Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Treatment with clopidogrel after myocardial infarction (MI) is recommended to almost all patients for 12 months.
There is interpatient variation in the effect of clopidogrel treatment, one possible explanation for this being low initiation or poor persistence with therapy.
WHAT THIS STUDY ADDS
We found remarkably high initiation and persistence with clopidogrel treatment among MI patients treated with percutaneous coronary intervention (PCI); interpatient variation cannot be explained by poor initiation and persistence in these patients.
Among MI patients without PCI and among MI patients with concomitant heart failure we found substantial underuse; therefore, focus on these groups is important.
AIMS
To identify possible underuse by analysing initiation and persistence with clopidogrel treatment in an unselected population of patients admitted with myocardial infarction (MI) with or without subsequent percutaneous coronary intervention (PCI).
METHODS
Patients admitted with first-time MI from 2000 to 2005 and subsequent prescription claims of clopidogrel were identified by individual-level linkage of nationwide administrative registries in Denmark. Independent factors affecting initiation and persistence with treatment were analysed by multivariable logistic regression models and Cox proportional hazard models.
RESULTS
A total of 46 190 MI patients were included in the study, of whom 14 939 were treated with PCI. From 2000 to 2005 initiation of clopidogrel increased from 80.4 to 93.7% among MI patients with PCI and from 2.8 to 39.3% among MI patients without PCI. MI patients with concomitant heart failure received less treatment [odds ratio (OR) 0.49, confidence interval (CI) 0.43, 0.56 among patients with PCI and OR 0.90, CI 0.81, 0.99 among patients without PCI in 2002–2003, and OR 0.89, CI 0.80, 1.00 in 2004–2005, respectively]. Of MI patients with PCI, 77.5% completed 9 months’ clopidogrel treatment in 2004–2005, the corresponding figures for MI patients without PCI being 53.9%.
CONCLUSIONS
Initiation and persistence with clopidogrel treatment is high in MI patients with PCI. However, we found substantial underuse among MI patients without PCI and in MI patients with heart failure.
Keywords: Clopidogrel, Acute Myocardial Infarction, initiation, persistence, percutaneous coronary intervention
Introduction
Treatment of acute coronary syndrome has improved markedly over the last decade, and dual antiplatelet therapy with acetylsalicylic acid (ASA) and clopidogrel are now cornerstones of treatment [1]. After non-ST-elevation infarction (NSTEMI), clopidogrel treatment is recommended for 12 months in both European and American guidelines [2, 3]. In patients treated with percutaneous coronary intervention (PCI) and stents, the recommended duration of clopidogrel treatment differs according to stent type, with 1–3 months’ treatment in patients treated with a bare metal stent (BMS) and 6–12 months in patients treated with a drug-eluting stent (DES) [3–5].
Clopidogrel is a thienopyridine, a platelet inhibitor binding irreversibly to the platelet P2Y12 receptor. Several studies have shown interpatient variability in response to inhibition of platelet function during clopidogrel treatment among both healthy individuals and patients with known cardiovascular disease. The variability is seen in patients treated with or without ASA added to clopidogrel treatment and has a dose-dependent component [6, 7]. It is estimated that 4–34% have low response [8]. In the context of increased cardiovascular events after discharge, it has been suggested that a component of the low response could be due to poor persistence with treatment [8, 9].
To our knowledge, it is unclear to what extent clopidogrel treatment is given as recommended to patients outside randomized clinical trials and to what extent patients persist with therapy as recommended. To illuminate the question ‘does poor persistence with clopidogrel treatment cause low response to clopidogrel’, we studied initiation and persistence with clopidogrel treatment in a population of patients admitted with first-time myocardial infarction (MI) with or without subsequent PCI.
Methods
In Denmark all citizens have a personal civil registration number that enables individual linkage of information across registries. The National Patient Register keeps information about all admissions to Danish Hospitals since 1978. Each admission is registered by a primary and secondary diagnosis coded according to the International Classification of Diseases (ICD). The Danish Register of Medicinal Product Statistics holds information on all prescriptions dispensed from pharmacies in Denmark. Each prescription is coded according to the Anatomical Therapeutical Chemical (ATC) classification and includes information about the date of each dispensing, quantity dispensed and the strength of the tablets. Prescribed daily dosage is not recorded. As part of partial coverage of drug expenses by the Danish healthcare system, pharmacies are requested to register all dispensed prescriptions, ensuring complete registration nationwide [10]. In Denmark cardiovascular pharmaceuticals require a prescription, except for ASA, which is dispensed as an over-the-counter (OTC) drug.
