Abstract
The increases in atmospheric carbon dioxide (CO2) concentrations can enhance plant growth and change their nutrient demands. We report that when tomato (Lycopersicon esculentum ‘Zheza 809’) plants were grown in iron (Fe)-limited medium (with hydrous ferric iron oxide) and elevated CO2 (800 μL L−1), their biomass and root-to-shoot ratio were greater than plants grown in ambient CO2 (350 μL L−1). Furthermore, the associated increase in Fe concentrations in the shoots and roots alleviated Fe-deficiency-induced chlorosis. Despite the improved nutrient status of plants grown in Fe-limited medium under elevated CO2, the Fe-deficiency-induced responses in roots, including ferric chelate reductase activity, proton secretion, subapical root hair development, and the expression of FER, FRO1, and IRT genes, were all greater than plants grown in the ambient CO2. The biomass of plants grown in Fe-sufficient medium was also increased by the elevated CO2 treatment, but changes in tissue Fe concentrations and Fe deficiency responses were not observed. These results suggest that the improved Fe nutrition and induction of Fe-deficient-induced responses in plants grown in Fe-limited medium under elevated CO2 are caused by interactions between elevated CO2 and Fe deprivation. Elevated CO2 also increased the nitric oxide (NO) levels in roots, but treatment with the NO scavenger cPTIO inhibited ferric chelate reductase activity and prevented the accumulation of LeFRO1, LeIRT1, and FER transcripts in roots of the Fe-limited plants. These results implicate some involvement of NO in enhancing Fe-deficiency-induced responses when Fe limitation and elevated CO2 occur together. We propose that the combination of elevated CO2 and Fe limitation induces morphological, physiological, and molecular responses that enhance the capacity for plants to access and utilize Fe from sparingly soluble sources, such as Fe(III)-oxide.
Carbon dioxide (CO2) is one of the most important greenhouse gases contributing to global warming (Intergovernmental Panel on Climate Change, 2001). Human activity has increased the concentration of atmospheric CO2 from about 280 μL L−1 at the beginning of the nineteenth century to 367 μL L−1 at the end of the twentieth century (Bolin and Kheshgi, 2001), and the concentration is estimated to reach 490 to 1,260 μL L−1 by 2100 (Intergovernmental Panel on Climate Change, 2001). Increases in CO2 concentration will likely have a profound impact on plant growth. Previous studies have shown that elevated CO2 increases net photosynthesis rate in C3 plants because higher CO2 suppresses ribulose-1,5-bisphosphate oxygenase activity, decreases photorespiration, and increases carbon assimilates for plant growth and development (Lawlor and Mitchell, 2000). As a consequence, elevated CO2 treatments generally increase the biomass of C3 plants (Dijkstra et al., 2002). For example, Kimball and Mauney (1993) demonstrated that cotton plants (Gossypium hirsutum) grown under 550 μL L−1 CO2 had a 35% higher biomass, 40% higher fruit weight, and 60% higher lint yield than plants grown under 350 μL L−1 CO2.
The enhancement of plant growth by elevated CO2 will also increase their demand for nutrients. For example, the growth response of Japanese red pine (Pinus densiflora) seedlings to phosphate (Pi) was saturated at 0.1 mm Pi in ambient CO2 (350 μL L−1), whereas in the elevated CO2 (700 μL L−1), the growth response to Pi supply did not saturate even at 0.2 mm Pi supply (Kogawara et al., 2006). Iron (Fe) is an essential micronutrient for plant growth and development. Although the total Fe content in soil regularly exceeds plant requirements, its bioavailability to plants is often limited (Guerinot and Yi, 1994), particularly in calcareous soils, which represent 30% of the earth's surface (Imsande, 1998). Therefore, Fe nutrition in plants is likely to be affected by the continued elevation of atmospheric CO2, which, in turn, will affect crop production. Sasaki et al. (1998) demonstrated that both ferric reductase activity and Fe uptake capacity of the marine alga Chlorococcum littorale cultured in Fe-limited medium were significantly enhanced by extremely high concentrations of CO2. However, little is known about how elevated CO2 influences Fe nutrition in higher plants, which undergo specific morphological and physiological changes in response to Fe deficiency (Marschner, 1995). Römheld and Marschner (1986) classified these changes as strategy I and strategy II mechanisms. Strategy I occurs in nongraminaceous monocots and dicots, and strategy II occurs in graminaceous monocots. For strategy I plants, Fe deficiency enhances the activity of the plasmalemma NADPH-ferric chelate reductase (FCR; Robinson et al., 1999; Yi and Guerinot, 1996), enhances the expression of plasmalemma Fe(II) transporters (Eide et al., 1996; Vert et al., 2002), increases acidification of the extracellular medium, and increases the subapical development of root hairs.
We investigated how elevated CO2 affects the Fe status of the strategy I species tomato (Lycopersicon esculentum) grown with a soluble Fe source or the sparingly soluble hydrous Fe(III)-oxide. We demonstrate that elevated CO2 can improve plant Fe nutrition under Fe-limited conditions by inducing morphological, physiological, and molecular changes that enhance Fe uptake.
RESULTS
Effect of Elevated CO2 on Chlorophyll Synthesis, Plant Growth, and Uptake of Fe
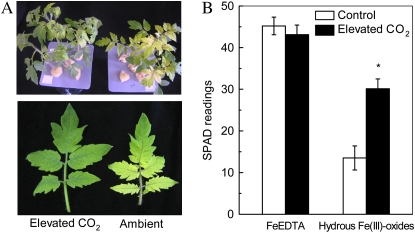
After 7-d growth in ambient CO2 and medium containing hydrous Fe(III)-oxide as the sole Fe source (Fe-limited medium), the newly formed tomato leaves were severely chlorotic (Fig. 1A) with a SPAD reading of 13.5. By comparison, leaves of plants grown in the same nutrient conditions, but at elevated CO2, had SPAD readings at approximately 30 (Fig. 1B), indicating that the elevated CO2 treatment significantly improved the chlorophyll synthesis of the plants grown in the Fe-limited medium. However, the chlorophyll content of plants grown with plant-available Fe (20 μm FeEDTA) was nearly the same in both ambient and elevated CO2 treatments with a SPAD reading of approximately 45 (Fig. 1B), indicating that elevated CO2 did not affect chlorophyll synthesis in Fe-sufficient plants.
