serves as a tethered ligand. Short synthetic peptidomimetics of the new N-terminus sequences generated by thrombin (SFLLR and GYPGQV for PAR-1 and PAR-4, respectively) can activate these receptors and reproduce most of the platelet action of thrombin in platelets.61 Although activation of either PAR-1 or PAR-4 can trigger platelet secretion and aggregation, PAR-1 is probably the most important receptor in thrombin responsiveness. Thus, blockade of PAR-1 with antibodies or specific antagonists abolishes platelet activation at low concentration (1 nM) of thrombin, whereas similar blockade of PAR-4 has no inhibitory effect.61 However, blockade of both PAR-1 and PAR-4 are required for complete abrogation of platelet responses to higher thrombin concentration (30 nM). Thus, a dual-receptor signaling model for thrombin-induced platelet activation is proposed, in which PAR-1 is the primary mediator of activation at low concentrations of the protease and PAR-4 has a role as a back-up receptor. Indeed, there are qualitative differences in the dynamics of activation of PAR-1 and PAR-4, which might be relevant for sustained optimal platelet responses to thrombin. Thus, PAR-4 mediated Ca2+ mobilization is slower and more prolonged than that of PAR-1, and the former receptor is switched off more slowly.73 Both PAR-1 and PAR-4 couple to Gqα and G12/13α, and PAR-1 also seems capable of activating Giα. Through these coupling mechanisms, thrombin gains access to the major network of platelet signaling pathways, thus causing full platelet activation.61
To our knowledge, no patient has been identified with congenital deficiencies of PAR receptors. However, mouse models have been developed that strengthen the relevance of PARs in platelet function.48 Mouse platelets express PAR-3 instead of PAR-1, but the latter is found in mice in tissues such as endothelium. It is to be noted that PAR-1−/− mice show normal hemostasis and respond to thrombin, consistent with PAR-3 assuming in mice the role of PAR-1 in human platelets and with a major role of PAR-4 in thrombin responsiveness. However, about half of PAR-1−/− mice die in the uterus with signs of deficient vasculogenesis. It seems that expression of PAR-1 and normal thrombin signaling in the endothelium, together with actions of other upstream coagulation factors, are critical for appropriate angiogenesis and vascular development.74 Platelets from PAR-4−/− mice display a complete loss of responsiveness to even high concentrations of thrombin despite a normal expression of PAR-3, thus supporting the two-receptor model of thrombin activation, with PAR-4 as the major signaling receptor in mouse.75 While PAR-3 and PAR-4 knockout mice are protected against thrombosis and demonstrate defective thrombus formation,75,76 direct PAR-1 inhibition in primate models also abrogates arterial thrombosis.77 In humans, PARs may also be significantly implicated in atherothrombosis by acting in other cells.61 For instance, PAR-1 mediated activation of endothelial cells by thrombin triggers release of VWF and surface expression of P-selectin, facilitating the rolling and adhesion of platelets and leukocytes. Also the protease induces the production of bioactive compounds (PAF, prostaglandins, chemokines, etc.) from the various cells in the diseased scenario promoting inflammation.61
In the light of their critical role in hemostasis and thrombosis, PARs are looked at as a potential target for new antithrombotics. Several peptide and non-peptide antagonists have been developed, and clinical studies are underway in the context of coronary artery disease and percutaneous coronary intervention.39,61 Other potential antithrombotic strategies targeting the thrombin signaling pathway include inhibitors of cell-surface promoters of thrombin generation, or direct thrombin inhibitors.
Although the contribution of catecholamines to the hemostatic/thrombotic process is generally thought to occur through its constrictive action on the vascular wall, circulating or locally secreted epinephrine also favors platelet activation in the growing platelet plug. A reduced number of epinephrine receptors has been related to mild bleeding disorders in a few patients.78 In contrast to other agonists, epinephrine is considered to be a weak agonist unable to directly activate PLC or to cause shape change. However, it acts sinergically with many other agonists at low concentrations significantly increasing their activatory effect. This potentiating action of epinephrine is due to its capacity to inhibit cAMP formation throughout the coupling of its platelet α2A-adrenergic receptor to the Giα family member Gzα.79 Recent studies in α2A-adrenergic-deficient mice have shown variable tail bleeding time, normal in vitro platelet response to thrombin or collagen, and normal thrombus formation over collagen-coated surface. However, these mice display defective responses to epinephrine which, even at high concentration, is incapable of potentiating the activation response to ADP or TxA2 analogs. Moreover, these mice are protected against lethal pulmonary thromboembolism induced by injection of collagen/epinephrine. In two in vivo models of thrombosis, FeCl3 induced injury of mesenteric arterioles and mechanical firm compression of the aorta, α2A-adrenergic-deficient mice displayed increased embolus formation suggesting a certain degree of thrombus instability.80 Variable responsiveness to epinephrine in relation to factors such as age, strenuous exercise or pathological conditions such as heart disease or myeloproliferative syndromes has been related to changes in the platelet expression level of α2A-adrenergic receptors.81,82 Some polymorphisms in the α2A-adrenergic receptor influence in vitro shear mediated platelet function.83 Additionally, it has been shown that inherited robust aggregation to a submaximal concentration of epinephrine establishes a true hyperreactive platelet phenotype which may influence the risk for arterial thrombosis.84,85.All these findings strengthen the relevance of signaling initiated by epinephrine through the α2A-adrenergic receptor on thrombus formation.
