Abstract
OBJECTIVE
For clinical applications of biomarkers, there is a need for multiplex assays using high throughput platforms. The objective of this study was to determine the efficacy of Luminex Multianalyte Profiling (xMAP) technology for measurement of salivary proteins and to evaluate whether multiplex assays are as effective as single-plex assays and enzyme-linked immunosorbent assay (ELISA).
RESULTS
The average levels of interleukin-8 (IL-8) from the single-plex assay were 3313.2 ± 3759.8 pg ml−1 [oral squamous cell carcinoma (OSCC), n = 20] and 1061.7 ± 1978.8 pg ml−1 (control, n = 20). The IL-1β average levels from the single-plex assay were 945.2 ± 1134.8 pg ml−1 (OSCC, n = 20) and 314.2 ± 444.8 pg ml−1 (control, n = 20). The average levels of IL-8 from the multiplex assay were 2834.9 ± 3385.6 pg ml−1 (OSCC, n = 20) and 947.3 ± 2036.8 pg ml−1 (control, n = 20). The IL-1β average levels from the multiplex assay were 1013.5 ± 1221.1 pg ml−1 (OSCC, n = 20) and 376.3 ± 576.3 pg ml−1 (control, n = 20). The correlation coefficient between Luminex and ELISA assay for IL-8 (n = 19) and IL-1β (n = 19) was 0.91 and 0.84, respectively.
CONCLUSION
Luminex xMAP single-plex and multiplex assays are as effective as ELISA assays for quantification of proteins in saliva. Both IL-8 and IL-1β were expressed at significantly higher levels in OSCC subjects than in the matched healthy control subjects.
Keywords: Luminex, saliva diagnostics, immunobead-based assay, oral cancer, OSCC, ELISA
Introduction
Oral cancer, predominantly oral squamous cell carcinoma (OSCC), is a high impact disease in the oral cavity, affecting more than 34 000 people in the United States each year (American Cancer Society, 2007). OSCC tumors arise through a series of molecular mutations that lead to uncontrolled cellular growth from hyperplasia to dysplasia to carcinoma in situ followed by invasive carcinoma. Major risk factors for OSCC include tobacco and alcohol consumption along with environmental and genetic factors (Figuerido et al, 2004; Brinkman and Wong, 2006; Turhani et al, 2006; Hu et al, 2007). OSCC is usually detected at late stages when the cancer has advanced and therefore results in poor prognosis and survival. Presently, surgery and radiotherapy are the primary treatments, but because of the location of OSCC in the head and neck; this usually results in postoperative defects and functional impairments in patients (Thomson and Wylie, 2002). Therefore, early disease detection is imperative because it can result in more effective treatment with superior results.
Saliva has gained notable attention as a diagnostic fluid because of its simple collection and processing, minimal invasiveness and low costs. Many researchers have studied salivary proteins as potential diagnostic markers for various diseases such as breast cancer, ovarian cancer, Sjögrens syndrome, hepatocellular carcinoma, leukoplakia and oral cancer (Yio et al, 1992; Streckfus et al, 2000; Gorelik et al, 2005; Rhodus et al, 2005; Brailo et al, 2006; Ryu et al, 2006; Hu et al, 2007). These potential disease markers, if successfully developed, can lead to simple clinical tools for early detection and the monitoring of disease prognosis and treatment in saliva, a non-invasive body fluid (Kingsmore, 2006; Hu et al, 2005).
Luminex Multianalyte Profiling (xMAP) technology, previously known as FlowMetrix and LabMAP (Elshal and McCoy, 2006), is a multiplex bead-based flow cytometric assay that is gaining recognition as a method for analyte quantitation. This technology utilizes 5.6-micron polystyrene beads that are internally dyed with different intensities of red and infrared fluorophores. Currently, there are 100 beads, each with a unique spectral make up, which allows the mixing of several bead sets and, in theory, enabling the detection of up to 100 different analytes per assay (Vignali, 2000). The beads can be bound by various capture reagents such as antibodies, oligonucleotides, and peptides, therefore facilitating the quantification of various proteins, ligands, DNA and RNA (Fulton et al, 1997; Kingsmore, 2006; Nolan and Mandy, 2006). The assays are run on a 96-well plate format, followed by detection on a Luminex 100 instrument. As the beads run through the instrument, the internal dyes are excited by a laser, which results in the classification of each bead. Another laser excites the reporter dye which is directly proportional to the amount of analyte bound to each bead (Vignali, 2000; Ray et al, 2005). The resulting fluorescence is recorded by the instrument which then provides the median fluorescence unit obtained from measuring 100 beads.
Luminex xMAP technology has many applications including protein expression profiling, gene expression profiling, genotyping, immunodiagnostics, and genetic disease diagnostics. Although single-plex bead-based assays have been available for a long time; technological developments have enhanced the development of multiplex bead-based assays enabling the utilization of this method for quantitation of a panel of protein markers simultaneously (Prabhakar et al, 2002; Linkov et al, 2007). The advantage of Luminex xMAP technology lies in its high sensitivity, throughput and efficiency (Vignali, 2000; duPont et al, 2005). Significant reduction in time and costs results from multiplexing when compared with enzyme-linked immunosorbent assay (ELISA). ELISA is more expensive and time-consuming to perform when many proteins are to be measured using many single-plex protein specific assays (de Jager and Rijkers, 2006). On the contrary, many protein analytes can be measured by the multiplexed bead-based assay with a single plate. This is extremely important for clinical studies where sample volumes are limited (Liu et al, 2005). Bead-based assay is more accurate because the median fluorescence is obtained from the readout of at least 50–100 beads. Thus, each bead functions as a duplicate making this assay more reliable (Kettman et al, 1998; Vignali, 2000).
