Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
There is increasing concern about the use of those medicines in children which have not been fully studied and licensed for childhood use. Such use is not uncommon, due in large part to a lack of availability of fully licensed products and formulations that are suitable for children.
There is little published information on the views of the public on this important area of paediatric care.
WHAT THIS STUDY ADDS
A survey of 1000 members of the public in Northern Ireland indicated that such use of medicines in children is not well known.
However, when informed about this practice, the majority believed that it would compromise safety and increase the likelihood of adverse effects. They also believed that parents/guardians should be told if their child was prescribed a medicine that had not been fully tested in children.
Participants in the survey indicated that they would be reluctant to involve their child in a clinical trial to help with the licensing process unless the child was suffering from a life-threatening illness.
AIMS
To explore awareness and views of the general public on unlicensed use of medicines in children and on the participation of children in clinical trials.
METHODS
Members of the public completed a questionnaire survey administered by face-to-face interview in public areas in N. Ireland. The main outcome measures were the views on unlicensed use of medicines in children and on clinical trials in children.
RESULTS
One thousand participants (59.2% female) took part; 610 were parents. Most participants (86%) had no previous knowledge about unlicensed use of medicines in children. Being a parent did not influence this nor did being a parent of a child who suffered from a health problem (P > 0.05). Most participants (92%) felt that parents should be told about unlicensed use of medicines, with the doctor most frequently selected as the person who should inform parents. At the outset, only 1.8% of participants felt that the use of medicines in children was unsafe. However, having been informed about unlicensed use of medicines, this proportion increased dramatically (62.4%; P < 0.001). Views on whether participants would enter a child of their own into a clinical trial varied according to the health status of the child (P < 0.05) i.e. a child in good health (3.9%) vs a child with a life-threatening condition (41.9%).
CONCLUSIONS
There is limited public knowledge of unlicensed use of medicines in children and a general reluctance to involve children in clinical trials unless the child to be involved has a life-threatening condition.
Keywords: unlicensed medicines, off-label medicines, clinical trials, children, consent
Introduction
Children have been described as ‘therapeutic orphans’ because they frequently do not have access to medicines, which have been appropriately researched and licensed for use within their age group [1]. As a result, a significant proportion of the medicines used to treat children are used off-label (e.g. the use of a medicine outside its license with respect to dose, route of administration or age of the recipient) or as an unlicensed preparation (e.g. tablets of an adult medicine crushed and made up into a suspension for oral administration to a child). At least one third of children in paediatric medical and surgical wards [2, 3] and 90% of children in neonatal intensive care units [4, 5] are prescribed unlicensed/off-label medicines. This practice is referred to throughout the paper as unlicensed use of medicines.
Since such use of medicines is necessary in paediatric practice, healthcare professionals have in certain instances been forced to ‘guestimate’ the doses to be administered. This situation has been alleviated somewhat in recent years with the publication of the compendium ‘Medicines for Children’[6] and more recently through the publication of the BNF for Children [7].
There still remains, however, a general concern since a number of high profile cases of dosing errors, some of which have caused fatalities, have been reported following the unlicensed use of medicines in children [8]. Furthermore, medication errors in children receiving such medicines have three times the potential to cause harm when compared with similar errors in adults [9], there is increased frequency and severity of adverse drug reactions [10, 11] and there is a general lack of long-term safety and efficacy data [12–15]. There is also the potential for treatment failure, e.g. clinicians may be over cautious in using appropriately high doses. Although in most cases the benefits of the unlicensed use of medicines outweigh the risks [16], such prescribing is expected to be the exception, rather than the rule [10].
It has been suggested that the lack of licensed medicines for children is a breach of their basic human rights [2, 17]. This, together with the problems associated with the unlicensed use of medicines, points to the need for more clinical trials in children. Such trials, however, are hampered by ethical concerns, practical issues around repeated blood sampling, particularly in neonates [18, 19], and by financial barriers [15, 16].