Stabilization phase
The last phase in the formation of an effective thrombus that arrests blood loss at the site of vascular injury has been named stabilization or perpetuation. It refers to the late wave of signaling events promoted by the close contact between recruited platelets once aggregation has started. Despite there being no evidence that these platelets form tight junctions, as do endothelial cells for instance, activated platelets within the forming plug come into sufficiently close contact (with gaps below 50 nm) to allow direct or indirect bridges between adjacent platelets and to allow paracrine action of platelet released molecules, favoring the transfer of information as in a neurological or immunological synapse. This narrow contact also restricts the diffusion of plasma factors within the gaps, preventing for instance a premature fibrinolytic action of plasmin over the growing thrombus. These late events consolidate the stability of the forming thrombus avoiding early disaggregation and/or embolization.
The most relevant, or at least the best known, contact-dependent signaling events during this stabilization phase is outside-in signaling through integrins, particularly αIIbβ3. This consists of those signals emanating from αIIbβ3 once ligand binding, predominantly fibrinogen, has occurred, which trigger essential events for thrombus growth and stabilization, such as cytoskeletal reorganization, formation and stabilization of large platelet aggregates, development of a procoagulant surface and a clot retraction that helps to narrow the gaps between platelets and to increase the local concentration of soluble platelet agonists.24,46 αIIbβ3 is a proven therapeutic target for antithrombotic therapy in patients undergoing percutaneous coronary interventions (PCI) and/or treatment for unstable angina, but ongoing studies are still underway to improve on the currently available drugs.86
Apart from αIIbβ3 clustering, much evidence indicates that integrin outside-in signaling relays on tyrosine phosphorylation of αIIbβ3 upon inside-out signals, and on the formation of large protein signaling complexes between the cytoplasmic domains of αIIbβ3 and intraplatelet proteins such as FAK, talin, myosin, β3-endonexin, CIB1, Shc, Src and Syk, the PRP-1b tyrosine phosphatase and PKCα, among others.87 The importance of αIIbβ3 outside-in signaling in the enhancement of platelet aggregation was demonstrated by the generation of knock-in mice where tyrosine residues Tyr-747 and Tyr-759 were mutated to phenylalanine. These so-called DiYF mice displayed selective impairment of outside-in signaling, resulting in the formation of unstable aggregates. The specific mechanisms of the links of intraplatelet proteins with αIIbβ3 and their physiological relevance for integrin outside-in signaling are now starting to be revealed.87
Integrins are by no means the only actors in the scene of perpetuation of the platelet plug. Thus, platelets express junctional adhesion molecules (JAM-A and JAM-C) which are thought to support cohesive and signaling interactions between adjacent platelets and between platelets and leukocytes favoring thrombus stabilization.88 Similarly, SLAM (CD150), a member of the CD2 family of adhesion molecules, is expressed in platelets and is tyrosine phosphorylated during platelet aggregation. Analysis of SLAM−/− mice revealed an impaired aggregation response to collagen and PAR-4, and female deficient mice displayed a marked decrease in platelet plug formation in a mesenteric vascular injury model.88
The CD40 ligand (CD40L, CD154), a protein member of the TNF family present on the surface of activated platelets, also seems to be important in this context. This protein, progressively shed from the platelet surface producing a soluble form, sCD40L, can bind to its receptor CD40 but also to αIIbβ3 through a KGD domain (or RGD in mice) favoring the outside-in integrin signaling.89 It is noteworthy that CD40L deficient mice show delayed occlusion following vascular injury and decreased thrombus stability.90
The family of Eph receptor tyrosine kinases and their ligands, known as ephrins, provide another mechanism of signaling promoted by direct contact between platelets.88,91 Human platelets express EphA4, EphB1 and ephrinB. The former is constitutively associated with αIIbβ3 in both resting and activated platelets. Clustering of either EphA4 or ephrinB1 causes platelet adhesion to immobilized fibrinogen. In contrast, blockade of Eph/ephrin interaction hampers clot retraction by impairing β3 phosphorylation, inhibits platelet aggregation at low concentrations of agonists, and results in smaller thrombi on collagen–coated surfaces under arterial flow conditions.88
Two additional ligand/receptor interactions generated by close platelet-platelet contact are binding of semaphorin 4D (Sema4D) and Gas-6 to their platelet receptors. Sema4D (CD100) is a type I membrane GP with a previously recognized role in T-cell activation also expressed in human platelets. Binding of Sema4D to its receptors, CD72 and plexin-B1, also seems to contribute to the regulation of thrombus formation as suggested by studies in Sema4D−/− mice. These mice exhibit impaired platelet responses to collagen, but normal to ADP and PAR-4, and decreased occlusive thrombi in various arterial thrombosis models.88,92 In addition to promoting thrombus formation, an ADAMTS17 cleaved fragment of Sema4D acts on endothelial cells favoring tissue repair. Gas-6, the product of the growth arrest-specific gene 6, is a vitamin K-dependent protein implicated in cell growth, adhesion, and migration, through its interactions with the Axl, Tyro 3 and Mer tyrosine kinase receptors. In mouse, Gas-6 is found in plasma and in the α-granules from which it is secreted upon activation. Mice deficient in Gas-6 or in any of its receptors show abnormal platelet responses to agonists and are protected against thrombosis, suggesting a major role of this axis in thrombus formation and vascular wall homeostasis.93,94 Biochemical studies have shown that Gas-6 mediated signaling reinforces αIIbβ3 outside-in signaling by activation of PI3-K and Akt, and promotes β3 phosphorylation and therefore clot retraction. Due to these actions, inhibition of Gas-6 signaling has been proposed as an attractive target for novel antithrombotic drugs.39 However, the role of Gas-6 in human platelet function must still be fully clarified since while the protein was demonstrated to be present in plasma, it has not been detected in human platelets.95