The disadvantage of xMAP technology is the possible cross-reactivity between antibodies. Sensitivity may also be compromised because of the increasing number of beads per well. In addition, the performance in the multiplex assays can be variable as a result of the multipurpose diluent, which may not optimize each analyte to the same extent as in the case with single analyte measurements by ELISA (Carson and Vignali, 1999). In the laboratory, interleukin-8 (IL-8) and IL-1β protein biomarkers are measured by ELISAs. Previous studies have shown significantly higher levels of these proteins among OSCC patients than control subjects (Hoffmann et al, 2007). In preparation for clinical applications, there is a need to perform multiplex biomarker assays using high throughput platforms. Although Luminex multiplex bead-based assay kits have been optimized to measure protein levels in serum and cell lines, the purpose of this study was to examine if this technique can be used to measure IL-8 and IL-1β levels in the saliva of OSCC and matched healthy control subjects.
Materials and methods
Patients
All participants in this study signed the University of California-Los Angeles Institutional Review Board-approved consent form agreeing to donate saliva for experiments. Twenty Caucasian OSCC patients with ages ranging from 32 to 79 years were recruited from the Division of Head and Neck Surgery / Otolaryngology at the University of California Los Angeles (UCLA) Medical Center. A majority of the OSCC patients were diagnosed with early stage oral cancer, as shown in the patient demographics in Table 1. No patient had a history of prior malignancy, immunodeficiency, auto-immune disorders, hepatitis or HIV infection as well as prior treatment in the form of chemotherapy, radio-therapy, surgery or alternative medicine. All the 20 healthy control subjects were Caucasian ranged in age from 24 to 75 years (Table 1). To examine if IL-8 is significantly elevated in periodontitis patients, we also included 10 periodontitis patients and 10 healthy control subjects recruited at the UCLA School of Dentistry for this study.
Table 1.
Demographics of patients (a) and healthy control subjects (b)
| Patient | Diagnosis | Staging | TNM staging | Location | Age (years) | Ethnicity | Sex | Tobacco | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||||
| 1 | OSCC | I | T1N0M0 | Tongue | 32 | Caucasian | M | Yes | |||
| 2 | OSCC | I | T1N0M0 | Tongue | 55 | Caucasian | M | Yes | |||
| 3 | OSCC | I | T1N0M0 | Tongue | 63 | Caucasian | F | No | |||
| 4 | OSCC | I | T1N0M0 | Right alveolar ridge | 79 | Caucasian | F | Yes | |||
| 5 | OSCC | I | T1N0M0 | Right tonsil | 66 | Caucasian | F | Yes | |||
| 6 | OSCC | II | T1N1M0 | Tongue | 53 | Caucasian | M | No | |||
| 7 | OSCC | I | T2N0M0 | Tongue | 49 | Caucasian | F | No | |||
| 8 | OSCC | II | T2N0M0 | Tongue | 66 | Caucasian | F | No | |||
| 9 | OSCC | II | T2N0M0 | Floor of mouth | 69 | Caucasian | F | No | |||
| 10 | OSCC | II | T2N0M0 | Left Tonsil | 55 | Caucasian | M | Yes | |||
| 11 | OSCC | II | T2N0M0 | Base of tongue | 58 | Caucasian | M | No | |||
| 12 | OSCC | II | T2N0M0 | Left tonsil | 59 | Caucasian | M | No | |||
| 13 | OSCC | II | T2N0M0 | Right tongue | 77 | Caucasian | M | Yes | |||
| 14 | OSCC | II | T2N1M0 | Base of tongue | 40 | Caucasian | M | No | |||
| 15 | OSCC | III | T2N1M0 | Left tonsil | 65 | Caucasian | M | Yes | |||
| 16 | OSCC | IV | T2N2aM0 | Right tonsil | 43 | Caucasian | F | Yes | |||
| 17 | OSCC | IV | T2N2aM0 | Base of tongue | 53 | Caucasian | M | Yes | |||
| 18 | OSCC | III | T2N2bM0 | Base of tongue | 65 | Caucasian | M | Yes | |||
| 19 | OSCC | III | T2N2M0 | Pharynx | 62 | Caucasian | M | Yes | |||
| 20 | OSCC | IV | T4N2M0 | Tongue and floor of mouth | 73 | Caucasian | F | No | |||
| Subject | Diagnosis | Age (years) | Ethnicity | Sex | Tobacco | ||||||
| (b) | |||||||||||
| 1 | Normal | 31 | Caucasian | F | No | ||||||
| 2 | Normal | 34 | Caucasian | F | Yes | ||||||
| 3 | Normal | 59 | Caucasian | F | Yes | ||||||
| 4 | Normal | 59 | Caucasian | F | No | ||||||
| 5 | Normal | 75 | Caucasian | F | No | ||||||
| 6 | Normal | 24 | Caucasian | M | No | ||||||
| 7 | Normal | 24 | Caucasian | M | No | ||||||
| 8 | Normal | 25 | Caucasian | M | No | ||||||
| 9 | Normal | 26 | Caucasian | M | No | ||||||
| 10 | Normal | 30 | Caucasian | M | Yes | ||||||
| 11 | Normal | 39 | Caucasian | M | Yes | ||||||
| 12 | Normal | 55 | Caucasian | M | Yes | ||||||
| 13 | Normal | 24 | Caucasian | M | No | ||||||
| 14 | Normal | 24 | Caucasian | M | No | ||||||
| 15 | Normal | 52 | Caucasian | M | Yes | ||||||
| 16 | Normal | 41 | Caucasian | M | Yes | ||||||
| 17 | Normal | 40 | Caucasian | M | Yes | ||||||
| 18 | Normal | 38 | Caucasian | M | Yes | ||||||
| 19 | Normal | 26 | Caucasian | M | Yes | ||||||
| 20 | Normal | 49 | Caucasian | F | Yes | ||||||
OSCC, oral squamous cell carcinoma; TNM, TNM classification of malignant tumors, T: size of primary tumor, N: degree of spread to regional lymph nodes, M: presence of metastasis.
