Abstract
In plants, the vascular tissue contains the enucleated sieve tubes facilitating long-distance transport of nutrients, hormones, and proteins. In addition, several mRNAs and small interfering RNAs/microRNAs were shown to be delivered via sieve tubes whose content is embodied by the phloem sap (PS). A number of these phloem transcripts are transported from source to sink tissues and function at targeted tissues. To gain additional insights into phloem-delivered RNAs and their potential role in signaling, we isolated and characterized PS RNA molecules distinct from microRNAs/small interfering RNAs with a size ranging from 30 to 90 bases. We detected a high number of full-length and phloem-specific fragments of noncoding RNAs such as tRNAs, ribosomal RNAs, and spliceosomal RNAs in the PS of pumpkin (Cucurbita maxima). In vitro assays show that small quantities of PS RNA molecules efficiently inhibit translation in an unspecific manner. Proof of concept that PS-specific tRNA fragments may interfere with ribosomal activity was obtained with artificially produced tRNA fragments. The results are discussed in terms of a functional role for long distance delivered noncoding PS RNAs.
In general, the vascular transport system of plants delivers nutrients and small signal molecules throughout the plant body. It is well established that RNA viruses and viroids can hitchhike on the phloem transport system to systemically infect the plant body. More recently, however, underlining the complexity of a systemic signaling system established by the phloem, mRNA, and small interfering RNAs (siRNAs)/microRNAs (miRNAs) were identified as potential long-distance signals moving via the sieve tubes (Lucas et al., 2001; Ruiz-Medrano et al., 2004; Lough and Lucas, 2006). The long-distance transport of RNA molecules adds another regulatory system allowing transcripts produced in leaves to be functional in distant tissues. Hundreds of mRNA transcripts potentially transported via the phloem were identified in pumpkin (Cucurbita maxima; Ruiz-Medrano et al., 1999), melon (Cucumis melo; Omid et al., 2007), maize (Zea mays; Nakazono et al., 2003), and Ricinus communis (Doering-Saad et al., 2006). For example, a systemic and specific phloem-delivered homeodomain protein transcript was shown to alter the size of potato (Solanum tuberosum) tubers (Chen et al., 2003), and transcripts of transcriptional regulators involved in gibberellic acid signaling were shown to modulate leaf shape in Arabidopsis (Arabidopsis thaliana), tomato (Solanum lycopersicum), and pumpkin (Haywood et al., 2005). More than 1000 siRNAs and four miRNAs (miR156, miR159, miR167, and miR171) were found in the phloem sap (PS) of pumpkin plants (Yoo et al., 2004). In the PS of Brassica napus, 32 annotated plant miRNAs belonging to 18 different families could be identified. In particular, the phosphate starvation-induced miRNA399 was shown to move across graft junctions, confirming a signaling function of the phloem-delivered miRNA (Buhtz et al., 2008; Pant et al., 2008).
The phloem transport pathway is established by the companion cell-sieve elements system. Via the companion cells, transcripts move as RNA-protein complexes into enucleated sieve elements using the symplasmic intercellular channels formed by plasmodesmata. Sieve elements establish the sieve tube system, which is formed by elongated cells devoid of nuclei. Once passed through plasmodesmata, RNA molecules have the potential to move systemically following the source-sink flow of the phloem stream and may be unloaded in sink tissues (for review, see Lucas et al., 2001; Lough and Lucas, 2006).
Given the essential role played by RNAs and the phloem delivery system, plants may well have established a multifunctional and complex RNA long-distance signaling system. In this study, we identified and characterized small noncoding RNA molecules of a size between 30 and 90 bases present in the PS of pumpkin. The identified small RNAs represent phloem-specific fragments of ribosomal RNAs (rRNA), specific tRNAs, spliceosomal RNAs, protein transport-associated RNA, and RNAs of unknown function. We provide functional evidence in the form of nonselective inhibition of translation mediated by phloem RNA. Our results are consistent with the notion that vascular plants evolved a phloem delivery system for RNA molecules acting as long-distance signals.
RESULTS
Cucurbit PS Contains Small RNA Distinct from Systemic si/miRNA
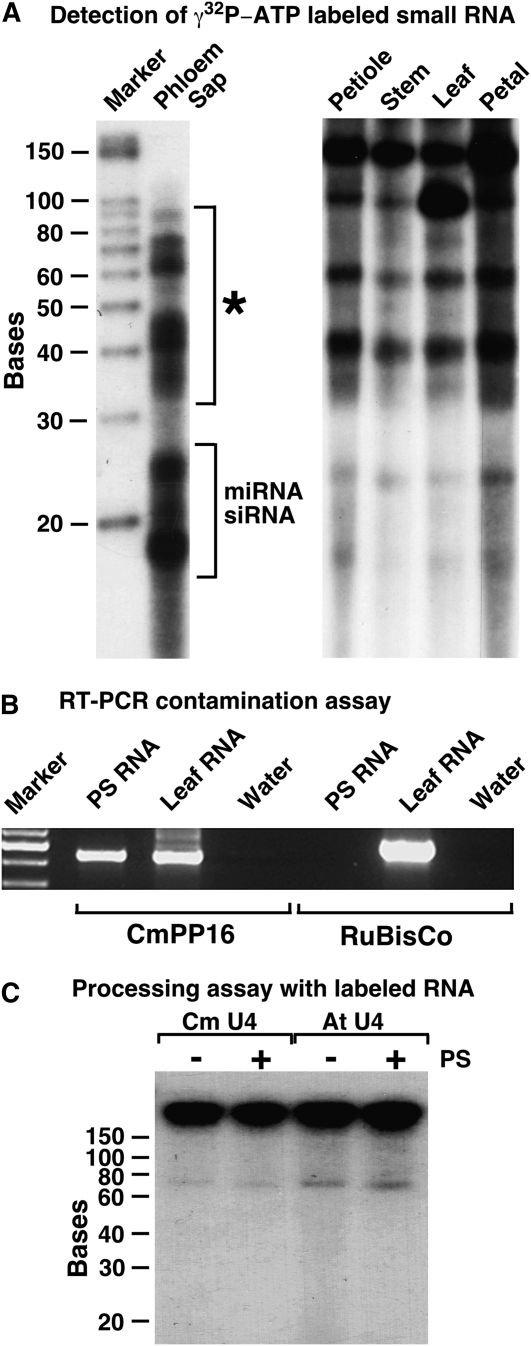
Earlier efforts to identify PS RNA molecules focused on small PS RNAs with a size below 30 bases, which resemble siRNA and miRNA, and on large polyadenylated mRNAs. Both classes of RNA molecules are suggested to serve as systemic signaling agents delivered over long distances via the phloem tissue (Lucas et al., 2001; Ruiz-Medrano et al., 2004; Yoo et al., 2004; Buhtz et al., 2008). However, the occurrence of small RNAs in the size range from 30 to 90 bases was not addressed. To evaluate the presence of such small RNAs, we used pumpkin from which analytical quantities of PS could be collected (Golecki et al., 1999; Ruiz-Medrano et al., 1999; Yoo et al., 2004). We harvested PS from cut petioles and stem tissues, isolated the RNA molecules, and submitted 5′ 32P-labeled RNA to denaturing PAGE. By this means, PS RNA compared to petiole, stem, leaf, and petal tissue RNA, we detected distinct and relatively high amounts of small RNA molecules larger than 30 bases (Fig. 1A). As shown by reverse transcription (RT)-PCR, green tissue-specific small subunit (SSU) of Rubisco mRNA was absent and phloem-specific CmPP16 mRNA (Xoconostle-Cazares et al., 1999) was present in the used PS RNA sample. Thus, the detected small PS RNAs were not a contamination from surrounding tissues due to the used PS harvesting method (Fig. 1B). RNA stability assays were employed to rule out the possibility that the detected small PS RNAs were degradation products made by RNA-processing enzymes activated during PS collection (Fig. 1C). Here, in vitro-transcribed U4 spliceosomal RNAs from Arabidopsis and pumpkin were added to PS droplets appearing on the cut petiole, incubated, and coharvested with endogenous PS RNA. No fragmentation or degradation of the labeled RNA probes was observed (Fig. 1C). Collectively, these results support the concept that small RNA fragments exist in the sieve tubes and are not produced by the used harvesting method.
