Dickkopf-1 is an inhibitor of Wnt signaling, which is crucial for osteoblast differentiation. This study shows that serum levels of Dicckopf-1 are increased in patients with thalassemia and osteoporosis. Interestingly, in a placebo-controlled, randomized therapeutic trial a 12-months treatment with zoledronic acid reduced serum Dickkopf-1 levels and increased bone mineral density in these patients.
Keywords: thalassemia, osteoporosis, Dickkopf-1, osteoblast, zoledronic acid
Abstract
Dickkopf-1 is an inhibitor of Wnt signaling, which is crucial for osteoblast differentiation. We evaluated serum levels of Dickkopf-1 in 66 patients with thalassemia-induced osteoporosis who received therapy with zoledronic acid in a placebo-controlled, randomized trial. At baseline, thalassemia patients had increased serum levels of Dickkopf-1 that correlated with reduced bone mineral density of the lumbar spine and the distal radius. High Dickkopf-1 also correlated with increased bone resorption and reduced bone formation markers. Zoledronic acid produced a reduction in serum Dickkopf-1, which was associated with bone mineral density increase after 12 months of therapy. On the contrary, placebo group showed a borderline increase of Dickkopf-1, which was higher in patients who showed deterioration in pain scores. These results suggest that Dickkopf-1 is implicated in the pathogenesis of osteoporosis in thalassemia and reveal Dickkopf-1 as a possible target for the development of novel agents for the management of thalassemia-induced osteoporosis (ClinicalTrials. govIdentifier: NCT00346242).
Introduction
Osteoporosis represents an important cause of morbidity in adult patients with thalassemia. Its pathogenesis is multifactorial, and includes mainly bone marrow expansion, endocrine dysfunction and iron overload.1 Osteoclast activity is elevated in thalassemia osteoporosis,2 and thus bisphosphonates have been used for its management.3–5 Moreover, osteoblasts are also deregulated in thalassemia osteoporosis1,6 but there is very limited data for the underlying mechanisms of this deregulation.
Dickkopf-1 (Dkk1) is a soluble inhibitor of Wingless type (Wnt)/β-catenin signaling required for the development of embryonic head-inducing tissues in amphibian embryos.7 Dkk1 is implicated in the regulation of osteoblast differentiation8,9 being a negative regulator of normal bone homeostasis in vivo.10 Dkk1 overexpression in osteoblasts causes osteopenia and inhibits fracture repair,11 while Dkk1 activation in osteoblasts seems to participate in the pathogenesis of gluco-corticoid- and estrogen deficiency-mediated osteoporosis.12 Furthermore, Dkk1 is implicated in the pathogenesis of cancer related bone disease11 and Dkk1 serum levels are elevated in patients with multiple myeloma and breast cancer with bone metastasis.13,14 However, there is no information for the serum levels of Dkk1 in human osteoporosis of any benign etiology, including thalassemia-induced osteoporosis. The aim of this study was to evaluate, for the first time, the serum levels of Dkk1 in patients with thalassemia and osteoporosis who received therapy with zoledronic acid (ZOL) and evaluate possible correlations with clinical and laboratory data in an attempt to clarify if Dkk1 has any role in the pathogenesis of osteoporosis in thalassemia.
Design and Methods
Sixty-six patients (22M/44F, median age 42 years) with thalassemia-induced osteoporosis who participated in a phase II, randomized, placebo-controlled trial (ClinicalTrials.gov Identifier: NCT00346242) were studied. The characteristics of the patients and the study protocol have been previously described.4,5 In summary, patients were blindly randomized to receive ZOL at a dose of 4 mg, iv, in 15 min infusion, every six months (group A, n=23) or every three months (group B, n=21), or to receive placebo every three months (group C, n=22) for a 12-month period.
