Abstract
The contrasting hydraulic properties of wheat (Triticum aestivum), narrow-leafed lupin (Lupinus angustifolius), and yellow lupin (Lupinus luteus) roots were identified by integrating measurements of water flow across different structural levels of organization with anatomy and modeling. Anatomy played a major role in root hydraulics, influencing axial conductance (Lax) and the distribution of water uptake along the root, with a more localized role for aquaporins (AQPs). Lupin roots had greater Lax than wheat roots, due to greater xylem development. Lax and root hydraulic conductance (Lr) were related to each other, such that both variables increased with distance from the root tip in lupin roots. Lax and Lr were constant with distance from the tip in wheat roots. Despite these contrasting behaviors, the hydraulic conductivity of root cells (Lpc) was similar for all species and increased from the root surface toward the endodermis. Lpc was largely controlled by AQPs, as demonstrated by dramatic reductions in Lpc by the AQP blocker mercury. Modeling the root as a series of concentric, cylindrical membranes, and the inhibition of AQP activity at the root level, indicated that water flow in lupin roots occurred primarily through the apoplast, without crossing membranes and without the involvement of AQPs. In contrast, water flow across wheat roots crossed mercury-sensitive AQPs in the endodermis, which significantly influenced Lr. This study demonstrates the importance of examining root morphology and anatomy in assessing the role of AQPs in root hydraulics.
The expression of a large number of aquaporins (AQPs) occurs predominantly in roots (for review, see Bramley et al., 2007b). These membrane integral proteins form water-conducting channels, which are considered responsible for the variable hydraulic conductivity of root systems (Lpr; Javot and Maurel, 2002). Reverse genetics has demonstrated that AQP activity is linked to the hydraulics of some species during abiotic perturbation, as the water relations of antisense or mutant plants tends to be more sensitive to the perturbation (Martre et al., 2002; Siefritz et al., 2002; Aharon et al., 2003; Yu et al., 2005; Jang et al., 2007). However, reverse genetics can also alter the expression of other AQPs that were not targeted (Jang et al., 2007) and possibly other transporters involved in osmoregulation. These combined effects may cause changes in the morphology of the root system. For example, antisense Arabidopsis (Arabidopsis thaliana) and tobacco (Nicotiana tabacum) had larger root masses (Kaldenhoff et al., 1998; Martre et al., 2002; Siefritz et al., 2002). The increase in root mass may be a compensatory effect, because a lower expression of plasma membrane AQPs reduced the water permeability of some cells (Martre et al., 2002; Siefritz et al., 2002). However, it is not known if the anatomy of antisense roots was also altered or if water uptake was localized to specific regions of the root. These features would also have an important influence on Lpr.
To fully understand root system hydraulics and the contribution of native AQPs, comprehensive studies at different scales are required (cells, organs, and whole roots). This should be coordinated with examinations of anatomy to identify features that will influence the rate of water flow through the root. Comparative studies on species from different evolutionary lines and with differing root hydraulic properties may also further elucidate AQP behavior. To address these issues, we examined the hydraulic properties of roots of two contrasting species: wheat (Triticum aestivum) and narrow-leafed lupin (Lupinus angustifolius). Wheat develops a root system of several extensively branched individual roots (O'Brien, 1979), whereas narrow-leafed lupin has a dominant taproot that can develop many primary lateral roots (Clements et al., 1993). Under the same field conditions, wheat has a much greater total root length density than narrow-leafed lupin (Hamblin and Tennant, 1987; Gallardo et al., 1996; Gregory and Eastham, 1996). However, both species have similar aboveground biomass and extract similar volumes of water from the soil profile (Hamblin and Tennant, 1987). Per unit of root length, narrow-leafed lupin appears to take up and transport water at a greater rate than wheat (Hamblin and Tennant, 1987; Gallardo et al., 1996). The reason for these differing hydraulics was not previously investigated but was speculated to be because wheat and lupin roots have different xylem development (see below; Hamblin and Tennant, 1987; Gallardo et al., 1996). Other differences in anatomy and AQP activity could also be the cause of the disparity.
To investigate more subtle differences in root hydraulics, we also examined the hydraulic properties of a closely related lupin species: yellow lupin (Lupinus luteus ‘Wodjil’). Water transport in yellow lupin roots has not been measured previously. However, anecdotal evidence indicates that the hydraulic properties differ between the two lupin species. Yellow lupin is more tolerant of waterlogging than narrow-leafed lupin due to characteristics of its roots (Davies et al., 2000a, 2000b), which may be related to AQP activity (Bramley et al., 2007b). In addition, comparative measurements of leaf water potential in the field (Palta et al., 2004) suggest that yellow lupin may have greater conductance to water flow than narrow-leafed lupin.
The aim of this study was to identify the influence of root structure, anatomy, and AQP activity on the rate of water transport in wheat, narrow-leafed lupin, and yellow lupin roots. The rationale and main hypotheses were as follows. (1) Lupin roots have larger and more abundant xylem vessels than wheat (Hamblin and Tennant, 1987). If water flow through xylem vessels is analogous to flow through pipes, then the axial conductance (Lax) of lupin roots should be greater than that of wheat. (2) The transport of water through the radial flow path, not the axial path, is considered the greatest limitation to the rate of water flow through the whole root (Steudle and Peterson, 1998). Therefore, the total hydraulic conductance (Lr) of wheat and lupin roots should be less than their Lax. (3) Lupin roots have abundant root hairs over the whole root system, in comparison with wheat, where root hairs are more localized to the apical region (Hamblin and Tennant, 1987). This implies that the absorbing region differs between the species and that lupin roots may absorb water more evenly along the length of the root compared with wheat. (4) Structural differences and AQP activity in the radial pathway should be the primary factors determining Lpr of wheat and lupin. In the radial path, water crosses a series of tissues from the root surface to the vascular tissue in the stele. Water can flow through the apoplast (cell walls and intercellular spaces) and/or through cells. The relative contribution of the radial pathways depends on their relative Lr. Both conductances are proportional to their cross-sectional area and inversely proportional to their path length (Steudle and Frensch, 1996). The radial path length was expected to be longer in lupin than in wheat, because lupin roots are two to three times thicker. If the radial path length is longer in lupin roots, then lupin roots should have fewer anatomical barriers to water flow in the apoplast and/or greater AQP activity in the cell-to-cell path, facilitating greater overall root Lr. AQP activity can only influence radial conductance if water crosses membranes. In the apoplast, flow can be restricted by the deposition of suberin in cell walls, such as Casparian bands and suberin lamellae in the exodermis and endodermis (Steudle and Peterson, 1998). (5) Because the two lupin species are closely related, the anatomy of yellow lupin roots was expected to be very similar to that of narrow-leafed lupin roots. Therefore, AQPs were expected to mediate differences in Lpr between the species.
To test these hypotheses, the rate of water flow was measured across whole root systems, individual roots, and root cell membranes of seedlings grown in sand. Anatomical features in different regions of the root were related to the water flow measurements to identify the structural components influencing root water uptake and transport. Cell size, radial path length, and the developmental state of the endodermis and exodermis (if present) were examined. Cross sections of roots were stained for Casparian bands and suberin lamellae (Brundrett et al., 1988, 1991). To determine whether water uptake is localized to a particular region of the root, the Lr of root segments and root systems was compared against root length. Assuming that water can enter the root anywhere along the root surface, Lr should be proportional to root length. The Lax of small root segments was measured and related to changes in xylem vessel development as well as total root Lr. To estimate the contribution of the radial pathways, a radial profile of the hydraulic conductivity of cells in each cell layer of the root was created. The profile was applied to a model, modified from Jones et al. (1988), describing the root as a series of concentric cylindrical membranes. Water flow across the cell-to-cell pathway was inhibited with an AQP blocker to test the model and the contribution of AQP activity. Here, we assumed that blocking the cell-to-cell pathway would not increase the hydraulic conductivity of the apoplastic pathway. By adopting this integrated approach, we identified the contributions of root morphology, anatomy, and AQPs to root water flow in the three contrasting species.
RESULTS
The Total Length of Wheat Roots Was Greater Than That of Lupin Roots, But They Did Not Transport More Water to the Shoot
Fourteen days after sowing (DAS), wheat root systems comprised four or five seminal roots (Fig. 1). The longest seminal root was 360 to 490 mm long, with the second and third seminal roots 20 to 50 mm shorter. The remaining roots grew to lengths of up to 200 mm. No nodal roots developed in these young plants. The basal half of the wheat root system also contained many fine lateral roots. The root systems of both lupin species were physically similar at 14 DAS. Lupins had a sturdy taproot with lateral roots in the basal region (Fig. 1) and a few small nitrogen-fixing nodules close to the base of the stem. Wheat root systems were more than twice the total length of lupin root systems (Table I). However, wheat roots were smaller in diameter than lupin roots, which resulted in no significant difference in surface area of root systems between the three species (P = 0.26; Table I).
Figure 1.
The root systems of wheat and narrow-leafed lupin at 14 DAS. The root systems were stained for 1 h with 0.5% methylene blue to enhance the image contrast. The insets show freehand, transverse sections of the root (A and F) or stele (B–E and G–K). Sections A and F were stained with 0.05% toluidine blue. Sections stained with Sudan Red and viewed under white light (B and D) or phase contrast (G and J) show suberin lamellae in the walls of the endodermis (en). Fluorescence in sections stained with the berberine-aniline blue procedure and viewed under UV light (C, E, H, and K) show Casparian bands (E and K; indicated by white arrows) in the endodermis and lignified cell walls, phloem, and xylem vessels (C, E, H, and K). Bars = 50 μm.
Table I.
