Abstract
We report the isolation and expression of the Hox gene, Cnox-2, in Hydractinia symbiolongicarpus, a hydrozoan displaying division of labor. We found different patterns of aboral-to-oral Cnox-2 expression among polyp polymorphs, and we show that experimental conversion of one polyp type to another is accompanied by concordant alteration in Cnox-2 expression. Our results are consistent with the suggestion that polyp polymorphism, characteristic of hydractiniid hydroids, arose via evolutionary modification of proportioning of head to body column.
Among the most heated of 19th-century zoological debates was that focused on the individuality of polymorphic hydroids (1, 2). The most striking examples of polyp polymorphism is found in the hydroid family Siphonophora, where complex free-living colonies are composed of functionally specialized and morphologically distinct polyps and medusae. The specialized polyps and medusae appeared as organs of an individual to Huxley (3), who was impressed by the exquisite division of labor among polymorphs. Yet, Agassiz (4) maintained that the polyps and medusae are parts of colonies, if one considers their ancestry. Haeckel (5) found support for both views when he considered their ontogenies. The challenge, then and now, for interpreting the individuality of polymorphic hydroids is to understand the morphological transformations that have occurred between the various polyps and medusae.
Recent findings in developmental biology suggest that insight into the evolution of the division of labor in hydroid colonies may be found in the study of Hox genes. Hox genes specify positional information along the anterior–posterior axes of developing embryos of mice, fruit flies, and nematodes (6–8). Shenk et al. (9, 10) extended these findings to the aboral–oral axis of the near-basal metazoan phylum Cnidaria. They demonstrated that the Hox gene, Cnox-2, is expressed at high levels in the foot and body-column epithelia of the solitary hydroid, Hydra, but at low levels in the head (hypostome and tentacles). The same authors also showed that experimental alteration of the aboral–oral axis is accompanied by corresponding changes in Cnox-2 expression. These results suggest Cnox-2 as a promising candidate for the study of patterning events in those colonial hydroids, which unlike Hydra, display a division of labor among polyp types that differ in their aboral–oral patterning.
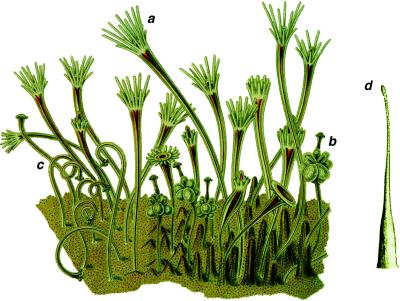
Colonies of Hydractinia symbiolongicarpus display four distinct polyp types. The gastrozooid is the feeding polyp and is functionally and structurally similar to that of Hydra (Fig. 1a), possessing an aboral region comprising the body column and an oral region comprising the hypostome, mouth, and tentacles. The gonozooid and dactylozooid are polyps specialized for sexual reproduction and for capturing the eggs of their hermit crab host, respectively (Fig. 1 b and c). Both lack a hypostome and tentacles and hence may represent an expansion of the body column to the exclusion of oral regions of the gastrozooid (11–13). By contrast, the tentaculozooid, believed to play a role in colony defense, resembles a single tentacle of a gastrozooid (Fig. 1d), suggesting an expansion of an oral structure to the exclusion of the body column and polyp base (11–13).
Figure 1.
Polymorphic polyps of Hydractinia, with three of the polyp types shown in a colony and the fourth polyp type shown detached. (a) gastrozooid; (b) gonozooid; (c) dactylozooid; and (d) tentaculozooid. Modified from refs. 13 and 21.
It has long been asserted that the division of labor in hydroid colonies arose via evolutionary modifications in the axial patterning of the gastrozooid to produce morphologically distinct and functionally specialized polyp types (3, 5, 14–17). If this is the case, and the Hydra system is generalizable to other hydroid species, then polyp polymorphs should display altered patterns of oral-to-aboral Cnox-2 expression. To test this, we determined Cnox-2 expression patterns among Hydractinia polyp polymorphs.
MATERIALS AND METHODS
Polyclonal Antibody Production.