It is generally considered that it is the role of the prescriber to convey information to parents/guardians about unlicensed use of medicines in their children without causing undue confusion or distress [20]. Unfortunately, such reporting can lead to lack of trust and as such influence the treatment negatively [21]. The situation, therefore, is that parents/guardians are often not specifically told about the unlicensed use of medicines. Furthermore there is a distinct lack of research on the views and knowledge of the general public about unlicensed use of medicines in children and in particular the views of parents [12]. The aim of the present study was to investigate the latter together with views of the public on the participation of children in clinical trials.
Methods
A draft survey instrument was prepared to collect the required information. The draft questionnaire was examined for fitness for purpose and face validity in a focus group involving six parents. Following this review, the final version of questionnaire (Appendix) was piloted in a sample of 20 members of the public prior to moving on to the main survey. Throughout the research, the questionnaire was administered to members of the public using a structured interview technique (face to face) by one trained interviewer (TLM). The target sample size was 1000 as in previous public surveys carried out in N. Ireland [22, 23]. The study received ethical approval from the School of Pharmacy, Queen's University, Research Ethics Committee.
All interviews were conducted in public areas, between August and November 2006, either in Belfast or in provincial towns in Northern Ireland (six study sites in total with at least two visits made to each location). Interviews were carried out on all the days of the week (including weekends). Members of the public, who appeared to be over 16 years of age, were approached by the interviewer and invited to participate. Each interview required approximately 10 min to complete. After asking some preliminary questions on medicine safety in children and whether such medicines go through a similar testing process as is the case with adult medicines, participants were told that some medicines were used in children without being fully studied or licensed for use in children. Participants were then asked a further series of questions, having been given this information. In those questions which specifically related to ‘own children’, participants who had no children were asked to assume that they had a child of their own. At the end of the survey, to avoid any concerns, participants were assured that all medicines, regardless of their licence status, were selected with the utmost care for all children.
Responses were coded and entered into a customized database in SPSS, version 14, for statistical analysis (Chi squared test and sign tests).
Results
Demographics
Of the 1000 members of the public interviewed, 408 were male (40.8%) and 592 were female (59.2%); 610 (61%) had one or more children and of those 16.9% had children suffering from a chronic health condition. A small proportion (9.2%) of the participants had children who were now over 18 years of age. The majority of participants were 20–40 years old (60.8%), while 4.5% were under 20 and 5.9% were over 60 years old.
Participants’ knowledge about children's medicines
The majority of participants (62.5%) thought that all medicines for children had gone through a similar testing and licensing process for use in children as is the case for adult medicines before they are prescribed either by a GP (63%) or in the hospital (62%). When told about unlicensed use of medicines in children (Appendix), 86% of participants claimed that they had never heard or read about this. This lack of knowledge was not influenced by demographic parameters including parents vs non-parents and parents who had children suffering from a health problem vs those parents of a healthy child (P > 0.05; Chi squared test).
Views about the safety of children's medicines
There was a marked change in the participants’ views on medicine safety pre- and post- being informed about the unlicensed use of medicines in children (Figure 1). This figure represents data relating to hospital care. At the outset of the questionnaire, only 1.8% of the study population felt that the use of medicines in children was unsafe. However, having been told about unlicensed use of medicines, this proportion increased dramatically (62.4%; P < 0.001; sign test). The data were almost identical for primary care (20.7, 64.9, 0.6, 1.1, 12.75 vs 0, 18.9, 52.7, 10.2, 18.2%; same categories as Figure 1). Concerning adverse drug reactions, 89.6% of participants felt that unlicensed use of medicines would increase the extent of side-effects, e.g. rash, drowsiness, headache etc. in children. Within the group of parents, those feeling this way were more likely to be parents of children with a health problem (96% vs 91%; P < 0.05; Chi squared test).
Figure 1.
