Abstract
OBJECTIVE
To assess the technical feasibility of using a retrievable, closed cell intracranial stent delivered through a microcatheter for safe removal of foreign bodies or clot.
METHODS
In vitro and in vivo testing were performed to demonstrate the feasibility of using retrievable intracranial stents for foreign body or clot removal. In vitro testing was performed in an anatomically correct silicone vascular replica by partially deploying the stent around a coil, then retracting the stent into the microcatheter to trap the coil. Withdrawal of the stent delivery system into the guide catheter resulted in coil removal. Subsequently, the technique was evaluated in a porcine model of intracranial aneurysms, wherein both fresh clot and herniated coils were extracted from the carotid arteries.
RESULTS
In these experimental procedures, both herniated coils and fresh clot were safely and easily removed from the in vitro and in vivo models. No periprocedural adverse events were observed.
CONCLUSION
These in vitro and in vivo studies suggest the potential use of retrievable stents for the removal of foreign bodies or clot from the intracranial circulation.
Keywords: Aneurysm, Angiography, Clot, Coil, Endovascular treatment, Retrieval, Stent, Stroke
Endovascular coiling has become an accepted treatment for intracranial aneurysms, offering protection from rebleeding and satisfactory clinical outcomes (7). However, a recent review of a large case series has demonstrated that thromboembolic complications arising from coil embolization remain a significant concern for the endovascular treatment of intracranial aneurysms. In the treatment of 681 patients with ruptured aneurysms, procedural complications occurred in approximately 6% of patients (14). Of these complications, 80% were due to thromboembolism (14). An increase in thromboembolic complications has been reported with the use of temporary occlusion balloons to support the embolization of wide-neck aneurysms (11, 14). Moreover, detachable coil dislodgement from the aneurysmal sac or protrusion into the parent vessel could be a source of thromboembolic complications. These events are managed by surgical recanalization, use of stents to pin the material to the vessel wall, or use of various endovascular devices to retrieve the coil (2, 4, 9, 12, 13).
In addition, a long series of devices has been proposed for mechanical thrombectomy in acute stroke, culminating with the MERCI Retrieval System (Concentric Medical, Mountain View, CA), which has been approved by the Food and Drug Administration for intracranial clot extraction (8, 10). The MERCI device has offered patients with acute stroke a second opportunity for treatment if the narrow time window for pharmacological intervention has lapsed. However, the device is not optimal; successful vessel recanalization is achieved only in approximately half of the cases (10). In a different strategy to reconstitute flow in patients with acute stroke, Levy et al. (5, 6) demonstrated in a small patient series that intracranial stenting may be a viable option in patients resistant to standard acute stroke treatments.
The technique we report demonstrates the potential use of a retrievable closed cell intracranial stent, beyond its approved use in stent-assisted coiling of wide-neck intracranial aneurysms, to extract both foreign material and thrombus in an in vivo aneurysm model.
MATERIALS AND METHODS
In Vitro Model
In the Elastrat human vascular model (Elastrat Sàrl, Geneva, Switzerland), which mimics the vasculature extending from the femoral arteries to the cerebrovascular system, a retrievable, closed cell intracranial stent (Enterprise Vascular Reconstruction Device; Cordis Neurovascular, Miami Lakes, FL) was used to remove platinum coils. In three separate trials, a 4-mm × 8-cm Guglielmi Detachable Coil Ultra Soft (Boston Scientific Neurovascular, Fremont, CA) was deployed in the superior (M2) division of the right middle cerebral artery (MCA) (n = 2) (Fig. 1A) and in the M1 segment of the right MCA(n = 1). A peristaltic pump using saline at 37°C as a working fluid was used to drive the flow within the model. In a coaxial technique, a microcatheter (Prowler Plus; Cordis Neurovascular) was navigated to the level of the coils. The stent was partially deployed beyond the microcatheter, distal to the protruding coils (Fig. 1B). Subtle retraction of the stent caused the coil to become trapped within the stent struts, at which point the stent was resheathed (Fig. 1C). Next, the system was retracted, along with the coil, into the guide catheter positioned in the internal carotid artery (Fig. 1D).
FIGURE 1.