Cnox-2 antigen was produced from a 19-aa HPLC-purified synthetic peptide (W. M. Keck, Biotechnology Resource Center, Yale University) which comprised region 112–130 (PREGEAAPSQKIYPFGRDS) located outside the homeodomain, toward the amino terminus. The synthetic peptide was coupled to ovalbumin as a carrier protein and purified over a size-exclusion column (Pierce). Rabbits were immunized with 200 μg of antigen and boosted twice with 100 μg. Crude sera recognized synthetic peptide alone and synthetic peptide conjugated to an alternate carrier protein, KLH (Pierce) on a dot blot (data not shown). IgG fractions were prepared by protein A chromatography (Bio-Rad) according to the manufacturer’s instructions. Protein A-purified antibodies were found to bind to a protein of the predicted size on a Western blot from Hydractinia total protein fractionated by SDS/PAGE (data not shown).
Antibody Competition with Peptide.
Protein A-purified anti-Cnox-2 antibodies were incubated with either a 5× molar excess of the Cnox-2 synthetic peptide or a 5× molar excess of the carrier protein (ovalbumin) at 4°C for 1 hour. Preincubated peptide/anti-Cnox-2 antibodies, ovalbumin/anti-Cnox-2 antibodies, and untreated anti-Cnox-2 antibodies were subjected to whole-mount immunolocalization as described below.
Whole-Mount Immunolocalization.
Hydractinia colonies were relaxed in menthol and fixed in 4% paraformaldehyde at 4°C overnight. Colonies were washed 3 × 10 min in Tris-buffered saline with 0.25% Triton X-100 (TBST), dehydrated, and stored in absolute MeOH at −20°C. Colonies were rehydrated in TBST; individual polyps were removed, washed 3 × 10 min in TBST, and incubated for 2 h at room temperature in 10% goat normal serum/TBST. Protein A (Bio-Rad)-purified anti-Cnox-2 antibodies were diluted to 3.5 μg/ml in 10% goat normal serum/TBS and added to polyps for incubation overnight at 4°C. Polyps were washed 10 × 10 min at room temperature in TBST. Alkaline phosphatase-conjugated goat anti-rabbit antibody (Sigma) was diluted 1:800 in 10% goat normal serum/TBS and added to polyps for incubation at room temperature for 2 h. Polyps were washed in TBST overnight at 4°C and at room temperature for 10 × 10 min. Polyps were washed 3 × 10 min. in AP buffer (100 mM Tris, pH9.5/100 mM NaCl/50 mM MgCl2/2 mM levimisole) and incubated in substrate solution (4.5 μl/ml NBT/3.5 μl/ml X-phosphate in AP buffer) (Boehringer) for 2–4 h. Color reaction was stopped by washing 3 times in TBS/50 mM EDTA. Background was removed by washing polyps 3 times in MeOH for 30 min. Polyps were rehydrated and mounted in 10% stop solution/glycerol. All observations reported here were repeated in multiple replicate trials.
Dactylozooid-To-Gastrozooid Transformation.
Colonies encrusting gastropod shells occupied by the hermit crab Pagurus longicarpus were collected at Lighthouse Point, New Haven, CT. Portions of the colonies surrounding the aperture of the shell were removed with a scalpel and divided into “explants” of roughly 5 mm2, from which all polyps other than dactylozooids were excised. Explants were maintained at 15–16°C for 4–8 days and observed daily for transformation. Transformants were fed either whole or partial nauplii of Artemia salina to insure that transformed polyps were functionally competent gastrozooids. Polyps were fixed and stained for Cnox-2 as described above.
Quantitative Measures of Cnox-2 Expression.
All specimens were digitized for convenience in counting nuclei. For all Cnox-2-stained gastrozooids (normal and transformed), the entire hypostome and an equivalent area in the midbody region were counted for Cnox-2 staining. In the gonozooids, dactylozooids, and tentaculozooids, the anteriormost region and equivalent area in the midbody column was used for counting Cnox-2 staining. Counts were corrected for differences in cell density between oral and aboral regions, by using 4′,6-diamidino-2-phenylindole (DAPI)-stained material.
RESULTS
Expression of Cnox-2 in Polyp Polymorphs.