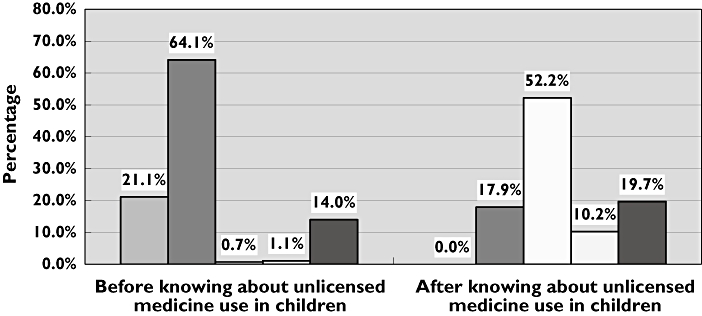
Change in the views of participants on the safety of medicines prescribed for children in hospital after knowing that children can be prescribed medicines that have not been fully tested for use in children. Extremely Safe ( ); Safe (
); Safe ( ); unsafe (
); unsafe ( ); Extremely unsafe (
); Extremely unsafe ( ); I don't know (▪)
); I don't know (▪)
Communication between the doctor and parents/guardians
The majority of participants (92.1%) felt that parents should be told about unlicensed use of medicines in their child. Within the hospital setting, participants felt that this information should be provided by the doctor (82.3%), pharmacist (15.1%) or nurse (2.6%) while in primary care, the providers of the information were selected as follows: the GP (79.8%), the community pharmacist (12.9%) and others (7.3%). In contrast, 7.9% of participants thought that the parents/guardians should not be told if their child was being prescribed a medicine that had not been fully studied or licensed for use in children, the majority basing their answer on the view that such knowledge could lead to undue concern. When participants were asked what they would do if ‘their’ child (those without children were asked to assume that they had a child of their own) was prescribed such a medicine, 41.9% indicated they ‘would use the medicine in the child but check the child carefully for side-effects’, 39.9% indicated that they would ‘ask the doctor to change the medicine to one that had been fully tested and licensed for use in children’, while 18.2% indicated that they would ‘simply accept that the doctor knows best’. Those participants who attended University were less likely to indicate that they would ask the doctor to change the medicine (P < 0.05; Chi squared test). When asked whether they thought that doctors would knowingly prescribe a medicine that had not been fully tested for use in children, 84.2% of participants thought that they would. When asked about the reason, 76.1% agreed that the doctor would feel that the benefit of such a medicine would outweigh the risk of using it, 15.6% felt that this would occur when there was no alternative licensed medicine while 9.2% said that doctors had enough experience to prescribe any medicine for children.
Clinical trials in children
A range of scenarios was presented to participants to allow comparison of their views and attitudes concerning allowing their children to participate in clinical trials (Figure 2). Participants’ willingness to allow their child to be entered into clinical trials to help with the licensing process varied according to the status of the child, i.e. if the child was in good health only 3.9% were willing to allow their child to participate; corresponding data for the other categories were as follows: life-threatening condition, e.g. heart failure, and the medicine being tested was used for that condition (41.9%); child already in hospital (28.5%); minor condition, e.g. hay fever, and the medicine being tested was used for that condition (19.1%); serious but not life threatening condition, e.g. asthma or epilepsy, and the medicine being tested was being used for that condition (16.9%). Variations in the responses were statistically significant (P < 0.05; Chi squared test).
Figure 2.