The current list of molecules that have a specific role in thrombus stability is probably far from complete. Novel proteomic or genomic strategies are being used to identify new candidate molecules phosphorylated upon platelet-platelet interaction. Thus, proteomic assays and molecular profiling of platelet RNA in custom arrays has served to recognize CD84, a protein homologous to SLAM, and PEAR-1 (platelet endothelial aggregation receptor 1), as new tyrosine/serine phosphorylated receptors. Recently, it has been shown that genetic variants in PEAR-1 associate with increasing overall platelet aggregation and reduced responsiveness to aspirin in subjects with premature cardiovascular disease.96 Signaling by these new receptors seems to be dependent on αIIbβ3 mediated platelet-platelet contacts, again resembling the immune synapse model.97
The formation of a fibrin network upon activation of the coagulation cascade is generally considered the last critical event contributing to thrombus stability. Recent studies with the laser-injury induced thrombosis model in mice expressing a low level of tissue factor (TF) have shown that this fibrin formation depends on the monocyte-derived TF carried out by microparticles, with minimal contribution of vessel wall TF.98 These microparticles are captured by the thrombus through the interaction between P-selectin expressed on the surface of activated platelets and PSGL-1 present on the microparticles.99 Thus, mice deficient in either PSGL-1 or P-selectin display thrombi with little TF and thrombin. However, certain questions are raised. First, other studies using models of photochemical induced injury in the carotid artery and vena cava ligation have found that vessel wall TF plays a critical role in thrombin generation inside the thrombus.100 Second, since circulating microparticles are present in blood, a mechanism must exist to prevent the initiation of blood coagulation. A suggested hypothesis is that TF normally is in a latent or encrypted form which lacks coagulant activity. How TF is encrypted and transformed to the active form is not yet fully known, but there is evidence that it may involve formation/disruption of disulfide bonds within the molecule. It is proposed that at the site of vascular injury, TF can be activated by the action of disulphide isomerase released from activated platelets and endothelial cells. Adding controversy to this issue, plateles themselves can carry or synthesize TF which can also play a role in thrombus formation.101,102
Negative regulation of platelet activation and thrombus growth
Arterial thrombus formation in vivo is a dynamic process, with developing thrombus at the site of injury gradually building up over time before either entirely occluding the blood vessel, or reaching a state of surface passification where thrombus growth is limited and thrombus size is stabilized. The essential roles of nitric oxide (NO) and prostacyclin (PGI2) in the negative regulation of platelets to prevent uncontrolled thrombosis have been well established.103,104 However, platelet activation can also be inhibited by signaling through the adhesion molecule PECAM-1 (also known as CD31). Like GP VI, PECAM-1 is a member of the Ig superfamily, with 6 extracellular Ig domains, transmembrane domain, and cytoplasmic tail. The cytoplasmic domain of PECAM-1 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) which becomes phosphorylated upon stimulation by homophilic interactions and/or clustering, facilitating the recruitment of tyrosine, serine/threonine or possibly lipid phosphatases, and the consequent inhibition of kinase-dependent signaling. PECAM-1 plays a role in attenuating thrombus formation involving GP VI, GP Ib, and thrombin-mediated platelet activation, as well as normal integrin αIIbβ3-mediated platelet function.105–108 Like PECAM-1, ESAM, a molecule on the platelet surface, seems to have a restraining role on thrombus growth and stability.88
Recent data also suggest that activation of αIIβb3, and likely that of other integrins, is not an irreversible process but rather a dynamic and bi-directional process, in particular for some agonists such as ADP and for strongly activated procoagulant or coated platelets with prolonged calcium elevation.36 Shifting activated integrins back to their resting state is a newly identified mechanism to control thrombus growth, and may represent a novel antithrombotic strategy. Moreover, balance between signaling through activatory adhesion receptors and platelet-surface sheddases, particularly of the metalloproteinase-disintegrin (ADAM) family, provides layers of regulation (proteinase and receptor), and a higher order of control of cellular function. Thus, ectodomain shedding of platelet adheso-signaling receptors, GP Ibα and GP VI, would provide an attractive mechanism for limiting thrombus growth and stability.109 Another mechanism contributing to restrict thrombus growth under flow is the cleavage by ADAMS13 of VWF engaged in thrombi surface, as suggested by recent perfusion experiments in the presence of a function-blocking antibody against this metalloprotease.110
Conclusions
Over the last few years we have seen impressive advances in our understanding of the mechanisms of hemostasis and thrombosis. There is now ample evidence that platelets play a paramount role in all stages of the complex, dynamic process of thrombus formation. Platelet receptors, signaling events and release/shedding of platelet proteins and inflammatory substances act in an orchestrated manner with the vascular endothelium and other blood cells and coagulation factors to permit the initial tethering of platelets over the vascular injury, to promote the growth of the platelet plug by recruiting and aggregating new platelets, and to consolidate the thrombus by means of post-aggregation signaling events, clot retraction, and controlled formation of a fibrin network. Compensatory molecules and signaling events have the opposite effect to limit undesirable platelet accumulation and pathological thrombosis. Translation of this knowledge to drug development will guarantee novel therapeutic approaches in the prevention of bleeding and thrombosis.