Dimensions and mean water transport parameters of whole roots used in the pressure chamber measurements
At 14 d after sowing, ΔP of 0.3, 0.5, and 0.7 MPa was applied to detopped root systems to induce a water flux. The flow rate at each pressure was plotted against ΔP, and the slope of the linear regression gave Lr. Normalizing flow rate by surface area gave Lpr. Required ΔP is the pressure that was needed to induce a flow rate through detopped root systems that was equivalent to midday transpiration. Values are means ± se (n = 6–8). Asterisks denote significant differences by Tukey's test (P < 0.05).
| Parameter | Narrow-Leafed Lupin | Yellow Lupin | Wheat |
|---|---|---|---|
| Total length (m) | 2.3 ± 0.1 | 1.9 ± 0.2 | 5.2 ± 0.5* |
| Surface area (m2 × 10−3) | 8.2 ± 0.6 | 7.1 ± 0.6 | 7.0 ± 0.5 |
| Transpiration (g h−1) | 0.6 ± 0.05 | 0.4 ± 0.04* | 0.6 ± 0.01 |
| Required ΔP (MPa) | 0.31 ± 0.045* | 0.25 ± 0.05* | 0.41 ± 0.09* |
| Lr (m3 s−1 MPa−1 × 10−10) | 10.61 ± 1.02 | 8.24 ± 0.88 | 3.96 ± 0.58* |
| ΔP intercept (kPa) | 180* | 100* | −3* |
| Lpr (m s−1 MPa−1 × 10−8) | 13.12 ± 1.7 | 11.66 ± 1.1 | 5.74 ± 0.8* |
Midday transpiration of yellow lupin plants, measured gravimetrically, was approximately one-third lower than that of the other species (P = 0.004; Table I). However, transpiration of wheat and narrow-leafed lupin plants was not significantly different (P > 0.05; Table I).
Lr Increased with Root Length in Lupin But Not in Wheat
The hydraulic properties of whole root systems were measured with a pressure chamber. Preliminary measurements indicated that the pressure range applied to root systems induced water flux that would have been sufficient to meet the demands of transpiration (Table I). The rate of water transport, per unit pressure gradient, by whole roots was similar for both lupin species, which resulted in a similar mean Lr (P > 0.05; Table I). However, the abscissa intercepts of the linear regression between flow rate and applied pressure (ΔP) differed significantly between the lupin species and were greater than zero (P < 0.05; Table I). The ΔP intercept of narrow-leafed lupin was greater than that of yellow lupin, indicating that a greater driving force was required in narrow-leafed lupin roots to induce the same flux as in yellow lupin (P = 0.812; Table I). The average Lr of lupin root systems was more than 2-fold greater than that of wheat (P < 0.001; Table I). Because the roots of all species had a similar surface area, Lpr of lupin roots was approximately twice that of wheat roots (P < 0.001; Table I), but if flow rate was expressed on a per unit root length basis, Lpr of lupin roots was approximately 6-fold greater than that of wheat roots (data not shown).
Root segments (with the tip intact) between 60 and 180 mm long connected to a root pressure probe were used to determine whether Lr varied with root length. Root segments shorter than 60 mm were generally too delicate to connect to the probe, and lateral roots prevented segments longer than 180 mm from sealing to the pressure probe. After connecting the excised end of the detached root segment to the root pressure probe, measurements began when root pressure (Pr) was stable. The Pr of the two lupin species was not significantly different, but Pr of wheat roots was approximately twice that of lupin roots (Table II).
Table II.
The dimensions and hydraulic properties of detached root segments at 14 d after sowing
Root segments, excised from the taproot of lupin or the longest seminal root of wheat, were connected to a root pressure probe. When Pr was stable, the Lr was measured. Lr was normalized by dividing by the surface area of the root to give Lpr. Because of the dependence of some hydraulic parameters on root length (see text for details), ranges are given with means ± se in parentheses. Asterisks denote significant differences between species (P < 0.001).
| Property | Narrow-Leafed Lupin | Yellow Lupin | Wheat |
|---|---|---|---|
| Diameter (mm)a | 1.1–1.9 | 0.7–1.3 | 0.5–0.9 |
| (1.5 ± 0.05*) | (1.0 ± 0.03*) | (0.7 ± 0.03*) | |
| Length (mm) | 75–181 | 82–170 | 69–179 |
| (129 ± 6) | (120 ± 5) | (120 ± 8) | |
| No. of cortical cell layers (including endodermis) | 9–11 | 8–10 | 5–7 |
| Pr (kPa) | 20–119 | 26–103 | 77–172 |
| (48.5 ± 5.1) | (52.8 ± 3.4) | (114.9 ± 7.3*) | |
| Lr (m3 s−1 MPa−1 × 10−11) | 2.0–7.0 | 1.4–3.6 | 1.2–4.1 |
| (3.8 ± 0.3*) | (2.3 ± 0.1) | (2.5 ± 0.2) | |
| Lpr (m s−1 MPa−1 × 10−8) | 3.7–8.6 | 3.8–8.9 | 5.3–18.4 |
| (6.0 ± 0.4) | (5.9 ± 0.3) | (10.6 ± 1.0*) | |
| No. of measured roots | 20 | 22 | 17 |
Diameter was measured at the excised end of the root.
The Lr of narrow-leafed lupin root segments tended to be greater than that of yellow lupin and wheat root segments (Table II). Lr of lupin root segments increased linearly with length of the segment (P < 0.0001, r2 = 0.61 for narrow-leafed lupin and P < 0.0001, r2 = 0.59 for yellow lupin; Fig. 2, A and B). In comparison, Lr of wheat root segments was almost constant with segment length (P = 0.937, r2 = 0.0004; Fig. 2C). Because root segments of lupin were larger in diameter than those of wheat (Table II), normalizing Lr by surface area resulted in the average Lpr of lupin being about half that of wheat root segments (Table II). There was no significant difference in Lpr between the two lupin species (Table II). In addition, because Lr was not related to length in wheat, normalizing by surface area resulted in Lpr decreasing linearly with length of the root segment (P = 0.017, r2 = 0.32; Fig. 2F).
Figure 2.
The relationship between root length and Lr (A–C) or Lpr (D–F) of detached root segments (black symbols) and whole root systems (white symbols) of narrow-leafed lupin (A and D), yellow lupin (B and E), and wheat (C and F). Note the log scale of the root length and Lr axes. Comparison of linear regression for root segments and whole root systems resulted in the same linear fit (P > 0.05) between Lr and root length for narrow-leafed lupin (A) and yellow lupin (B).
Lr of root segments was more than 1 order of magnitude smaller than Lr of whole root systems (Tables I and II). This disparity in Lr for both lupin species was because Lr of whole root systems was related to total root length, with the same regression applying for whole root systems as distal root segments (P > 0.05; Fig. 2, A and B). The increase in water transport with increasing length of lupin roots resulted in an almost constant Lpr with length within each of the two methods of measuring Lr, but whole root systems had on average a higher Lpr than distal root segments (Fig. 2, D and E; Tables I and II). In comparison, although Lr of wheat root systems was greater than for distal root segments (Fig. 2C), this was not related to total root length. Instead, Lpr of distal root segments tended to decrease with length, to a lower constant value in whole roots (Fig. 2F).
Xylem Development and Lax
Lignified xylem vessels fluoresced bright yellow/white with the aniline blue procedure, which indicated xylem maturity (Brundrett et al., 1988). The development of primary xylem vessels began within 5 mm of the root tip in all species. The seminal roots of wheat developed a single large central xylem vessel (59–82 μm diameter) surrounded by five to eight smaller vessels (16–23 μm diameter; Fig. 1). Walls of the small vessels became lignified within 10 mm of the root tip (Fig. 1E). In some roots, the central vessel and/or several smaller vessels divided into two. Secondary thickening of the central vessel wall occurred 80 to 100 mm from the root tip, but lignification of the wall was not observed until more than 100 mm from the tip (Fig. 1C).
Xylem vessels developed in a diarch pattern in the roots of both lupin species, initially consisting of two groups of five or six small vessels (Fig. 1K). New vessels developed centripetally, forming a continuous band across the center of the stele between 100 and 120 mm from the root tip (Fig. 1J). Xylem vessel walls became lignified as lupin roots matured (Fig. 1H). The abundance and size of lupin xylem vessels continued to increase with distance from the tip, resulting in a larger and more circular stele (Fig. 1G).
Using the fluorescence images, the cross-sectional area of mature xylem vessels (Ax) was measured along the length of wheat seminal roots and the taproot of lupins. For wheat, Ax was constant along the length of the root until at least 100 mm from the root tip, where Ax increased to a larger constant value (Fig. 3A). Distal to 100 mm from the root tip, Ax of a wheat seminal root was approximately half that of lupin taproots, and as roots matured the difference in Ax increased between the species (Fig. 3A). For lupin, Ax increased with distance from the root tip in the form of logistic growth curves, which were significantly different between the species (P < 0.0001; Fig. 3A). Ax of yellow lupin tended to increase more with distance from the root tip than that of narrow-leafed lupin.
Figure 3.
The relationship between distance from the root tip and the cross-sectional area of lignified xylem as indicated by fluorescence (Ax; A) or Lax (B) for narrow-leafed lupin (black circles), yellow lupin (white circles), and wheat (inverted black triangles). Lax was measured on 10-mm-long root segments after measuring Lr with the root pressure probe. The curves represent best statistical fits. The error bars in A represent se (n = 9), and each point in B represents a segment from an individual root.