Dkk1 was measured at baseline and after 12 months of therapy, using ELISA methodology (Biomedica Medizinprodukte, No. BI-20412, Gesellschaft GmbH, Wien, Austria; in this ELISA 1 pmol/L=28.68 pg/mL), along with a series of serum bone indices: i) bone resorption markers [C-telopeptide of type-I collagen (CTX), tartrate-resistant acid phosphatase isoform-5b (TRACP-5b)], ii) bone formation markers [bone-alkaline phosphatase (bALP), osteocalcin (OC) and C-terminal propeptide of collagen type-I (CICP)], and iii) osteoclast regulators [receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG), and osteopontin], as previously described.4 Bone mineral density (BMD) of the lumbar spine (L1–L4), femoral neck (FN) and distal radius (R) was determined using Dual-Energy X-ray Absorptiometry (DXA; LUNAR, PRODIGY Version 8.60.006/SYSTEM GE medical system LUNAR USA 726, Heartland Trail, Madison, WI 53717, USA) before and 12 months post-ZOL treatment.4
The above bone markers were also evaluated in 30, age- and gender-matched, healthy controls (11M/19F, median age 44 years, range: 21–55 years). All controls had BMD measurements (L1–L4/FN/R) to exclude osteopenia/osteoporosis of other etiology, and were examined to ensure that there was no evidence of bone disease (i.e. osteoarthritis) and no receipt of medication that could alter the normal bone turnover during the previous six months.
Statistical analysis
The Mann-Whitney test and paired samples t-tests were applied to evaluate the differences between patients and controls while the Wilcoxon signed rank test was used to evaluate differences between baseline and values of the studied parameters at the various time points. Differences between patients of three groups were evaluated using the Mann-Whitney test and the one-way ANOVA. The correlation between changes of various biochemical parameters and BMD was evaluated with the Spearman’s (rs) correlation coefficient. Variables found to be statistically significant at the p<0.05 level for the presence of severe osteoporosis (Z-score< −4.0) in at least one studied site were entered into a multivariate model using Cox regression analysis to identify the most statistically significant model. All p values are two sided, the level of significance is <0.05 and confidence intervals refer to 95% boundaries.
Results and Discussion
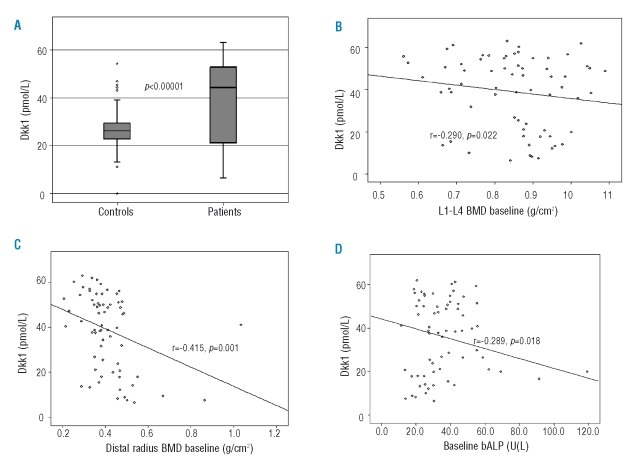
At baseline, thalassemia patients had increased serum levels of Dkk1 (mean±SD: 39±17.1 pmol/L) compared with controls (27.4±9.7 pmol/L; p<0.0001; Figure 1A). Furthermore, thalassemia patients had increased values of CTX (0.81±0.56 vs. 0.32±0.19 ng/mL; p<0.0001), TRACP-5b (1.29±0.94 vs. 0.58±0.34 U/L; p<0.01), bALP (35.5±12.2 vs. 19.8±13.5 U/L; p<0.001), CICP (81.3±31.0 vs. 56.7±32.2 ng/mL; p=0.003), and sRANKL/OPG ratio (0.39±0.18 vs. 0.12±0.19; p=0.001) compared with controls. Serum Dkk1 correlated with L1–L4 BMD (r=−0.290, p=0.022; Figure 1B) and R-BMD (r=−0.415, p=0.001; Figure 1C), but also with TRACP-5b (r=0.310, p=0.011), CTX (r=0.323, p=0.01) and bALP levels (r=−0.289, p=0.018; Figure 1D). There was also a weak correlation between FN-BMD and serum Dkk1 (r=−0.212, p=0.072). The univariate analysis showed that CTX, TRACP-5b, bALP, CICP, sRANKL/OPG ratio and Dkk1 correlated with the presence of severe osteoporosis (Z-score <−4.0) in at least one of the studied sites (p<0.04 for all markers). However, the Cox regression analysis revealed that only serum Dkk1 as a continuous variable had an independent value for the presence of severe osteoporosis (Z-score <−4.0) in our patients (Hazard ratio 1.055; 95% CI 1.016–1.095; p=0.002).