Study population
From the National Patient Register we identified all patients aged ≥30 years who were discharged alive after first admission for MI (ICD-10 codes I21 and I22; as primary or secondary diagnoses) in the period 2000–2005. The diagnosis of MI in the National Patient Register has been validated with a positive predictive value of >93% [11]. Patients were categorized into MI patients with or without PCI performed within 30 days of admission. Patients with a previous history of PCI were excluded. In addition, patients living outside of Denmark and patients lost to follow-up (n = 80) were excluded. Comorbidity was determined according to the modified Ontario Acute Myocardial Infarction Mortality Prediction Rules by diagnosis from the index admission and 1 year prior to admission [12, 13]. Use of antidiabetic medication (ATC code A10) was used as a proxy for the diagnosis of diabetes. As diagnosis of heart failure has a low sensitivity in the National Patient Register, we used loop diuretics (ATC code C03C) as a proxy for heart failure, as done by Gislason et al. [14]. Use of acetylsalicylic acid (ATC B01AC06 and N02BA01) was determined from 90 days before to 90 days after admission to hospital. Use of other cardiovascular drugs [β-blockers, angiotensin converting enzymes inhibitors (ACE-i) and statins] within 30 days of discharge was determined. Hospitals were grouped into three categories: tertiary cardiac hospital (an invasive hospital with specialized cardiac functions), main regional hospital (a larger non-invasive hospital with a department of cardiology) and local hospital (a smaller non-invasive hospital with a department of internal medicine) [13].
Clopidogrel use
Initiation of clopidogrel treatment (ATC code B01AC04) was defined as a claimed prescription within 30 days from discharge of first MI; identified from the Register of Medicinal Product Statistics. Persistence with therapy was calculated from subsequent prescription claims. We assumed the daily dosage was one tablet (75 mg), hence dispensing of 30 tablets yielded treatment for 30 days. Excess tablets were allowed to accumulate for up to three consecutive prescriptions. This method allows us to determine whether clopidogrel was available any day during the study period. Persistence with therapy was defined as days where tablets were available; days with no tablet available were registered as a break in therapy. Breaks of ≥30 days were defined as nonpersistence with treatment. For patients admitted to a Danish hospital during the observation period, the days of hospital stay were left out of the calculations, since patients in Denmark receive medical treatment at the hospital while admitted. Due to changes in guidelines throughout the study period, we divided the study population into three time periods according to admission year: 2000–2001, 2002–2003 and 2004–2005.
Statistical analysis
Descriptive statistics and baseline variables are presented as percentage, means with standard deviation (SD), or medians with range. Factors affecting initiation of clopidogrel treatment were analysed by multivariable logistic regression analysis. PCI was the most pronounced independent factor for initiation of clopidogrel treatment (with significant interaction); we therefore stratified the patients into two groups according to PCI status: MI patients with PCI and MI patients without PCI. Each group was analysed separately. Analyses were adjusted for age, sex, concomitant medical treatment (dichotomous covariates), comorbidity, and type of hospital. In the group of MI patients without PCI each time period was analysed separately, since there was no recommendation for clopidogrel in the first time period (except for patients with allergies to ASA). The univariate relationship between MI groups and time to 30 days break in clopidogrel treatment were examined by the Kaplan–Meier method. Cox proportional hazard models were used to estimate association between covariates affecting nonpersistence with clopidogrel treatment (breaks in therapy ≥30 days) in the last time period, 2004–2005. The Cox proportional hazard models were adjusted for age, gender, concomitant medical treatment, comorbidity, PCI status, and type of hospital. Proportion of days covered were calculated as the percentage of days covered of the first 360 days after start of treatment. All statistical calculations were performed using the SAS statistical software package, version 9.1 for windows (SAS Institute Inc., Cary, NC, USA).
Ethics
The Danish Data Protection Agency approved this study (ref. 2003-54-1269). In Denmark ethical approval is not required for retrospective registry studies. Data were made available to us in such a way that no individuals could be identified.
Results
Between 2000 and 2005 a total of 57 032 patients were admitted with first-time MI in Denmark, of whom 46 190 (81.0%) survived ≥30 days and were included in the study. Of these, 14 939 (32.3%) were treated with a PCI within 30 days of discharge. In 2000–2001 74.0% of patients in the PCI group were treated with stents, whereas 82.9% were treated with stents during 2004–2005. Distributions of baseline characteristics over the three time periods are shown in Table 1.