Figure 1.
Size distribution of isolated small PS RNA and harvesting control. A, Denaturing PAGE of pumpkin. Shown are PS, petiole, stem, leaf, and petal small RNA labeled with [γ32P]ATP. Indicated PS RNA fragments (asterisk) were eluted after PAGE, cloned, and sequenced. B, Contamination control by RT-PCR. PS RNA (approximately 0.5 μg) and leaf RNA (approximately 0.5 μg) were submitted to 30 RT-PCR cycles using primers amplifying phloem-specific CmPP16 mRNA or green tissue-specific Rubisco SSU mRNA. Note that with the PS RNA sample, no amplification of Rubisco SSU mRNA occurred. C, PS RNA harvesting and RNA processing control: in vitro-transcribed, [α32P]-labeled Cm U4 snRNA and At U4 snRNA were added to PS droplets appearing on cut stem tissue, harvested, submitted to RNA isolation protocol, and analyzed via denaturing PAGE. −, RNA incubated with buffer; +, RNA added to PS droplet and re-isolated. Note that no processing or degradation of the RNA was observed.
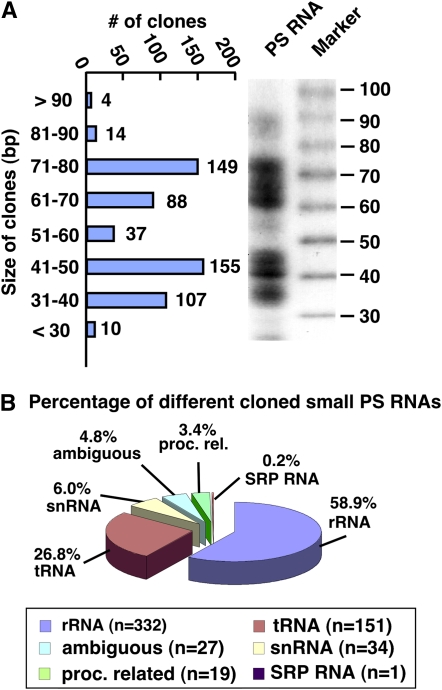
To obtain the sequence information of the small PS RNAs, we produced corresponding cDNAs, which were directionally cloned by a modified SAGE approach (Supplemental Materials S1). PS RNAs within a size ranging from 30 to 90 bases (Fig. 1A) were excised from gels after PAGE, cloned as concatamerized cDNAs, and sequenced. By this means, we got the sequence information of 564 PS cDNAs resembling the isolated PS RNA molecules. Bioinformatic analysis revealed that the size distribution of the cloned cDNAs corresponds to that of PS RNAs appearing on PAGE (Fig. 2A). Using the BLAST algorithm, we identified 537 RNA fragments, which were identical or highly similar (>90%) to known cDNAs. The remaining and ambiguous RNA sequences (n = 27) either did not match any published DNA sequence or could not be assigned to a particular sequence. This approach allowed us to categorize the identified PS RNA fragments into six groups that consist of related DNA sequences, such as rRNAs (n= 332), tRNAs (n = 151), small nucleosomal RNAs (snRNAs; n = 34), prokaryotic-related RNAs, including mitochondrial and chloroplastic rRNAs and tRNAs (n = 19), signal recognition particle RNA (n = 1) involved in ER protein import, and ambiguous RNAs (n = 27; Fig. 2B; Supplemental Materials S2 and S3).
Figure 2.
Size distribution and assignment of cloned small PS RNAs. A, The absolute number and size distribution of cloned PS RNA fragments compared to their appearance after denaturing PAGE. B, Pie graph showing the different classes and relative ratio of cloned sequences in percent according to sequence similarity. The cloned small PS RNAs could be grouped into six classes: rRNA, tRNA, snRNA, processing-related small RNA (proc. rel.), signal recognition particle RNA (SRP RNA), and ambiguous RNA with no meaningful similarity to know sequences.
Distinct rRNA and snRNA Fragments Appear in the PS
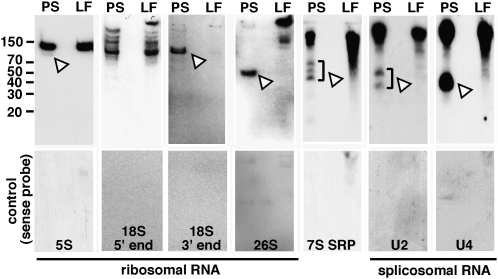
To substantiate that the identified PS RNA fragments smaller than full-length ncRNAs were not cloning artifacts, we used northern assays. Total RNA extracted from pumpkin PS and pumpkin leaves were transferred after PAGE onto membranes and probed with radioactively labeled oligonucleotides specific to 5S rRNA, 18S rRNA, 26S rRNA, U2 snRNA, and U4 snRNA. As presented in Figure 3, all probed PS RNAs appear as truncated and, with the exception of 26S rRNA, also as full-length molecules in the PS RNA extract. The combined results confirm the identity and size of the cloned small ncRNA fragments and suggest that the PS compared to leaf tissue specifically accumulates high amounts of rRNA and snRNA fragments with distinct sizes.
Figure 3.
Northern assays confirm the presence of truncated rRNAs, snRNAs, and 7S signal recognition particle RNA in PS exudate. Approximately 5 μg PS RNA (PS) and pumpkin leaf RNA (LF) were transferred on nitrocellulose membranes after denaturing PAGE and incubated with specific oligonucleotide probes. Arrowheads, RNA fragments appearing specifically in the PS exudate.