After the measurement of Lr, the root in the pressure probe was excised at the seal and the conductance of the segment remaining in the seal (10 mm long) was measured. Here, radial flow would be negligible because the segment was surrounded by the seal; hence, the measurement is defined as the Lax. Lax varied with distance from the root tip (Fig. 3B) and followed a similar pattern to the development of Ax in all species (Fig. 3A). Lax increased nonlinearly with distance from the tip in lupin roots, with different regressions fitting the two lupins (P = 0.011; Fig. 3B). In comparison, Lax increased only marginally with distance from the tip of wheat roots (Fig. 3B). Lax of wheat for segments excised less than 100 mm from the tip was approximately one-third that of lupins, but values of Lax diverged further between the species as the root matured (Fig. 3B).
Lax was up to 2 orders of magnitude greater than Lr, particularly for lupin root segments (Fig. 4). Lax was highly correlated with Lr in lupin root segments, with linear regressions that were significantly different between the two species (P = 0.004). The regression coefficient for narrow-leafed lupin (9.9 ± 1.4 × 10−3) was more than twice that of yellow lupin (4.1 ± 1.0 × 10−3). Therefore, narrow-leafed lupin has a greater radial conductance than yellow lupin for the same axial Lr. There was no correlation between the two hydraulic parameters in wheat (Fig. 4).
Figure 4.
The correlation between the Lr of a detached root segment and its Lax. Lax values are shown in Figure 3B, where distance from the root tip denotes the length of the original root that Lr was measured on. The correlation is significant for narrow-leafed lupin (black circles; P < 0.0001, r2 = 0.81) and yellow lupin (white circles; P = 0.002, r2 = 0.56) but not for wheat (inverted black triangles; P = 0.712). Each point represents an individual root segment.
Radial Anatomy
Root diameter was related to the number of cell layers and cell sizes in the cortex (Tables I and III; Fig. 1). The number of cell layers across the cortex was constant in all species along the length of the root for at least 250 mm. Suberin was not detected in the outer cortex of any species, indicating that none of the species developed an exodermis when grown in sand. The Casparian strip in the endodermis had developed at 5 mm from the tip in all species, but suberin lamellae developed in the endodermis much closer to the root tip in wheat than in lupin (Fig. 1). Around 40 mm from the root tip, endodermal cells of wheat contained suberin lamellae and the inner tangential walls of the endodermis and walls of stellar cells became thickened (Fig. 1D). In the endodermis of lupins, only a few cells contained suberin lamellae at 100 to 120 mm from the tip (Fig. 1J). At 200 mm from the root tip, all lupin endodermal cell walls contained suberin lamellae, except cells adjacent to xylem poles (Fig. 1G). No secondary thickening of cell walls occurred in roots of either lupin species.
Table III.
Dimensions of root cells in each cell layer
Cell layers are numbered from the epidermis (1) toward the root axis. Values are means ± se (n = 22–59) from 10 roots. The data were combined for cell layers 4 to 6 of yellow lupin and cell layers 2 to 4 of wheat because no significant differences could be found between these cell layers. Volumes were only determined for those cell layers punctured by the cell pressure probe.
| Cell Layer | Narrow-Leafed Lupin
|
Yellow Lupin
|
Wheat
|
|||
|---|---|---|---|---|---|---|
| Diameter | Volume | Diameter | Volume | Diameter | Volume | |
| μm | pL | μm | pL | μm | pL | |
| 1 | 14 ± 1 | 15 ± 1 | 16 ± 0 | 18 ± 1 | 27 ± 1 | 51 ± 2 |
| 2 | 23 ± 1 | 50 ± 3 | 26 ± 1 | 63 ± 4 | 51 ± 1 | 279 ± 15 |
| 3 | 37 ± 2 | 151 ± 22 | 40 ± 1 | 242 ± 19 | 51 ± 1 | 279 ± 15 |
| 4 | 46 ± 3 | 269 ± 30 | 54 ± 1 | 626 ± 37 | 51 ± 1 | 279 ± 15 |
| 5 | 52 ± 2 | 404 ± 33 | 54 ± 1 | 626 ± 37 | 34 ± 2 | 215 ± 7 |
| 6 | 63 ± 3 | 640 ± 60 | 54 ± 1 | 626 ± 37 | 30 ± 4 | 226 ± 10 |
| 7 | 60 ± 4 | 492 ± 51 | 36 ± 2 | 316 ± 34 | ||
| 8 | 52 ± 3 | 421 ± 46 | 22 ± 1 | 94 ± 8 | ||
| 9 | 41 ± 1 | 13 ± 0 | ||||
| 10 | 22 ± 1 | |||||
| 11 | 17 ± 0 | |||||
Hydraulic Properties of Root Cells
Since Lr ≪ Lax, radial flow was the limiting factor in root water transport for all of the species. Therefore, the hydraulic conductivity of cells (Lpc) in each radial cell layer was measured with the cell pressure probe in order to identify whether the disparity in root water transport between the species was due to different hydraulic properties at the cell level. Due to their small size and possibly different composition of cell sap, epidermal cells were the most challenging to measure, particularly in lupin roots. Although turgor pressure could be measured, constant blockages of the microcapillary or sudden losses of turgor pressure meant that Lpc was not determined in sufficient lupin epidermal cells for statistical analysis.
There was no significant difference in turgor pressure of root cells between the lupin species (P > 0.05), but the cells of the epidermis had a lower turgor pressure (0.26 ± 0.03 MPa) than all other cell layers (0.37 ± 0.01 MPa). The average turgor pressure of wheat root cells (0.61 ± 0.01 MPa) was greater than that of lupin but was independent of the radial location in the root (P = 0.119).
Lpc of root cells ranged between 0.3 × 10−6 and 3.5 × 10−6 m s−1 MPa−1, and the average Lpc was not significantly different between the three species (P = 0.12). The location of the cell within the cortex influenced Lpc such that Lpc tended to increase toward the root axis (Fig. 5A), although due to the variability between roots and cells, this trend was not statistically significant in the lupin species (P = 0.072 for narrow-leafed lupin and P = 0.081 for yellow lupin). For wheat roots, the trend was more pronounced and the inner cell layers of the cortex (cell layers 5 and 6) were significantly different from those of the epidermis (cell layer 1; P < 0.05).
Figure 5.
Radial profiles of the mean Lpc (A) and adjusted Lpc (Lpca; B) for each cortical cell layer, 30 to 50 mm from the tips of narrow-leafed lupin (black circles, solid lines), yellow lupin (white circles, dotted lines), and wheat (inverted black triangles, dashed lines) roots. Lpca was calculated from Equation 1 to account for the decrease in area of each cell layer toward the root axis. The cell layers are numbered from the outside of the root (epidermis = 1) toward the root axis. Error bars represent se (n = 4–16 cells per layer, except for the epidermis of both lupin species, where Lpc was not measured).
The increased Lpc near the axis could be compensating for the decreasing area for water flow toward the axis. When Lpc was adjusted by a factor for the decrease in area (C) for each jth cell layer (Eq. 1), Lpc was almost constant across the cortex for all species (Fig. 5B).
 |
(1) |
Estimation of the Contribution of the Radial Pathways with the Concentric Membrane Model
The radial profile of Lpc (Fig. 5A) was used to predict Lr and Lpr by applying a model that describes the root as a series of concentric cylindrical membranes (see “Materials and Methods” for the parameters). The model assumes that radial water flow is primarily via the cell-to-cell flow path and that, after entering the root, water crosses each cell layer in series to reach the stele. The root diameters and number of cell layers used in the predictions are shown in Table IV. Each cell layer has two concentric membranes, which can be included independently of each other in the model. Where Lpc was not measured in lupin, the value of Lpc used was the same as the closest measured cell layer.
Table IV.
Comparison of the estimated values for various root water flow parameters from the concentric membrane model and the values measured with the root pressure probe
The Lr of roots with an average length of 120 mm was calculated from Equation 2, assuming that all of the root length, except 5 mm at the apex, was absorbing water and that all radial water flow occurred through the cell-to-cell flow path. The Lpr was calculated from Equation 3 using the same assumptions. To compare the linear regressions between Lr and root length, the length of the concentric membranes was varied to coincide with the range of root length segments measured with the root pressure probe. n/a, Not applicable.
| Parameter | Type of Value | Narrow-Leafed Lupin | Yellow Lupin | Wheat |
|---|---|---|---|---|
| Root diameter (μm) | 1,537 | 1,050 | 660 | |
| No. of cell layers including endodermis | 11 | 9 | 6 | |
| Lr for 120-mm-long root (m3 s−1 MPa−1 × 10−11) | Predicted | 3.0 | 2.1 | 1.9 |
| Measured | 3.4 | 2.3 | 2.5 | |
| Regression coefficient for Lr versus root length (m2 s−1 MPa−1 × 10−11) | Predicted | 26.0 | 18.4 | 16.2 |
| Measured | 43.3 | 19.2 | 0.68a | |
| Root length when Lr = 0 (mm) | Predicted | 5 | 5 | 5 |
| Measured | 40.5 | 2.1 | n/a | |
| Lpr (m s−1 MPa−1 × 10−8) | Predicted | 5.4 | 5.6 | 7.8 |
| Measured | 6.0 | 5.9 | 10.6 | |
| P (predicted versus measured Lpr) | 0.106 | 0.271 | 0.015 | |
| Reduction in Lpr after 50 μm HgCl2 (%) | Predicted | 33 | 85 | 77 |
| Measured | 10 | 14 | 41 |
Regression does not significantly deviate from zero (P = 0.937).