Figure 1.
Effects of elevated CO2 treatment on chlorophyll synthesis. A, Tomatoes grown in hydrous Fe(III)-oxide medium. B, SPAD readings of tomatoes grown in FeEDTA or hydrous Fe(III)-oxide medium. Tomatoes were grown under ambient (350 μL L−1) or elevated (800 μL L−1) CO2. The image was taken on the seventh day of treatment, and the SPAD readings were recorded. Data are means ± sd (n = 8). *, Significant differences (P < 0.05) between ambient and elevated CO2 treatments.
Plant growth was increased by elevated CO2 in both Fe-sufficient and Fe-limited media. Shoot fresh weight was increased by 22% and 44%, respectively, and root fresh weight by 43% and 97%, respectively, compared with plants grown in ambient CO2 (Table I). The root-to-shoot ratio of Fe-sufficient and Fe-limited plants was also greater in elevated CO2, but the changes were not statistically significant for Fe-sufficient plants (Table I). Fe concentrations in the Fe-sufficient plants were not altered by CO2 treatment, but significant increases in Fe concentrations occurred in Fe-deficient plants under elevated CO2 (Table I).
Table I.
Effects of elevated CO2 treatment on biomass, root-to-shoot ratio, and Fe concentration of tomatoes grown in FeEDTA or hydrous Fe(III)-oxide medium
Tomatoes were grown under ambient (350 μL L−1) or elevated (800 μL L−1) CO2. On the seventh day of treatment, biomass and Fe concentration were analyzed. Data are means ± sd (n = 5). *, Significant differences (P < 0.05) between ambient and elevated CO2 treatments. DW, Dry weight.
| Fe Source | Treatment | Fresh Biomass
|
Root-to-Shoot Ratio | Fe Concentration
|
||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||
| g plant−1 | μg g−1 DW | |||||
| FeEDTA | Ambient | 3.354 ± 0.242 | 0.442 ± 0.073 | 0.132 ± 0.014 | 106.07 ± 9.40 | 338.28 ± 35.95 |
| Elevated CO2 | 4.078 ± 0.309 * | 0.631 ± 0.079 * | 0.155 ± 0.021 | 107.46 ± 4.24 | 327.42 ± 31.05 | |
| Hydrous Fe(III)-oxide | Ambient | 2.461 ± 0.431 | 0.351 ± 0.059 | 0.143 ± 0.024 | 58.74 ± 2.07 | 101.81 ± 12.23 |
| Elevated CO2 | 3.539 ± 0.458 * | 0.690 ± 0.101 * | 0.194 ± 0.013 * | 77.36 ± 5.25 * | 156.42 ± 15.27 * | |
Effect of Elevated CO2 on Fe-Deficiency-Induced Physiological and Morphological Responses
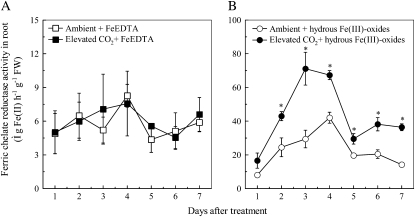
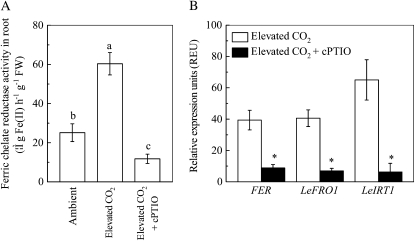
Fe deficiency can induce specific physiological and morphological responses in strategy I plants (Römheld and Marschner, 1986). Root FCR activity in Fe-sufficient plants was similar in ambient and elevated CO2 treatments (Fig. 2A). However, FCR activity was strongly induced in plants grown in Fe-limited medium and this induction was more marked when Fe deficiency was combined with elevated CO2 (Fig. 2B).
Figure 2.
Effects of elevated CO2 treatment on FCR activity of roots from the plants grown in FeEDTA (A) or hydrous Fe(III)-oxide (B) medium. Treatments are the same as in Figure 1. Data are means ± sd (n = 4). *, Significant differences (P < 0.05) between ambient and elevated CO2 treatments at each time point. FW, Fresh weight.
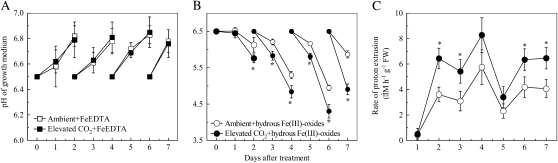
pH changes in the nutrient medium were also affected by CO2 concentration. Whereas the pH of the nutrient solution increased steadily in both ambient and elevated CO2 treatments under Fe-sufficient growth conditions (Fig. 3A), the pH decreased significantly when Fe was limited and the changes were greater when Fe limitation was combined with elevated CO2 (Fig. 3B). This indicates that elevated CO2 enhances proton extrusion from roots under Fe-limited conditions. To control for the larger root biomass under elevated CO2 (Table I), we analyzed proton extrusion from Fe-limited plants. Proton extrusion rate was increased by elevated CO2 after 2 d of treatment, although this change was not statistically significant on days 4 and 5 (Fig. 3C).
Figure 3.