Saliva collection
Unstimulated whole saliva samples were collected between 9 am and 10 am. Patients were asked to refrain from eating, drinking, smoking, and oral hygiene procedures for at least 1 h before saliva collection. Samples were collected by asking subjects to first rinse their mouth with water and then expectorate saliva into a 50-ml centrifuge tube kept on ice for 10 min. The oral cancer and healthy control subjects were not formally screened for periodontal disease but were asked not to donate saliva if they suffered from bleeding gums. Samples which contained visible traces of blood were discarded from this study. Protease inhibitor cocktail (1 µl ml−1 of aprotonin, 10 mg ml−1 of phenylmethyl-sulfonyl fluoride, 400 mM sodium orthovanadate; Sigma-Aldrich, St. Louis, Mo, USA) was added immediately after sample collection to minimize protein degradation. Briefly, 2 ml of clear whole saliva was obtained from patients after centrifugation at 2600 g for 15 min to remove cell pellets and debris. The samples were then divided into 20 µl aliquots to reduce freeze thaw cycles; consequently, the samples used for this study was freeze thawed twice.
Bead-based assays
Human IL-8 and IL-1β Fluorokine MultiAnalyte Profiling systems (Fluorokine MAP) were performed according to R&D systems protocol (R&D systems, Minneapolis, MN, USA). Saliva samples were diluted five times with calibrator diluent for the IL-1β assay and eight times for the IL-8 assay. Initially, the 96-well filter bottom plate were prewet. Fifty microlitre of diluted microparticle solution and 50 µl of sample were added to each well in duplicate. Next the plate was incubated for 3 h and washed three times with wash buffer. Afterwards, 50 µl of diluted biotin antibody was added to each well and incubated for 1 h. The plate was then washed as described above and 50 µl of diluted Streptavidin-PE was added to each well and incubated for 30 min. All incubations were performed at room temperature on an orbital shaker set at 15 g. Finally, the plate was washed again with 100 µl of wash buffer. The median relative fluorescence units were measured using the Luminex 100 analyzer (Luminex, Austin, TX, USA). For the multiplexed assays, the same procedure was followed except that the IL-8 and IL-1β microparticles were pooled and then subsequently added to each well.
ELISA
Enzyme-linked immunosorbent assay (Pierce, Rockford, IL, USA) was performed to determine the IL-8 levels in the saliva samples of OSCC (n = 40) and control patients (n = 42). Samples were diluted 1:8 in sample diluent and 50 µl was loaded, in duplicate, onto a 96-microwell Plate coated with anti-human IL-8 antibodies. Similarly, the IL-1β ELISA assay (Pierce, Rockford, IL, USA) was performed in the saliva samples of OSCC (n = 36) and control patients (n = 42) with a dilution factor of 1:5. After incubation for 1 h on a rotator (2.5 g), the microwell strips were washed three times with approximately 300 µl of washing buffer, followed by the addition of 50 µl of biotinylated antibody reagent to each well. The plate was incubated again for 1 h followed by a wash (3×) with the washing buffer.
Subsequently, 100 µl of streptavidin-HRP solution was added to all wells and incubated for 30 min. After another wash (3×), 100 µl of premixed 3,3′,5,5′-tetra-methylbenzidine substrate solution was added to each well and incubated, in the dark, for 30 min. Finally, stop solution (100 µl) was added to each well and the absorbance was measured. The IL-8 ELISA assay was also performed on the saliva samples of periodontitis (n = 10) and control subjects (n = 10) using the same method described above.
Statistical analysis
Single-plex and multiplex raw data were transformed by natural logarithm to obtain normal distribution. Student t-test was employed for the comparison of single-plex and multiplex data and the critical alpha level of 0.05 was defined for statistical significance. The Pearson correlation coefficient of log transformed single-plex and multiplex data was calculated and represented by R2 values. Using this set of data, we conducted receiver operating characteristic (ROC) curve analyzes to evaluate overall performance of the predictive power of each of the biomarkers. The optimal cut-point was determined for each biomarker by searching for those that yielded the maximum corresponding sensitivity and specificity. ROC curves were then plotted on the basis of the set of optimal sensitivity and specificity values. Area under the curve was computed via numerical integration of the ROC curves. All statistical data analysis was performed by the statistical software packages r 2.5.0 and Bioconductor.