A Specific Subset of Full-Length and Truncated tRNAs Is Present in the PS
We observed a nonequal distribution of tRNA sequences in the cloned PS cDNA pool. For example, 57 Asp-tRNA, three Arg-tRNA, and no Ile-tRNA clones were found in the PS cDNA library (Table I; Supplemental Materials S3). Also, we found a high number of tRNA fragments. Therefore, we inspected the presence of all tRNA anticodon families in the PS by northern assays (Fig. 4A; Table I). All probed tRNA species could be detected in the PS extract except Ile-tRNA and Thr-tRNA, which produced no or only a faint signal, respectively. The lack of a specific Ile-tRNA species in the PS supports the notion that, similar to other phloem-specific RNAs (Yoo et al., 2004; Zhong et al., 2008), tRNA molecules are also selectively transferred to the sieve tube system.
Table I.
tRNAs detected in the phloem exudate and codon usage frequency in pumpkin
n/a, Not analyzed.
| tRNA (Codon) | No. of Clonesa | cDNA Clone Size in nt (tRNA Region)b | Presence in PS | Processed Form in PS (Sizes in nt) | Codon Usage Frequencyc | Probes Usedd |
|---|---|---|---|---|---|---|
| tRNA-Asp (GAC) | 57 | 30–47 (3′) | ++ | Yes (∼38) | 25.2 | 41–60 |
| tRNA-Lys (AAG) | 45 | 38–46 (3′) | ++ | Yes (∼60) | 39.2 | 36–55 |
| tRNA-Gly (GGA) | 16 | 31–41 (5′) | + | Yes (∼31/∼37) | 21.4 | 6–25 |
| tRNA-Met (AUG) | 12 | 32–45 (5′) | + | Yes (∼33/∼44/∼68) | 24.7 | 8–27/53–72 |
| tRNA-Glu (GAG) | 6 | 58–60 (3′) | + | Yes (∼58) | 39.8 | 22–41 |
| tRNA-Pro (CCA) | 4 | 38–41 (3′) | + | Yes (∼42) | 14.9 | 47–66 |
| tRNA-Ser (AGC) | 2 | 42/47 (3′) | + | None | 12.0 | 56–75 |
| tRNA-Arg (CGU) | 1 | 41 (3′) | + | None | 12.0 | 31–50 |
| tRNA-Gln (CAA) | 0 | n/a | + | Yes (∼37/∼66) | 21.2 | 31–50 |
| tRNA-Ala (GCU) | 0 | n/a | + | Yes (∼35) | 24.2 | 31–50 |
| tRNA-Asn (AAC) | 0 | n/a | + | Yes (∼40) | 27.2 | 5–24/51–70 |
| tRNA-Trp (UGG) | 0 | n/a | + | Yes (∼50) | 17.1 | 30–49 |
| tRNA-Tyr (UAC) | 0 | n/a | + | Yes (∼31/∼44/∼60) | 15.6 | 11–30 |
| tRNA-Phe (UUC) | 0 | n/a | + | Yes (∼65) | 26.3 | 11–30 |
| tRNA-Cys (UGC) | 0 | n/a | + | None | 8.1 | 31–50 |
| tRNA-Leu (CUC) | 0 | n/a | + | None | 12.8 | 31–50 |
| tRNA-His (CAC) | 0 | n/a | + | None | 12.4 | 31–50 |
| tRNA-Val (GUU) | 0 | n/a | + | None | 22.0 | 46–65 |
| tRNA-Thr (ACU) | 0 | n/a | −/+ | Yes (∼36/∼49/∼61) | 13.4 | 31–50 |
| tRNA-Ile (AUA) | 0 | n/a | + | n/a | 12.4 | 46–65 |
| tRNA-Ile (AUU) | 0 | n/a | − | None | 24.3 | 31–50 |
Number of clones similar to tRNA found in the PS cDNA library.
Length of the cloned cDNA fragment in bases.
Codon usage frequency in pumpkin calculated per thousand codons according to release 2007 (http://www.kazusa.or.jp/codon/).
Region used to probe tRNAs with oligonucleotides.
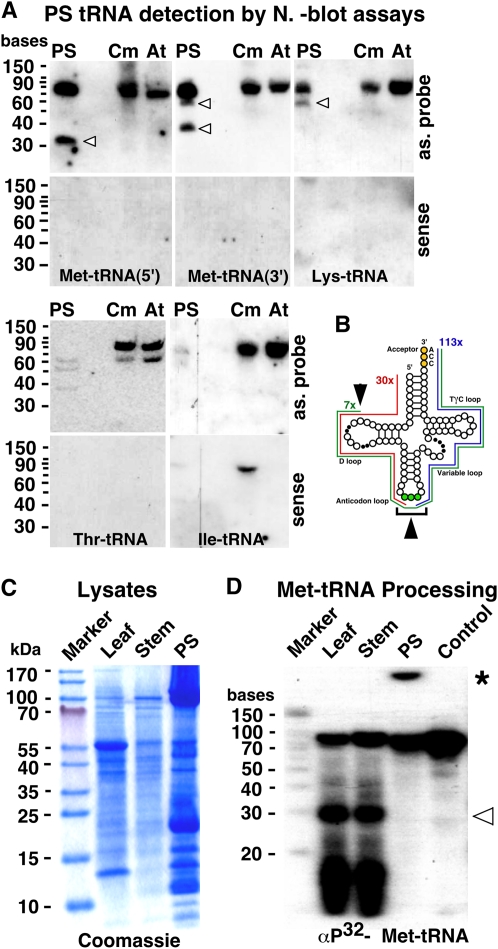
Figure 4.
PS exudate tRNA fragments are processed outside the phloem. A, Northern-blot assays. Approximately 5 μg RNA from pumpkin PS, leaves (Cm), and Arabidopsis rosette leaves (At) were probed with labeled oligonucleotides hybridizing with specific tRNAs. Arrowheads indicate tRNA fragments detected exclusively in the PS. B, tRNA model indicating the number and size of tRNA fragments identified in the cDNA library. C, SDS-PAGE of leaf, stem, and PS protein extracts stained with Coomassie Brilliant Blue. D, Processing of in vitro-produced, [α32P]ATP-labeled Met-tRNA by protein lysates as shown in C. Note that Met-tRNA processing was not observed with PS protein lysate. Arrowhead, Met-tRNA fragments formed after incubation with leaf or stem lysate. *, Shifted RNA band observed due to presence of phloem RNA-binding proteins.
All 18 detected tRNAs appeared as full-length mature tRNA molecules within the predicted size range of 70 to 80 bases. In addition, 12 of the 20 probed tRNAs were also detected as smaller PS-specific fragments, which were not present in leaf tissue RNA extracts (Fig. 4; Table I). In all sequenced PS tRNA clones for which the 3′ sequence information was available, we found a posttranscriptional 3′-cytidine-cytidine-adenosine (CCA) modification typical for mature aminoacylated tRNAs (Supplemental Materials S2). Thus, the identified tRNAs and their fragments derived from edited and functional aminoacylated tRNA molecules capable of transferring amino acids to the protein translation apparatus.
tRNAs Are Processed Prior to Transport into Sieve Elements
The detected PS tRNA fragments seem to result from a specialized RNA endonuclease activity cutting specifically at the tRNA anticodon or D loop. For example, all cloned Asp-tRNA PS fragments match the 3′ half following the anticodon, whereas the sequences similar to Met-tRNA match the 5′ tRNA region prior to the anticodon loop (Supplemental Materials S3). In summary, the cloned tRNA fragments covered approx. three quarters or the 5′ and 3′ halves of the tRNA sequences, respectively (Fig. 4B).