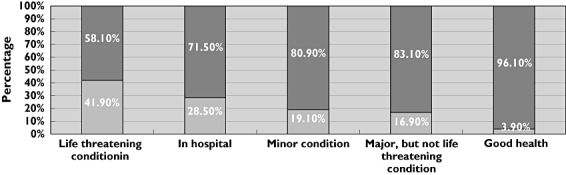
Views on whether participants* would enter their child into a clinical trial depending on the situation in relation to the child (range: child had life threatening condition to child in good health). *Participants who were not parents were asked to assume that they had a child of their own in answering the questions. yes ( ); no (
); no ( )
)
Of the 1000 participants in the survey, 610 were parents with one or more children. When parents were compared against non-parents to see if the assumed health status (scenarios) influenced them in volunteering their child for participation in a clinical trial, it was noted that when the child was suffering from a life threatening condition or already in hospital, non-parents were more willing to consent for ‘their’ child to enter a clinical trial (Table 1). Parents were more likely than non-parents to volunteer their child for a clinical trial if the child was suffering from a minor condition. Of the 610 parents, 103 (16.9%) had a child with a health problem while 507 (83.1%) were parents of healthy children. A repeat evaluation involving these two latter sub-groups of parents was also performed (Table 1). The only statistically significant difference between the two groups occurred in the scenario relating to the child suffering from a life threatening condition, where parents of a child with an existing health problem were more likely to support entry of their child into a clinical trial (P < 0.05; Chi squared test).
Table 1.
Influence of being a parent (vs a non-parent) and being a parent of a healthy child (vs parent of a child with a health problem) on offering their child's participation in a clinical trial
| Participant categorization | Participant categorization | |||||
|---|---|---|---|---|---|---|
| Assumed health condition | Parentsn (%) | Non-parentsn (%) | P value Chi squared test | Parents of healthy childn (%) | Parents of child with health problem*n (%) | P valueChi squared test |
| Child suffering from a life threatening condition | 240 (39.3) | 179 (45.9) | 0.024 | 194 (38.3) | 55 (53.4) | 0.002 |
| Child suffering from a chronic but not life threatening condition | 112 (18.4) | 57 (14.6) | 0.072 | 101 (19.9) | 14 (13.6) | 0.107 |
| Child suffering from a minor condition | 130 (21.3) | 61 (15.6) | 0.015 | 110 (21.7) | 23 (22.3) | 0.584 |
| Child in hospital | 159 (26.2) | 126 (32.3) | 0.020 | 156 (30.8) | 31 (30.1) | 0.484 |
| Child in good health | 27 (4.4) | 12 (3.1) | 0.183 | 24 (4.7) | 6 (5.8) | 0.145 |
diabetes (42%), asthma (31%), epilepsy (20%) and congenital disorder (7%).
The willingness of participants to allow their child to participate in a clinical trial was also influenced, to a statistically significant degree (P < 0.05; Chi squared test), by the action advocated following the prescription of a medicine that had not been fully tested for use in children (Table 2). For ease of data interpretation, the participants were dichotomized into those who favoured changing the medicine and those who would use the medicine. For each health status scenario, those participants who favoured changing the medicine to a licensed product were more willing to agree ‘their’ child's participation in a clinical trial. Being a parent did not influence these latter findings (P > 0.05; Chi squared test).
Table 2.
Influence of action suggested by participants (n = 1000) after child is prescribed a medicine that has not been fully tested for use in children, on participants’ offering their child's participation in a clinical trial
| Participant categorization | |||
|---|---|---|---|
| Assumed health condition | Change the medicine*n (%) | Accept the medicine†n (%) | P value Chi squared test |
| Child suffering from a life threatening condition | 195 (48.3) | 224 (37.6) | 0.001 |
| Child suffering from a chronic but not life threatening condition | 80 (19.8) | 89 (14.9) | 0.044 |
| Child suffering from a minor condition | 111 (27.5) | 80 (13.4) | <0.001 |
| Child in hospital | 168 (41.6) | 117 (19.6) | <0.001 |
| Child in good health | 28 (6.9) | 11 (1.8) | <0.001 |
Ask the doctor to change the medicine to one that has been fully tested and licensed for use in children.
Combined categories: Simply accept that the doctor knows best and use the medicine in the child but check carefully for side-effects.