Results
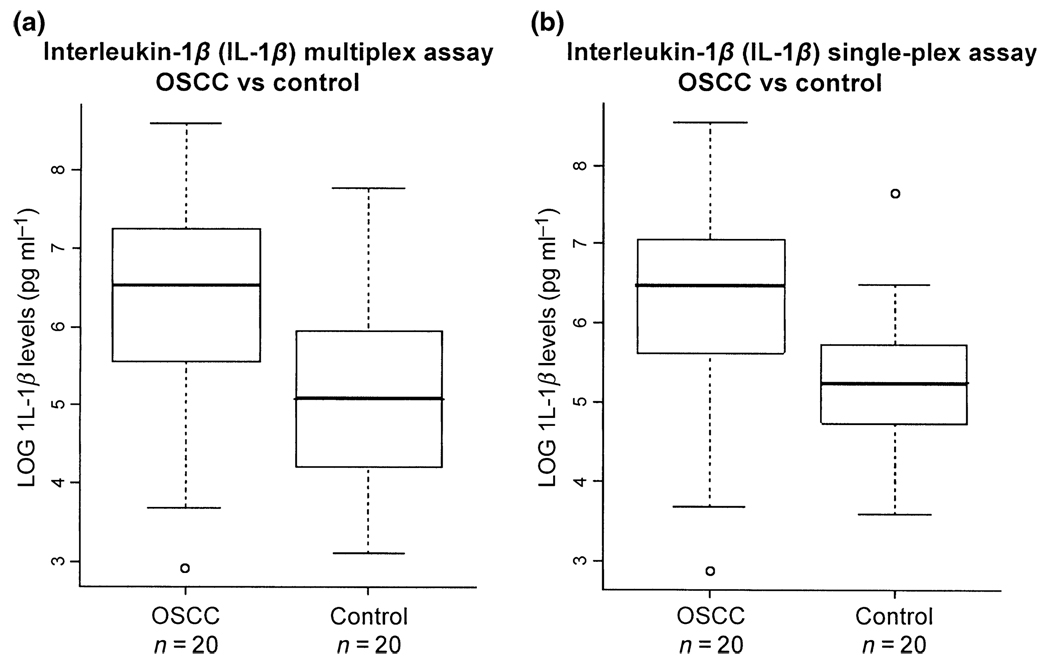
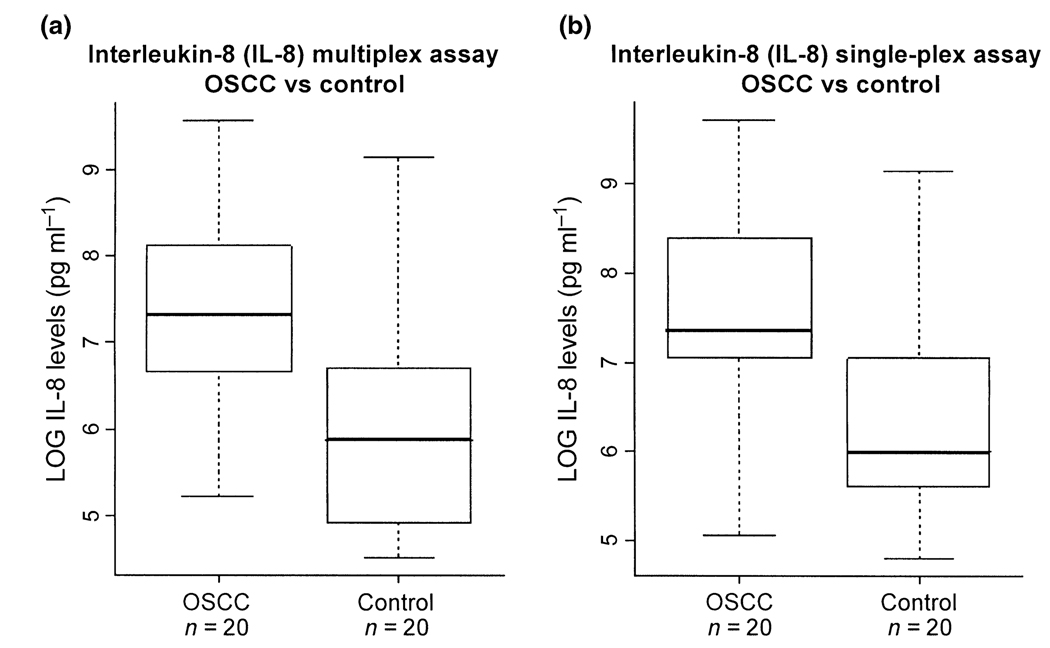
We demonstrated that both single-plex and multiplex assays could be used to determine salivary IL-8 and IL-1β levels using xMAP. IL-1β level in saliva was found to be statistically higher in OSCC patients than in control subjects. The boxplot in Figure 1b shows the distribution of IL-1β levels in OSCC and control subjects. The average level of IL-1β from the single-plex assay was determined as 945.2 ± 1134.8 pg ml−1 for OSCC subjects (n = 20) and 314.2 ± 444.8 pg ml−1 for matched control subjects (n = 20). ROC analysis resulted in an ROC value of 0.77 with a sensitivity of 75% and specificity of 80%, respectively. The IL-8 concentration in saliva was also statistically higher in OSCC than in control (Figure 2b). The average level of IL-8 from the single-plex assay was 3313.2 ± 3759.8 pg ml−1 for OSCC (n = 20) and 1061.7 ± 1978.8 pg ml−1 for controls (n = 20). The ROC analysis showed an ROC value of 0.80 with a sensitivity of 75% and specificity of 80%, respectively. These results confirmed the use of IL-8 and IL-1β as biomarkers for OSCC detection (St John et al, 2004).
Figure 1.