Table 1.
Baseline characteristics: patients admitted with first time myocardial infarction (MI) from 2000 to 2005
| 2000–2001* | 2002–2003* | 2004–2005* | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total* | MI + PCI | MI − PCI | MI + PCI | MI − PCI | MI + PCI | MI − PCI |
| Total patients | 46 190 | 2760 (18.1) | 12 516 (81.9) | 5621 (34.3) | 10 772 (75.7) | 6558 (45.2) | 7963 (54.8) |
| Women* | 17 478 (37.8) | 776 (28.1) | 5 082 (40.6) | 1598 (28.4) | 4 579 (42.5) | 1809 (27.6) | 3633 (45.6) |
| Age, women† | 72.8 ± 12.7 | 63.7 ± 11.6 | 74.0 ± 12.0 | 65.9 ± 12.2 | 75.1 ± 12.2 | 67.3 ± 12.3 | 75.9 ± 12.2 |
| Men* | 28 712 (62.2) | 1984 (71.9) | 7 433 (59.4) | 4023 (71.6) | 6 193 (57.5) | 4749 (72.4) | 4330 (54.4) |
| Age, men† | 65.6 ± 12.8 | 59.5 ± 11.0 | 67.2 ± 12.6 | 61.0 ± 11.5 | 68.8 ± 12.7 | 61.7 ± 11.7 | 69.6 ± 12.9 |
| Comorbidity | |||||||
| Cerebral vascular disease | 2 529 (5.5) | 69 (2.5) | 758 (6.1) | 126 (2.2) | 796 (7.4) | 159 (2.4) | 621 (7.8) |
| Diabetes with complications | 2 606 (5.6) | 97 (3.5) | 737 (5.9) | 210 (3.7) | 756 (7.0) | 230 (3.5) | 576 (7.2) |
| Cardiac dysrythmias | 5 333 (11.6) | 174 (6.3) | 1 513 (12.1) | 406 (7.2) | 1 557 (14.5) | 459 (7.0) | 1224 (15.4) |
| Acute renal failure | 467 (1.0) | 10 (0.4) | 129 (1.0) | 26 (0.5) | 160 (1.5) | 23 (0.4) | 119 (1.5) |
| Chronic renal failure | 700 (1.5) | 22 (0.8) | 182 (1.5) | 37 (0.7) | 207 (1.9) | 41 (0.6) | 211 (2.7) |
| Malignacy | 1 568 (3.4) | 42 (1.5) | 472 (3.8) | 102 (1.8) | 477 (4.4) | 109 (1.7) | 366 (4.6) |
| Shock | 500 (1.1) | 25 (0.9) | 123 (1.0) | 37 (0.7) | 145 (1.3) | 35 (0.5) | 135 (1.7) |
| Pulmonary oedema | 766 (1.7) | 20 (0.7) | 247 (2.0) | 31 (0.6) | 248 (2.3) | 31 (0.5) | 189 (2.4) |
| Concomitant medical treatment | |||||||
| β-Blockers | 35 515 (76.9) | 2439 (88.4) | 9 056 (72.4) | 4984 (88.7) | 7 664 (71.1) | 5871 (89.5) | 5501 (69.1) |
| ACE inhibitors | 21 042 (45.6) | 1070 (38.8) | 4 958 (39.6) | 2912 (51.8) | 4 828 (44.8) | 3629 (55.2) | 3655 (45.9) |
| Statins | 29 216 (63.3) | 1965 (71.2) | 5 073 (40.5) | 5046 (89.8) | 5 856 (54.4) | 6276 (95.7) | 5000 (62.8) |
| Loop diuretics‡ | 18 114 (39.2) | 613 (22.2) | 5 776 (46.2) | 1239 (22.0) | 5 153 (47.8) | 1441 (22.0) | 3892 (48.9) |
| Antidiabetic drugs§ | 5 681 (12.3) | 236 (8.6) | 1 606 (12.8) | 550 (9.8) | 1 475 (13.7) | 644 (9.8) | 1170 (14.7) |
| Vitamin-K antagonist | 3 391 (7.3) | 135 (4.9) | 941 (7.5) | 309 (5.5) | 934 (8.7) | 348 (5.3) | 724 (9.1) |
| Acetylsalicylic acid | 38 410 (83.2) | 2264 (82.0) | 9 585 (76.6) | 5069 (90.2) | 8 747 (81.2) | 6096 (93.0) | 6649 (83.5) |
| Clopidogrel¶ | 19 632 (42.5) | 2220 (80.4) | 349 (2.8) | 5158 (91.8) | 2 631 (24.4) | 6142 (93.7) | 3132 (39.3) |
| Type of hospital: | |||||||
| Local hospital | 6 017 (13.0) | 406 (14.7) | 2 376 (19.0) | 628 (11.2) | 1 681 (15.6) | 337 (5.1) | 589 (7.4) |
| Main regional hospital | 29 655 (64.2) | 1435 (52.0) | 8 404 (67.2) | 2716 (48.3) | 7 469 (69.3) | 3506 (53.5) | 6125 (76.9) |
| Tertiary cardiac hospital | 10 518 (22.8) | 919 (33.3) | 1 736 (13.9) | 2277 (40.5) | 1 622 (15.1) | 2715 (41.4) | 1249 (15.7) |
Numbers in parentheses are percentages of total number of patients in the group.