To learn whether the tRNA fragments are produced within the phloem tissue, we exposed in vitro-produced, [α32P]ATP-labeled Met-tRNA to tissue lysate or PS exudate prepared following a protocol used for assaying RNA processing (Tang et al., 2003). The transcript was incubated with leaf, stem, or phloem protein extracts (Fig. 4C) and RNA processing was analyzed after 3 h (Fig. 4D). A truncated Met-tRNA fragment was produced by leaf or stem extracts but not by PS extracts. The apparent size of approximately 33 bases of the processed Met-tRNA fragment was similar to that observed on northern blots (Fig. 4A). Thus, the tRNA fragments observed in the PS seem to be produced in leaf tissues prior to their allocation to sieve elements.
PS RNA But Not PS Proteins Inhibit Translation
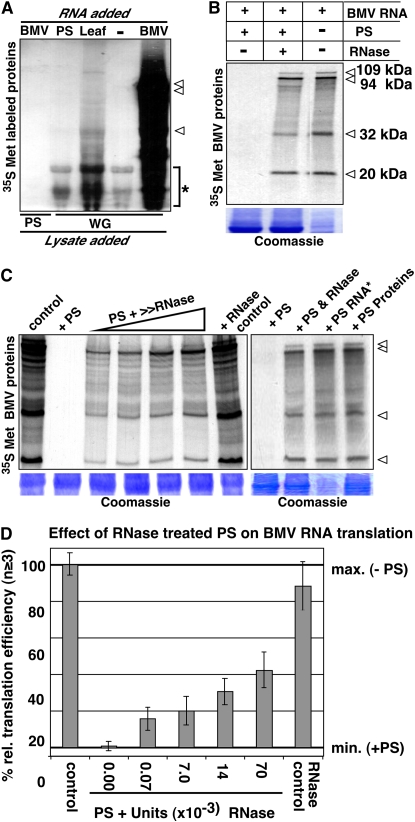
It is generally accepted that the sieve tubes are devoid of ribosomes and, thus, lack translational activity. However, high amounts of mRNA (Ruiz-Medrano et al., 1999; Omid et al., 2007) and si/miRNA (Yoo et al., 2004; Buhtz et al., 2008) were reported to be present in and delivered via the phloem transport system. Our analysis on pumpkin suggests that in addition, high levels of RNA molecules involved in translation, such as rRNA and tRNA, exist in the sieve tubes. Thus, we asked whether translational activity could be detected in PS extracts supplemented with brome mosaic virus (BMV) RNA and a buffer system facilitating in vitro translation. Translation was determined by incorporation of [35S]-labeled Met in nascent peptides (Fig. 5A). First, we mixed freshly harvested PS with BMV RNA and, in parallel, intended as a positive control, PS RNA, leaf RNA, or BMV RNA was added to wheat germ (WG) lysate. In contrast to the positive control with WG lysate, even after long incubation (4 h), no BMV RNA translation was detected in the PS extract. In WG lysate incubated with leaf RNA, low amounts of newly synthesized labeled proteins were detected. However, the addition of PS extract to WG lysate strongly interfered with BMV RNA translation, which normally would produce three major bands with a size of 20, 32, and 94 kD and one minor band with a size of 109 kD (Fig. 5B). To ensure that proper conditions were used and that the PS-mediated translational inhibition was not a result of interfering ions or sugars present in PS extracts, we tested the effect of RNA-depleted PS on translation efficiency of BMV RNA (Fig. 5, B and C). Protein production recovered when PS pretreated with increasing amounts of rRNase A was added to the WG in vitro translation system (Fig. 5, B and C). However, this recovery due to degradation of the PS RNA content contradicts the results obtained with denatured PS RNA added to the WG system, the extracted PS RNA did not inhibit BMV RNA translation (Fig. 5C).
Figure 5.
PS RNAs, not PS proteins, inhibit in vitro translation. Translation reactions were performed in the presence of [35S]Met and protein production was determined by detecting labeled protein(s) after denaturing PAGE. A, Test of PS exudate translational activity. No translational activity of BMV RNA with PS lysate, weak translational activity after adding leaf RNA to WG; −, negative control avoiding RNA and positive control with BMV RNA (BMV) in WG extract. Note that the gel was overexposed to detect minor quantities of proteins produced in the translation assays. *, Unspecific background. B, PS exudate treated with RNase allows translation. PS exudate (5 μL) inhibited translation, whereas RNase-treated PS exudate allowed BMV RNA translation similar to the positive control without PS. C, Depletion of PS RNA. Compared to the + control with WG and BMV RNA, addition of PS exudate effectively inhibited BMV RNA translation. Increasing quantities of rRNase A (0.00007–0.014 units) added to the PS exudate resulted in an increased BMV RNA translation. Note that the added rRNase A was inactivated after incubation and did not affect BMV RNA translation (+ RNase control). Denatured PS RNA (RNA*) and PS proteins did not decrease BMV RNA translation. Arrowheads, BMV RNA-translated proteins with their expected sizes of 20 kD (coat protein), 32 kD (movement protein), 94 kD (RNA-dependent RNA polymerase), and 109 kD (methyltransferase/helicase). Bottom panel, Coomassie Brilliant Blue-stained protein-loading control. D, Statistical analysis of the relative translation efficiency in the presence of untreated PS (+PS), rRNase A-treated PS, and controls without PS (−PS). Number of repeated experiments, n ≥ 3. Error bars = sd of the means.
Native PS RNA Inhibits Translation
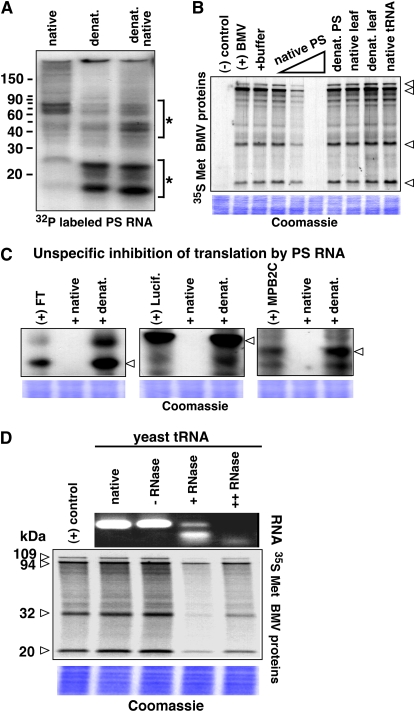
The lack of translation inhibition by PS RNA, which was isolated using a harshly denaturing guanidinium thiocyanate/phenol (Trizol) extraction protocol, raised the question whether the RNA isolation method interferes with RNA function. Thus, we avoided Guanidinium thiocyanate and extracted the PS RNA in a lightly acidic (pH 5.2) environment reported to stabilize the tertiary structure of RNA (Nixon and Giedroc, 2000; Flinders and Dieckmann, 2001; Wadkins et al., 2001; Biala and Strazewski, 2002). First, we compared the size distribution and capacity to inhibit translation of denatured (Trizol-treated) to native (acidic phenol-treated) PS RNA (Fig. 6).