Figure 1.
Patients with thalassemia and osteoporosis have increased levels of serum Dkk1 compared with normal controls (A). Increased Dkk1 levels correlated with reduced BMD of the lumbar spine (B) and the wrist (C) but also with reduced bALP (bone formation marker) (D).
Dkk1 protein is implicated in osteoblast differentiation and bone remodeling in healthy people and in patients with bone disorders.9–14 Our results show, for the first time, that Dkk1 is increased in the serum of thalassemia patients with osteoporosis. The correlation observed between serum Dkk1 and BMD of the R and L1–L4 as well as the independent value of Dkk1 in revealing the presence of severe osteoporosis further suggests that Dkk1 is implicated, at least partially, in the pathogenesis of osteoporosis in thalassemia. We found a better correlation between serum Dkk1 and R-BMD compared to L1–L4 or FN-BMD. This may be explained by the severity of osteoporosis in the R observed in our cohort of patients. More specifically, the median Z-score of R-BMD was lower (−3.55) compared with that of the L1–L4 (median Z-score: −2.4) and the FN, which had the lower level of bone loss in this cohort of patients (median Z-score: −1.55; p (ANOVA) <0.01].4
Dkk1 also correlated with increased bone resorption (as assessed by CTX and TRACP-5b measurements) in our patients; an observation which indicates that Dkk1 is able to enhance osteoclast activity in thalassemia. This phenomenon has been already described in myeloma where Dkk1 produced by myeloma cells reduces osteoblast function but also increases the production of RANKL and decreases the production of OPG by stromal cells, and thus leads to increased bone resorption.15 Furthermore, in patients with rheumatoid arthritis, Dkk1 directly impaired new bone formation through the reduction of osteoblast function and the decreased production of OPG, which shifted the RANKL/OPG ratio in favor of bone resorption.16 Thus it seems that Dkk1 inhibits bone formation while promoting bone resorption in different bone disorders and supports the notion that Dkk1 is a key molecule in bone biology. The negative correlation between Dkk1 and bALP (an osteoblast product) found in our study is another indication that Dkk1 plays a significant role in the biology of abnormal bone remodeling in thalassemic osteoporosis.