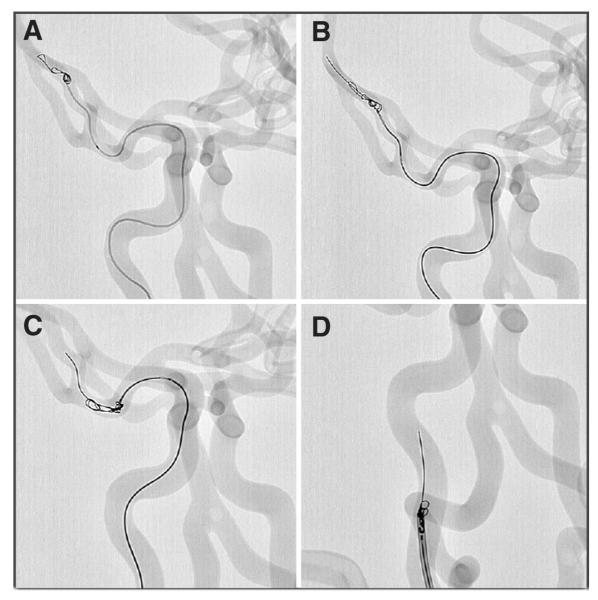
A, coil deployed into the superior division of the right middle cerebral artery. B, stent partially deployed distal to the coil. C, captured coil being retrieved, leading to capture within the guide catheter (D).
In the first experiment, the coil was removed after three attempts of stent resheathing in the right M2 segment of the model. The stent was examined after removal of the coil, and no damage to the struts was observed. In the second trial using the same microcatheter and stent, increased friction of the system was experienced. The microcatheter was relaced, which alleviated the friction, and the coil was removed after two passes of the system. In the third trial, in which the coil was floated and eventually trapped in the M1 segment of the right MCA, the first pass of the stent led to capture of the coil. During the retraction of the system, however, the coil fractured into two parts. The stent was redeployed, capturing both coil fragments, and they were removed successfully in a single attempt.
Animal Model
After successful removal of bare platinum coils with the stent from an in vitro model, this procedure was tested in a preclinical aneurysm model. All procedures described were approved by the Institutional Care and Use Committee at the University of Massachusetts Medical School. One female Yorkshire swine weighing 25 kg was premedicated with intramuscularly administered glycopyrrolate (0.01 mg/kg) and anesthetized with an intramuscular injection of a cocktail containing Telazol (5 mg/kg; Fort Dodge Laboratories, Fort Dodge, IA), xylazine (2.5 mg/kg), and ketamine (2.5 mg/kg). Anesthesia was maintained with mechanical ventilation of oxygen containing 2% isoflurane. Bilateral venous pouch aneurysms were created on the common carotid arteries (CCAs) using standard surgical technique (3). A heparin bolus (100 U/kg) was intravenously administered, and anticoagulation was sustained with maintenance administration of 500 units every hour.
Foreign Body Removal
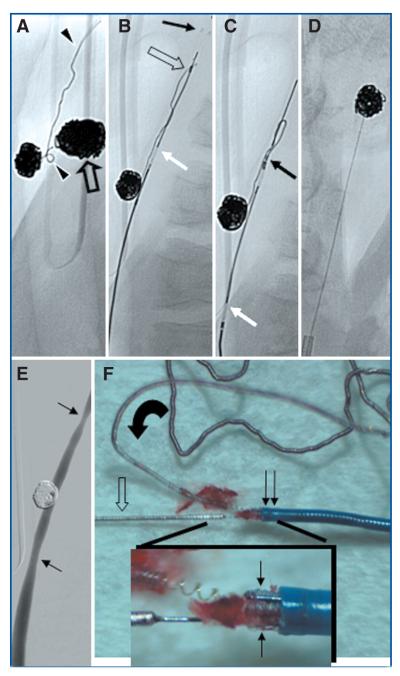
After angiography was performed (Allura FD20; Philips Medical Systems, Best, The Netherlands), the aneurysms were embolized with detachable coils. During coil embolization, parts of two coils herniated into the CCA from the left aneurysm (Fig. 2A). A 6-French guide catheter was placed into the CCA. Using the same technique described for the in vitro experiments, the stent was partially deployed beyond the microcatheter distal to the protruding coils (Fig. 2B). After a single attempt, the stent was used to capture (Fig. 2C) and remove (Fig. 2D) the coil. Both coils were successfully removed using this technique. Control angiography revealed a patent parent vessel (Fig. 2E). A macroscopic view of the trapped coil within the struts showed no stent fractures or damage to the microcatheter (Fig. 2F). The device was preserved in fine condition for further use.
FIGURE 2.
A, two coil loops (arrowheads) herniating into the left common carotid artery (CCA) and coil. Coil mass within contralateral aneurysm (open arrow). B, placement of microcatheter tip at the level of the coil (open arrow). Partial deployment of the stent; depicted are the distal markers (black arrow). White arrow indicates the positioning marker that indicates the final point for stent retrieval. Note that the second coil had been retrieved previously using the same technique and device. C and D, captured coil loop being removed by pulling back the system. E, final control angiography with mild vasospasm at the former site of vascular clips placed surgically for temporary aneurysm construction. F, macroscopic image of captured and unraveled coil loops (curved arrow) trapped within the stent distal markers (arrows) and microcatheter (double arrow). Note the stent delivery wire (open arrow).