Figure 6.
Native PS RNA and truncated tRNAs inhibit translation. A, Comparison of small PS RNA size distribution among native isolated PS RNA, denatured PS RNA, and native re-isolated denatured PS RNA. *, RNA fragments appearing in different amounts. B, Inhibition of translation. Increasing amounts of native PS RNA, denatured PS RNA, native leaf RNA, denatured pumpkin leaf total RNA, and native yeast tRNA. Arrowheads, BMV RNA-translated proteins. C, Sequence specificity and translation inhibition by native PS RNA. FT, luciferase (Lucif.), and AtMPB2C RNA translation was inhibited by native (middle lanes), but not by denatured (denat.), PS RNA (right lanes). D, Native tRNA fragments produced by rRNase A inhibit translation. Top, Ethidium bromide agarose gel of yeast tRNA used in the translation assays. Bottom, SDS-PAGE of translated [35S]Met-labeled BMV proteins. Note that yeast tRNA fragments inhibit BMV RNA translation, whereas full-length tRNA or fully degraded tRNA had no effect. B to D, Arrowheads indicate BMV RNA-translated proteins (for further details, see Fig. 5 legend). Lower panel, Coomassie Brilliant Blue-stained protein-loading control.
In comparison to denatured PS RNA, native PS was enriched with approximately 70- to 80-base-long RNA fragments, which resemble the size fraction of tRNAs, and contained fewer si/miRNA molecules (Fig. 6A). In contrast to denatured PS RNA, native leaf RNA, and native yeast (Saccharomyces cerevisiae) tRNA fractions, native isolated PS RNA and protease treated PS efficiently inhibited in vitro translation of BMV RNA (Fig. 6B; Supplemental Materials S4). The native PS RNA inhibition was also observed with FLOWERING LOCUS T (FT), Luciferase, and Arabidopsis MOVEMENT PROTEIN BINDING PROTEIN 2C (AtMPB2C) RNA (Fig. 6C), demonstrating sequence-independent PS RNA imposed inhibition of translation.
PS tRNA Fragments May Contribute to Translational Inhibition
In general, aberrant DNA and RNA fragments have the potential to interfere with ribosomal activity, resulting in a loss of mRNA translation (Dao et al., 1994; Piepenburg et al., 2000; Bakowska-Zywicka and Twardowski, 2007). As it was technically not feasible to isolate pure PS tRNAs in high amounts, we used yeast tRNA to evaluate the possibility that PS tRNA fragments mediate translation inhibition. By rRNase A treatment, we produced tRNA fragments and tested their effect on in vitro translation (Fig. 6D). Full-length (untreated) tRNA and nucleotides from fully digested tRNA molecules had no effect on translation, whereas fragmentized tRNA molecules isolated under native conditions were effectively blocking translation. Taken together, these results are consistent with a view that tRNA fragments present in the PS might be one of the RNA components interfering with ribosomal activity.
DISCUSSION
In this study, we demonstrated that high quantities of small ncRNAs are present in PS extracts. Small fragments from rRNAs (e.g. 25S rRNA), tRNAs (e.g. Met-tRNA), and snRNAs (e.g. spliceosomal U4 RNA) were exclusively detected in the PS RNA population and not in leaf RNA extracts. RNA degradation assays indicate that the detected small RNA fragments are not produced during the harvesting process by activation of an RNase (Fig. 1). Additional controls in the form of RT-PCR assays on Rubisco mRNA confirmed that RNA from surrounding tissues did not contaminate the isolated PS RNA fractions.
Implicating a specific RNA transfer to the enucleated sieve tube system, all essential tRNAs except Ile tRNA and Thr tRNA seem to be present in relatively high amounts in the phloem exudate (Table I; Fig. 4). Our in vitro tRNA processing assays suggest the presence of a specific RNA endonuclease activity in surrounding tissues rather than in the phloem system. This observation is consistent with a notion that PS tRNA fragments produced in leaves are specifically transferred to the phloem tissue via plasmodesmata. However, we cannot exclude the possibility that the observed uneven distribution of tRNA molecules (Fig. 4; Table I) is a result of a PS RNAse activity degrading subsets of RNAs within the sieve tube system. Alternatively, it could well be that the PS tRNA fragments are incomplete degradation remnants from differentiating sieve tube cells loosing their nuclei. Because degradation of aberrant tRNAs seems to occur independent of their identity (LaCava et al., 2005), we consider this scenario as unlikely. Consistent with the idea that PS RNA originates from surrounding source tissues, we propose that ncRNAs and fragments thereof are produced in leaf tissues and transferred selectively into sieve elements. This notion is supported by the existence of a phloem-specific transport system in pumpkin, which selectively delivers small RNA molecules from companion cells to sieve elements (Yoo et al., 2004).
In contrast to leaf RNA and to RNA-depleted PS protein extracts, PS RNA interferes with translation (Fig. 5 and Fig. 6). The observed inhibition of translation follows a complex reaction curve suggesting a multi-component inhibition system (Supplemental Materials S4). These experiments allowed us to exclude the possibility that proteins such as the rRNA depurinating ribosome-inactivating proteins (Taylor et al., 1994; Mansouri et al., 2006) mediate the inhibition of translation.
Potential Function of PS tRNA Halves
In general, mature tRNAs are made from precursor tRNAs by cleaving off 5′ leader and 3′ trailer sequences and, if they contain an intron, by splicing. In addition, specific enzymes modify a number of bases, and three nucleotides CCA are added at the 3′ end. A correctly edited tRNA is aminoacylated prior to export from the nucleus (Hopper and Phizicky, 2003). Aberrant tRNAs potentially interfering with cellular function are completely degraded in the nucleus by an exosome complex (LaCava et al., 2005). Regarding mature PS tRNA, it is possible that in the cytosol of mature sieve elements, specific amino acids from aminoacylated PS tRNAs are used to form polypeptides at ribosomal complexes. The majority of the cloned PS tRNAs from which we obtained sequence information of the 3′ end carry the three nucleotides CCA (Supplemental Materials S3). Thus, functional aminoacylated tRNAs capable of facilitating translation seem to exist in the phloem. However, ultrastructural observations (Sjolund and Shih, 1983), amino acid loading experiments (Fisher et al., 1992), and proteomic analysis of the PS content from several species such as Cucumis sativus (Walz et al., 2004), Triticum aestivum (Fukuda et al., 2005), or B. napus (Giavalisco et al., 2006) showed that a translatory system based on ribosomes using tRNAs is not present in sieve elements. In addition, supporting the notion that peptide synthesis cannot occur in the sieve tube system, we observed that native PS RNA and truncated tRNAs effectively inhibit translation.