The Lr of each concentric cylindrical membrane (Lmembrane) when water uptake is distributed evenly along the length of 120-mm-long roots (excluding 5 mm at the apex) is shown in Figure 6. The model predicted that Lr of narrow-leafed lupin would be greater than that of yellow lupin, which increased with root length (Fig. 7). The predicted Lr for both lupin species was close to the measured Lr for a root segment 120 mm long (Table IV). The disparity between predicted and measured values for narrow-leafed lupin will increase with longer root lengths, because the measured Lr increased more rapidly with root length than predicted (compare regression coefficients in Table IV). Extrapolating the regression for the measured Lr and root length to the abscissa indicated that the first 40 mm of narrow-leafed lupin roots was not involved in water uptake, but the intercept for yellow lupin was close to that set in the model (Table IV). Normalizing Lr by the surface area of lupin roots resulted in a predicted Lpr that was not significantly different from the measured Lpr (Table IV). The closeness of the predicted and measured Lpr values for lupins indicated that the majority of radial water flow could occur across each cell layer through the cell-to-cell pathway.
Figure 6.
Lmembrane of each cell layer predicted by the concentric membrane model, for a 120-mm-long root of narrow-leafed lupin (black circles, solid line), yellow lupin (white circles, dotted line), and wheat (inverted triangles, dashed lines). For wheat, the predictions are shown for all of the root length involved in water uptake (inverted black triangles) or if water uptake predominantly occurs between 5 and 40 mm from the root tip (inverted white triangles).
Figure 7.
Lr predicted by the concentric membrane model in relation to root length for narrow-leafed lupin (black circles, solid line), yellow lupin (white circles, dotted line), and wheat (inverted triangles, dashed lines). For wheat, the predictions are shown for all of the root length involved in water uptake (inverted black triangles) or if water uptake predominantly occurs between 5 and 40 mm from the root tip (inverted white triangles). The regression coefficients for the linear regressions that were significantly greater than zero (P < 0.01) are given in Table IV.
For wheat, the concentric membrane model predicted a smaller Lr than for lupin for the same root length (Fig. 7). The predicted Lr for a 120-mm-long wheat root was close to the measured value (Table IV). However, unlike the measured Lr, predicted Lr increased with length (Fig. 7), which implied that a specific region of wheat roots determines Lr. To explore this observation, the model was adjusted for anatomical structures in the cell-to-cell path that could influence Lr. To incorporate the effect of suberization of the endodermis, the model was adjusted to allow for water uptake only between 5 and 40 mm from the tip. Limiting the length of the absorbing region resulted in a predicted Lr independent of root length (Fig. 7). However, Lmembrane (Fig. 6), and consequently predicted Lr, was reduced to a value that was only one-quarter of the measured Lr. Therefore, not all cylindrical membranes in the radial pathway contribute equally to water flow. Changing the model to allow for cell-to-cell water flow across only two cylindrical membranes resulted in a predicted Lr close to the measured Lr. For example, using Lmembrane for the epidermis (both inward- and outward-facing membranes) predicted Lr of 2.95 × 10−11 m3 s−1 MPa−1 (Lpr of 10.7 × 10−8 m s−1 MPa−1), and Lmembrane for the endodermis predicted Lr of 2.5 × 10−11 m3 s−1 MPa−1 (Lpr of 10.4 × 10−8 m s−1 MPa−1), which were very similar to the measured values (Table IV).
Test of the Model by Inhibiting Water Flow through AQPs with HgCl2
Mercury had no significant effect on Lpr of whole root systems or root segments of either lupin species (Fig. 8, A and B). However, Lpr of wheat root systems and individual roots was reduced by up to half after mercury treatment (P = 0.0002; Fig. 8, A and B).
Figure 8.
The effect of mercury on Lpr and Lpc of whole root systems (A), individual roots (B), and root cortical cells (C) of narrow-leafed lupin (NL), yellow lupin (YL), and wheat (W). Measurements were conducted before (black bars) and after (white bars) treatment of roots with 50 μm HgCl2. Asterisks denote significant differences due to treatment at the 5% (*), 1% (**), and 0.1% (***) levels of significance. Error bars represent se (n = 4–7 for roots and n = 10–14 for cells).
On average, mercury reduced Lpc of cells from treated roots by 33% in narrow-leafed lupin, by 86% in yellow lupin, and by 77% in wheat (Fig. 8C). The outer cell layers of narrow-leafed lupin roots were particularly sensitive to mercury treatment, and measurements of Lpc were only obtained from cells deeper than the third cell layer. Mercury treatment did not affect the volumetric elastic modulus of any species (P = 0.147); therefore, changes in the rate of water exchange across the cell were due to the effect of mercury on Lpc.
If water flow occurs entirely through cells by crossing membranes, the inhibition in Lpc should also be reflected in Lpr. However, the concentric membrane model predicted a greater reduction in Lpr than the measured value, particularly for lupin (Table IV). The values in Table IV are based on the assumptions that the entire root length and all cell layers are involved. Adjusting the model for wheat, using the same parameters above (length of absorbing region of 35 mm and cellular flow across two membranes) resulted in predicted values lower than measured values. A 77% reduction in both Lmembrane of the endodermis resulted in Lpr of 2.44 × 10−8 m s−1 MPa−1. However, if only the exterior membrane of the endodermis was inhibited, Lpr was reduced by just over half (4.4 × 10−8 m s−1 MPa−1) compared with the 43% reduction in Lpr actually measured.
DISCUSSION
The main hydraulic properties of wheat and lupin roots were identified by integrating measurements of water flow, across different structural levels of organization, with anatomy and modeling. An important observation from this study was the major role of root structure and anatomy on root water transport and the localized influence of AQPs. AQP activity was ubiquitous in the cortex and epidermal cells of all species, based on the high values of Lpc and the strong inhibition by Hg2+. The similar magnitudes and profiles of Lpc across the root for each species pointed toward a major role for AQPs in root water transport. However, the contrasting behaviors and the weaker inhibition of water flow by Hg2+ in root tissue and root systems demonstrated that AQPs have localized influences on root hydraulics. At the cell level, AQPs considerably influence Lpc of all three species. At the level of the root system, the control of bulk water flow by AQPs was limited to a small region of the endodermis in wheat. For lupins, bulk water flow occurred predominantly through the apoplast without the influence of AQPs. Without manipulation of the rhizosphere, the benefit of these contrasting behaviors can only be speculated upon, but they may indicate a tradeoff between the overall rate and the potential to rapidly regulate the rate of water flow.
The Total Length of Wheat Roots Was Greater Than That of Lupin, But Wheat Roots Did Not Transport More Water to the Shoot
The features described here for wheat and lupin roots grown in sand-filled pots are consistent with those previously described for roots grown in the field (Greacen et al., 1976; Passioura, 1980; Hamblin and Tennant, 1987). Even at the relatively early stage of growth used in this study (14 DAS), the total length of wheat roots was greater than that in lupin roots. Wheat root systems appear to have more rapid early growth and greater proliferation of roots than lupins (Gregory and Eastham, 1996). Whole plant transpiration confirmed that the shorter root systems of lupin supplied the shoots with equivalent volumes of water as wheat. Eudicots commonly use the same amount of water as monocots, despite having smaller root length density (Mason et al., 1983). Therefore, the contrasting hydraulic properties of wheat and lupin roots may be more extensive among herbaceous species.
Wheat Roots Predominantly Absorb Water in a Region Close to the Root Tip, But Lupin Roots Absorb Water More Evenly along Their Whole Length
If the root is analogous to a leaky pipe, where water can enter anywhere along the length of this pipe, then Lr should increase with root length. Hence, Lpr should be constant with length. In this study, wheat root systems consisted of up to five seminal roots. Lr of an individual, unbranched, seminal root did not vary between 70 and 200 mm from the tip, resulting in a decrease in Lpr with root length. This indicated that water was not uniformly absorbed along the entire root but instead occurred predominantly in a distal region. The region coincided with a less mature endodermis. Farther from the tip, the endodermis was in the tertiary state of development, containing suberin lamellae and thickened cell walls. Water uptake by a region close to the root tip appears to be a common feature of cereal roots (Greacen et al., 1976). Lr of whole root systems was more than 10-fold greater than that of seminal root, indicating that more than one root contributes to water uptake in whole root systems. Using the 95% confidence interval of Lr from root segments (1.95 × 10−11 to 2.88 × 10−11 m3 s−1) and assuming that water uptake was confined to apical regions, 10 to 26 root tips contributed to flow in wheat root systems. At 14 DAS, each of the three longest seminal roots had more than 10 lateral roots greater than 10 mm long (Fig. 1). Therefore, there may be variability between root tips in their contribution to flow. This variability may depend on maturity and heterogeneity between local root environments.
For both lupin species, Lr of unbranched taproot segments and whole root systems increased proportionally with root length. This implies that water was absorbed more evenly along the entire root length, including the lateral roots. Unlike wheat roots, the endodermis of lupins did not become as heavily suberized and there was no secondary thickening. The presence of suberin lamellae in the endodermis did not appear to restrict water flow, as there was no corresponding change in Lr with the development of this structure. However, a large region of the endodermis, adjacent to xylem poles, did not become suberized. Lateral roots may also prevent the endodermis from forming a tight barrier in more mature roots as they emerge from this unsuberized region. Eudicots undergo secondary growth, so more mature lupin roots may develop a periderm. If a periderm does develop, then the impermeability of this tissue to water may alter the relationship between Lr and root length. Hamblin and Tennant (1987) did not describe a periderm in field-grown lupin roots but observed abundant root hairs on all parts of roots, suggesting that water uptake also potentially occurs along the length of more mature root systems.