Effects of elevated CO2 treatment on proton extrusion of roots. A, pH of the FeEDTA growth medium. B, pH of hydrous Fe(III)-oxide growth medium. C, Root proton extrusion rate of the plants grown in hydrous Fe(III)-oxide medium. Treatments are the same as in Figure 1 and measurement of the proton extrusion rate was conducted as described in “Materials and Methods.” Data are means ± sd (n = 4 for pH data; n = 8 for proton extrusion rate). *, Significant differences (P < 0.05) between ambient and elevated CO2 treatments at each time point. FW, Fresh weight.
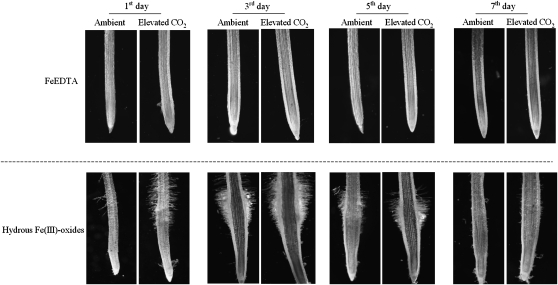
The development of subapical root hairs and subapical root swelling was observed on the Fe-limited tomatoes, but the growth was greater at elevated CO2 than at the ambient CO2 treatment, especially on day 3 (Fig. 4).
Figure 4.
Effects of elevated CO2 treatment on subapical root hair development of the plant grown in FeEDTA (A) or hydrous Fe(III)-oxide (B) medium. Treatments are the same as in Figure 1.
Effect of Elevated CO2 on the Expression of Genes Involved in Fe Uptake
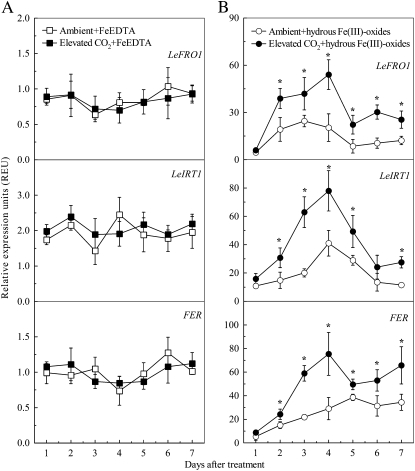
Ferric Fe reduction and the transport of ferrous Fe across the plasma membrane with FCR and the Fe(II) transport system are pivotal steps involved in the Fe uptake by strategy I plants. LeFRO1 and LeIRT, respectively, encode FCR and the Fe(II) transporter in tomato. The expression of both genes was increased significantly in roots by Fe deficiency (Fig. 5) and these changes were greater under elevated CO2 especially between 2 to 4 d of treatment (Fig. 5B). By contrast, elevated CO2 did not affect the expressions of these two genes in Fe-sufficient plants (Fig. 5A).
Figure 5.
Effects of elevated CO2 treatment on expression levels of FER, LeFRO1, and LeIRT1 in tomato roots cultured with FeEDTA (A) or hydrous Fe(III)-oxide (B) medium. Relative expression levels were calculated and normalized with respect to α-tubulin mRNA. Treatments are the same as in Figure 1. Data are means ± sd (n = 5). *, Significant differences (P < 0.05) between ambient and elevated CO2 treatments at each time point.
The basic helix-loop-helix (bHLH) protein FER regulates the expression of LeFRO1 and LeIRT1 genes, as well as other responses to Fe deficiency in tomato roots (Ling et al., 2002; Bereczky et al., 2003; Li et al., 2004). The expression of the FER gene in roots of plants grown in Fe-limited medium was significantly induced as well, and this change was even greater after 2 d in elevated CO2 (Fig. 5B). However, the elevated CO2 did not affect the FER expression in roots of Fe-sufficient plants (Fig. 5A).
Possible Role of Nitric Oxide in the Regulation of the Enhanced Plant Responses to Fe Deficiency under Elevated CO2
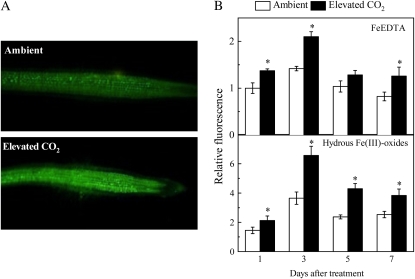
Nitric oxide (NO) was recently demonstrated to be a signal molecule involved in regulating the gene expression during Fe deficiency (Graziano and Lamattina, 2007). We measured NO levels in the roots using diaminofluorescein-FM diacetate (DAF-FM DA) and found that the NO level was always higher in Fe-limited plants, and elevated CO2 produced significant increase of the NO level in Fe-limited plants under both Fe-sufficient and Fe-limited conditions except on the fifth day for Fe-sufficient plants (Fig. 6). The interesting thing is that NO production occurs in Fe-sufficient plants without causing the same effects as in Fe-deficient plants, suggesting that NO production is necessary, but not sufficient, to induce the up-regulated Fe acquisition response under elevated CO2 conditions.
Figure 6.
Effect of elevated CO2 treatment on NO production in roots. A, Photographs of NO production shown as green fluorescence in representative roots after 3 d of growth in hydrous Fe(III)-oxide medium. B, Time course of NO production expressed as relative fluorescence. Treatments are the same as in Figure 1. NO production was visualized with DAF-FM DA dye. Photographs were analyzed with Photoshop software (Adobe Systems) and fluorescence intensity was estimated by measuring the average pixel intensity. Data are means ± sd (n = 20). *, Significant differences (P < 0.05) between ambient and elevated CO2 treatments at each time point.
When Fe-limited plants grown with elevated CO2 were treated with the NO scavenger cPTIO, the FCR activity was significantly inhibited to levels below those measured in ambient CO2 (Fig. 7A). Furthermore, the enhanced expressions of LeFRO1, LeIRT1, and FER genes by the elevated CO2 were all strongly inhibited by the cPTIO treatment (Fig. 7B).
Figure 7.