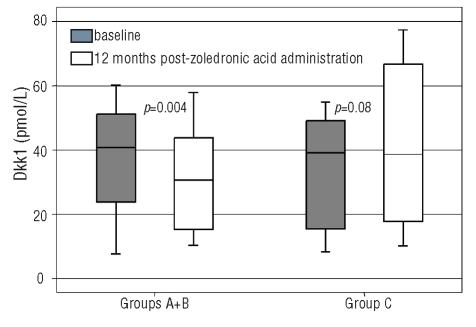
Patients in group B experienced an increase of L1–L4 BMD, while no other alterations in BMD were observed in the three groups during the study period. ZOL groups had a reduction in markers of bone resorption and formation, as previously reported.4 ZOL patients (groups A+B) showed a reduction of Dkk1 after 12 months of therapy (from 39.6±16.6 to 28.9±16.3 pmol/L; p=0.004; Figure 2); indeed they almost normalized Dkk1 levels (p=0.436 compared to control values). Furthermore there was no difference between percentage reduction of serum Dkk1 between patients of groups A and B (median % Dkk1 reduction was 24.7% and 27.2% for groups A and B, respectively; p=0.321). A significant negative correlation was observed between percentage change of Dkk1 and percentage change of L1–L4 BMD (r=−0.458, p<0.01) and R-BMD (r=−0.512, p<0.01) in ZOL patients. Dkk1 percentage reduction also correlated with percentage reduction of TRACP-5b (r=0.426, p<0.01) and CTX (r=0.448, p<0.01) in ZOL groups. On the contrary, patients of the placebo group showed a borderline increase of Dkk1 (from 33.1±16.8 to 40.1±23.2 pmol/L, p=0.08) after 12 months of ZOL. Nine patients of the placebo group showed a deterioration of pain scores during the study period.4 These patients had higher levels of Dkk1 at 12-months compared with all others (48.3±11.9 vs. 36.7±17.8 pmol/L, p=0.035).
Figure 2.
Zoledronic acid administration produced a reduction in Dkk1 serum levels but in placebo group a borderline increase of Dkk1 was observed.
The significant correlation between percentage changes of Dkk1 and bone resorption markers after 12 months of ZOL therapy is another strong indicator that Dkk1 interferes with bone resorption in thalassemia. This increased bone resorption is due to increased osteoclast function mainly through the RANKL/OPG pathway.2,17,18 Therefore, bisphosphonates that are potent inhibitors of osteoclast function have been used for the treatment of thalassemia osteoporosis.1,3–5 ZOL, a third generation aminobisphosphonate, has shown very encouraging results in this setting.4,19,20 It increases BMD of L1–L4/R/FN even after its discontinuation.5 ZOL mode of action includes mainly osteoclast inhibition. In this study, Dkk1 levels reduced post-ZOL therapy. This reduction was actually due to ZOL as the placebo group showed a borderline increase of Dkk1. As Dkk1 is a negative bone regulator its reduction post-ZOL, which correlates with BMD improvement, is an important finding and reveals another possible mode of action of ZOL. ZOL, which is able to reduce osteoblast function indirectly (we have seen a reduction of both bALP and OC in ZOL groups in this study)4 may also reduce Dkk1 production by primary osteoblast cells. However, this is only a hypothesis and further studies are needed to explain the Dkk1 reduction post-ZOL.
In conclusion, our study shows that Dkk1 is increased in the serum of patients with thalassemia and osteoporosis, correlates with their BMD and bone resorption markers, and is reduced post-ZOL therapy, suggesting a pathogenetic role of Dkk1 in thalassemia osteoporosis. These results also give evidence that Dkk1 enhances bone resorption and reveal another mechanism for increased bone resorption in this setting. The implication of Dkk1 in the pathogenesis of bone loss in thalassemia is of high importance as pre-clinical studies have shown that neutralizing Dkk1 antibodies and/or enhancing Wnt/β-catenin signaling may prove effective in treating bone pathologies, and phase II studies with such antibodies have been started in myeloma- and other cancer-related bone disease. Thus the confirmation of our results will reveal Dkk1 as a possible target for the development of novel agents against osteopenia and osteoporosis in thalassemia.
Footnotes
Authorship and Disclosures
EV and ET designed the study, analyzed the data, and wrote the paper. EV, KV and AB followed-up the patients. DC, CX, GB, AP, and ET performed all laboratory parameters of the study. DC and EK performed the statistical analysis.
The paper has been presented as an oral presentation at the 2008 European Hematology Association Annual Meeting [Abstract N. 900].
The authors reported no potential conflicts of interest.