Lax Is Greater in Lupin Roots because of Greater Xylem Development
Hamblin and Tennant (1987) speculated that lupin roots have higher specific rates of water uptake than wheat roots due to increased xylem development. In this study, Lax of the roots of both lupin species was greater than that in wheat, which was related to the area of lignified xylem. Larger vessels have greater potential for water flow, since according to the Hagen-Poiseuille equation, Lr varies to the fourth power of the vessel radius. In lupin roots, the number and diameter of xylem vessels increased nonlinearly with maturity. The logistic growth curves imply that the amount of lignified xylem will increase in the taproot until around 450 mm from the tip. However, this extrapolation does not take into account secondary xylem development in mature roots. The increase in xylem may be beneficial in the region of lateral root development (Steudle and Peterson, 1998). The strong relationship between Lax and Lr indicates that increasing xylem development in lupin roots may also compensate for more water arriving at the xylem as water is absorbed along the length of the taproot.
Because monocots do not undergo secondary growth, the development of xylem vessels in wheat roots is determined at an early stage in root growth. Each seminal root contains a large central vessel surrounded by several smaller vessels. Fluorescence imagery indicated that the small vessels become lignified at least 100 mm closer to the root tip than the central vessel. However, the central vessel does not appear to be functional up to 200 mm from the root tip, as there was no abrupt change in Lax that corresponded with fluorescence of the vessel. According to the Hagen-Poiseuille equation, the Lax of a 10-mm-long wheat root segment (average of seven vessels of 21 μm diameter) is 3.7 × 10−9 m3 s−1 MPa−1, if we assume that the central vessel is nonfunctional. This is almost 5-fold greater than the average measured Lax of a 10-mm root segment. Although the measured Lax in this study may be artificially high, flow through xylem vessels is not as ideal as the assumptions used in calculating Lax with the Hagen-Poiseuille equation; therefore, measured Lax values are typically less than calculated (Greacen et al., 1976; Steudle and Peterson, 1998). The central vessel may become functional at greater distances from the root tip, where lateral roots develop. However, the presence of lateral roots prevented the sealing of root segments to the root pressure probe to test this hypothesis.
Yellow Lupin May Be More Conservative in Its Water Use Than Narrow-Leafed Lupin
Yellow lupin roots tended to have a greater cross-sectional area of xylem vessels but had similar Lax to narrow-leafed lupin. This disparity between the two species was manifested in the relationship between Lax and Lr. The regressions in Figure 4 imply that yellow lupin has a greater capacity to carry water axially relative to the radial path. For narrow-leafed lupin, the radial conductance increases at a greater rate per Lax, so greater water potential gradients may exist in the xylem. Corresponding to this, yellow lupin tends to maintain a higher leaf water potential than narrow-leafed lupin, but it also tends to have lower rates of stomatal conductance and transpiration (Davies et al., 2000a; Palta et al., 2004). In this study, the transpiration of yellow lupin plants was also less than that of narrow-leafed lupin plants. Minimizing the water potential gradient driving water flow will reduce the risk of cavitation, particularly because wide vessels are more vulnerable to cavitation (Tyree et al., 1994).
The Flow of Water Radially Determines Lr
Lax was 2 orders of magnitude greater than Lr, indicating that the rate of water flow through the radial pathway was limiting to Lr for all three species. Similar differences in magnitude between axial and radial conductance have been reported for a wide variety of species (Steudle and Peterson, 1998). The radial pathway of all three species has the same fundamental structure, with the main differences being the length of the pathway (distance across the cortex) and the development of the endodermis. None of the species developed a subepidermal layer that was different in structure from the other cortical cells. An exodermis may develop in older root systems under field conditions or in adverse environments. However, an exodermis was not induced in lupin by growing the roots in aeroponic culture, as has been demonstrated for maize (Zea mays; Zimmermann et al., 2000; Hartung et al., 2002). In addition, Hamblin and Tennant (1987) did not identify an exodermis in mature root systems of wheat or narrow-leafed lupin grown under the same field conditions, but the cortex of wheat roots deteriorated.
The conductance of the radial pathway is inversely proportional to the length of the flow path or the number of cell layers, since the cells are arranged in series (Steudle and Frensch, 1996). The length of the radial flow path was constant with distance from the root tip for all species, even though the development of the stele caused lupin roots to increase in diameter. The length of the flow path, from the root surface to the xylem, was greatest in narrow-leafed lupin, followed by yellow lupin and then wheat. However, Lr was lowest in wheat roots; therefore, the width of the cortex was not the only factor determining Lr.
There Was Strong Evidence for Predominantly Cell-to-Cell Water Flow
Water flow from the root surface to the vascular tissue can occur through two parallel pathways: the apoplast and the cell-to-cell pathway. Water passing through cells may cross into adjacent cells through connecting plasmodesmata and/or across membranes. The symplastic connection through plasmodesmata has been demonstrated with tracer dyes and predominantly occurs within a few millimeters of the root tip (Hukin et al., 2002). Plasmodesmata have also been observed in regions farther from the root tip (Ma and Peterson, 2001), but their involvement in symplastic transport is less documented. Measurements of Lpc with the cell pressure probe are a composite value of the plasma membrane, tonoplast, and plasmodesmata. To determine the contribution of the cell-to-cell and apoplastic pathways for each species, radial profiles of Lpc were created. The profiles of Lpc for all of the species were similar in magnitude and behavior. Lpc tended to increase from the root surface toward the endodermis. As water travels across the root, the surface area for flow decreases. Lpc increased proportionally to the decrease in area, implying a compensatory effect. The trend in Lpc also suggested that water flow might occur predominantly through the cell-to-cell pathway. This possibility was tested by the concentric membrane model.
A model describing the root as a series of concentric membranes has been used previously to predict Lpr of barley (Hordeum distichon), wheat, and maize roots (Jones et al., 1983, 1988; Steudle and Jeschke, 1983). However, the length of the absorbing region was not taken into consideration in these previous models, which has an important influence on Lr. Here, we expanded the model for a root composed of membrane cylinders to calculate Lr and included the radial profile of Lpc (instead of an average value of Lpc). The model assumes that water flow occurs predominantly through the cell-to-cell pathway. If this assumption is correct, then the measured and predicted values of Lr should be similar. Using membrane cylinders, the influence of the length of the absorbing region, anatomy, and, hence, specific membranes could be investigated.
For both lupin species, the predicted Lr values were similar to the measured Lr if water crossed all cell layers through the cell-to-cell pathway. Lr was also predicted to increase with root length. For wheat, the predicted and measured values were also in close agreement, but only when the length of the absorbing region was confined to the distal region, where the endodermis was less developed and only two cylindrical membranes were included in the flow path. Although suberization of the endodermis did not appear to influence Lr of lupin roots, state III development of the endodermis (suberin lamellae and tertiary thickening) has been correlated with a decline in water uptake of cereal roots (Sanderson, 1983). It is not apparent from the model alone which cell layer controls radial water flow, but the most likely candidate is the endodermis, because Lr was influenced by its anatomy and in older wheat roots the cortex shrivels and deteriorates (Greacen et al., 1976; Hamblin and Tennant, 1987). We also considered the epidermis, because the cortex of wheat roots apparently shrinks when the water potential of the root decreases (Greacen et al., 1976). This implies that it is not buffered against large changes in water potential in the xylem and, therefore, the greatest resistance to radial water flow could occur in the epidermis. Although the epidermis had smaller Lpc, Lpc was proportional to the surface area for transport; therefore, the conductivity of the epidermis was no less than that of the endodermis.
Water Flow Occurs Predominantly through the Apoplast in Lupin Roots But Is Controlled by AQPs in the Endodermis in Wheat Roots
Because the model provided strong evidence for a large component of cell-to-cell flow, we further tested the predictions by inhibiting flow through the cell-to-cell pathway. Despite the shortcomings with Hg2+ being relatively nonspecific in its mechanism of inhibition of water transport (Zhang and Tyerman, 1999), the inhibition is evidence for the activity and control of water flow by AQPs in root cell membranes. If water transport occurred across specific cylindrical membranes and/or through all cell layers, then the effect of mercury at the root level should have been proportional to the effect at the cell level. However, Lpc was reduced more than Lr.
Mercury failed to affect Lr of lupin root segments or root systems, despite dramatic reductions of Lpc in all cell layers. Therefore, water primarily flowed around cells in lupin roots, without crossing membranes. This also implies that water did not cross membranes in the endodermis, despite the Casparian strip and the later partial development of suberin lamellae. The Casparian strip in the endodermis is also not a significant barrier to apoplastic water flow in maize roots (Steudle et al., 1993). It is possible that mercury failed to permeate lupin roots sufficiently to reach the endodermis. AQPs in the endodermis may also be insensitive to mercury, which has been reported for some plasma membrane intrinsic proteins (PIPs) and tonoplast intrinsic proteins (TIPs; Daniels et al., 1994; Biela et al., 1999).
For wheat roots, the model and the inhibition of Lr and Lpc indicated that Lr is regulated by AQPs in a small region of the endodermis. At the endodermis, it is unlikely that mercury could permeate fully because of the Casparian strip. If only the exterior “membrane” of the endodermis was inhibited by mercury, a similar inhibition in Lr was predicted by the model to what was measured. Some studies have removed cell layers to measure Lpr in conjunction with mercury treatment, because of the presence of Casparian bands and suberin lamellae (Martre et al., 2001; North et al., 2004). However, the delicate nature of wheat roots prevents this type of manipulation. AQPs were previously suspected to be involved in root hydraulics of wheat because of the high water permeability of cells. Lpc of wheat equates to osmotic water permeability of 35 to 491 μm s−1, which is within the range of AQP-facilitated water transport reported for membrane vesicles of wheat and membrane vesicles and protoplasts of a range of other species (Niemietz and Tyerman, 1997; Ramahaleo et al., 1999; Suga et al., 2003; Alleva et al., 2006). In addition, Lpr varies diurnally and changes rapidly in response to abiotic perturbation (Carvajal et al., 1996; Clarkson et al., 2000). The first major characterization of AQP genes in wheat has identified 24 PIPs and 11 TIPs (Forrest and Bhave, 2008), although their expression patterns and regulation of their transcripts and proteins are not yet known. However, most PIPs of wheat contain up to four conserved Cys residues, which could be involved in the mercury inhibition (Forrest and Bhave, 2008).