Mean value ± SD years.
Loop diuretics are used as a proxy for heart failure.
Antidiabetic medication used as a proxy of diabetes.
Patients disclaiming a receipt <30 days after discharge.
Initiation of clopidogrel treatment
Initiation of clopidogrel treatment increased from 80.4% in 2000–2001 to 93.7% in 2004–2005 among MI patients treated with PCI, and from 2.8% in 2000–2001 to 39.3% in 2004–2005 among MI patients not treated with PCI (Figure 1). There were no differences in initiation rates or persistence with treatment if we divided the MI patients with PCI into groups receiving early (day 0–1) or late (day 2–29) PCI treatment; we therefore studied MI with PCI as one group within each time period.
Figure 1.
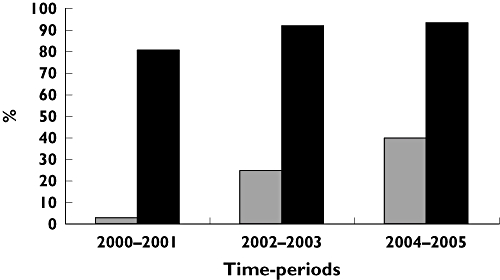
Dispensing a prescription of clopidogrel within 30 days of discharge. MI without PCI ( ); MI with PCI (▪)
); MI with PCI (▪)
Multivariable logistic regression analysis identified factors affecting initiation of therapy (Table 2). Among all patients concomitant medical treatment with ASA, β-blockers and statins was a predictor of a higher initiation rate, whereas treatment with vitamin-K antagonist predicted a lower initiation rate. Patients with heart failure undergoing PCI were less likely to initiate clopidogrel treatment. This tendency was present, but less clear, among MI patients without PCI having concomitant heart failure, with an odds ratio of 0.90 (0.81, 0.99) during 2002–2003 and 0.89 (0.80, 1.00) during 2004–2005 for initiating clopidogrel treatment. Among patients not treated with PCI, women received less treatment during 2004–2005. MI patients without PCI were more likely to initiate clopidogrel treatment when admitted to a tertiary cardiac hospital.
Table 2.