Role of NO in regulating the stimulative effect of elevated CO2 treatment on plant Fe uptake. A, Effects of cPTIO treatment on root FCR activity. FW, Fresh weight. B, Effects of cPTIO treatment on expression levels of LeFRO1, LeIRT1, and FER in tomato roots. After 2 d of growth under elevated CO2, plants were subjected to 200 μm cPTIO treatment for 24 h, and then the FCR activity and expression of genes were analyzed. Relative expression levels were calculated and normalized with respect to α-tubulin mRNA. Data are means ± sd (n = 5). Different letters or asterisks indicate significant differences (P < 0.05) among the treatments.
DISCUSSION
Kläring et al. (2007) proposed that plant growth is stimulated by CO2 concentrations up to 800 to 1,000 μL L−1, suggesting that the current ambient CO2 concentration of approximately 350 μL L−1 is suboptimal. Thus, plant growth is likely to increase as the CO2 concentration reaches the 490 to 1,260 μL L−1 levels predicted to occur by 2100 (Intergovernmental Panel on Climate Change, 2001). Previous studies have examined how the CO2-dependent stimulation in growth is likely to enhance the requirements for macronutrients like nitrogen (N) and phosphorus (Baxter et al., 1994; Kogawara et al., 2006), but none have considered the changing demand for micronutrients. Although this extra demand for nutrients may be partially compensated by increasing nutrient-use efficiency (Conroy et al., 1992; Newbery et al., 1995), the stimulation in growth associated with elevated CO2 is unlikely to be sustained without a concomitant increase in nutrient supply. In other words, either more fertilizer will need to be applied to avoid nutrient deficiencies or plants will have to become more efficient at acquiring those nutrients from the soil. Furthermore, because Fe deficiency is a major nutritional disorder in humans, affecting an estimated 2 billion people (Baynes and Bothwell, 1990), the Fe status of food crops will be increasingly important in the future. In this study, we found that elevated CO2 not only increased the biomass accumulation of plants cultured in the Fe-limited medium, but also significantly improved their Fe status.
Elevated CO2 Favors Plant Growth under Fe-Limited Condition
The increased biomass accumulation of plants grown in elevated CO2 is largely attributed to the increase of net photosynthesis, but nutrient limitation has generally been found to suppress this response (Conroy 1992; McKee and Woodward 1994; Lloyd and Farquhar 1996; Stitt and Krapp, 1999). For examples, when birch (Betula pendula; Pettersson et al., 1993; Silvola and Ahlholm, 1995), loblolly pine (Pinus taeda; Gebauer et al., 1996), rice (Oryza sativa; Ziska et al., 1996), cotton (Rogers et al., 1993), wheat (Triticum aestivum; G.S. Rogers et al., 1996), and tobacco (Nicotiana tabacum; Geiger et al., 1999) were grown at various N supplies, elevated CO2 led to large increases of biomass at the highest N supply, small increases at a moderately limiting N supply, and no increase, or even a slight decrease, at the lowest N supply. Therefore, nutrient supply and, consequently, the nutrient status of plants should be a critical factor determining growth responses to the elevated CO2. We have shown that the elevated CO2 treatments significantly increased the Fe concentrations in tomato leaves (Table I) and alleviated the Fe-deficiency-induced chlorosis (Fig. 1) when grown in Fe-limited medium. Furthermore, the relative increases in biomass at elevated CO2 in Fe-limited plants were greater than the increases measured in Fe-sufficient plants (Table I). Therefore, the increase in plant biomass under elevated CO2 and restricted Fe supply (Fig. 2) cannot be attributed to increased photosynthesis alone, but also to the improved Fe nutrition of the plants.
Elevated CO2 Enhances Fe Acquisition from the Fe-Limited Medium
How, then, does the elevated CO2 increase Fe status of plants grown in Fe-limited medium alleviate the Fe-deficiency-induced chlorosis? Increased activities of FCR and the Fe(II) transporter IRT1 in roots are indispensable for plant adaptation to Fe deficiency (Robinson et al., 1999; Vert et al., 2002; Curie and Briat, 2003) and we found that both FCR activity and LeFRO1 and LeIRT1 expression in Fe-limited plants were significantly enhanced by the elevated CO2 (Figs. 2B and 5B). Graziano and Lamattina (2007) claimed that, while these two responses are essential responses to Fe deficiency, they are not sufficient to confer a noticeable increase of Fe concentration in plants under Fe-limited conditions. Their argument was based on two observations: (1) NO-stimulated FCR activity and expression of LeIRT1 did not significantly increase the Fe concentration of the plants grown in low Fe medium (0.1 μm FeEDTA; Graziano and Lamattina, 2007); (2) Arabidopsis (Arabidopsis thaliana) plants overexpressing AtIRT1 or AtFRO2 and grown in Fe-deficient medium do not accumulate more Fe than the wild-type plants (Connolly et al., 2002, 2003). However, the similar Fe concentrations in the transgenic and wild-type plants may have been caused by the fact that Fe was not available for reduction and uptake in the Fe-deficient treatment used in those experiments (Connolly et al., 2002, 2003). This explanation may also be true for the finding of Graziano and Lamattina (2007) because 0.1 μm FeEDTA is very low for plant growth and can be quickly depleted from nutrient medium. Recently, Yuan et al. (2008) reported that the overexpression of FIT (the Arabidopsis ortholog of FER; Bauer et al., 2007) with either AtbHLH38 or AtbHLH39 in Arabidopsis, which results in constitutive accumulation of AtIRT1 protein and high FCR activity in roots, significantly increases Fe accumulation in shoots. Therefore, we propose that the increase of FCR activity and expression of the LeIRT1 gene found in this study contributes, at least in part, to the increased Fe concentration of plants grown under elevated CO2 and limited Fe.