What Are AQPs for in Lupin Roots?
Although AQPs in narrow-leafed lupin or yellow lupin have not yet been characterized, the high values of Lpc and the effect of mercury demonstrated high AQP activity in both lupin species. In addition, the increase in Lpc toward the root axis suggests that AQP activity increases to compensate for a concentrating effect of the root geometry. For the measurements in this study, water flow was induced by hydrostatic pressure, and under these conditions, bulk water flow may bypass cells. Hence, water flow generated by transpiration also probably travels predominantly through the apoplast. However, water flow induced by osmotic gradients must cross membranes, so this may be where AQP activity influences water flow across lupin roots. Lpr of lupin roots measured using hydrostatic pressure is at least twice the Lpr measured using an osmotic gradient, in comparison with wheat, where Lpr was the same under both types of gradient (Bramley et al., 2007a). This suggests that water flow may follow different radial pathways in lupin roots when influenced by different driving forces. According to the composite transport model, water flow should primarily cross the cell-to-cell pathway when induced osmotically (Steudle and Peterson, 1998). Therefore, mercury should have a greater inhibitory effect on osmotically induced Lpr of lupin, but this remains to be tested. Lupin roots are particularly sensitive to mercury treatment, which has adverse effects when combined with an osmotic experiment causing Pr to decline without subsequent recovery (data not shown).
One of the hypotheses of this study was that the hydraulics of narrow-leafed lupin and yellow lupin differed because of AQP activity. Here, AQPs were not found responsible for Lr under these water-sufficient conditions. However, AQPs may be required for osmoregulatory processes (Tyerman et al., 1999) and during drought as lupin roots undergo osmotic adjustment to maintain turgor pressure (Jensen et al., 1989). During water deficit, roots often become more suberized (Vandeleur et al., 2009), which could change the radial pathway for water flow and lead to the involvement of AQPs.
Contrasting Root Hydraulics
The apoplast should have a higher conductivity to water flow than the cell-to-cell pathway because of the absence of membranes. Therefore, by having a greater proportion of water flow via the apoplast, lupins are able to achieve the same or superior radial conductance as the thinner wheat roots. However, the main consequence of predominantly apoplastic water flow is that Lr is invariable in the short term. Lupins may rely on anatomical and growth-related changes to regulate water flow at the root level and/or manipulate the driving force through closure of stomata. Stomatal conductance of lupins decreases under drought, as more of the root system is exposed to the drying soil and root water uptake decreases (Jensen et al., 1989). In addition, without increasing the gradient to drive water flow, the apical part of lupin roots may become hydraulically isolated when surface soils become dry, unless the radial permeability in the older parts of the root is reduced (e.g. through suberization).
In comparison with lupins, wheat root systems are not dependent on growth-related changes in anatomy to vary Lpr. Wheat can rapidly adjust Lpr because flow through a seminal root is predominantly controlled by AQPs. Under the growing conditions of this study, the control occurs in the endodermis in the apical region. It is not known which specific AQPs are expressed in the endodermis, but the expression of different AQPs can be localized to specific tissues (for review, see Bramley et al., 2007b) and even oriented within the tissue (Hachez et al., 2006). Because the wheat root system consists of several individual roots, it is possible that Lpr of each of these roots can be adjusted independently. For example, after excising four of the seminal roots in durum wheat (Triticum durum), water flow in the remaining root increased, maintaining the water supply to the shoot (Vysotskaya et al., 2004). A feature like this may provide an advantage in heterogeneous or fluctuating environments.
CONCLUSION
The hydraulic properties of wheat and lupin roots are highly contrasting, which suggests a balance between root diameter, Lr, and the ability to rapidly adjust to prevailing conditions. By absorbing water more evenly along the root length, which is related to increasing xylem development, lupin roots have a high capacity to transport water, although yellow lupin may be more conservative than narrow-leafed lupin in its water use strategy. In addition, predominantly apoplastic flow endows lupin roots with the same or superior radial conductance as thinner wheat roots, but it provides little ability to rapidly adjust Lr in the short term. However, osmotically induced water flow must cross membranes, which could be facilitated by AQPs. In comparison, preferentially absorbing water in the distal region and low Lax diminish the potential for high water flow in wheat. However, because a significant component of water flow crosses at least two membranes, which is controlled by AQPs, wheat has the ability to rapidly adjust Lr.
The strong evidence of AQP activity in all cell layers of the cortex of all species implies that their role is more extensive than solely transporting water from the surface of the root to the vascular tissue. The similarity in root cortex profile of Lpc yet contrasting hydraulics of whole roots demonstrate the importance of examination of root structure and anatomy in assessing the role of AQPs in whole root transport.
A cautionary note should be included about the use of Lpr. Because of problems of scale, measurements of Lpr are generally normalized by root dimensions, such as length, surface area, or mass. Water uptake is limited by the absorbing area; therefore, normalization by the entire root size may be inappropriate for some species. The form of the normalization is particularly important when comparing the effects of treatments that alter the dimensions or structure of the root system, especially if the absorbing area is unaltered. In these cases, it may be more meaningful to normalize hydraulic conductivity by another parameter such as leaf area.
MATERIALS AND METHODS
Plant Material
The details of growing wheat and lupin seedlings were described previously by Bramley et al. (2007a). For all experiments, seedlings were used at 14 DAS. Seedlings were used because the growth of root systems was not constrained by pot size and roots could be removed without damage. Briefly, seeds of narrow-leafed lupin (Lupinus angustifolius ‘Merrit’), yellow lupin (Lupinus luteus ‘Wodjil’), and wheat (Triticum aestivum ‘Kulin’) were germinated and planted into individual pots (75 mm diameter, 350 mm depth) containing medium to fine river sand. Lupin seeds were inoculated with Bradyrhizobium (Nitrogerm 100; Adelaide Seed Company) at the time of planting. Plants were watered daily with nutrient solution, pH 6.0 (Davies et al., 2000a).
For measurements on whole root systems, seeds were planted individually into specially designed pots that fitted into a pressure chamber, as described by Gallardo et al. (1996). To force the cotyledons to grow above the top of the pot surface and enable the lupin stem and wheat mesocotyl to protrude through the lid of the pressure chamber, the seeds were planted beneath a small collar of sand constructed in the lid of the pot. The collar was removed when the seedling had emerged. Plants were grown in a growth cabinet with a 12-h-light/12-h-dark photoperiod (photosynthetically active radiation of 450–500 μmol m−2 s−1), temperature of 21°C/17°C ± 1°C, and 68% relative humidity.
Whole Plant Transpiration
The volume of water transpired per plant was measured gravimetrically. Plants were grown as described above. At the start of the experiment, the pots were watered early in the morning and allowed to freely drain for 1 h. The pots were then covered to prevent further drainage or soil evaporation. Pots were weighed hourly between 10:00 am and 2:00 pm, with the average volume transpired recorded from five individual plants of each species.
Water Flow through Whole Root Systems
The Lr of whole root systems was measured by the pressure chamber technique. Before a measurement, the pot was watered with nutrient solution and drained on absorbent paper for 0.5 h. Shoots were excised below the cotyledon of lupin and just above the crown of wheat. The pot was sealed (Lab Putty; Halas Dental Supplies) in the pressure chamber with the cut lupin stem or wheat mesocotyl protruding through the lid of the chamber.
Pressurizing the chamber induced water flow through the root system, and the water flux was determined from the rate of exuding sap. Preliminary measurements indicated that the flux-pressure relationship was linear between 0.2 and 0.7 MPa. In addition, upon initial pressurization at 0.7 MPa, the exudation rate of sap gradually increased until becoming steady after 2 to 3 h. The water flux at each subsequent pressure was stable within 0.3 h. Thereafter, all root systems were initially pressurized at 0.7 MPa for 3 h, before collecting exudates, to ensure steady-state conditions (Markhart and Smit, 1990). Experiments commenced at the same time each day to eliminate possible confounding diurnal effects (Passioura and Munns, 1984).
After the initial 3-h equilibration period, the pressurization protocol followed that of Gallardo et al. (1996), collecting exudate at 0.7, 0.5, and 0.3 MPa. Lr (in m3 s−1 MPa−1) of the root system was calculated from the linear regression of water flux against applied pressure. Lr was converted into Lpr (in m s−1 MPa−1) by dividing by the unit surface area of the root system.
The total lengths and surface areas of the root systems were measured after washing roots from pots and staining them for 1 h in 0.5% methylene blue. Roots were scanned using a flatbed scanner (600 pixels mm−1; ScanJet; Hewlett-Packard Australia) covered with a thin film of water. Images were analyzed using Rootedge version 2.3b software (National Soil Tilth Laboratory, U.S. Department of Agriculture). Roots were assumed cylindrical for converting image areas to surface areas.
Radial and Axial Lr of Individual Roots
Roots were carefully washed from the sand, and the longest seminal root of wheat or the taproot of lupin was excised at various distances from the root tip, below emerging lateral roots. The root was connected to a root pressure probe (Steudle, 1993) and supported inside a glass tube (5 mm diameter × 140 mm length) in which aerated nutrient solution was continuously circulated. When Pr was constant (2–12 h), the rate of water flow through the root was measured with the pressure-clamp technique (Bramley et al., 2007a). Lr was calculated from the linear regression of flow rate against applied pressure and normalized to Lpr by dividing by the surface area of the root segment.