Footnotes
Authorship and Disclosures
All authors contributed equally to the manuscript.
The authors reported no potential conflict of interest.
Funding: Research of the authors’ group is supported by grants from Ministerio de Educación, Ciencia y Tecnología & FEDER (SAF 2006-06212), Fundación Séneca (04515/GERM/06, 03116/PI/05), and Red RECAVA (RD06/0014/0004). Leyre Navarro-Núñez is a fellow from Ministerio de Educación y Ciencia (BES-2005-7496).
References
- 1.Clemetson KJ, Clemetson JM. Platelet receptors. In: Michelson AD, editor. Platelets. 2nd ed. San Diego, CA: Elsevier/Academic Press; 2007. pp. 117–43. [Google Scholar]
- 2.George JN. Platelets. Lancet. 2000;355:1531–9. doi: 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 4.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 5.Prandoni P. Links between arterial and venous disease. J Intern Med. 2007;262:341–50. doi: 10.1111/j.1365-2796.2007.01815.x. [DOI] [PubMed] [Google Scholar]
- 6.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 7.Cosemans JMEM, Munnix ICA, Wetzker R, Heller R, Jackson SP, Heemskerk JWM. Continuous signalling via PI3K isoforms β and γ is required for platelet ADP receptor function in dynamic thrombus stabilization. J Thromb Haemost. 2006;108:3045–52. doi: 10.1182/blood-2006-03-006338. [DOI] [PubMed] [Google Scholar]
- 8.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–66. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SP, Nesbitt WS, Kulkarni S. Signaling event underlying thrombus formation. J Thromb Haemost. 2003;1:1602–12. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 10.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–50. [PubMed] [Google Scholar]
- 11.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. 2007;104:7899–903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera J, Lozano ML, Corral J, González-Conejero R, Martínez C, Vicente V. Platelet GP Ib/IX/V complex: physiological role. J Physiol Biochem. 2000;56:355–65. doi: 10.1007/BF03179804. [DOI] [PubMed] [Google Scholar]
- 13.Luo SZ, Mo X, Afshar-Kharghan V, Srinivasan S, Lopez JA, Li R. Glycoprotein Ibalpha forms disulfide bonds with 2 glycoproteins Ibbeta subunits in the resting platelets. Blood. 2007;109:603–9. doi: 10.1182/blood-2006-05-024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmeier W, Piffath CL, Goerge T, Cifuni SM, Ruggeri ZM, Ware J, et al. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci USA. 2006;103:16900–5. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117:953–60. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Vanhoorelbeke K, Cauwenberghs N, Meiring M, Depraetere H, Kotze HF, et al. Inhibition of the von Willebrand (VWF)-collagen interaction by an antihuman VWF monoclonal antibody results in abolition of in vivo arterial platelet thrombus formation in baboons. Blood. 2002;99:3623–8. doi: 10.1182/blood.v99.10.3623. [DOI] [PubMed] [Google Scholar]
- 17.Fontayne A, Vanhoorelbeke K, Pareyn I, Van Rompaey I, Meiring M, Lamprecht S, et al. Rational humanization of the powerful antithrombotic anti-GPIbalpha antibody: 6B4. Thromb Haemost. 2006;96:671–84. [PubMed] [Google Scholar]
- 18.Ozaki Y, Asazuma N, Suzuki-Inoue, Berndt MC. Platelet GPIb/IX/V-dependent signaling. J Thromb Haemost. 2005;3:1745–51. doi: 10.1111/j.1538-7836.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- 19.Du X. Signaling and regulation of the glycoprotein Ib/IX/V complex. Curr Opin Hematol. 2007;14:262–9. doi: 10.1097/MOH.0b013e3280dce51a. [DOI] [PubMed] [Google Scholar]
- 20.López JA, del Conde I, Shrimpton CN. Receptors, rafts, and microvesicles in thrombosis and inflammation. J Thromb Haemost. 2005;3:1737–44. doi: 10.1111/j.1538-7836.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 21.Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–25. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 22.Savage B, Ginsberg MH, Ruggeri ZM. Influence of fibrillar collagen structure on the mechanisms of platelet thrombus formation under flow. Blood. 1999;94:2704–15. [PubMed] [Google Scholar]