Thrombus Removal
After a prolonged coiling procedure in the contralateral CCA aneurysm, spontaneous and occlusive clotting of the distal CCA occurred (Fig. 3A). The microcatheter was navigated distal to the clot, and the stent was partially deployed (Fig. 3B). The stent was partially resheathed, and the system was withdrawn into the guide catheter. Control angiography revealed that after a single pass of the device, the clot was cleared and the parent vessel was patent with some mild vasospasm (Fig. 3C). The stent system with removed clot was imaged under the microscope (Fig. 3D).
FIGURE 3.
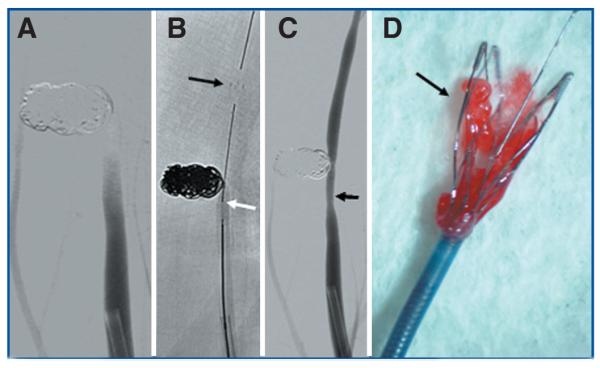
A, coiled aneurysm of the right CCA. Clot formation within the artery adjacent to the coil mass. B, placement of microcatheter tip into the clot. Partial deployment of the stent; depicted are the distal markers (black arrow). White arrow indicates the positioning marker (see also Fig. 1). C, complete removal of clot mass with reconstitution of blood flow. Arrow indicates mild residual vasospasm. D, macroscopic view of captured clot within the stent struts.
DISCUSSION
Our in vitro and in vivo experiments suggest that it may be feasible to remove both foreign bodies and clot material safely, effectively, and easily with a new generation of resheathable closed cell intracranial stents. The ability to navigate the system through a microcatheter to the most distal parts of the cerebral vasculature makes the device attractive for removal of dislodged coils. Currently, only a few foreign body retrieval devices are able to access distal cerebral vessels (4). In our experiment using the swine aneurysm model, which is susceptible to vasospasm and rapid thrombus formation, the CCAs remained patent after partial deployment of the device and extraction of the clot and coils. Although it is not demonstrated with the data presented in this article, we hypothesize that the device and technique described do not produce significant endothelial damage because the device is not dragged along the vessel wall, but rather resheathed by advancing the microcatheter. This hypothesis is supported by recent clinical observation with up to 6 months of angiographic follow-up, wherein the stent was used in the treatment of intracranial aneurysms (15).
Because this stent is retrievable, a partial deployment of the stent into the clot with or without adjunctive antiplatelet or fibrinolytic therapy should be evaluated in subsequent studies. If Thrombolysis In Myocardial Infarction II or III flow is obtained, the stent can be retrieved. However, the benefit of using this system could be a final deployment of the stent in the event that sufficient flow is not achieved. Stenting of clot in acute stroke has been reported with successful recanalization in up to 80% of cases with both balloon-expandable and self-expanding stents (5, 6). Likewise, should this technique be unsuccessful when removing a herniated coil, the coil mass may be pinned to the parent vessel wall by deploying the stent completely (2).
The swine aneurysm model used has limitations, including the lack of tortuosity. It should be noted that when resheathing the device within a tight curve, the friction experienced may increase. Because of this limitation, a tortuous human vascular replica was used in a series of in vitro experiments. In these experiments, multiple attempts were required in two of three trials to remove the coil successfully. This is likely because the silicone model is tacky, and the dislodged coil has the tendency to adhere to the inner surface of the tubing (1). Furthermore, we observed that after multiple passes of the stent system through the microcatheter, the increase in friction necessitated the use of a new microcatheter, likely because of damage to the hydrophilic coating. Throughout the series of in vitro experiments, including seven deployments, the same stent was used and did not show any signs of strut fracture. In one case, the coil was fractured after having been removed three times. However, both fragments were easily removed, likely because of the intact stretch-resistant member of the coil system.
The stent used in this study, available under a humanitarian device exemption, is not indicated for the described application and is quite an expensive tool at present. Moreover, new devices, including the Penumbra system (Penumbra, Inc., San Leandro, CA) for mechanical thrombectomy and the Alligator Retrieval Device (Chestnut Medical Technologies, Inc., Menlo Park, CA) for foreign body removal, are presently being evaluated clinically for their respective indications. Further evaluation of the present intracranial stent and the technique described is required to explore the possibility of expanding its indication, thus increasing the arsenal of tools for foreign body and clot removal.