The final question in the questionnaire asked participants to consider whether the age of their child would influence their views on the testing of medicines in children. Taking account of the full 1000 participants, only 28.4% indicated that age would influence their decision. Reasons given by these latter participants focused on the view that older children would be more resilient and should be involved in preference to younger children. There were no differences in response rates between parents and non-parents (P > 0.05; Chi squared test) to this question.
Discussion
This study found that 86% of the general public surveyed lacked knowledge about the unlicensed medicine use in children, with no difference in knowledge between parents and non-parents. This was not surprising since the situation regarding unlicensed use of medicines in children has achieved relatively little media attention and intuitively one would consider that children would be afforded more protection than adults regarding evidence based medicine. Furthermore awareness in the public would be expected to be limited since doctors and pharmacists admit preferring not to tell parents that their children are being prescribed an unlicensed/off-label medicine [24, 25]. Once parents knew of this practice, they wished to be told if their child was prescribed such a treatment. This is not surprising since increasingly patients wish to become involved in their own healthcare [26] and presumably also in the healthcare of their children. Furthermore, a significant minority (39.9%) indicated that they would ask the doctor to change the medicine to a licensed product. This was likely due to concerns about side effects, and indeed providing information on the unlicensed use of medicines in children significantly decreased participant confidence in drug safety. This could become a particular issue with regard to patient adherence with prescribed medication and as such presents a dilemma to both doctors who prescribe the medicine and pharmacists who dispense the medicine. However, since the majority of participants felt that parents/guardians should be told if a child is prescribed a medicine which has not been fully tested for use in children, healthcare professionals must be aware of the need to convey such information sensitively without causing distress or confusion so as to avoid it impacting negatively on medicine use [20]. This latter concern was expressed by a small minority of the present participants (7.9%) who felt that parents should not be informed about unlicensed medicines use in their children. To avoid heightening concerns, it will also be important that there is consistency in the ‘messages’ given by different healthcare professionals regarding unlicensed use of medicines.
The results demonstrate that there is a general reluctance to involve children in clinical trials unless the child to be involved has a life-threatening condition. Again this is understandable since parents may not wish to subject their child to become what they may view as a ‘guinea pig’ in clinical trials, which from time to time receive significant adverse publicity relating to safety [27], unless the condition is life-threatening. Such involvement is borne out in clinical practice, for example, in the management of childhood cancer. Typically, after a period of aggressive chemotherapy has failed to cure the disease, parents are faced with a number of choices, that may include enrolling their child in a phase I/II clinical trial [28]. Indeed more than half of all American children with cancer, and 46% of such children in the UK, take part in clinical trials [29–31]. Views on this matter are, however, not consistent, perhaps reflecting differences between chronic vs acute illness. A study carried out in Nottingham, for example, reported that the major reasons given by parents for having their children take part in a research study (management of pneumonia in previously well children) was to benefit children in the future (31%) and to contribute to science (27%), while only 18% said that the major reason they allowed participation was benefit to their own child [31]. Although this selfless approach may have been in the mind of the present respondents when responding to questions, there was little support for entering healthy children into clinical trials i.e. only 5% of participants. Although the comparisons presented in Table 1, highlighted statistically significant differences between parents and non-parents, the differences were generally quite small, indicating a relatively universal view by all participants. The results in Table 2 on the other hand showed more marked and consistent differences. Participants who favoured replacing unlicensed medicine use with a licensed product were more willing to agree ‘their’ child's participation in a clinical trial, regardless of the child's health status. Although not definitive, these data suggest that participants understood that to have more licensed products available for general use in children, more clinical trials involving children will be required (including potential participation of their own children).
The present survey sheds some light on issues that need to be addressed with parents/guardians within this important area of clinical practice and also the likely views of parents that researchers will be faced with when undertaking clinical trials in children. Further qualitative work would, however, be helpful to explore some of the issues in more depth. Work is also required on the views of children themselves, in particular, what they would wish to be told if prescribed a medicine that had not been fully tested/licensed in children and how they would feel about participating in clinical trials for the benefit of others.