Comparison of multiplex (a) and single-plex (b) assays for the measurement of IL-1β in the saliva of oral squamous cell carcinoma (OSCC) versus control subjects. For the multiplex assay (a), the average levels of IL-1β are 1013.5 pg ml−1 in OSCCs (n=20) and 376.3 pg ml−1 in controls (n = 20). For the single-plex assay (b), the average levels of IL-1β are 945.2 pg ml−1 in OSCCs (n = 20) and 314.2 pg ml−1 in controls (n = 20)
Figure 2.
Comparison of multiplex (a) and single-plex (b) assays for the measurement of IL-8 in the saliva of OSCC versus control subjects. For the multiplex assay (a), the average levels for OSCCs (n = 20) and controls (n = 20) are 2834.9 and 947.3 pg ml−1, respectively. For the single-plex assay (b), the average levels for OSCCs (n = 20) and controls (n = 20) are 3313.2 and 1061.7 pg ml−1, respectively
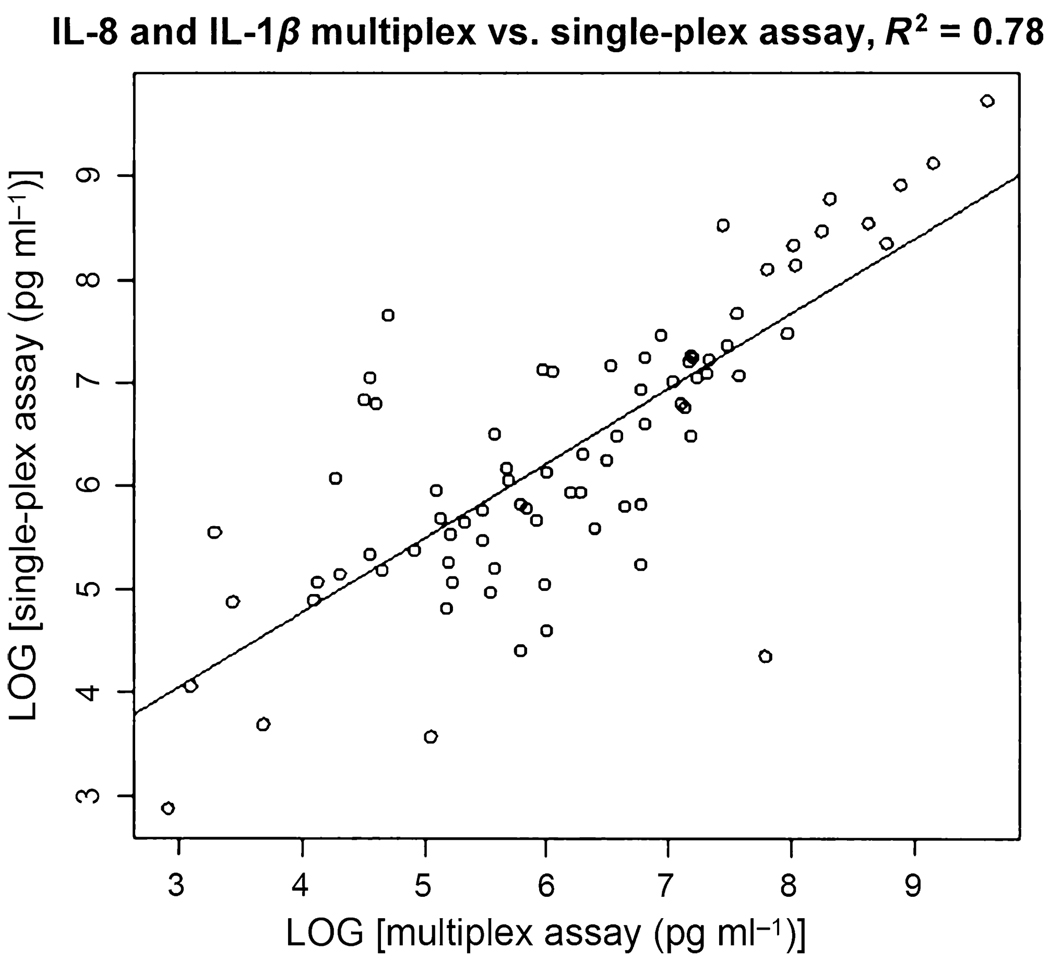
Similar results were obtained for both IL-1β and IL-8 from the multiplexed assays (Figure 1a and Figure 2a). The average levels of IL-1β were 1013.5 ± 1221.1 pg ml−1 in OSCC subjects (n = 20) and 376.3 ± 576.3 pg ml−1 in control subjects (n = 20). The ROC analysis resulted in an ROC value of 0.74 with a sensitivity of 80% and a specificity of 65%, respectively. The average levels of IL-8 were 2834.9 ± 3385.6 pg ml−1 in OSCC subjects (n = 20) and 947.3 ± 2036.8 pg ml−1 in control subjects (n = 20). The ROC analysis revealed an ROC value of 0.81 with a sensitivity of 75% and specificity of 80%, respectively. These results are summarized in Table 2. The multiplex and single-plex Luminex assays show a high correlation coefficient of R2 = 0.9025 (Figure 3).
Table 2.