CONCLUSION
Preliminary experimentation has demonstrated a novel application of a retrievable closed cell intracranial stent for foreign body and clot removal.
Acknowledgments
There are no financial disclosures related to the submission of the data reported in the article. Financial support of our research laboratory from Philips Medical Systems and the National Institutes of Health (Grant 1R21EB007767–01) is gratefully acknowledged.
Contributor Information
Ajay K. Wakhloo, Departments of Radiology, Neurosurgery, and Neurology, University of Massachusetts Medical School, Worcester, Massachusetts
Matthew J. Gounis, Department of Radiology, University of Massachusetts Medical School, Worcester, Massachusetts
REFERENCES
- 1.Asakura F, Yilmaz H, Abdo G, Sekoranja L, Millan D San, Augsburger L, Sztajzel R, Ruefenacht DA, Perren F, Lovblad KO, Goto K. Preclinical testing of a new clot-retrieving wire device using polyvinyl alcohol hydrogel vascular models. Neuroradiology. 2007;49:243–251. doi: 10.1007/s00234-006-0181-1. [DOI] [PubMed] [Google Scholar]
- 2.Fessler RD, Ringer AJ, Qureshi AI, Guterman LR, Hopkins LN. Intracranial stent placement to trap an extruded coil during endovascular aneurysm treatment: Technical note. Neurosurgery. 2000;46:248–251. [PubMed] [Google Scholar]
- 3.German WJ, Black SP. Experimental production of carotid aneurysms. N Engl J Med. 1954;250:104–106. doi: 10.1056/NEJM195401212500303. [DOI] [PubMed] [Google Scholar]
- 4.Henkes H, Lowens S, Preiss H, Reinartz J, Miloslavski E, Kühne D. A new device for endovascular coil retrieval from intracranial vessels: Alligator retrieval device. AJNR Am J Neuroradiol. 2006;27:327–329. [PMC free article] [PubMed] [Google Scholar]
- 5.Levy EI, Ecker RD, Horowitz MB, Gupta R, Hanel RA, Sauvageau E, Jovin TG, Guterman LR, Hopkins LN. Stent-assisted intracranial recanalization for acute stroke: Early results. Neurosurgery. 2006;58:458–463. doi: 10.1227/01.NEU.0000199159.32210.E4. [DOI] [PubMed] [Google Scholar]
- 6.Levy EI, Mehta R, Gupta R, Hanel RA, Chamczuk AJ, Fiorella D, Woo HH, Albuquerque FC, Jovin TG, Horowitz MB, Hopkins LN. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28:816–822. [PMC free article] [PubMed] [Google Scholar]
- 7.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 8.Nesbit GM, Luh G, Tien R, Barnwell SL. New and future endovascular treatment strategies for acute ischemic stroke. J Vasc Interv Radiol. 2004;15:S103–S110. doi: 10.1097/01.rvi.0000112578.95689.66. [DOI] [PubMed] [Google Scholar]
- 9.Schellhammer F, Zahringer M, Lackner K. Nitinol micro-forceps for retrieval of intravascular objects: First in vitro experiences. Invest Radiol. 2002;37:577–579. doi: 10.1097/00004424-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 11.Soeda A, Sakai N, Sakai H, Iihara K, Yamada N, Imakita S, Nagata I. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: Evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2003;24:127–132. [PMC free article] [PubMed] [Google Scholar]
- 12.Standard SC, Chavis TD, Wakhloo AK, Ahuja A, Guterman LR, Hopkins LN. Retrieval of a Guglielmi detachable coil after unraveling and fracture: Case report and experimental results. Neurosurgery. 1994;35:994–999. doi: 10.1227/00006123-199411000-00038. [DOI] [PubMed] [Google Scholar]
- 13.Thornton J, Dovey Z, Alazzaz A, Misra M, Aletich VA, Debrun GM, Ausman JI, Charbel FT. Surgery following endovascular coiling of intracranial aneurysms. Surg Neurol. 2000;54:352–360. doi: 10.1016/s0090-3019(00)00337-2. [DOI] [PubMed] [Google Scholar]
- 14.van Rooij WJ, Sluzewski M, Beute GN, Nijssen PC. Procedural complications of coiling of ruptured intracranial aneurysms: Incidence and risk factors in a consecutive series of 681 patients. AJNR Am J Neuroradiol. 2006;27:1498–1501. [PMC free article] [PubMed] [Google Scholar]
- 15.Weber W, Bendszus M, Kis B, Boulanger T, Solymosi L, Kühne D. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: Initial clinical and angiographic results in 31 aneurysms. Neuroradiology. 2007;49:555–561. doi: 10.1007/s00234-007-0232-2. [DOI] [PubMed] [Google Scholar]