Dicotyledons also acidify the rhizosphere as part of the strategy I responses to Fe deficiency (Römheld and Marschner, 1986). In well-aerated soils, the solubility of the inorganic Fe depends on the reversible dissolution and precipitation of the ferric oxides in the soil and a decrease of one pH unit will theoretically increase the Fe solubility by 1,000-fold (Lindsay and Schwab, 1982). Therefore, the solubility of Fe strongly depends on the proton activity in the medium. The increase in soluble Fe concentration in these experiments can be estimated by GEOCHEM-PC (Parker et al., 1995). In the growth medium containing hydrous Fe(III)-oxide, the greater acidification in the elevated CO2 treatment (Fig. 3) would lead to a 12-fold greater concentration of soluble Fe compared to the ambient CO2 treatment. This response may contribute to the increased Fe accumulation in plants under the elevated CO2 environment and Fe-limited medium.
Development of dense subapical root hairs is another typical morphological response of strategy I plants to Fe deficiency (Römheld and Marschner, 1986; Schmidt, 1999). Longer root hairs or a greater root hair density greatly enlarges the root surface area and allows for a larger soil volume to be explored. The formation of root hairs in response to Fe deficiency is associated with cell-specific accumulation of transcripts that are involved in Fe acquisition (Santi and Schmidt, 2008). Therefore, subapical root hair development enhanced by elevated CO2 (Fig. 4) may also contribute partly to the increased Fe accumulation in plants grown in the Fe-limited medium. In this study, however, we found that the subapical root region of Fe-limited plants did not develop root hairs in both the ambient and elevated CO2 treatments on day 7 of the treatment (Fig. 4), even though FCR activity of roots was higher in the elevated CO2 treatment (Fig. 2B). This indicates that the subapical root hair development may only play a minor role in increasing Fe uptake of plants under the combined conditions of Fe limitation and elevated CO2. Importantly, the role of root hairs is likely to have been underestimated in these hydroponic experiments because the dissolved nutrients can easily diffuse to the roots. The role of root hairs will be more important in soil experiments.
Elevated CO2 stimulated root growth and resulted in a significantly greater root-to-shoot ratio (Table I). Similar changes in root-to-shoot ratio have previously been interpreted as a mechanism by which plants can take up more nutrients when growth is stimulated by the elevated CO2 concentration (H.H. Rogers et al., 1996).
Taken together, the elevated CO2 under Fe-limited conditions enhances root growth, root hair development, proton release, root FCR activity, and expressions of LeFRO1 and LeIRT1 genes, all of which enable plants to access and accumulate more Fe. Importantly, elevated CO2 did not induce these responses in plants that were well supplied with Fe (Figs. 2A, 3A, 4A, and 5A) and the Fe concentrations in those plants remained unchanged (Table I). A combination of limited Fe availability and elevated CO2 was required to induce the symptoms typical of Fe deficiency.
A Possible Role for NO
Then, how does the elevated CO2 enhance the Fe-deficiency-induced responses of plants under Fe-limited conditions? It is clear that this enhancement should not be related to the extent of Fe deficiency because the plant Fe nutrient status was better in the elevated than ambient CO2 (Fig. 1; Table I). Recently, NO was shown to be a general signal molecule involved in inducing the adaptive responses of roots to Fe-deficient conditions, including enhancing the expressions of genes involved in Fe uptake (Graziano and Lamattina, 2007). We found in this study that NO levels in roots in response to Fe deficiency were consistently greater in elevated CO2 (Fig. 6) and that the NO scavenger cPTIO inhibited the usual changes associated with Fe deficiency that affect FCR activity and LeFRO1 and LeIRT1 expression (Fig. 7). These results suggest that the adaptive responses induced by elevated CO2 and Fe deficiency may be controlled by NO. Interestingly, although the elevated CO2 treatment significantly increased NO levels in roots of Fe-sufficient plants, previous studies have shown that the Fe deficiency responses were not induced in those plants (Graziano and Lamattina, 2007). This may also be the reason why the elevated CO2 only specifically enhanced the Fe-deficiency-induced responses of the Fe-limited tomatoes.
The bHLH protein FER has been demonstrated to regulate the responses to Fe deficiency in tomato roots (Ling et al., 2002; Bereczky et al., 2003; Li et al., 2004). Moreover, the overexpression of FIT confers the increased FCR activity in Fe-deficient plants (Jakoby et al., 2004). We also found that the expression of FER was increased by a combination of Fe deficiency and elevated CO2 (Fig. 5B) and that cPTIO treatment prevented the accumulation of FER transcripts (Fig. 7B). Furthermore, the FER protein is necessary to mediate the regulatory function of NO in Fe-deficiency-induced responses (Graziano and Lamattina, 2007).
The question of how elevated CO2 increases NO levels in roots remains open. CO2 could enhance the activity of nitrate reductase in plants (Buchanan et al., 2000), which is a major enzyme in NO synthesis through the nitrite reduction (Yamasaki et al., 1999; Meyer et al., 2005). Alternatively, elevated CO2 could increase auxin levels (Li et al., 2002; Teng et al., 2006), which then induces NO accumulation (Du et al., 2008). These issues require further investigation, but we propose that elevated CO2 first increases NO levels in roots by enhancing the nitrate reductase activity and then enhances FER expression. These changes strengthen the molecular and physiological responses to Fe deprivation and induce the morphological changes in roots.
We have demonstrated that a combination of elevated CO2 and Fe limitation can induce a set of morphological, physiological, and molecular responses in plants that improve their Fe status by enabling them to better access Fe from sparingly soluble sources. NO may be a signaling molecule that controls these processes.