In Arabidopsis, 81 nuclear-encoded cytosolic tRNAs are reported to harbor an approximately 10- to 13-base-long intron downstream of the anticodon loop: 70 of 76 Tyr-tRNAs (GTA) and 11 of 24 Met-tRNAs (http://lowelab.ucsc.edu/GtRNAdb/Athal/; Akama and Kashihara, 1996; Lowe and Eddy, 1997). However, all cloned PS tRNAs belong to plant tRNA gene classes lacking introns. Thus, aberrant splicing could not be the source of the tRNA fragments/halves detected in the PS.
Evidence for a potential function of tRNA halves was found in studies on infectious filamentous fungi Aspergillus fumigatus small ncRNAs. tRNAs halves corresponding to the 5′ or 3′ parts of 16 tRNAs are generated during development of asexual spores (Jochl et al., 2008). A similar phenomenon was observed in the prokaryote Streptomyces coelicolor upon developmental switch (Haiser et al., 2008). tRNA halves were also detected in Tetrahymena during early amino acid starvation (Lee and Collins, 2005) and in yeast, Arabidopsis, and human cells upon oxidative stress (Kawaji et al., 2008; Thompson et al., 2008). All these tRNA halves seem to originate from mature tRNAs cleaved in the anticodon loop by an unidentified RNase. Although no experimental evidence was provided, the authors speculate whether these tRNA fragments could regulate protein synthesis (Lee and Collins, 2005; Jochl et al., 2008). In favor of this idea, we observed that heterologous tRNA fragments added to the in vitro translation system effectively inhibited translation (Fig. 6).
Phloem-Delivered tRNA Halves as a Potential Long-Distance Signal
An additional functional aspect for phloem-allocated tRNAs may be that they serve as a source for cytokinins. tRNA:isopentenyltransferases target cis-hydroxy isopentenylated adenosine residues immediately 3′ to the anticodon of the group NNA tRNAs (Eisenberg et al., 1979), such as Cys, Leu, Phe, Try, Trp, and Ser tRNAs, which we detected in the PS. In Arabidopsis, two such tRNA-modifying genes are highly expressed in sink tissues such roots or young leaves, which are described as a source for cis-Zeatin type cytokinin (Miyawaki et al., 2004; Miyawaki et al., 2006). Thus, phloem-delivered tRNAs could be a systemic source for the paracrine plant hormone cytokinin with numerous, graft-transmitted, regulatory functions such as nutrient sink-strength increase, senescence delay, lateral bud growth stimulation, and inhibition of cell elongation (Mok and Mok, 2001).
Fragmentation of Met-RNA seems to depend on leaf-specific enzymes not present in phloem exudates, and these Met-tRNA fragments apparently accumulate in the PS (Fig. 4). From a functional point of view, allocation of tRNA fragments might serve as a protection mechanism to deplete tRNA fragments from translational active tissues where they would interfere with translation. Alternatively, mature and halved tRNAs could serve as a long-distance signal informing sink tissues (e.g. root and shoot apices) about the metabolic status of source tissues (expanded leaves). Aberrant tRNA halves produced in leaves would down-regulate protein synthesis, whereas full-length aminoacylated tRNA would increase translational activity. Thus, the relative amount of full-length versus halved tRNAs may determine the pace of growth in sink tissues. Another potential function of PS RNA-mediated inhibition of translation may be as a systemic apoptotic signal, which triggers differentiation of provascular tissue. In the phloem-unloading zone below apical meristems, the stalling of ribosomal activity may assist a developmental program leading to enucleated vascular cells forming the sieve tube system and xylem vessels.
MATERIALS AND METHODS
Plant Materials and PS Harvest
Pumpkin (Cucurbita maxima) Dutch. cv Big Max plants were grown in the greenhouse under natural daylight conditions with mid-day light intensity in the range of 1,200 to 1,500 μmol m−2 s−1 and day/night temperatures of approximately 26°C/22°C. Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 plants were grown in controlled-environment chambers under long-day conditions (16 h light, 1,000 μmol m−2 s−1, and day/night temperatures of 22°C). PS was collected from flowering plants as described (Ruiz-Medrano et al., 1999; Yoo et al., 2004). In short, after cutting the stem/petiole, the liquid appearing on the surface was removed four to five times with heat-sterilized filter paper (Whatman M3). Avoiding physical contact with the plant tissue, 100 μL PS was harvested with 10-μL pipette tips and transferred into tubes containing 1 μL β-mercaptoethanol and 1 μL RNAsin RNase inhibitor (40 units μL−1, Promega) and stored on ice until further use.
PS Protein and RNA Isolation and Detection
PS protein extracts were prepared as described (Aoki et al., 2002; Yoo et al., 2004). PS RNA was extracted from 250 μL freshly harvested PS under either fully denaturing conditions with 1 mL Trizol LS (guanidinium thiocyanate-phenol, pH 4.5; Invitrogen) and 200 μL chloroform as described (Ruiz-Medrano et al., 1999) or relatively mild (native) phenol conditions following a modified protocol (Varshney et al., 1991a, 1991b) by adding 50 μL 3 m NaOAC, pH 5.2, 200 μL 1× TE buffer (10 mm Tris, pH 7.4, 1 mm EDTA) to 250 μL PS and submitting to the standard phenol extraction protocol (25:24:1, pH 5.0 ± 0.2). To precipitate the native PS RNA, 2 volumes of 99% ethanol were added and incubated for 12 h at −20°C. After centrifugation (16,000g, 30 min at 4°C), the pellet was washed twice with 70% ethanol/(+10 mm NaOAC, pH 5.2, for native conditions), once with 99% ethanol, air dried, and resuspended in 50 μL acidic buffer (10 mm NaOAC, pH 5.2, 1 mm EDTA). The usual yield was 20 to 40 ng RNA/100 μL PS.
Labeling of PS RNA with [γ32P]ATP was done via a phosphate exchange reaction as described (Yoo et al., 2004). In short, approximately 8 ng PS RNA was mixed with 1× polynucleotide kinase buffer (Ambion) containing 5% PEG6000 (Sigma), 5 nm ATP (Sigma), 0.1 mm ADP (Sigma), 10 μCi [γ32P]ATP (NEN), and 10 units T4 polynucleotide kinase (Ambion), incubated at 37°C for 30 min, and passed through a G-25 column (Amersham). Labeled PS RNA was submitted to denaturing 7 m urea 15% PAGE as described (Yoo et al., 2004).