Acknowledgments
Competing interests: None
The authors wish to thank Dr Chris Patterson, Department of Epidemiology and Public Health, Queen's University Belfast for his statistical support.
Appendix
Survey on the use of prescribed medicines for children – views of the general public
Children medicines
I would like to ask you some questions about the use of prescribed medicines for children. When I say prescribed medicines here I am referring only to medicines that are prescribed for children by a doctor either in hospital or by a family doctor (GP). I am not including those medicines that you can buy without a prescription. I am also not including complimentary medicines e.g. homeopathy, herbal remedies etc.
-
My first question is, do you have any children?
yes
-
no
If yes, how many ___________
If yes, what are their ages: 1. all older than 18
2. Other (ages) ________
________________________________________________
________________________________________________
________________________________________________
-
In your opinion, how safe are medicines that are prescribed for children in hospital?
Extremely safe
Safe
Unsafe
Extremely unsafe.
I don't know
-
In your opinion, how safe are medicines that are prescribed for children by a family doctor (GP)?
Extremely safe
Safe
Unsafe
Extremely unsafe.
I don't know
All adult medicines go through a testing process in adults before they are given a license and put on the market.
-
Do you think that all medicines prescribed for children have gone through a similar testing and licensing process for use in children before they are prescribed for children in hospital?
Yes
No
I don't know
-
Do you think that all medicines prescribed for children have gone through a similar testing and licensing process for use in children before they are prescribed for children by a family doctor (GP)?
Yes
No
I don't know
Although it may come as a surprise, the actual situation is that some medicines that are routinely prescribed for children have not been fully studied or licensed for use in children. Also sometimes the dose used has to be increased beyond that which has been recommended by the manufacturer.
-
Have you ever read or heard about this?
Yes
No
-
Do you think that parents should be told every time their child is prescribed a medicine that has not been fully tested for use in children?
Yes (go to question 8)
No (go to question 9)
-
If yes, who should tell the parent that their child is getting such a medicine?
In hospital Outside hospital a. the doctor a. the family doctor (GP) b. the hospital pharmacist b. the pharmacist (chemist) c. A nurse c. other d. other -
If no, could you please give some reasons for your answer?
________________________________________________
________________________________________________
________________________________________________
________________________________________________
-
Do you think that a doctor would knowingly prescribe a medicine for a child that has not been fully tested for use in children?
Yes (go to question 11)
No (go to question 12)
-
Why do you think a doctor would prescribe a medicine that has not been fully tested for use in children? (You can choose more than one answer)
The doctor felt that the benefit of the medicine would outweigh the risks of using it.
Doctors have enough experience to prescribe all medicines.
There is no alternative fully tested medicine for children.
-
Do you think that the use of medicines that have not been fully tested for use in children would increase the risk of side effects in children, e.g. rash, drowsiness, headache…etc.?
Strongly agree
Agree
Disagree
Strongly disagree
The majority of the prescribing of medicines for children which have not been fully tested for use in children takes place in hospitals, but sometimes such prescribing is carried out by the family doctor (GP).
Having had this discussion:
-
In your opinion, how safe are medicines that are prescribed for children in hospital?
Extremely safe
Safe
Unsafe
Extremely unsafe.
I don't know
-
In your opinion, how safe are medicines that are prescribed for children by a family doctor (GP)?
Extremely safe
Safe
Unsafe
Extremely unsafe.