MATERIALS AND METHODS
Plant Culture
The tomato (Lycopersicon esculentum ‘Zheza 809’) seeds were germinated in 0.5 mm CaSO4 solution. Seven days after sowing, seedlings of similar size were transferred to 1-L pots (four holes per seedling holder, and one seedling per hole) filled with aerated, full-strength complete nutrient solution. The nutrient solution had the following composition (in μm): KH2PO4 250, MgSO4 500, KNO3 1,000, Ca(NO3)2 500, H3BO3 10, MnSO4 0.5, ZnSO4 0.5, CuSO4 0.1, (NH4)6Mo7O24 0.1, FeEDTA 20. The solution pH was adjusted to 6.5 using 1 m NaOH. The nutrient solutions were renewed every 3 d. All plants were grown in the controlled-environment growth chambers at a humidity of 70%, with a daily cycle of a 28°C, 14-h day and a 22°C, 10-h night. The daytime light intensity was 180 μmol photons m−2 s−1.
After 13 d of growth in the complete nutrient solution, one-half of the plants were transferred to an otherwise identical nutrient solution with the FeEDTA replaced with 0.3 g/L hydrous Fe(III)-oxide, and another one-half of the plants were continuously cultured in the 20 μm Fe EDTA contained nutrient solution. Meantime, CO2 treatments were also initiated by growing the above plants in the chambers with a CO2 concentration of either 350 (ambient) or 800 (elevated CO2) μL L−1. The hydrous Fe(III)-oxide, which consisted of hematite together with a trace amount of by-produced goethite, was prepared according to Schwertmann and Cornell (1991). To avoid the hydrous Fe(III)-oxide adhering to the root surface during cultivation, the hydrous Fe(III)-oxide was placed in a 36-mm-diameter dialysis bag (Puyi) with 12,000 to 18,000 Mr cutoff, which could ensure that smaller molecules like Fe ions and their chelates pass freely in or out of the bag. The initial soluble Fe concentration in the hydrous Fe(III)-oxide nutrient solution was 0.06 μm, which could be taken as Fe-limited medium. The nutrient solution was renewed every 3 d and was supplemented to 1 L with deionized water daily.
cPTIO Treatment
As we found that the NO levels of roots in hydrous Fe(III)-oxide medium were increased by elevated CO2, a NO scavenger, cPTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide], was used to investigate the possible role of NO in regulating the enhancement of Fe-deficient responses induced by elevated CO2. After 2 d of growth in the hydrous Fe(III)-oxide-containing nutrient solution under elevated CO2, part of plants were transferred to an otherwise identical growth solution containing 200 μm cPTIO. After 24 h, FCR activity and mRNA levels of FER, LeFRO1, and LeIRT1 in roots were analyzed following the methods described below.
Chlorophyll Synthesis and Biomass Analysis
After 7 d of growth under elevated CO2, the chlorophyll content of the newly formed leaves was analyzed with a chlorophyll meter (SPAD-502; Minolta) and recorded as a SPAD reading. After chlorophyll content recording, the plants were separated into shoots and roots with scissors. Roots were washed with deionized water and blotted dry with a paper towel. The shoots and roots were weighed and dried in a 75°C oven to a constant weight for elements content analysis.
Analysis of Proton Extrusion Rate
After the plants were subjected to ambient and elevated CO2 treatments, the pH in nutrient solution was measured every day with a pH electrode (METROHM). The proton extrusion rate was analyzed following the method of Römheld et al. (1984). Briefly, the individual plants precultured with hydrous Fe(III)-oxide nutrient solution under ambient or elevated CO2 treatment were transferred to 300-mL pots filled with Fe-omitted nutrient solution and were continuously grown under ambient or elevated CO2 conditions. After 12 h, each solution was filtered and the volume was adjusted to the initial value. The net release of protons was determined by titrating the solution with 10−3 m NaOH. Prior to the determination of the fresh weight of roots, the excess water was removed by blotting with filter paper. The aim of omitting hydrous Fe(III)-oxide from nutrient solution in this experiment was to avoid the reaction of protons with hydrous Fe(III)-oxide so as to more accurately estimate the proton extrusion rate.
Determination of FCR Activity
FCR activity was determined following our previously research (Jin et al., 2007). Briefly, 1 g of the excised roots (<10 cm from the root tips) was placed in an Erlenmeyer flask filled with 50 mL of an assay solution consisting of 0.5 mm CaSO4, 0.1 mm MES, 0.1 mm 4,7-diphenyl-1,10-phenanhroline-disufonic acid, and 100 μm Fe EDTA at pH 5.5 adjusted by 1 m NaOH. The flasks were placed in a dark room at 25°C for 1 h, with periodic hand swirling at 15-min intervals. The absorbance of the assay solutions was recorded by a spectrophotometer at 535 nm, and the concentration of Fe(II)[BPDS]3 was quantified using an extinction coefficient of 22.14 mm−1 cm−1.
Root Morphology Observation
Observation of the root subapical root hair patterns was performed using light microscopy with differential interference contrast optics. Photomicrographs were recorded on a CCD camera (Nikon Eclipse E600).
In Situ Measurement of NO in the Root
NO was imaged using DAF-FM DA and epifluorescence microscopy. Roots were loaded with 5 μm DAF-FM DA in 20 mm HEPES-NaOH buffer (pH 7.4) for 30 min, washed three times in fresh buffer, and observed under a microscope (Nikon Eclipse E600; Nikon; excitation 488 nm, emission 495–575 nm). Twenty roots of each treatment were measured each time. The signal intensities of green fluorescence in the images of the young root hair zone (3–10 mm from root tip) were quantified according to the method of Guo and Crawford (2005) by using Photoshop software (Adobe Systems). Data are presented as the mean of fluorescence intensity relative to the roots of day 1 ambient treatment.