- 23.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–61. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 24.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and intregrin αIIbβ3 signaling in platelets. J Thromb Haemost. 2005;3:1752–62. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 25.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, et al. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–7. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 26.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, et al. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–41. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Lockyer S, Concepcion A, Gong X, Takizawa H, Guertin M, et al. The Fab fragment of a novel anti-GPVI monoclonal antibody, OM4, reduces in vivo thrombosis without bleeding risk in rats. Arterioscler Thromb Vasc Biol. 2007;27:1199–205. doi: 10.1161/ATVBAHA.107.140590. [DOI] [PubMed] [Google Scholar]
- 28.Corral J, González-Conejero R, Rivera J, Ortuño F, Aparicio P, Vicente V. Role of the 807 C/T polymorphism of the alpha2 gene in platelet GP Ia collagen receptor expression and function-effect in thromboembolic diseases. Thromb Haemost. 1999;81:951–6. [PubMed] [Google Scholar]
- 29.Kritzik M, Savage B, Nugent DJ, Santoso S, Ruggeri ZM, Kunicki TJ. Nucleotide polymorphisms in the α2 gene define multiple alleles that are associated with differences in platelet α2 β1 density. Blood. 1998;92:2382–8. [PubMed] [Google Scholar]
- 30.Nieuwenhuis HK, Akkerman JW, Houdijk WP, Sixma JJ. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985;318:470–2. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- 31.Holtkötter O, Nieswandt B, Smyth N, Müller W, Hafner M, Schulte V, et al. Integrin α 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–94. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 32.Grüner S, Prostredna M, Schulte V, Krieg T, Eckes B, Brakebusch C, et al. Multiple integrin-ligand interactions synergize in shear-resistant platelet adhesion at sites of arterial injury in vivo. Blood. 2003;102:4021–7. doi: 10.1182/blood-2003-05-1391. [DOI] [PubMed] [Google Scholar]
- 33.He L, Pappan LK, Grenache DG, Li Z, Tollefsen DM, Santoro SA, et al. The contributions of the alpha2 beta1 integrin to vascular thrombosis in vivo. Blood. 2003;102:3652–7. doi: 10.1182/blood-2003-04-1323. [DOI] [PubMed] [Google Scholar]
- 34.Kuijpers MJ, Pozgajova M, Cosemans JM, Munnix IC, Eckes B, Nieswandt B, et al. Role of murine integrin alpha2beta1 in thrombus stabilization and embolization: contribution of thromboxane A2. Thromb Haemost. 2007;98:1072–80. [PubMed] [Google Scholar]
- 35.Van de Walle GR, Schoolmeester A, Iserbyt BF, Cosemans JM, Heemskerk JW, Hoylaerts MF, et al. Activation of αIIbβ3 is a sufficient but also an imperative prerequisite for activation of α2β1 on platelets. Blood. 2007;109:595–602. doi: 10.1182/blood-2005-11-011775. [DOI] [PubMed] [Google Scholar]
- 36.Cosemans JM, Iserbyt BF, Deckmyn H, Heemskerk JW. Multiple ways to switch platelet integrins on and off. J Thromb Haemost. 2008;6:1253–61. doi: 10.1111/j.1538-7836.2008.03041.x. [DOI] [PubMed] [Google Scholar]
- 37.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer DA, Kahn ML. GPVI and α2β1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood. 2005;106:1268–77. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein VI-related clinical defects. Br J Haematol. 2007;139:363–72. doi: 10.1111/j.1365-2141.2007.06799.x. [DOI] [PubMed] [Google Scholar]
- 39.Barret NE, Jones L, Kaiser WJ, Moraes LA, Rana R, Sage T, et al. Future innovations in anti-platelet therapies. Br J Pharmacol. 2008;154:918–39. doi: 10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–11. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, et al. 2006. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr Biol. 2006;16:1796–806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–30. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 43.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, et al. LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–82. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, van den Berg TK, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood . 2008 Dec 8; doi: 10.1182/blood-2008-10-182154. [Epub ahead of print as doi: 10.1182/blood-2008-10-–182154] [DOI] [PubMed] [Google Scholar]
- 45.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 46.Woulfe D, Yang J, Prevost N, O’Brien P, Fortna R, Tognolini M, et al. Signaling receptors on platelets and megakaryocytes. Methods Mol Biol. 2004;273:3–31. doi: 10.1385/1-59259-783-1:003. [DOI] [PubMed] [Google Scholar]