References
- 1.Voskaridou E, Terpos E. New insights into the pathophysiology and management of osteoporosis in patients with β thalassaemia. Br J Haematol. 2004;127:127–39. doi: 10.1111/j.1365-2141.2004.05143.x. [DOI] [PubMed] [Google Scholar]
- 2.Voskaridou E, Kyrtsonis MC, Terpos E, Voskaridou E, Kyrtsonis MC, Terpos E, et al. Bone resorption is increased in young adults with thalassaemia major. Br J Haematol. 2001;112:36–41. doi: 10.1046/j.1365-2141.2001.02549.x. [DOI] [PubMed] [Google Scholar]
- 3.Voskaridou E, Terpos E, Spina G, Skordili M, Palermos J, Rahemtulla A, et al. Pamidronate is an effective treatment for osteoporosis in patients with beta-thalassaemia. Br J Haematol. 2003;123:730–7. doi: 10.1046/j.1365-2141.2003.04657.x. [DOI] [PubMed] [Google Scholar]
- 4.Voskaridou E, Anagnostopoulos A, Konstantopoulos K, Stoupa E, Spyropoulou E, Kiamouris C, et al. Zoledronic acid for the treatment of osteoporosis in patients with β-thalassemia: results from a single-center, randomized, placebo-controlled trial. Haematologica. 2006;91:1193–202. [PubMed] [Google Scholar]
- 5.Voskaridou E, Christoulas D, Konstantinidou M, Tsiftsakis E, Alexakos P, Terpos E. Continuous improvement of bone mineral density two years post zoledronic acid discontinuation in patients with thalassemia-induced osteoporosis: long-term follow-up of a randomized, placebo-controlled trial. Haematologica. 2008;93:1588–90. doi: 10.3324/haematol.12849. [DOI] [PubMed] [Google Scholar]
- 6.Lala R, Chiabotto P, Di Stefano M, Isaia GC, Garofalo F, Piga A. Bone density and metabolism in thalassaemia. J Pediatr Endocrinol Metabol. 1998;11 (Suppl 3):785–90. [PubMed] [Google Scholar]
- 7.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Wang Y, Li X, Zhang J, Mao J, Li Z, et al. The LRP5 high-bone mass G171V mutation disrupts LRP5 interaction with Mesd. Mol Cell Biol. 2004;24:4677–84. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawadi G, Vayssière B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–53. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–9. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, et al. The role of Dickkopf-1 in bone development, homeostasis and disease. Blood. 2009;113:517–25. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang FS, Ko JY, Lin CL, Wu HL, Ke HJ, Tai PJ. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomor-phological study in ovariectomized rats. Bone. 2007;40:485–92. doi: 10.1016/j.bone.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Politou M, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, et al. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer. 2006;119:1728–31. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 14.Voorzanger-Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clézardin P, et al. Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer. 2007;97:964–70. doi: 10.1038/sj.bjc.6603959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–63. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 17.Voskaridou E, Terpos E. Osteoprotegerin to soluble receptor activator of nuclear factor κ-B ligand ratio is reduced in patients with thalassaemia-related osteoporosis who receive vitamin D3. Eur J Haematol. 2005;74:359–61. doi: 10.1111/j.1600-0609.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, et al. The “lively” cytokines network in β-thalassemia major-related osteoporosis. Bone. 2007;40:1588–94. doi: 10.1016/j.bone.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Gilfillan CP, Strauss BJ, Rodda CP, Bowden DK, Kean AM, Obaid M, et al. A randomized, double-blind, placebo-controlled trial of intravenous zoledronic acid in the treatment of thalassemia-associated osteopenia. Calcif Tissue Int. 2006;79:138–44. doi: 10.1007/s00223-006-0314-x. [DOI] [PubMed] [Google Scholar]
- 20.Perifanis V, Vyzantiadis T, Tziomalos K, Vakalopoulou S, Garipidou V, Athanassiou-Metaxa M, et al. Effect of zoledronic acid on markers of bone turnover and mineral density in osteoporotic patients with β-thalassaemia. Ann Hematol. 2007;86:23–30. doi: 10.1007/s00277-006-0180-7. [DOI] [PubMed] [Google Scholar]