Multivariable logistic regressions analysis: odds ratio for initiation of clopidogrel treatment within 30 days of discharge
| MI patients with PCI All time periods | MI patients without PCI 2000–2001 | MI patients without PCI 2002–2003 | MI patients without PCI 2004–2005 | |||||
|---|---|---|---|---|---|---|---|---|
| n (total) = 14 939, of these 13 520 initiated clopidogrel treatment | n (total) = 12 516, of these 349 initiated clopidogrel treatment | n (total) = 8141, of these 2631 initiated clopidogrel treatment | n (total) = 7963, of these 3132 initiated clopidogrel treatment | |||||
| OR* | P-value† | OR* | P-value† | OR* | P-value† | OR* | P-value† | |
| Time periods | ||||||||
| 2000–2001 | 1.00 | |||||||
| 2002–2003 | 2.38 (2.06, 2.2.75) | <0.001 | ||||||
| 2004–2005 | 2.91 (2.50, 3.39) | <0.001 | ||||||
| Women | 1.07 (0.94, 1.22) | NS | 0.83 (0.66, 1.05) | NS | 0.97 (0.88, 1.06) | NS | 0.86 (0.78, 0.95) | <0.001 |
| Men | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Age 30–64 years | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Age 65–74 years | 1.04 (0.90, 1.19) | NS | 1.04 (0.80, 1.35) | NS | 1.01 (0.89, 1.14) | NS | 1.03 (0.90, 1.18) | NS |
| Age >75 years | 1.05 (0.89, 1.24) | NS | 0.72 (0.53, 0.98) | 0.03 | 0.92 (0.82, 1.04) | NS | 0.98 (0.86, 1.11) | NS |
| Comorbidity | ||||||||
| Cerebral vascular disease | 0.48 (0.36, 0.63) | <0.001 | 1.15 (0.71, 1.85) | NS | 0.89 (0.74, 1.08) | NS | 0.81 (0.67, 0.98) | 0.03 |
| Diabetes with complications | 1.38 (0.98, 1.94) | NS | 0.78 (0.44, 1.38) | NS | 0.99 (0.80, 1.23) | NS | 1.11 (0.88, 1.39) | NS |
| Cardiac dysrythmia | 0.70 (0.58, 0.85) | <0.001 | 0.88 (0.59, 1.32) | NS | 1.04 (0.90, 1.20) | NS | 0.82 (0.71, 0.96) | 0.01 |
| Acute renal failure | 0.51 (0.26, 1.00) | 0.05 | 0.49 (0.11, 2.21) | NS | 0.99 (0.63, 1.55) | NS | 0.50 (0.30, 0.82) | 0.006 |
| Chronic renal failure | 1.15 (0.63, 2.08) | NS | 1.59 (0.65, 3.89) | NS | 0.92 (0.62, 1.35) | NS | 1.08 (0.78, 1.51) | NS |
| Malignancy | 0.76 (0.52, 1.09) | NS | 0.43 (0.17, 1.04) | NS | 0.75 (0.58, 0.96) | NS | 0.83 (0,65, 1,06) | NS |
| Shock | 0.50 (0.31, 0.81) | 0.005 | 0.34 (0.05, 2.51) | NS | 0.73 (0.45, 1.18) | NS | 0.76 (0.49, 1.18) | NS |
| Pulmonary oedema | 0.64 (0.37, 1.12) | NS | 0.29 (0.07, 1.20) | NS | 0.90 (0.65, 1.26) | NS | 0.80 (0.57, 1.11) | NS |
| Concomitant medical treatment | ||||||||
| β-Blocker | 2.14 (1.85, 2.48) | <0.001 | 1.27 (0.96, 1.68) | NS | 1.60 (1.43, 1.80) | <0.001 | 1.90 (1.69, 2.13) | <0.001 |
| ACE inhibitor | 1.16 (1.02, 1.31) | 0.02 | 1.30 (1.04, 1.63) | 0.02 | 1.05 (0.96, 1.16) | NS | 1.01 (0.91, 1.11) | NS |
| Statin | 120 (1.72, 2.32) | <0.001 | 1.76 (1.38, 2.25) | <0.001 | 2.39 (2.14, 2.67) | <0.001 | 2.51 (2.24, 2.83) | <0.001 |
| Loop diuretics‡ | 0.49 (0.43, 0.56) | <0.001 | 0.96 (0.75, 1.22) | NS | 0.90 (0.81, 0.99) | 0.03 | 0.89 (0.80, 1.00) | 0.04 |
| Antidiabetic treatment§ | 0.85 (0.69, 1.05) | NS | 1.08 (0.74, 1.58) | NS | 1.14 (0.97, 1.34) | NS | 0.91(0.77, 1.07) | NS |
| Vitamin-K antagonists | 0.52 (0.42, 0.64) | <0.001 | 0.48 (0.28, 0.82) | 0.008 | 0.53 (0.44, 0.64) | <0.001 | 0.48 (0.39, 0.57) | <0.001 |
| Acetylsalic acid | 1.68 (1.44, 1.96) | <0.001 | 0.44 (0.35, 0.55) | <0.001 | 1.16 (1.02, 1.32) | 0.02 | 1.86 (1.60, 2.16) | <0.001 |
| Type of hospital | ||||||||
| Local hospital | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Main regional hospital | 0.96 (0.79, 1.18) | NS | 1.27 (0.93, 1.75) | NS | 1.41 (1.23, 1.62) | <0.001 | 1.14 (0.95, 1.38) | NS |
| Tertiary cardiac hospital | 0.93 (0.76, 1.14) | NS | 2.05 (1.41, 2.99) | <0.001 | 2.16 (1.83, 2.56) | <0.001 | 1.33 (1.08, 1.65) | 0.009 |
Odds Ratio (+/− confidence interval).