Comparison of single-plex and multiplex assays for the measurement of interleukin (IL)-8 and IL-1β proteins in saliva of oral squamous cell carcinoma (OSCC) and matched control subjects
| Mean level (pg ml−1) | ||||||
|---|---|---|---|---|---|---|
| Protein | OSCC (n = 20) | Control (n = 20) | P-value | ROC | Sensitivity (%) | Specificity (%) |
| IL-8 (single-plex) | 3313.2 ± 3759.8 | 1061.7 ± 1978.8 | 0.02 | 0.80 | 75 | 80 |
| IL-8 (multiplex) | 2834.9 ± 3385.6 | 947.3 ± 2036.8 | 0.04 | 0.81 | 75 | 80 |
| IL-1β (single-plex) | 945.2 ± 1134.8 | 314.2 ± 444.8 | 0.03 | 0.77 | 75 | 80 |
| IL-1β (multiplex) | 1013.5 ± 1221.1 | 376.3 ± 576.3 | 0.04 | 0.74 | 80 | 65 |
ROC, receiver operating characteristic.
Figure 3.
Correlation between the multiplex and single-plex assays of interleukin (IL)-8 and IL-1β. The R2 value is 0.78
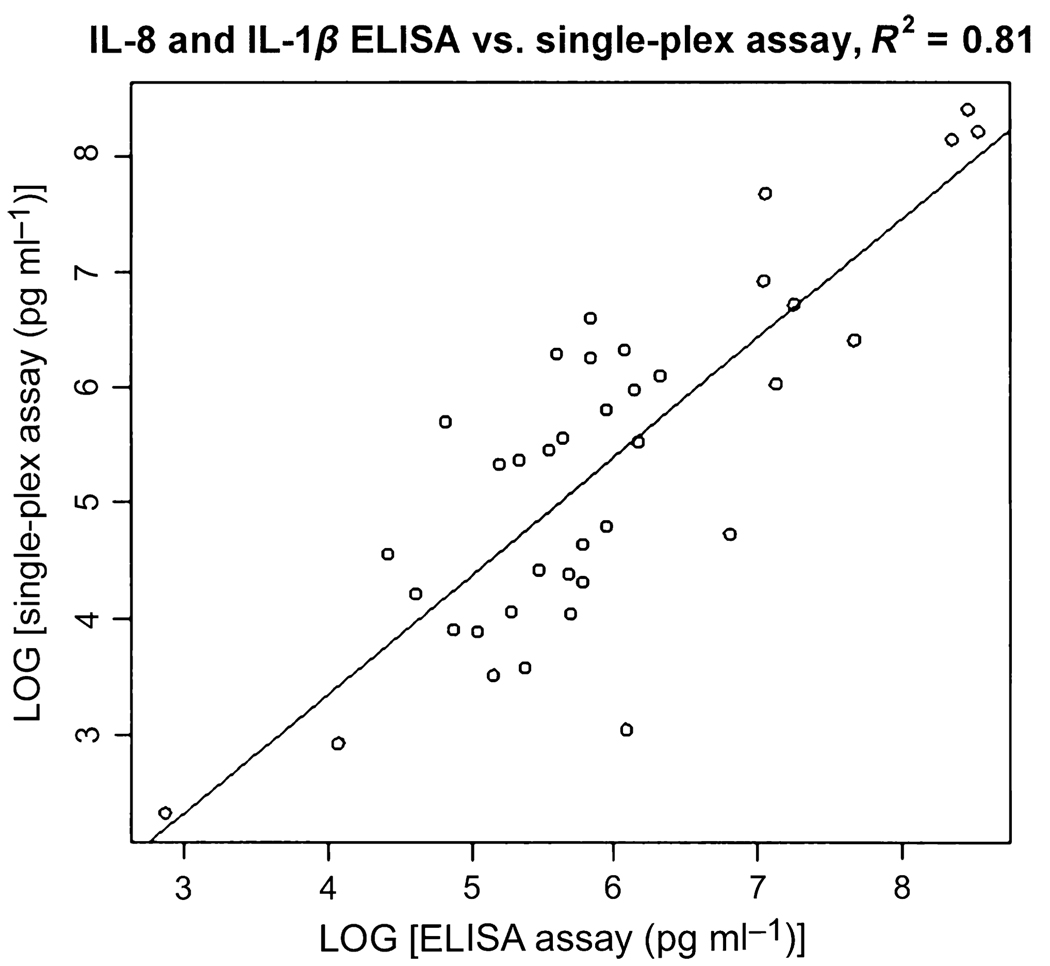
We also measured salivary IL-8 and IL-1β levels in the same patients with OSCC and control subjects using ELISA. The average levels of IL-8 using the ELISA assay were 3347.7 ± 2929 (OSCC, n = 40) and 759.4 ± 563 pg ml−1 (control, n = 42). The ROC analysis indicated an ROC value of 0.82 and a sensitivity and specificity of 87.5% and 64.3%, respectively. The average levels of IL-1β were 591.5 ± 618.7 pg ml−1 (OSCC, n = 36) and 79.6 ± 57.8 pg ml−1 (control, n = 42). The ROC analysis indicated an ROC value of 0.84 and a sensitivity and specificity of 63.9% and 100%, respectively. Luminex assay and ELISA gave highly correlated results. The correlation coefficient (R2) was 0.91 for IL-8 (n = 19) and 0.84 for IL-1β (n = 19). Figure 4 shows the correlation coefficient between the single-plex Luminex assay and ELISA for IL-8 and IL-1β combined is R2 = 0.8794.
Figure 4.