I don't know
Ask those without children (refer to Question 1) to assume that they have a child when answering questions 15–17
-
If you were told that your child was being prescribed a medicine that had not been fully tested for use in children, what action would you take?
ask the doctor to change the medicine to one that has been fully tested and licensed for use in children
simply accept that the doctor knows best
use the medicine in the child, but check child carefully for side-effects
-
For a drug to be licensed for use in children, it has to go through tests done on children themselves. Would you be willing to let your child participate in such tests, if:
Your child had a life threatening condition, e.g. heart failure, and the medicine being tested was used for that condition Y/N
Your child had a serious, but not life threatening condition. e.g. asthma, epilepsy, and the medicine being tested was being used for that condition? Y/N
Your child had a minor condition e.g. hay fever and the medicine being tested was used for that condition? Y/N
Your child was already in hospital Y/N
Your child was in good health (i.e. volunteer their involvement for the good of humanity) Y/N
-
Thinking back to the previous question, would the age of the child make a difference to your views on the testing of these medicines in children, bearing in mind that a child can be anything from an infant up to the age of 18 years?
yes
no
If yes, please explain
________________________________________________
________________________________________________
________________________________________________
________________________________________________
General information and demographics
-
Age group:
under 20 20–30 31–40 41–50 51–60 over 60 -
Sex
male
female
How long have you been living in Northern Ireland? ________ Years _______ Months.
-
Do you live in the:
City
Town
Village
Country
At what age did you leave school? _______ years
-
Did you attend a:
primary school
secondary school
grammar school
technical college
university
For those with children (see Question 1)
-
Do any of you children have a health problem?
f. yes
g. no
If yes, please give details.
Thank you very much for your help in answering the questions. Your help is much appreciated.
REFERENCES
- 1.Shirkey H. Editorial comment: identification of prescribed drugs on the label of the container. J Pediatr. 1967;71:592–4. doi: 10.1016/s0022-3476(67)80116-1. [DOI] [PubMed] [Google Scholar]
- 2.Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, Knoeppel C, Seyberth H, Pandolfini C, Raffaelli M, Rocchi F, Bonati M, Jong T, Anker J. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ. 2000;320:79–82. doi: 10.1136/bmj.320.7227.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy S, Newman C, Gudka S. Unlicensed and off label drug use in acute lymphoblastic leukaemia and other malignancies in children. Ann Oncol. 2003;14:42–7. doi: 10.1093/annonc/mdg031. [DOI] [PubMed] [Google Scholar]
- 4.Conroy S, McIntyre J, Choonara I. Unlicensed and off label drug use in neonates. Arch Dis Child Fetal Neonatal Ed. 1999;80:142–5. doi: 10.1136/fn.80.2.f142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy S, McIntyre J. The use of unlicensed and off-label medicines in the neonate. Semin Fetal Neonatal Med. 2005;10:115–22. doi: 10.1016/j.siny.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Royal College of Paediatrics. Medicines for Children. London: RCPCH; 1999. [Google Scholar]
- 7.Joint Formulary Committee. British National Formulary for Children. 1st edn. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2005. [Google Scholar]
- 8.Fernandez CV, Gillis-Ring J. Strategies for the prevention of medical errors in pediatrics. J Pediatr. 2003;143:155–62. doi: 10.1067/S0022-3476(03)00244-0. [DOI] [PubMed] [Google Scholar]
- 9.Fortescue EB, Kaushal R, Landrigan PC, McKenna KJ, Clapp MD, Federico F, Goldmann DA, Bates DW. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;111:722–9. doi: 10.1542/peds.111.4.722. [DOI] [PubMed] [Google Scholar]