Cnox-2 expression was detected by whole-mount immunolocalization with IgG-purified immune fractions of polyclonal antibodies and visualized by alkaline phosphatase staining. In immunoreactive cells, high-intensity staining was localized to the nuclei, as expected of a putative transcription factor. The pre-immune serum showed no nuclear staining (data not shown). A competition experiment was performed to ensure that the anti-Cnox-2 antibodies were specific to the Cnox-2 protein and not to the carrier protein ovalbumin, which the peptide was linked to before injection into the rabbit. Molar excess of either peptide or carrier protein was preincubated with the anti-Cnox-2 antibodies before whole-mount immunolocalization. The anti-Cnox-2 antibodies preincubated with the synthetic peptide failed to stain cells in whole-mount experiments. By contrast, those anti-Cnox-2 antibodies that were preincubated with carrier protein revealed patterns of immunolocalization identical to that of untreated antibodies (see below). These results indicate that the anti-Cnox-2 antibodies are specific to the Cnox-2 epitope and not to the carrier protein.
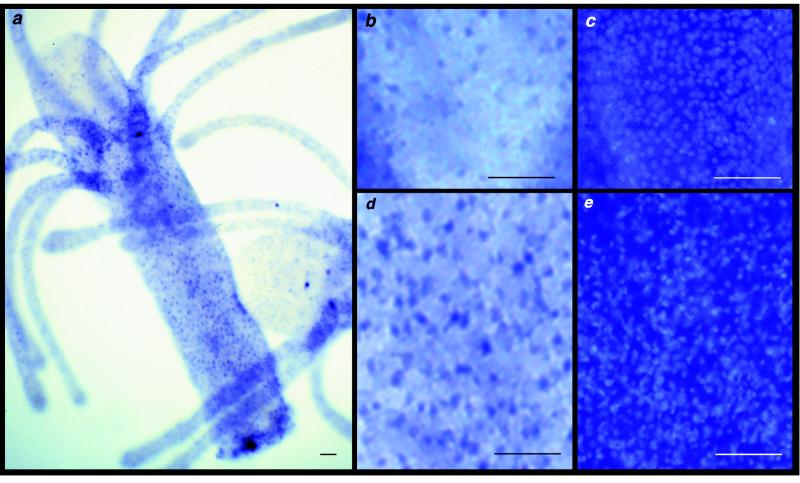
We determined Cnox-2 expression patterns in the four distinct polyp types in Hydractinia. In the gastrozooid (Fig. 2a), Cnox-2 displays weak expression in the oral regions of the hypostome and tentacles (Fig. 2b) and strong ectodermal expression in aboral regions of the polyp base and body column (Fig. 2d). This differential expression pattern is not an artifact of differences in cell density. A general nuclear stain actually reveals greater cell density in the oral region than in the body column (Fig. 2 c and e). Quantification of the density of cells expressing Cnox-2 revealed that intensely stained nuclei in the aboral ectoderm occur at frequencies roughly triple that found in oral regions (mean aboral/oral ratio = 3.35, SD = 0.65, n = 6).
Figure 2.
Whole-mount immunolocalization of Cnox-2 in the gastrozooid. Immunoreactive nuclei are stained blue. Larger, irregular blue shapes are nonspecific staining. (a) Lateral view of a gastrozooid. (b and d) Magnification of representative regions of a hypostome and body column. (c and e) 4′,6-diamidino-2-phenylindole (DAPI)-stained image of identical regions, showing total nuclei. (Bars = 0.1 mm.)
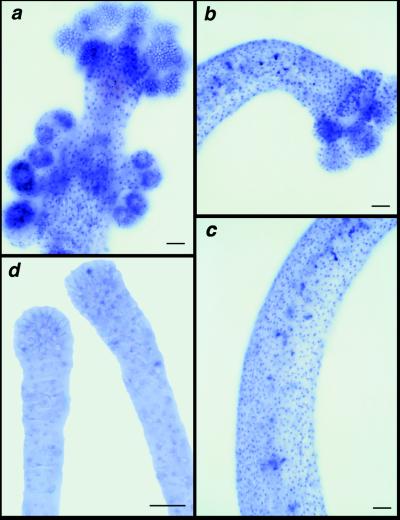
Cnox-2 expression in the gonozooid and dactylozooid differ from that of the gastrozooid but have identical patterns to one another. The gonozooid and dactylozooid display strong ectodermal expression throughout the entire length of the polyp, at levels approximately equivalent to that seen in the gastrozooid base. Quantification of the density of cells expressing Cnox-2 revealed that there was no detectable difference in Cnox-2 expression along the aboral–oral axis in either the gonozooid (Fig. 3a) or dactylozooid (Fig. 3 b and c) (aboral/oral ratio for dactylozooids, mean = 1.01, SD = 0.05, n = 3; and for gonozooids, mean = 0.94, SD = 0.42, n = 3). The tentaculozooid, which lacks a mouth, displays very weak expression from its basal region to the tip (Fig. 3d), at approximately the same levels seen in the tentacles of gastrozooids.