Correlation between the single-plex and ELISA assays of interleukin (IL)-8 and IL-1β. The R2 value is 0.81
Salivary IL-8 levels in periodontitis patients and matched controls were also measured using ELISA. The average level of IL-8 for the periodontitis patients (n = 10) was 818.82 ± 228.41 pg ml−1 and 589.24 ± 370.29 pg ml−1 for the control subjects (n = 10). The P-value between the periodontitis and control groups were determined as 0.098.
Discussion
The results of this study indicate that the Luminex xMAP technology is a useful platform for validation of salivary protein biomarkers with both single-plex and multiplex assays. Previous studies have reported achievable results using the multiplex bead-based assay for serum, plasma and cell culture supernatant samples (Oliver et al, 1998; Kellar et al, 2001; de Jager et al, 2003; Brailo et al, 2006; Allen et al, 2007; Linkov et al, 2007). Carson and Vignali reported that the multiplex assay performed as well as the single-plex assay for cytokines but provided better sensitivity than ELISA (Carson and Vignali, 1999). A recent study also found a high correlation between bead-based assays and ELISA (duPont et al, 2005). Therefore, multiplex bead-based assays may substitute the ELISA method when a large number of protein analytes need to be validated. The measured levels of IL-8 in the saliva of OSCC subjects from this study are also comparable with those measured by ELISA from another group of researchers (Rhodus et al, 2005). We also demonstrated that there was a high correlation between the Luminex assay data and the ELISA data, and the multiplex assays were found as effective as the single-plex assays for measuring salivary proteins.
The average level of IL-1β in OSCC patients obtained from the single-plex assay differed from the multiplexed assay by 7.0%, whereas the measurements in control subjects differed by 18.0% between the two assays. As for the measurements of IL-8, the average level of IL-8 in OSCC patients differed by 15.6% between the single-plex and multiplex assays whereas the difference between these two assays for IL-8 in control subjects was 11.4%. In our study, the IL-8 levels and the IL-1β levels obtained with the single-plex and multiplex assay are comparable to the levels obtained using ELISA. The high correlation between the Luminex assay and ELISA for IL-8 and IL-1β shows the effectiveness of the Luminex assays for detecting protein levels in saliva. Furthermore, the similar results obtained with the single-plex and multiplex assays confirm the benefits of multiplexing by Luminex xMAP technology without compromising the accuracy.
There has been concern for using inflammatory cytokine proteins such as IL-8 and IL-1β as biomarkers although previous studies have firmly shown significantly higher levels of these proteins among OSCC patients than control subjects (Hoffmann et al, 2007). Cytokines are intercellular signaling proteins which play a role in regulating growth, cellular proliferation, angiogenesis and tissue repair. They also function in immune responses to infection, injury and inflammation (Ray et al, 2005; de Jager and Rijkers, 2006). Several conditions can also give rise to increased levels of inflammatory proteins such as rheumatoid arthritis, periodontal disease, osteoporosis, Sjögren’s syndrome, chronic parotitis, severe exercise and diabetes. Therefore, an immunological disease control group may be included for further validation of IL-8 and IL-1β as truly discriminatory markers for OSCC. We assayed salivary IL-8 levels in patients with severe periodontal disease (n = 10) and found that while they have elevated IL-8 in saliva, patients with oral cancer have significantly higher saliva levels of IL-8. This confirms that although an immunological disease may increase cytokines levels, the levels are not as high as those found in OSCC patients. It should be noted that cytokines are one of the most low-abundant proteins in human saliva. Therefore, this validation platform should be applicable to most of the salivary proteins.
There are limitations in this study that need to be acknowledged. First, the sample size is relatively small. A larger sample cohort would allow for a greater statistical comparison between the cancer and healthy control groups. Another limitation of this study is that the multiplex assay had slightly lower values for measurement of IL-8 and IL-1B than the single-plex assay. The lower values can be attributed to the beads not being specific to one protein. A previous study comparing bovine plasma IL-1β levels using single-plex and multiplex assays also reported a difference of 6.4% between the assays (Dernfalk et al, 2007).
In summary, we have demonstrated that Luminex xMAP technology can be used to validate and quantitate protein levels in saliva. The high correlation between the Luminex assays and ELISA confirms that Luminex xMAP technology is a reliable method for quantification of salivary proteins. We have also showed that the multiplex assay provided comparable results to the single-plex assays therefore demonstrating the efficacy of this technology. Our results suggest that saliva can be a promising and valuable diagnostic fluid because it contains measurable proteins, such as IL-8 and Il-1β, at differential levels that can discriminate disease process. Future validation studies may need to be performed using a larger patient cohort, including periodontal disease group that should be included to validate firmly IL-8 and IL-1β as protein biomarkers for OSCC. The ability to engage a high throughput platform such as the Luminex xMAP for multiplex protein biomarkers detection in saliva is a significant technological advancement towards the eventual utilization of saliva as a clinical diagnostic fluid.
Acknowledgements
This work was supported by the PHS grants U01 DE016275 (D. Wong) and R03-DE017144 (S. Hu). M. Arellano-Garcia would like to acknowledge the Carl Storm Underrepresented Minority Fellowship established by the Gordon Research Conferences Board of Trustees for their support. The authors would like to thank Henry Chan for the technical support he provided with the Luminex 100 instrument.