Cloning of PS Small RNA and RNA Isolation from Tissues for RT-PCR
An adopted elution and cloning protocol (Lau et al., 2001) was used to isolate and clone small PS RNA fragments. In short, after denaturing PAGE, the gel was washed twice with RNase-free water, gel slabs containing the 30- to 90-nucleotide-long 3′ [32P]-labeled RNA fragments were harvested and cloned (Supplemental Materials S1). RNA from pumpkin tissues was harvested avoiding veins, ground up in liquid nitrogen, and submitted to native phenol extraction (see above) and precipitated by adding 1/3 volume 10 m LiCl for 12 h at 4°C and the resulting pellet was washed with 2.5 m LiCl, 80% and 99% ethanol. Then 0.5 μg of total RNAs from pumpkin leaves and from pumpkin PS were submitted to RT-PCR reactions using specific primers (Supplemental Materials S1).
In Vitro Transcription/Translation and RNA Stability Assays
For in vitro T7 transcription and WG translation reactions in the presence of [35S]Met (NEN), the manufacturer's protocols were followed (T7 Megascript, Ambion; Wheat Germ Extract Kit, Promega). To evaluate RNA stability, 30 ng labeled RNA was produced in the presence of [α32P]ATP, applied onto PS exudates appearing on the stem/petioles, and the resulting 200 μL PS/labeled RNA mixture was extracted following the denaturing phenol extraction protocol. T7 cDNA templates for KNOTTED1 (GenBank accession no. AY312169), FT (The Arabidopsis Information Resource [TAIR] accession no. AT1G65480), AtMPB2C (TAIR accession no.At5G08120), luciferase (GB accession no. E15166), AtU4 snRNA (TAIR accession no. AT3G06900), CmU4 snRNA, and pumpkin Met-tRNA were produced with T7 promoter primers (Supplemental Materials S1).
Preparation of Leaf and PS Lysates and RNA Processing Assays
The assays were performed as described (Tang et al., 2003). In short, pumpkin tissue was ground up in liquid N2 and approximately 170 mg tissue powder or 100 μL harvested PS was mixed with 500 μL lysis buffer (100 mm KOAc, 30 mm HEPES-KOH, pH 7.4, 2 mm MgOAc, 5 mm dithiothreitol, 1 mg/mL Pefabloc SC [Boehringer Mannheim]), vortexed for 5 min, and centrifuged (14,500g, 25 min, 4°C). The supernatant was stored at −80°C until use. For RNA processing assays, approximately 10 μL containing 2 μg RNA of in vitro-transcribed, [α32P]ATP (NEN)-labeled RNA probe was mixed with 10 μL reaction buffer (0.1 mm GTP, 0.5 mm ATP, 10 mm phosphocreatine, 10 mm creatine phosphokinase, 5 mm dithiothreitol, 1 unit RNAsin, 1 volume lysate), and incubated 3 h at 25°C. The reactions were stopped by adding 50 μL Protease K solution (140 μg Proteinase K [Roche] in 280 mm Tris-Cl, pH 8, 43.75 mm EDTA, 525 mm NaCl, 3.5% SDS) and incubation at 65°C for 15 min.
Depletion of RNA or Proteins Present in the PS Extract
To deplete PS RNA, 0.00007 units, 0.007 units, 0.014 units, or 0.7 units of rRNase A (Ambion) was added to 10 μL PS exudate and incubated at 25°C for 20 min. The reaction was stopped with 2 mm EDTA and 4 units RNAsin (Promega) and stored on ice until usage. Complete PS protein depletion was done by mixing extracted PS RNA with 20 μg Protease K and incubation at 25°C for 10 min.
Northern-Blot Assays
Northern hybridization assays were done as described (Yoo et al., 2004). In short, approximately 5 μg of RNA from pumpkin PS, leaves, and Arabidopsis ecotype Columbia-0 leaves were submitted to denaturing 7 m urea 15% PAGE, transferred (Semi-Dry Electrophoretic Transfer Cell system, Bio-Rad) onto a H-bond nylon membrane (Amersham), UV cross-linked (Bio-Rad), and stained with 0.0025% methylene blue to confirm that similar amounts of RNA were loaded. After prehybridization (UTRHYB solution, Ambion) for 1 h at 68°C, [γ32P]ATP-labeled oligonucleotide probes (Supplemental Materials S1) were added, and the membrane was incubated for 12 h at 37°C. After 2× washing with 5 mL/cm2 2× SSC buffer (300 mm NaCl, 30 mm Na3 citrate, pH 7.0) at 25°C for 2 min, the membrane was exposed to an x-ray film (Fuji) or to a phosphoimager system (Amersham).
In Silico Analysis of Cloned PS RNA
To find known DNA fragments similar or identical to the cloned PS RNA sequences, the BLAST (Altschul et al., 1990) algorithm was used. BLAST searches were performed against plant-specific databases accessible via TAIR (http://www.arabidopsis.org/Blast/index.jsp) and the nonredundant nucleotide GenBank database present at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Materials S1. Cloning of small PS RNA and oligonucleotides used in the study.
Supplemental Materials S2. Sequence comparisons and statistics of cloned small PS RNAs.
Supplemental Materials S3. Sequences of all cloned small PS RNAs.
Supplemental Materials S4. Dosage-dependent inhibitory effect on translation of native PS RNA and PS RNA protease treatment assays.
Supplementary Material
Acknowledgments
We thank Dave Jackson (Cold Spring Harbor Laboratory), Shmulik Wolf (Hebrew University), and Bill Lucas (University of California, Davis) for critical comments on the project; A. Hartig and A. Bachmair (University of Vienna) for helpful comments on the manuscript text; and N. Winter, D. Fichtenbauer, and K. Pranjic, members of the F.K. lab, for technical advice, discussions, and comments.
This work was supported by the Austrian Science Fund (grant no. P19682–B03 to F.K.).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Friedrich Kragler (friedrich.kragler@univie.ac.at).