Figure 3.
Whole-mount immunolocalization of Cnox-2 in polyp polymorphs. Immunoreactive nuclei are stained blue. Larger, irregular blue shapes are nonspecific staining. (a) Lateral view of a gonozooid. (b and c) Representative lateral views of the oral and aboral regions of the dactylozooid, respectively. (d) Lateral view of two tentaculozooids. (Bars = 0.1 mm.)
Experimental Transformation of Dactylozooids to Gastrozooids.
Developmental studies on this hydroid are greatly facilitated by the ease with which one polyp can be experimentally transformed into another. When dactylozooids are experimentally removed from a colony, they transform into gastrozooids at appreciable frequency (18, 19). To establish whether conversion of polyp type results in concomitant alterations in Cnox-2 expression, we removed dactylozooids from colonies and monitored Cnox-2 expression after conversion. Alteration in Cnox-2 expression followed morphological transformation. The sequence for altered Cnox-2 expression is as follows: Cnox-2 expression initially decreased in the hypostome region, followed by a decrease in the tentacle region and then at the base of the tentacles until ultimately, dactylozooids that fully transformed into gastrozooids displayed aboral–oral Cnox-2 expression patterns indistinguishable from that of normal gastrozooids (aboral/oral ratio for dactylozooid-to-gastrozooid transformants, mean = 3.34, SD = 0.55, n = 6).
DISCUSSION
The apportionment of tasks of feeding, reproduction, and colony defense among different polyp types is a repeated pattern within the phylum Cnidaria (20). The relative simplicity of the cnidarian body plan led 19th-century zoologists to claim that the ancestral polyp type, the gastrozooid, was comprised of different body regions/structures and that differential expansion or contraction of these regions can account for the different morphologies found in polyp polymorphs (3, 5, 14–17). If this were the case, then one would expect that molecular markers of body regions would display differential expression in a fashion consistent with the hypothesized alteration of body regions.
Cnox-2 protein was detected at low levels in the hypostome and tentacles and at high levels in the body column of the gastrozooid of Hydractinia (Fig. 2). The pattern detected was indistinguishable from that previously described for Hydra (9, 10), lending support to the classical notion that body regions of polyp types are conserved features. Further support is found in our observation that experimental transformation of dactylozooids into gastrozooids was accompanied by a modification of Cnox-2 expression from that characteristic of the dactylozooid to that of the gastrozooid. This finding suggests that Cnox-2 may not simply correlate with the relative extent of head and body column, but may be actively involved in its maintenance.
Because Cnox-2 expression delineates head and body regions in hydrozoans as distinct as Hydra and Hydractinia, a test of the classical interpretation that polyp polymorphs represent differential expansion or contraction of these body regions, proved possible. Dactylozooids and gonozooids, both of which lack a hypostome and tentacles, are hypothesized to represent polyp types in which the body column has been expanded at the expense of head structures. These were, therefore, predicted to lack the weak Cnox-2 expression seen in the head of gastrozooids, and the results in Fig. 3 a–c show this to be the case.
Whereas the dactylozooid and gonozooid appear to represent an expansion of the body column to the exclusion of the head on the basis of morphological and now expression data, the tentaculozooid represents the reverse condition. Tentaculozooids resemble a single tentacle of a gastrozooid, suggesting an expansion of a head structure to the exclusion of the body column. The Cnox-2 expression pattern documented (Fig. 3d) shows the predicted weak expression characteristic of the tentacles of gastrozooids.