References
- Allen C, Duffy S, Teknos T, et al. Nuclear factor-{kappa} B-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:3182–3190. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts and Figures 2007. Atlanta: American Cancer Society; 2007. [Google Scholar]
- Brailo V, Vucicevic-Boras V, Cekic-Arambasin A, Alajbeg IZ, Milenovic A, Lukac J. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006;42:370–373. doi: 10.1016/j.oraloncology.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Brinkman BMN, Wong DT. Disease mechanism and biomarkers of oral squamous cell carcinoma. Curr Opin Oncol. 2006;18:228–233. doi: 10.1097/01.cco.0000219250.15041.f8. [DOI] [PubMed] [Google Scholar]
- Carson RT, Vignali DAA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- Dernfalk J, Persson Waller K, Johannisson A. The xMAP(TM) technique can be used for detection of the inflammatory cytokines IL-1 beta, IL-6 and TNF-alpha in bovine samples. Vet Immunol Immunopathol. 2007;118:40–49. doi: 10.1016/j.vetimm.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figuerido ML, Kim Y, Xiaofen CZ, Myers JN, Wong DT. Molecular mechanisms of head and neck cancer. Drug Discov Today: Dis Mechan. 2004;1:273–281. [Google Scholar]
- Fulton RJ, McDade RL, Smith PL, Kienker LJ, Kettman JR., Jr Advanced multiplexed analysis with the FlowMetrix ™ system. Clin Chem. 1997;43:1749–1756. [PubMed] [Google Scholar]
- Gorelik E, Landsittel DP, Marrangoni AM, et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- Hoffmann TK, Sonkoly E, Homey B, et al. Aberrant cytokine expression in serum of patients with adenoid cystic carcinoma and squamous cell carcinoma of the head and neck. Head Neck. 2007;29:472–478. doi: 10.1002/hed.20533. [DOI] [PubMed] [Google Scholar]
- Hu S, Xie Y, Ramachandran P, et al. Large-scale identification of proteins in human salivary proteome by liquid chromatography / mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics. 2005;5:1714–1728. doi: 10.1002/pmic.200401037. [DOI] [PubMed] [Google Scholar]
- Hu S, Wang J, Meijer J, et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007;56:3588–3600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager W, Rijkers GT. Solid-phase and bead-based cytokine immunoassay: a comparison. Methods. 2006;38:294–303. doi: 10.1016/j.ymeth.2005.11.008. [DOI] [PubMed] [Google Scholar]
- de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane B-E. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kettman JR, Davies T, Chandler D, Oliver KG, Fulton RJ. Classification and properties of 64 multiplexed microsphere sets. Cytometry. 1998;33:234–243. [PubMed] [Google Scholar]
- Kingsmore SF. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat Rev Drug Discov. 2006;5:310–321. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkov F, Lisovich A, Yurkovetsky Z, et al. Early detection of head and neck cancer: development of a novel screening tool using multiplexed immunobead-based biomarker profiling. Cancer Epidemiol Biomarkers Prev. 2007;16:102–107. doi: 10.1158/1055-9965.EPI-06-0602. [DOI] [PubMed] [Google Scholar]
- Liu MY, Xydakis AM, Hoogeveen RC, et al. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 System. Clin Chem. 2005;51:1102–1109. doi: 10.1373/clinchem.2004.047084. [DOI] [PubMed] [Google Scholar]
- Nolan JP, Mandy F. Multiplexed and microparticle-based analyses: quantitative tools for the large-scale analysis of biological systems. Cytometry A. 2006;69A:318–325. doi: 10.1002/cyto.a.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KG, Kettman JR, Fulton RJ. Multiplexed analysis of human cytokines by use of the FlowMetrix system. Clin Chem. 1998;44:2057–2060. [PubMed] [Google Scholar]
- duPont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of Luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–191. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP™ assay. J Immunol Methods. 2002;260:207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- Ray CA, Bowsher RR, Smith WC, et al. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. J Pharma Biomed Anal. 2005;36:1037–1044. doi: 10.1016/j.jpba.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F. NF-kappa B dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29:42–45. doi: 10.1016/j.cdp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Ryu OH, Atkinson JC, Hoehn GT, Illei GG, Hart TC. Identification of parotid salivary biomarkers in Sjogren’s syndrome by surface-enhanced laser desorption / ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatol. 2006;45:1077–1086. doi: 10.1093/rheumatology/kei212. [DOI] [PubMed] [Google Scholar]
- St John MA, Li Y, Zhou X, et al. Interleukin 6 and Interleuking 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–935. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- Streckfus D, Bigler L, Tucci M, Thigpen JT. A preliminary study of CA15-3, c-erbB-2, epidermal growth factor receptor, cathepsin-D, and p53 in saliva among women with breast carcinoma. Cancer Invest. 2000;18:101–109. doi: 10.3109/07357900009038240. [DOI] [PubMed] [Google Scholar]
- Thomson PJ, Wylie J. Interventional laser surgery: an effective surgical and diagnostic tool in oral precancer management. Int J Oral Maxillofac Surg. 2002;31:145–153. doi: 10.1054/ijom.2001.0189. [DOI] [PubMed] [Google Scholar]
- Turhani D, Krapfenbauer K, Thurnher D, Langen H, Fountoulakis M. Identification of differentially expressed, tumor-associated proteins in oral squamous cell carcinoma by proteomic analysis. Electrophor. 2006;27:1417–1423. doi: 10.1002/elps.200500510. [DOI] [PubMed] [Google Scholar]
- Vignali DAA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Yio X, Jiang J, Yin F, Ruan K. Highly sensitive sandwich enzyme immunoassay for alpha-fetoprotein in human saliva. Ann Clin Biochem. 1992;29:519–522. doi: 10.1177/000456329202900505. [DOI] [PubMed] [Google Scholar]