- 10.Collier J. Paediatric prescribing: using unlicensed drugs and medicines outside their licensed indications. Br J Clin Pharmacol. 1999;48:5–8. doi: 10.1046/j.1365-2125.1999.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essex C, Rylance G. Children have rights to medicines. BMJ. 1997;316:62. doi: 10.1136/bmj.315.7099.62a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong I, Sweis D, Cope J, Florence A. Paediatric medicines research in the UK: how to move forward? Drug Saf. 2003;26:529–37. doi: 10.2165/00002018-200326080-00001. [DOI] [PubMed] [Google Scholar]
- 13.Horen B, Montastruc JL, Lapeyre-Mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol. 2002;54:665–70. doi: 10.1046/j.1365-2125.2002.t01-3-01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52:77–83. doi: 10.1046/j.0306-5251.2001.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner S, Longworth A, Nunn AJ, Choonara I. Unlicensed and off label drug use in paediatric wards: prospective study. BMJ. 1998;316:343–5. doi: 10.1136/bmj.316.7128.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Impicciatore P, Choonara I. Status of new medicines approved by the European Medicines Evaluation Agency regarding paediatric use. Br J Clin Pharmacol. 1999;48:15–8. doi: 10.1046/j.1365-2125.1999.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aynsley-Green A, Barker M, Burr S, Macfarlane A, Morgan J, Sibert J, Turner T, Viner R, Waterston V, Hall D. Who is speaking for children and adolescents and for their health at the policy level? BMJ. 2000;321:229–32. doi: 10.1136/bmj.321.7255.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parliament UK. Medicines (Children) House Commons Hansard Debate. 1999;329:329. [Google Scholar]
- 19.Kmietowicz Z. Drug industry is unwilling to run trials in children. BMJ. 2000;320:1362. [PMC free article] [PubMed] [Google Scholar]
- 20.Zachry WM, III, Ginsburg DB. Patient autonomy and the regulation of direct-to-consumer advertising. Clin Ther. 2001;2024:2022–3. doi: 10.1016/s0149-2918(01)80155-7. [DOI] [PubMed] [Google Scholar]
- 21.Sweis D, Wong IC. Giving medicines to children: understanding the parents’ views. Paediatr Drugs. 2004;6:67–9. doi: 10.2165/00148581-200406010-00005. [DOI] [PubMed] [Google Scholar]
- 22.Wazaify M, Shields E, Hughes CM, McElnay JC. Societal perspectives on over-the-counter (OTC) medicines. Fam Pract. 2005;22:170–6. doi: 10.1093/fampra/cmh723. [DOI] [PubMed] [Google Scholar]
- 23.Bell H, Hughes C, McElnay JC. Societal perspectives on the role of the community pharmacist and community-based pharmaceutical services. J Soc Adm Pharm. 2000;17:119–28. [Google Scholar]
- 24.Ekins-Daukes S, Helms PJ, Taylor MW, McLay JS. Off-label prescribing to children: attitudes and experience of general practitioners. Br J Clin Pharmacol. 2005;60:145–9. doi: 10.1111/j.1365-2125.2005.02397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart D, Rouf A, Snaith A, Elliott K, Helms PJ, McLay JS. Attitudes and experiences of community pharmacists towards paediatric off-label prescribing: a prospective survey. Br J Clin Pharmacol. 2007;60:90–5. doi: 10.1111/j.1365-2125.2007.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraenkel L, McGraw S. Participation in medical decision making: the patients’ perspective. Med Decis Making. 2007;27:553–8. doi: 10.1177/0272989X07306784. [DOI] [PubMed] [Google Scholar]
- 27.St Clair EW. The calm after the cytokine storm: lessons from the TGN1412 trial. J Clin Invest. 2008;118:1344–7. doi: 10.1172/JCI35382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bluebond-Langner M, Belasco JB, Goldman A. Understanding parents’ approaches to care and treatment of children with cancer when standard therapy has failed. J Clin Oncol. 2007;25:2414–9. doi: 10.1200/JCO.2006.08.7759. [DOI] [PubMed] [Google Scholar]
- 29.Clinical trials are proven to offer children with cancer the best chance of survival. Available at http://www.seattlecca.org/patientsandfamilies/WhatAreClinicalTrials/ChildrenClinicalTrials/ (last accessed: 29 September 2007.
- 30.Peden V, Choonara I, Gennery B, Done H. Recruiting children to a clinical trial. Paediatric and Perinatal Drug Therapy. 2000;4:75–8. [Google Scholar]
- 31.Sammons HM, Atkinson M, Choonara I, Stephenson T. What motivates British parents to consent for research? A questionnaire study. BMC Pediatr. 2007;7:12. doi: 10.1186/1471-2431-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