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Akama K, Kashihara M (1996) Plant nuclear tRNA(Met) genes are ubiquitously interrupted by introns. Plant Mol Biol 32 427–434 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215 403–410 [DOI] [PubMed] [Google Scholar]
- Aoki K, Kragler F, Xoconostle-Cazares B, Lucas WJ (2002) A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc Natl Acad Sci USA 99 16342–16347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowska-Zywicka K, Twardowski T (2007) Correlation of the structure and conformational changes of selected fragments of plant small ribosomal RNA within the steps of polypeptide chain elongation. J Plant Physiol 164 496–504 [DOI] [PubMed] [Google Scholar]
- Biala E, Strazewski P (2002) Internally mismatched RNA: pH and solvent dependence of the thermal unfolding of tRNA(Ala) acceptor stem microhairpins. J Am Chem Soc 124 3540–3545 [DOI] [PubMed] [Google Scholar]
- Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J (2008) Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J 53 739–749 [DOI] [PubMed] [Google Scholar]
- Chen H, Rosin FM, Prat S, Hannapel DJ (2003) Interacting transcription factors from the three-amino acid loop extension superclass regulate tuber formation. Plant Physiol 132 1391–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao V, Guenther R, Malkiewicz A, Nawrot B, Sochacka E, Kraszewski A, Jankowska J, Everett K, Agris PF (1994) Ribosome binding of DNA analogs of tRNA requires base modifications and supports the “extended anticodon”. Proc Natl Acad Sci USA 91 2125–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering-Saad C, Newbury HJ, Couldridge CE, Bale JS, Pritchard J (2006) A phloem-enriched cDNA library from Ricinus: insights into phloem function. J Exp Bot 57 3183–3193 [DOI] [PubMed] [Google Scholar]
- Eisenberg SP, Yarus M, Soll L (1979) The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J Mol Biol 135 111–126 [DOI] [PubMed] [Google Scholar]
- Fisher DB, Wu Y, Ku MS (1992) Turnover of soluble proteins in the wheat sieve tube. Plant Physiol 100 1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinders J, Dieckmann T (2001) A pH controlled conformational switch in the cleavage site of the VS ribozyme substrate RNA. J Mol Biol 308 665–679 [DOI] [PubMed] [Google Scholar]
- Fukuda A, Fujimaki S, Mori T, Suzui N, Ishiyama K, Hayakawa T, Yamaya T, Fujiwara T, Yoneyama T, Hayashi H (2005) Differential distribution of proteins expressed in companion cells in the sieve element-companion cell complex of rice plants. Plant Cell Physiol 46 1779–1786 [DOI] [PubMed] [Google Scholar]
- Giavalisco P, Kapitza K, Kolasa A, Buhtz A, Kehr J (2006) Towards the proteome of Brassica napus phloem sap. Proteomics 6 896–909 [DOI] [PubMed] [Google Scholar]
- Golecki B, Schulz A, Thompson GA (1999) Translocation of structural P proteins in the phloem. Plant Cell 11 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiser HJ, Karginov FV, Hannon GJ, Elliot MA (2008) Developmentally regulated cleavage of tRNAs in the bacterium Streptomyces coelicolor. Nucleic Acids Res 36 732–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood V, Yu TS, Huang NC, Lucas WJ (2005) Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J 42 49–68 [DOI] [PubMed] [Google Scholar]
- Hopper AK, Phizicky EM (2003) tRNA transfers to the limelight. Genes Dev 17 162–180 [DOI] [PubMed] [Google Scholar]
- Jochl C, Rederstorff M, Hertel J, Stadler PF, Hofacker IL, Schrettl M, Haas H, Huttenhofer A (2008) Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic Acids Res 36 2677–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaji H, Nakamura M, Takahashi Y, Sandelin A, Katayama S, Fukuda S, Daub CO, Kai C, Kawai J, Yasuda J, et al (2008) Hidden layers of human small RNAs. BMC Genomics 9 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D (2005) RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121 713–724 [DOI] [PubMed] [Google Scholar]
- Lau NC, Lim LP, Weinstein EG, Bartel DP (2001) An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294 858–862 [DOI] [PubMed] [Google Scholar]
- Lee SR, Collins K (2005) Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J Biol Chem 280 42744–42749 [DOI] [PubMed] [Google Scholar]
- Lough TJ, Lucas WJ (2006) Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol 57 203–232 [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Yoo BC, Kragler F (2001) RNA as a long-distance information macromolecule in plants. Nat Rev Mol Cell Biol 2 849–857 [DOI] [PubMed] [Google Scholar]
- Mansouri S, Nourollahzadeh E, Hudak KA (2006) Pokeweed antiviral protein depurinates the sarcin/ricin loop of the rRNA prior to binding of aminoacyl-tRNA to the ribosomal A-site. RNA 12 1683–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37 128–138 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA 103 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DW, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52 89–118 [DOI] [PubMed] [Google Scholar]
- Nakazono M, Qiu F, Borsuk LA, Schnable PS (2003) Laser-capture microdissection, a tool for the global analysis of gene expression in specific plant cell types: identification of genes expressed differentially in epidermal cells or vascular tissues of maize. Plant Cell 15 583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon PL, Giedroc DP (2000) Energetics of a strongly pH dependent RNA tertiary structure in a frameshifting pseudoknot. J Mol Biol 296 659–671 [DOI] [PubMed] [Google Scholar]
- Omid A, Keilin T, Glass A, Leshkowitz D, Wolf S (2007) Characterization of phloem-sap transcription profile in melon plants. J Exp Bot 58 3645–3656 [DOI] [PubMed] [Google Scholar]
- Pant BD, Buhtz A, Kehr J, Scheible WR (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenburg O, Pape T, Pleiss JA, Wintermeyer W, Uhlenbeck OC, Rodnina MV (2000) Intact aminoacyl-tRNA is required to trigger GTP hydrolysis by elongation factor Tu on the ribosome. Biochemistry 39 1734–1738 [DOI] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cazares B, Kragler F (2004) The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr Opin Plant Biol 7 641–650 [DOI] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cazares B, Lucas WJ (1999) Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development 126 4405–4419 [DOI] [PubMed] [Google Scholar]
- Sjolund RD, Shih CY (1983) Freeze-fracture analysis of phloem structure in plant tissue cultures. I. The sieve element reticulum. J Ultrastruct Res 82 111–121 [DOI] [PubMed] [Google Scholar]
- Tang G, Reinhart BJ, Bartel DP, Zamore PD (2003) A biochemical framework for RNA silencing in plants. Genes Dev 17 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Massiah A, Lomonossoff G, Roberts LM, Lord JM, Hartley M (1994) Correlation between the activities of five ribosome-inactivating proteins in depurination of tobacco ribosomes and inhibition of tobacco mosaic virus infection. Plant J 5 827–835 [DOI] [PubMed] [Google Scholar]
- Thompson DM, Lu C, Green PJ, Parker R (2008) tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14 2095–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney U, Lee CP, RajBhandary UL (1991. a) Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J Biol Chem 266 24712–24718 [PubMed] [Google Scholar]
- Varshney U, Lee CP, Seong BL, RajBhandary UL (1991. b) Mutants of initiator tRNA that function both as initiators and elongators. J Biol Chem 266 18018–18024 [PubMed] [Google Scholar]
- Wadkins TS, Shih I, Perrotta AT, Been MD (2001) A pH-sensitive RNA tertiary interaction affects self-cleavage activity of the HDV ribozymes in the absence of added divalent metal ion. J Mol Biol 305 1045–1055 [DOI] [PubMed] [Google Scholar]
- Walz C, Giavalisco P, Schad M, Juenger M, Klose J, Kehr J (2004) Proteomics of curcurbit phloem exudate reveals a network of defence proteins. Phytochemistry 65 1795–1804 [DOI] [PubMed] [Google Scholar]
- Xoconostle-Cazares B, Xiang Y, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Franceschi VR, Lucas WJ (1999) Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283 94–98 [DOI] [PubMed] [Google Scholar]
- Yoo BC, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, Lee YM, Lough TJ, Lucas WJ (2004) A systemic small RNA signaling system in plants. Plant Cell 16 1979–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Archual AJ, Amin AA, Ding B (2008) A genomic map of viroid RNA motifs critical for replication and systemic trafficking. Plant Cell 20 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.