Real-Time Reverse Transcription-PCR Analyses
Root samples were frozen in liquid nitrogen immediately after collection and stored at −80°C. About 100 mg of tissue were ground in liquid nitrogen and total RNA was extracted by TRIzol (Invitrogen), and then the first-strand cDNA was synthesized with the total RNA by PrimeScript reverse transcription (RT) reagent kit (TaKaRa). All RNA samples were checked for DNA contamination before cDNA synthesis. The mRNA levels of FER, LeFRO1, and LeIRT1 were detected by the SYBR Green RT-PCR kit (TaKaRa) with the following pairs of gene-specific primers: FER fw, 5′-TGAATCTTCTGGCACAACG-3′; rev, 5′-CCAATGATGGAGGCTTTATC-3′; LeFRO1 fw, 5′-GCAAGACACCAGAAATCCTAC-3′; rev, 5′-ATCAGATGGGTTGGGCTT-3′; and LeIRT1 fw, 5′-AGCACTTGGGATAGCATTG-3′; rev, 5′-ACTGACATTCCACCAGCAC-3′. The RT-PCR analysis was performed with the ABI 7300 real-time PCR system (Applied Biosystems) with the following cycling conditions: 10 s at 95°C, 35 cycles of 95°C for 5 s, 60°C for 30 s. A pair of housekeeping genes of α-tubulin was used for a control PCR: fw, 5′-CCTGAACAACTCATAAGTGGC-3′; rev, 5′-AGATTGGTGTAGGTAGGGCG-3′. Each cDNA sample was run in triplicate. Amplification of PCR products was monitored via intercalation of SYBR-Green. Relative expression units (REU) were calculated according to the equation of Meleshkevitch et al. (2006) and multiplied by 100:
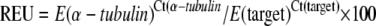 |
The efficiency (E) of each primer set was determined by plotting template dilutions against Ct values and is equal to 10[−1/slope]. Ct refers to the cycle number at which the fluorescence rises above a set threshold.
Fe Content Analysis
The dried root and shoot samples were wet digested in the concentrated HNO3 at 120°C until there was no brown NO gas emitting, then further digested with HNO3/HClO4 at 180°C until the solution became transparent. Digestates were diluted by ultrapure water and the concentration of Fe in the digestates was analyzed by inductively coupled plasma-mass spectrometry (Agilent 7500a).
Statistics
All statistical analyses were conducted with SAS software (SAS Institute). Means were compared by t test or Fisher's LSD test at P < 0.05 in all cases.
Acknowledgments
We thank the anonymous reviewers for their constructive suggestions, as well as Dr. Peter Ryan from CSIRO for critical reading and revision of the manuscript.
This work was supported by the Natural Science Foundation of China (grant no. 30625026) and the China Postdoctoral Science Foundation (grant no. 20080440204).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Shao Jian Zheng (sjzheng@zju.edu.cn).
Open access articles can be viewed online without a subscription.
References
- Bauer P, Ling HQ, Guerinot ML (2007) FIT, the FER-like iron deficiency induced transcription factor in Arabidopsis. Plant Physiol Biochem 45 260–261 [DOI] [PubMed] [Google Scholar]
- Baxter R, Gantley M, Ashenden TW, Farrar IF (1994) Effects of elevated carbon dioxide on three grass species from montane pasture. II. Nutrient uptake, allocation and efficiency of use. J Exp Bot 278 1267–1278 [Google Scholar]
- Baynes RD, Bothwell TH (1990) Iron deficiency. Annu Rev Nutr 10 133–148 [DOI] [PubMed] [Google Scholar]
- Bereczky Z, Wang HY, Schubert V, Ganal M, Bauer P (2003) Differential regulation of nramp and irt metal transporter genes in wild type and iron uptake mutants of tomato. J Biol Chem 278 24697–24704 [DOI] [PubMed] [Google Scholar]
- Bolin B, Kheshgi HS (2001) On strategies for reducing greenhouse gas emissions. Proc Natl Acad Sci USA 98 4850–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD
- Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerino ML (2003) Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol 133 1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy JP (1992) Influence of elevated atmospheric CO2 concentrations on plant nutrition. Aust J Bot 40 445–456 [Google Scholar]
- Conroy JP, Milham PJ, Barlow EWR (1992) Effect of nitrogen and phosphorus availability on the growth response of Eucalyptus grandis to high CO2. Plant Cell Environ 15 843–847 [Google Scholar]
- Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54 183–206 [DOI] [PubMed] [Google Scholar]
- Dijkstra P, Hymus G, Colavito D, Vieglais DA, Cundari CM, Johnson DP, Hungate BA, Hinkle CR, Drake BG (2002) Elevate atmospheric CO2 stimulates aboveground biomass in a fire-regenerated scrub-oak system. Glob Change Biol 8 90–103 [Google Scholar]
- Du ST, Zhang YS, Lin XY, Wang Y, Tang CX (2008) Regulation of nitrate reductase by its partial product nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L. cv. Baoda). Plant Cell Environ 31 195–204 [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer RLE, Reynolds JF, Strain BR (1996) Allometric relations and growth in Pinus taeda: the effect of elevated CO2 and changing N availability. New Phytol 134 85–93 [Google Scholar]
- Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M (1999) Influence of nitrate and ammonium nitrate supply on the response of photosynthesis, carbon and nitrogen metabolism, and growth to elevated carbon dioxide in tobacco. Plant Cell Environ 22 1177–1199 [Google Scholar]
- Graziano M, Lamattina L (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52 949–960 [DOI] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. Plant Physiol 104 815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-Induced senescence. Plant Cell 17 3436–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J (1998) Iron, sulfur, and chlorophyll deficiencies: a need for an integrative approach in plant physiology. Physiol Plant 103 139–144 [Google Scholar]
- Intergovernmental Panel on Climate Change (2001) The scientific basic. In JT Houghton, Y Ding, DJ Griggs, M Noguer, PJ van der Linden, X Dai, K Maskell, CA Johnson, eds, Climate Change. Cambridge University Press, Cambridge, UK, pp 7–16
- Jakoby M, Wang HY, Reidt W, Weisshaar B, Bauer P (2004) FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Lett 577 528–534 [DOI] [PubMed] [Google Scholar]