After measurements of Lr, the root was cut from the probe near the seal. Lax was measured on the segment of root remaining within the seal (10 mm), where radial flow was negligible. The rate of decline in Pr when the root was cut from the probe and applied hydrostatic pressure pulses were used to determine Lax according to Frensch and Steudle (1989) and Melchior and Steudle (1993). It should be noted that the measurement of Lax on this small segment of root could potentially overestimate the real Lax of the root, because xylem vessels will be less developed near the tip than where Lax was measured and vessel lengths may exceed 10 mm.
Radial Profile of the Lpc
The cell pressure probe (Steudle, 1993) was used to measure the water transport parameters of cells in each of the radial cell layers of the root (epidermis and cortical). Microcapillaries for the cell pressure probe were made from borosilicate glass with 1 mm o.d. × 0.58 mm i.d. (GC 100-15 Harvard Apparatus; SDR Clinical Technology). Tips were pulled to external diameters of 5 to 10 μm using one pull on a capillary puller (Narishige PP-83). The microcapillary was filled with silicon oil and connected to the probe using nitrile rubber seals. The cell pressure probe was mounted on a micromanipulator (Narishige MX1) with 1-μm increments so that the depth of the microcapillary tip, within the root, could be measured.
Roots were excised 80 mm from the root tip and secured inside a small Perspex chamber (Zhang and Tyerman, 1991). The tip of the microcapillary was introduced into the root perpendicular to the root axis using the micromanipulator. When the microcapillary punctured a cell, turgor pressure forced cell sap into the tip of the microcapillary and a meniscus formed between the sap and oil. The meniscus in the capillary was observed at 144× magnification with a stereo microscope (SZX12; Olympus Australia) with illumination from a fiber-optic light source (LG-PS2; Olympus Australia). The root was punctured in the root hair zone, 30 to 50 mm behind the root tip. The location of the cell in the cortex was estimated from the depth of the microcapillary tip from the root surface and the pulses of the meniscus within the microcapillary when the tip impaled the cells. Since the angle of the microcapillary was determined visually to be perpendicular to the root axis, the error of selecting a specific cell layer increased toward the center of the root. The tip of the microcapillary was assumed located in the correct cell layer by allowing for a 5° error and a radius of 25 μm, which gave a distance into the root from the outside surface of 285 μm. Therefore, cells were measured in each cell layer from the epidermis to the endodermis of wheat roots but only to the seventh or eighth cell layer of lupin roots because of their larger diameter.
The methodology for measuring Lpc was described previously by Zhang and Tyerman (1991). Changes in pressure were limited to approximately 0.05 MPa to prevent the inhibition of water permeability caused by large pressure pulses (Wan et al., 2004).
Estimation of the Contribution of the Cell-to-Cell Flow Path to Radial Flow
The contribution to radial flow of the cell-to-cell flow path was estimated by modifying the model of Kedem and Katchalsky (1963) that considers the permeability of concentric membranes in series. In this study, the root is described as a cylinder composed of concentric, cylindrical “membranes.” Water crosses each cylindrical membrane in series before reaching the vascular tissue (Fig. 9). This simplified model assumes that radial water flow only occurs via the cell-to-cell pathway, and if this assumption is correct, then Lr and Lpr calculated from the model should be similar to the measured values.
Figure 9.
Diagram of the concentric membrane model used to calculate Lr and Lpr from radial profiles of Lpc. The diagram shows a cylindrical root segment consisting of five cell layers, from the epidermis (Ep) to the endodermis (En). Each cell layer consists of two membranes, inner and outer, the radii of which were determined from anatomical studies and measurements of cell diameters. Water crosses each cell layer in series to reach the vascular tissue in the stele. The model assumes that radial water flow occurs by the cell-to-cell flow path, except in the stele. The cell layers where cellular flow occurs and the length of the root segment can be varied to examine the effect on Lr.
Using the radial profile of Lpc for the hydraulic conductivity of the concentric membranes, Lr was calculated from
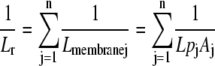 |
(2) |
where Lmembranej is the conductance, Lpj is the hydraulic conductivity, and Aj is the surface area of the jth membrane. To calculate A of each concentric membrane, the inner and outer radii of each cell layer were determined from root and cell diameters (Table III), measured 30 to 50 mm from the root tip, and the length of the concentric cylinders was varied according to the length of the root segment or absorbing region. Each individual membrane could be included/excluded in the model to determine how specific membranes influence Lr.
To normalize Lr by the surface area of the root, Lpr was calculated from
 |
(3) |
where rr and rj are the radius of the root and the jth membrane, respectively.
The following conditions were included in the model. (1) Lpc is a composite measurement for the tonoplast, plasma membrane, and plasmodesmata; therefore, the same value of Lpc was used for the two membranes, inner and outer, that enclosed each cell layer. (2) Water flow through the stele was considered apoplastic, because the cells in this tissue are very small and the apoplast constitutes a larger cross-sectional area than in the cortex (Steudle and Jeschke, 1983). (3) Where Lpc could not be measured (e.g. epidermis and endodermis of lupins), the average Lpc of the nearest measured cell layer was used. (4) The first 5 mm of the root apex was assumed not to be involved in the uptake of water, because this region typically has very low axial and radial conductances to water flow (Steudle, 2001) and the development of lignified (assumed to be functional) xylem occurred beyond this region.
Mercury Treatment
Lpr was measured on whole root systems before and after mercury treatment using the pressure chamber technique (Gallardo et al., 1996). After initial measurements (untreated), the pot was removed from the pressure chamber and the root system was rewatered with nutrient solution containing 50 μm HgCl2. So that the seal in the lid of the plant pot was not disturbed, 100 mL of mercury solution was injected into a small hole (5 mm diameter) located in the side of the pot, just below the top of the pot. A total of 100 mL was sufficient volume to wet the soil and drain from the bottom of the pot. The pot was drained for 0.5 h on absorbent paper before repressurizing the root system. The pressurization protocol described above was then repeated, collecting exudate from the cut stem when flow rate was constant. Repressurizing root systems after watering the root system without mercury resulted in linear regressions identical to initial regressions (data not shown). Therefore, any changes in Lpr were due to mercury treatment only.
Lpr of root segments was measured with the root pressure probe before and after mercury treatment. After initial measurements, the nutrient solution bathing the root was changed to include 50 μm HgCl2, and after 0.5 h, the measurements for Lpr were repeated.
Preliminary measurements with the cell pressure probe indicated that mercury had an effect on the half-time of the rate of water exchange across root cell membranes within 600 to 1,200 s of application, but treatments for more than 0.5 h could cause turgor pressure to decrease. Therefore, measurements to determine Lpc were conducted on roots pretreated for 600 s, with or without 50 μm HgCl2.
Anatomy
Freehand cross sections of roots were taken at 5, 10, and 20 mm from the tip, then every 20 mm up to 120 mm from the tip, and every 40 mm thereafter. Due to their small diameters, wheat roots were sectioned using the Parafilm method of freehand sectioning (Frohlich, 1984). Fluorescence under UV illumination, after staining root cross sections with the berberine/aniline blue procedure, indicated the presence of Casparian bands (Brundrett et al., 1988). Suberin lamellae were identified as red/brown cell walls after staining cross sections with Sudan Red 7B (Brundrett et al., 1991). Sections were observed with a Zeiss Axiophot microscope (Carl Zeiss) with video capture (Nikon DXM1200F digital camera and Nikon ACT-1 version 2.62 software; Coherent Life Sciences) using bright-field, phase-contrast, or epifluorescence optics. Ultraviolet illumination with filter number 1 (excitation BP365, chromatic beamsplitter FT396, emission LP397) was used for fluorescence microscopy.
Since the berberine-aniline blue procedure also stains lignified cell walls (Brundrett et al., 1988), the sections stained with berberine-aniline blue were also used to measure the cross-sectional area of functional xylem along the length of the root. Mature vessels were identified as those with lignified walls and hence fluorescing bright yellow-white under UV illumination (Brundrett et al., 1988). The cross-sectional area of fluorescing vessels was measured using Global Lab Image/2 version 3.0 (Data Translation, Total Turnkey Solutions). The same software was used to measure the dimensions of root cells (Table III) to calculate Lpc. In some instances, particularly for wheat roots, the dimensions of the cells actually punctured with the microcapillary could be delineated by using the probe to fill the cell with oil (Zhang and Tyerman, 1991).
Statistical Analyses
Statistical analyses were performed using SPSS version 11.0 and GraphPad Prism version 3.02. Data were log or inverse transformed, where necessary, to satisfy the requirements of normal distribution and homogeneity of variance for statistical analysis. For linear regression, plots of flow rate against applied pressure that were not significantly linear (P < 0.05) were eliminated from the analysis. Differences between species for regression coefficients and intercepts were tested by ANOVA with Tukey's posthoc test. t tests were used to examine whether the intercepts of the regression for whole root systems were significantly different from zero. Linear and nonlinear regression analyses were performed to examine the relationship between root length and root water transport, selecting the best fits that minimized the absolute sum of squares. To determine whether the axial and radial components of root water transport were related, correlation analysis was performed. To compare the water relations of cells from different cell layers, ANOVA with Tukey's posthoc test was used. Lpr of whole root systems or root segments, before and after mercury treatment, was compared in a paired t test. The effects of mercury on Lpc were tested with independent t tests.
Acknowledgments
Technical support was provided by Christiane Ludwig at CSIRO Plant Industry, Perth, and Wendy Sullivan at the University of Adelaide.