- 47.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost. 2008;99:466–72. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 48.Jirouskova M, Shet AS, Johnson GJ. A guide to murine platelet structure, function, assays, and genetic alterations. J Thromb Haemost. 2007;5:661–9. doi: 10.1111/j.1538-7836.2007.02407.x. [DOI] [PubMed] [Google Scholar]
- 49.Salles II, Feys HB, Iserbyt BF, De Meyer SF, Vanhoorelbeke K, Deckmy H. Inherited traits affecting platelet function. Blood Rev. 2008;22:155–72. doi: 10.1016/j.blre.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Hechler B, Zhang Y, Eckly A, Cazenave JP, Gachet C, Ravid K. Lineage-specific overexpression of the P2Y1 receptor induces platelet hyper-reactivity in transgenic mice. J Thromb Haemost. 2003;1:155–63. doi: 10.1046/j.1538-7836.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Niccoli G, Giubilato S, Russo E, Spaziani C, Leo A, Porto I, et al. Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention. Eur Heart J. 2008;29:1843–50. doi: 10.1093/eurheartj/ehn325. [DOI] [PubMed] [Google Scholar]
- 53.Gasparyan AY, Watson T, Lip GY. The role of aspirin in cardiovascular prevention: implications of aspirin resistance. J Am Coll Cardiol. 2008;51:1829–43. doi: 10.1016/j.jacc.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 54.Barstad RM, Orvim U, Hamers MJ, Tjønnfjord GE, Brosstad FR, Sakariassen KS. Reduced effect of aspirin on thrombus formation at high shear and disturbed laminar blood flow. Thromb Haemost. 1996;75:827–32. [PubMed] [Google Scholar]
- 55.Maalej N, Folts JD. Increased shear stress overcomes the antithrombotic platelet inhibitory effect of aspirin in stenosed dog coronary arteries. Circulation. 1996;9:1201–5. doi: 10.1161/01.cir.93.6.1201. [DOI] [PubMed] [Google Scholar]
- 56.Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Cir Res. 2007;100:1261–75. doi: 10.1161/01.RES.0000264509.36234.51. [DOI] [PubMed] [Google Scholar]
- 57.Huang JS, Ramamurthy SK, Lin X, Le Breton GC. Cell signaling through thromboxane A2 receptors. Cell Signal. 2004;16:521–33. doi: 10.1016/j.cellsig.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Nieswandt B, Aktas B, Mores A, Sachs UJH. Platelet in atherothrombosis: lesson from mouse models. J Thromb Haemost. 2005;3:1725–36. doi: 10.1111/j.1538-7836.2005.01488.x. [DOI] [PubMed] [Google Scholar]
- 59.Crawley JTB, Zarnardelli S, Chion CKN, Lane DA. The central role of thrombin in hemostasis. J Thromb Haemost. 2007;5 (Suppl 1):95–101. doi: 10.1111/j.1538-7836.2007.02500.x. [DOI] [PubMed] [Google Scholar]
- 60.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci USA. 2007;104:288–92. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–14. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 62.Mazzucato M, Marco LD, Masotti A, Pradella P, Bahou WF, Ruggeri ZM. Characterization of the initial alpha-thrombin interaction with glycoprotein Ib alpha in relation to platelet activation. J Biol Chem. 1998;273:1880–7. doi: 10.1074/jbc.273.4.1880. [DOI] [PubMed] [Google Scholar]
- 63.Celikel R, McClintock RA, Roberts JR, Mendolicchio GL, Ware J, Varughese KI, et al. Modulation of alpha-thrombin function by distinct interactions with platelet glycoprotein Ibα. Science. 2003;301:218–21. doi: 10.1126/science.1084183. [DOI] [PubMed] [Google Scholar]
- 64.Dumas JJ, Kumar R, Seehra J, Somers WS, Mosyak L. Crystal structure of the GpIbα-thrombin complex essential for platelet aggregation. Science. 2003;301:222–6. doi: 10.1126/science.1083917. [DOI] [PubMed] [Google Scholar]
- 65.Adam F, Guillin MC, Jandrot-Perrus M. Glycoprotein Ib-mediated platelet activation. A signaling pathway triggered by thrombin. Eur J Biochem. 2003;270:2959–70. doi: 10.1046/j.1432-1033.2003.03670.x. [DOI] [PubMed] [Google Scholar]
- 66.López JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier Syndrome. Blood. 1998;91:4397–418. [PubMed] [Google Scholar]
- 67.De Marco L, Mazzucato M, Masotti A, Fenton JW, 2nd, Ruggeri ZM. Function of glycoprotein Ib α in platelet activation induced by alpha-thrombin. J Biol Chem. 1991;266:23776–83. [PubMed] [Google Scholar]
- 68.Lozano ML, Rivera J, González-Conejero R, Moraleda JM, Vicente V. Loss of high-affinity thrombin receptors during platelet concentrate storage impairs the reactivity of platelets to thrombin. Transfusion. 1997;37:368–75. doi: 10.1046/j.1537-2995.1997.37497265336.x. [DOI] [PubMed] [Google Scholar]