- Jin CW, You GY, Tang CX, Wu P, Zheng SJ (2007) Iron-deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover (Trifolium pratense L.). Plant Physiol 144 278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball BA, Mauney JR (1993) Response of cotton to varying CO2, irrigation, and nitrogen: yield and growth. Agron J 85 706–712 [Google Scholar]
- Kläring HP, Hauschild C, Heißner A, Bar-Yosef B (2007) Model-based control of CO2 concentration in greenhouses at ambient levels increases cucumber yield. Agric Meteorol 143 208–216 [Google Scholar]
- Kogawara S, Norisada M, Tange T, Yagi H, Kojima K (2006) Elevated atmospheric CO2 concentration alters the effect of phosphate supply on growth of Japanese red pine (Pinus densiflora) seedlings. Tree Physiol 26 25–33 [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Mitchell RAC (2000) Crop ecosystem responses to climatic change: wheat. In KR Reddy, HF Hodges, eds, Climate Change and Global Crop Productivity. CAB International, Wallingford, UK, pp 57–80
- Li CR, Gan LJ, Xia K, Zhou X, Hew CS (2002) Responses of carboxylating enzymes, sucrose metabolizing enzymes and plant hormones in a tropical epiphytic CAM orchid to CO2 enrichment. Plant Cell Environ 25 369–377 [Google Scholar]
- Li L, Cheng X, Ling HQ (2004) Isolation and characterization of Fe(III)-chelate reductase gene LeFRO1 in tomato. Plant Mol Biol 54 125–136 [DOI] [PubMed] [Google Scholar]
- Lindsay WL, Schwab AP (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5 821–840 [Google Scholar]
- Ling HQ, Bauer P, Bereczky Z, Keller B, Ganal M (2002) The tomato fer gene encoding a bHLH protein controls iron-uptake responses in roots. Proc Natl Acad Sci USA 99 13938–13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J, Farquhar GD (1996) The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. I. General principles and forest ecosystems. Funct Ecol 10 4–32 [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, New York
- McKee IF, Woodward FI (1994) CO2 enrichment responses of wheat: interactions with temperature, nitrate and phosphate. New Phytol 127 447–453 [Google Scholar]
- Meleshkevitch EA, Assis-Nascimento P, Popova LB, Miller MM, Kohn AB, Phung EN, Mandal A, Harvey WR, Boudko DY (2006) Molecular characterization of the first aromatic nutrient transporter from the sodium neurotransmitter symporter family. J Exp Biol 209 3183–3198 [DOI] [PubMed] [Google Scholar]
- Meyer C, Lea US, Provan F, Kaiser WM, Lillo C (2005) Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth Res 83 181–189 [DOI] [PubMed] [Google Scholar]
- Newbery RM, Wolfenden J, Mansfield TA, Harrison AF (1995) Nitrogen, phosphorus and potassium uptake and demand in Agrostis capillaris: the influence of elevated CO2 and nutrient supply. New Phytol 130 565–574 [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL (1995) GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In RH Loeppert, AP Schwab, S Goldberg, eds, Chemical Equilibrium and Reaction Models, Special Publication 42. Soil Science Society of America, Madison, WI, pp 253–269
- Pettersson R, MacDonald JS, Stadenburg I (1993) Response of small birch plants (Betula pendula Roth.) to elevated CO2 and nitrogen supply. Plant Cell Environ 16 1115–1121 [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397 694–697 [DOI] [PubMed] [Google Scholar]
- Rogers GS, Milham PJ, Gillings M, Conroy JP (1996) Sink strength may be the key to growth and nitrogen response in N-deficient wheat at elevated carbon dioxide. J Plant Physiol 23 253–264 [Google Scholar]
- Rogers GS, Payne L, Milham P, Conroy J (1993) Nitrogen and phosphorus requirements of cotton and wheat under changing CO2 concentrations. Plant Soil 155 231–234 [Google Scholar]
- Rogers HH, Prior SA, Runios GB, Mitchell RJ (1996) Root to shoot ratio of crops as influenced by CO2. Plant Soil 187 229–248 [Google Scholar]
- Römheld V, Marschner H (1986) Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr 2 155–204 [Google Scholar]
- Römheld V, Muller C, Marschner H (1984) Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol 76 603–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Schmidt W (2008) Laser microdissection-assisted analysis of the functional fate of iron deficiency-induced root hairs in cucumber. J Exp Bot 59 697–704 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kurano N, Miyachi S (1998) Induction of ferric reductase activity and of iron uptake capacity in Chlorococcum littorale cells under extremely high-CO2 and iron-deficient conditions. Plant Cell Physiol 39 405–410 [Google Scholar]
- Schmidt W (1999) Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol 141 1–26 [Google Scholar]
- Schwertmann U, Cornell RM (1991) Iron Oxides in the Laboratory: Preparation and Characterization. VCH, Weinheim, Germany
- Silvola J, Ahlholm U (1995) Combined effects of carbon dioxide concentration and nutrient status on the biomass production and nutrient uptake of birch seedlings. Plant Soil 169 547–553 [Google Scholar]
- Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22 583–621 [Google Scholar]
- Teng NJ, Wang J, Chen T, Xu W, Wang Y, Li J (2006) Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol 172 92–103 [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Takahashi S (1999) An alternative pathway for nitric oxide production in plants, new features of an old enzyme. Trends Plant Sci 4 128–129 [DOI] [PubMed] [Google Scholar]
- Yi Y, Guerinot ML (1996) Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J 10 835–844 [DOI] [PubMed] [Google Scholar]
- Yuan YX, Wu HL, Wang N, Li J, Zhao WN, Du J, Wang DW, Ling HQ (2008) FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res 18 385–397 [DOI] [PubMed] [Google Scholar]
- Ziska LH, Weerakoon W, Namuco OS, Pamplona R (1996) The influence of nitrogen on the elevated carbon dioxide response in field grown rice. J Plant Physiol 23 45–52 [Google Scholar]