This work was supported by the Grains Research and Development Corporation of Australia and the Australian Research Council.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Helen Bramley (helen.bramley@afhe.ualberta.ca).
Open Access articles can be viewed online without a subscription.
References
- Aharon R, Shahak Y, Winiger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva K, Niemietz CM, Sutka M, Maurel C, Parisi M, Tyerman SD, Amodeo G (2006) Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J Exp Bot 57 609–621 [DOI] [PubMed] [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18 565–570 [DOI] [PubMed] [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD (2007. a) Comparison between gradient-dependent hydraulic conductivities of roots using the root pressure probe: the role of pressure propagations and implications for the relative roles of parallel radial pathways. Plant Cell Environ 30 861–874 [DOI] [PubMed] [Google Scholar]
- Bramley H, Turner DW, Tyerman SD, Turner NC (2007. b) Water flow in the roots of crop species: the influence of root structure, aquaporin activity, and waterlogging. Adv Agron 96 133–196 [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA (1988) A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146 133–142 [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA (1991) Efficient lipid staining in plant material with Sudan Red 7B or Fluoral Yellow 088 in polyethylene glycol-glycerol. Biotech Histochem 66 111–116 [DOI] [PubMed] [Google Scholar]
- Carvajal M, Cooke DT, Clarkson DT (1996) Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta 199 372–381 [Google Scholar]
- Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E (2000) Root hydraulic conductance: diurnal aquaporin expression and the effects of nutrient stress. J Exp Bot 51 61–70 [PubMed] [Google Scholar]
- Clements JC, White PF, Buirchell BJ (1993) The root morphology of Lupinus angustifolius in relation to other Lupinus species. Aust J Agric Res 44 1367–1375 [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106 1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CL, Turner DW, Dracup M (2000. a) Yellow lupin (Lupinus luteus) tolerates waterlogging better than narrow-leafed lupin (L. angustifolius). I. Shoot and root growth in a controlled environment. Aust J Agric Res 51 701–709 [Google Scholar]
- Davies CL, Turner DW, Dracup M (2000. b) Yellow lupin (Lupinus luteus) tolerates waterlogging better than narrow-leafed lupin (L. angustifolius). IV. Root genotype is more important than shoot genotype. Aust J Agric Res 51 729–736 [Google Scholar]
- Forrest K, Bhave M (2008) The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Funct Integr Genomics 8 115–133 [DOI] [PubMed] [Google Scholar]
- Frensch J, Steudle E (1989) Axial and radial hydraulic resistance to roots of maize (Zea mays L.). Plant Physiol 91 719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich MW (1984) Freehand sectioning with Parafilm. Stain Technol 59 61–62 [DOI] [PubMed] [Google Scholar]
- Gallardo M, Eastham J, Gregory PJ, Turner NC (1996) A comparison of plant hydraulic conductances in wheat and lupins. J Exp Bot 47 233–239 [Google Scholar]
- Greacen EL, Ponsana P, Barley KP (1976) Resistance to water flow in the roots of cereals. In OL Lange, L Kappen, E-D Schulze, eds, Water and Plant Life, Vol 19. Springer-Verlag, Berlin, pp 86–100
- Gregory PJ, Eastham J (1996) Growth of shoots and roots, and interception of radiation by wheat and lupin crops on a shallow, duplex soil in response to time of sowing. Aust J Agric Res 47 427–447 [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F (2006) Localization and quantification of plasma membrane aquaporin expression in maize primary root. Plant Mol Biol 62 305–323 [DOI] [PubMed] [Google Scholar]
- Hamblin A, Tennant D (1987) Root length density and water uptake in cereals and grain legumes: how well are they correlated? Aust J Agric Res 38 513–527 [Google Scholar]
- Hartung W, Leport L, Ratcliffe RG, Sauter A, Duda R, Turner NC (2002) Abscisic acid concentration, root pH and anatomy do not explain growth differences in chickpea (Cicer arietinum) and lupin (Lupinus angustifolius L.) on acid and alkaline soils. Plant Soil 240 191–199 [Google Scholar]
- Hukin D, Doering-Saad C, Thomas CR, Pritchard J (2002) Sensitivity of cell hydraulic conductivity to mercury is coincident with symplastic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta 215 1047–1056 [DOI] [PubMed] [Google Scholar]
- Jang J, Lee S, Rhee J, Chung G, Ahn S, Kang H (2007) Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Mol Biol 64 621–632 [DOI] [PubMed] [Google Scholar]
- Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot (Lond) 90 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CR, Henson IE, Turner NC (1989) Leaf gas exchange and water relations of lupins and wheat. II. Root and shoot water relations of lupin during drought-induced stomatal closure. Aust J Plant Physiol 16 415–428 [Google Scholar]
- Jones H, Leigh RA, Wyn Jones RG, Tomos AD (1988) The integration of whole-root and cellular hydraulic conductivities in cereal roots. Planta 174 1–7 [DOI] [PubMed] [Google Scholar]
- Jones H, Tomos AD, Leigh RA, Wyn Jones RG (1983) Water-relation parameters of epidermal and cortical cells in the primary root of Triticum aestivum L. Planta 158 230–236 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U (1998) Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. Plant J 14 121–128 [DOI] [PubMed] [Google Scholar]
- Kedem O, Katchalsky A (1963) Permeability of composite membranes. Part 3. Series array of elements. Trans Faraday Soc 59 1941–1953 [Google Scholar]
- Ma F, Peterson CA (2001) Frequencies of plasmodesmata in Allium cepa L. roots: implications for solute transport pathways. J Exp Bot 52 1051–1061 [DOI] [PubMed] [Google Scholar]
- Markhart AH III, Smit B (1990) Measurement of root hydraulic conductance. HortScience 25 282–287 [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130 2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P, North GB, Nobel PS (2001) Hydraulic conductance and mercury-sensitive water transport in roots of Opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiol 126 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WK, Meyer WS, Smith RCG, Barrs HD (1983) Water balance of three irrigated crops and fine textured soils of the riverine plain southeastern Australia. Aust J Agric Res 34 183–192 [Google Scholar]
- Melchior W, Steudle E (1993) Water transport in onion (Allium cepa L.) roots. Plant Physiol 101 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD (1997) Characterisation of water channels in wheat root membrane vesicles. Plant Physiol 115 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North GB, Martre P, Nobel PS (2004) Aquaporins account for variations in hydraulic conductance for metabolically active root regions of Agave deserti in wet, dry, and rewetted soil. Plant Cell Environ 27 219–228 [Google Scholar]
- O'Brien L (1979) Genetic variability of root growth in wheat (Triticum aestivum L.). Aust J Agric Res 30 587–595 [Google Scholar]
- Palta JA, Turner NC, French RJ (2004) The yield performance of lupin genotypes under terminal drought in a Mediterranean-type environment. Aust J Agric Res 55 449–459 [Google Scholar]
- Passioura JB (1980) The transport of water from soil to shoot in wheat seedlings. J Exp Bot 31 333–345 [Google Scholar]
- Passioura JB, Munns R (1984) Hydraulic resistance of plants. II. Effects of rooting medium, and time of day, in barley and lupin. Aust J Plant Physiol 11 341–350 [Google Scholar]
- Ramahaleo T, Morillon R, Alexandre J, Lassalles JP (1999) Osmotic water permeability of isolated protoplasts: modifications during development. Plant Physiol 119 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson J (1983) Water uptake by different regions of the barley root: pathways of radial flow in relation to development of the endodermis. J Exp Bot 34 240–253 [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. In JAC Smith, H Griffiths, eds, Water Deficits. Plant Responses from Cell to Community. Bios Science, Oxford, pp 5–36
- Steudle E (2001) The cohesion-tension mechanism and the acquisition of water by plant roots. Annu Rev Plant Physiol Plant Mol Biol 52 847–863 [DOI] [PubMed] [Google Scholar]
- Steudle E, Frensch J (1996) Water transport in plants: role of the apoplast. Plant Soil 187 67–79 [Google Scholar]
- Steudle E, Jeschke WD (1983) Water transport in barley roots. Planta 158 237–248 [DOI] [PubMed] [Google Scholar]
- Steudle E, Murrmann M, Peterson CA (1993) Transport of water and solutes across maize roots modified by puncturing the endodermis. Plant Physiol 103 335–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E, Peterson CA (1998) How does water get through roots? J Exp Bot 49 775–788 [Google Scholar]
- Suga S, Murai M, Kuwagata T, Maeshima M (2003) Differences in aquaporin levels among cell types of radish and measurement of osmotic water permeability of individual protoplasts. Plant Cell Physiol 44 277–286 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JAC (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 50 1055–1071 [Google Scholar]
- Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficacy for vulnerability to dysfunction? IAWA J 15 335–360 [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vysotskaya LB, Arkhipova TN, Timergalina LN, Dedov AV, Veselov SY, Kudoyarova GR (2004) Effect of partial root excision on transpiration, root hydraulic conductance and leaf growth in wheat seedlings. Plant Physiol Biochem 42 251–255 [DOI] [PubMed] [Google Scholar]
- Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. J Exp Bot 55 411–422 [DOI] [PubMed] [Google Scholar]
- Yu Q, Hu Y, Li J, Wu Q, Lin Z (2005) Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. Plant Sci 169 647–656 [Google Scholar]
- Zhang WH, Tyerman SD (1991) Effect of low O2 concentration and azide on hydraulic conductivity and osmotic volume of the cortical cells of wheat roots. Aust J Plant Physiol 18 603–613 [Google Scholar]
- Zhang WH, Tyerman SD (1999) Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiol 120 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann HM, Hartmann K, Schreiber L, Steudle E (2000) Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.). Planta 210 302–311 [DOI] [PubMed] [Google Scholar]