- 69.De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J Biol Chem. 2001;276:4692–8. doi: 10.1074/jbc.M008160200. [DOI] [PubMed] [Google Scholar]
- 70.Ramakrishnan V, Reeves PS, DeGuzman F, Deshpande U, Ministri-Madrid K, DuBridge RB, et al. Increased thrombin responsiveness in platelets from mice lacking glycoprotein V. Proc Natl Acad Sci USA. 1999;96:13336–41. doi: 10.1073/pnas.96.23.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ni H, Ramakrishnan V, Ruggeri ZM, Papalia JM, Phillips DR, Wagner DD. Increased thrombogenesis and embolus formation in mice lacking glycoprotein V. Blood. 2001;98:368–73. doi: 10.1182/blood.v98.2.368. [DOI] [PubMed] [Google Scholar]
- 72.Ramakrishnan V, DeGuzman F, Bao M, Hall SW, Leung LL, Phillips DR. A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V. Proc Natl Acad Sci USA. 2001;98:1823–8. doi: 10.1073/pnas.98.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Covic L, Gresser AL, Kuliopulos A. Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry. 2000;39:5458–67. doi: 10.1021/bi9927078. [DOI] [PubMed] [Google Scholar]
- 74.Griffin CT, Srinivasan Y, Zheng YW, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–70. doi: 10.1126/science.1061259. [DOI] [PubMed] [Google Scholar]
- 75.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signaling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–8. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 76.Weiss EJ, Hamilton JR, Lease KE, Coughlin SR. Protection against thrombosis in mice lacking PAR3. Blood. 2002;100:3240–4. doi: 10.1182/blood-2002-05-1470. [DOI] [PubMed] [Google Scholar]
- 77.Derian CK, Damiano BP, Addo MF, Darrow AL, D’Andrea MR, Nedelman M, et al. Blockade of the thrombin receptor protease-activated receptor-1 with a small-molecule antagonist prevents thrombus formation and vascular occlusion in nonhuman primates. J Pharmacol Exp Ther. 2003;304:855–61. doi: 10.1124/jpet.102.042663. [DOI] [PubMed] [Google Scholar]
- 78.Rao AK, Willis J, Kowalska MA, Wachtfogel YT, Colman RW. Differential requirements for platelet aggregation and inhibition of adenylate cyclase by epinephrine. Studies of a familial platelet α 2-adrenergic receptor defect. Blood. 1988;71:494–501. [PubMed] [Google Scholar]
- 79.Yang J, Wu J, Kowalska MA, Dalvi A, Prevost N, O’Brien PJ, et al. Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc Natl Acad Sci USA. 2000;97:9984–9. doi: 10.1073/pnas.180194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pozgajová M, Sachs UJ, Hein L, Nieswandt B. Reduced thrombus stability in mice lacking the α2A-adrenergic receptor. Blood. 2006;108:510–4. doi: 10.1182/blood-2005-12-4835. [DOI] [PubMed] [Google Scholar]
- 81.Wang JS, Cheng LJ. Effect of strenuous, acute exercise on alpha2-adrenergic agonist-potentiated platelet activation. Arterioscler Thromb Vasc Biol. 1999;19:1559–65. doi: 10.1161/01.atv.19.6.1559. [DOI] [PubMed] [Google Scholar]
- 82.Kaywin P, McDonough M, Insel PA, Shattil SJ. Platelet function in essential thrombocythemia. Decreased epinephrine responsiveness associated with a deficiency of platelet α-adrenergic receptors. N Engl J Med. 1978;299:505–9. doi: 10.1056/NEJM197809072991002. [DOI] [PubMed] [Google Scholar]
- 83.Yabe M, Matsubara Y, Takahashi S, Ishihara H, Shibano T, Miyaki K, et al. Identification of ADRA2A polymorphisms related to shear-mediated platelet function. Biochem Biophys Res Commun. 2006;347:1001–5. doi: 10.1016/j.bbrc.2006.06.180. [DOI] [PubMed] [Google Scholar]
- 84.Yee DL, Bergeron AL, Sun CW, Dong JF, Bray PF. Platelet hyperreactivity generalizes to multiple forms of stimulation. J Thromb Haemost. 2006;4:2043–50. doi: 10.1111/j.1538-7836.2006.02089.x. [DOI] [PubMed] [Google Scholar]
- 85.Bray PF, Mathias RA, Faraday N, Yanek LR, Fallin MD, Herrera-Galeano JE, et al. Heritability of platelet function in families with premature coronary artery disease. J Thromb Haemost. 2007;5:1617–23. doi: 10.1111/j.1538-7836.2007.02618.x. [DOI] [PubMed] [Google Scholar]
- 86.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin αIIbβ3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–25. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prévost N, Shattil JS. Outside-In signaling by integrin αIIβ3. In: Michelson AD, editor. Platelets. 2nd edition. San Diego, CA, USA: Elsevier/Academic Press; 2007. pp. 347–57. [Google Scholar]