The speculative evolutionary scenarios of A. Agassiz (14), Brooks (15), Haeckel (5), Huxley (3), Metschnikoff (17), and Garstang (16) all share the contention that the division of labor among polyp types arose by evolutionary modification of homologous regions. Cnox-2 expression delineates the aboral–oral body regions in the gastrozooid of two distantly related hydrozoans, and its expression was found to be correlated with the differential expansion and contraction of these body regions in polyp polymorphs. These findings support the classically held views on the homology of body regions in cnidarians and suggest that these and other such regions may be fruitfully delineated by patterns of Hox gene expression.
Acknowledgments
We thank S. Dellaporta, M. Dick, D. Green, D. Lackschewitz, M. Moreno, and A. Shenk for technical advice and assistance and N. Blackstone, H. Bode, D. Bridge, M. Dick, S. Rice, R. Steele, and G. Wagner for comments and discussion. The work was supported by National Science Foundation Grant IBN-9119437.
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF031953).
References
- 1.Gould S J. Nat Hist. 1982;93:20–29. [Google Scholar]
- 2.Winsor M P. Starfish, Jellyfish, and the Order of Life. New Haven, CT: Yale Univ. Press; 1976. [Google Scholar]
- 3.Huxley T H. The Oceanic Hydrozoa: A Description of the Calycophoridae and Physophoridae Observed During the Voyage of the H. M. S. “Rattlesnake” in the years 1846–1850: With a General Introduction. London: Ray Society; 1859. [Google Scholar]
- 4.Agassiz L. Contributions to the Natural History of the United States of America. Brown, Boston: Little; 1862. [Google Scholar]
- 5.Haeckel E. Report on the Siphonophorae Collected by H. M. S. Challenger During the Years 1873–76. London: The Challenger Reports; 1888. [Google Scholar]
- 6.Graham A, Papalopulu N, Krumlauf R. Cell. 1989;57:367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- 7.Duboule D, Dolle P. EMBO J. 1989;8:1498–1505. doi: 10.1002/j.1460-2075.1989.tb03535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B B, Muller-Immergluck M M, Austin J, Robinson N T, Chisholm A, Kenyon C. Cell. 1993;74:29–42. doi: 10.1016/0092-8674(93)90292-x. [DOI] [PubMed] [Google Scholar]
- 9.Shenk M A, Bode H R, Steele R E. Development. 1993;117:657–667. doi: 10.1242/dev.117.2.657. [DOI] [PubMed] [Google Scholar]
- 10.Shenk M A, Gee L, Steele R E, Bode H R. Dev Biol. 1993;160:108–118. doi: 10.1006/dbio.1993.1290. [DOI] [PubMed] [Google Scholar]
- 11.Berrill N J. J Morphol. 1953;92:241–272. [Google Scholar]
- 12.Burnett A L, Sindelar W, Diehl N. J Mar Biol Assoc UK. 1967;47:645–658. [Google Scholar]
- 13.Muller W. Wilhelm Roux’ Arch Entwicklungsmech Org. 1964;155:181–268. doi: 10.1007/BF00573905. [DOI] [PubMed] [Google Scholar]
- 14.Agassiz A. North American Acalephae. Bigelow, Cambridge, U.K.: University Press: Welsh; 1865. [Google Scholar]
- 15.Brooks W K. Mem Boston Soc Nat Hist. 1886;3:359–430. [Google Scholar]
- 16.Garstang W. Q J Microbiol Sci. 1946;87:103–193. [Google Scholar]
- 17.Metschnikoff E. Z Wiss Zool. 1874;24:1–15. [Google Scholar]
- 18.Muller W A. Wilhelm Roux’ Arch Entwicklungsmech Org. 1969;163:334–356. doi: 10.1007/BF00577020. [DOI] [PubMed] [Google Scholar]
- 19.Hazen A P. Am Nat. 1902;36:193–200. [Google Scholar]
- 20.Hyman L H. The Invertebrates: Protozoa through Ctenophora. New York: McGraw-Hill; 1940. [Google Scholar]
- 21.Allman G J. A Monograph of the Gymnoblastic or Tubularian Hydroids. London: Ray Society; 1872. [Google Scholar]