- 88.Brass LF, Zhu L, Stalker TJ. Novel therapeutic targets at the platelet vascular interface. Arterioscler Thromb Vasc Biol. 2008;28:s43–50. doi: 10.1161/ATVBAHA.107.161026. [DOI] [PubMed] [Google Scholar]
- 89.Hermann A, Rauch BH, Braun M, Schrör K, Weber AA. Platelet CD40 ligand (CD40L)-subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 2001;12:74–82. doi: 10.1080/09537100020031207. [DOI] [PubMed] [Google Scholar]
- 90.André P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, et al. CD40L stabilizes arterial thrombi by a β3 integrin-dependent mechanism. Nat Med. 2002;8:247–52. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 91.Prévost N, Woulfe DS, Jiang H, Stalker TJ, Marchese P, Ruggeri ZM, et al. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc Natl Acad Sci USA. 2005;102:9820–5. doi: 10.1073/pnas.0404065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA. 2007;104:1621–6. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–21. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 94.Saller F, Burnier L, Schapira M, Angelillo-Scherrer A. Role of the growth arrest-specific gene 6 (gas6) product in thrombus stabilization. Blood Cells Mol Dis. 2006;36:373–8. doi: 10.1016/j.bcmd.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 95.Balogh I, Hafizi S, Stenhoff J, Hansson K, Dahlbäck B. Analysis of Gas6 in human platelets and plasma. Arterioscler Thromb Vasc Biol. 2005;25:1280–6. doi: 10.1161/01.ATV.0000163845.07146.48. [DOI] [PubMed] [Google Scholar]
- 96.Herrera-Galeano JE, Becker DM, Wilson AF, Yanek LR, Bray P, Vaidya D, et al. A novel variant in the platelet endothelial aggregation receptor-1 gene is associated with increased platelet aggregability. Arterioscler Thromb Vasc Biol. 2008;28:1484–90. doi: 10.1161/ATVBAHA.108.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nanda N, Phillips DR. Novel targets for antithrombotic drug discovery. Blood Cells Mol Dis. 2006;36:228–31. doi: 10.1016/j.bcmd.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 98.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–7. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 99.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–8. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 101.López-Vilchez I, Escolar G, Diaz-Ricart M, Fuste B, Galan AM, White JG. Tissue factor-enriched vesicles are taken up by platelets and induce platelet aggregation in the presence of factor VIIa. Thromb Haemost. 2007;97:202–11. [PubMed] [Google Scholar]
- 102.Panes O, Matus V, Sáez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–50. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 103.Geiger J. Inhibitors of platelet signal transduction as anti-aggregatory drugs. Expert Opin Invest Drugs. 2001;10:865–90. doi: 10.1517/13543784.10.5.865. [DOI] [PubMed] [Google Scholar]
- 104.Radomski MW, Palmer RM, Moncada S. Endogenous nitric-oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 105.Wee JL, Jackson DE. The Ig-ITIM superfamily member PECAM-1 regulates the "outside-in" signalling properties of integrin αIIbβ3 in platelets. Blood. 2005;106:3816–23. doi: 10.1182/blood-2005-03-0911. [DOI] [PubMed] [Google Scholar]
- 106.Rathore V, Stapleton MA, Hillery CA, Montgomery RR, Nichols TC, Merricks EP, et al. PECAM-1 negatively regulates GPIb/V/IX signalling in murine platelets. Blood. 2003;102:3658–64. doi: 10.1182/blood-2003-06-1888. [DOI] [PubMed] [Google Scholar]
- 107.Thai le M, Ashman LK, Harbour SN, Hogarth PM, Jackson DE. Physical proximity and functional interplay of PECAM-1 with the Fc receptor FcgRIIa on the platelet plasma membrane. Blood. 2003;102:3637–45. doi: 10.1182/blood-2003-02-0496. [DOI] [PubMed] [Google Scholar]
- 108.Cicmil M, Thomas JM, Leduc M, Bon C, Gibbins JM. Platelet endothelial cell adhesion molecule-1 signaling inhibits the activation of human platelets. Blood. 2002;99:137–44. doi: 10.1182/blood.v99.1.137. [DOI] [PubMed] [Google Scholar]
- 109.Andrews RK, Karunakaran D, Gardiner EE, Berndt MC. Platelet receptor proteolysis. A mechanism for downregulating platelet reactivity. Arterioscler Thromb Vasc Biol. 2007;27:1511–20. doi: 10.1161/ATVBAHA.107.141390. [DOI] [PubMed] [Google Scholar]
- 110.Shida Y, Nishio K, Sugimoto M, Mizuno T, Hamada M, Kato S, et al. Functional imaging of shear-dependent activity of ADAMTS13 in regulating mural thrombus growth under whole blood flow conditions. Blood. 2008;111:1295–8. doi: 10.1182/blood-2007-09-110700. [DOI] [PubMed] [Google Scholar]