A value of P > 0.05 is considered to be nonsignificant (NS).
Loop diuretics is used as a proxy for heart failure.
Antidiabetic medication used as a proxy of diabetes.
Persistence with treatment
Among patients initiated on clopidogrel treatment, duration of treatment varied throughout the study period (Figure 2 and Table 3). The assumption of a daily dosage of 75 mg was confirmed by dosage calculations. We found persistence rates with clopidogrel treatment to be remarkably high among MI patients treated with PCI during the first 9 months. Factors affecting nonpersistence with treatment were analysed during the last time period (2004–2005), where guidelines clearly recommend treatment for 9–12 months. During this period, 26.3% of patients treated with PCI had a break of >30 days, whereas 26.9% of MI patients not treated with PCI experienced a break of >30 days. Among MI patients with PCI, re-initiation within the following 90 days occurred in 25.7%, the corresponding figure for MI patients without PCI being 21.1%. The results of the Cox proportional hazard analysis of risk of experiencing a break in therapy of ≥30 days are shown in Table 4.
Figure 2.
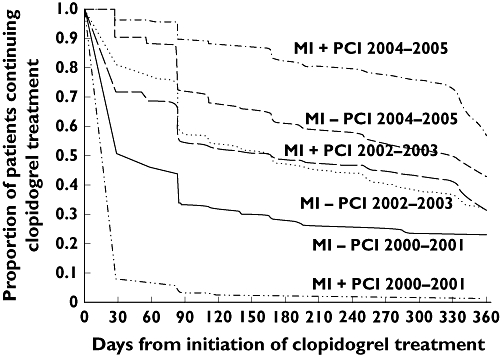
Myocardial infarction (MI) patients treated with clopidogrel: persistence with clopidogrel treatment during the first year after discharge
Table 3.
Persistence in treatment [proportion of days covered (PDC)]
| PDC*360 days after discharge | ||
|---|---|---|
| Mean (±SD) | Median (range) | |
| 2000–2001 | ||
| MI − PCI | 0.34 (±0.38) | 0.12 (0.99) |
| MI + PCI | 0.12 (±0.21) | 0.04 (0.99) |
| 2002–2003 | ||
| MI − PCI | 0.50 (±0.37) | 0.43 (0.98) |
| MI + PCI | 0.48 (±0.37) | 0.44 (0.98) |
| 2004–2005 | ||
| MI − PCI | 0.76 (±0.31) | 0.95 (0.96) |
| MI + PCI | 0.89 (±0.20) | 0.99 (0.96) |
If treated 30 days/year PDC = 0.083. 90 days/year PDC = 0.25. 180 days/year PDC = 0.5. 360 days/year PDC = 1.0. MI, myocardial infarction; PCI, percutaneous coronary intervention.
Table 4.
Cox regressions analysis: hazard ratio for having a break in clopidogrel treatment >30 days during 2004–2005, patients with or without percutaneous coronary intervention analysed together
| MI patients initiated on clopidogrel treatment 2004–2005* | Hazard ratio (95% CI) | P-value¶ |
|---|---|---|
| Women | 1.00 | |
| Men | 0.88 (0.78, 0.98) | 0.03 |
| Age 30–64 years | 1.00 | |
| Age 65–74 years | 1.14 (1.00, 1.31) | NS |
| Age >75 years | 1.03 (0.90, 1.19) | NS |
| Comorbodity: | ||
| Cerebral vascular disease | 0.85 (0.66, 1.08) | NS |
| Diabetes with complications | 1.10 (0.85, 1.44) | NS |
| Cardiac dysrythmia | 0.96 (0.81, 1.15) | NS |
| Acute renal failure | 1.32 (0.79, 2.22) | NS |
| Chronic renal failure | 0.81 (0.51, 1.29) | NS |
| Malignancy | 1.09 (0.83, 1.45) | NS |
| Shock | 1.36 (0.88, 2.10) | NS |
| Pulmonary oedema | 1.10 (0.72, 1.69) | NS |
| Percutaneous coronary intervention | 1.05 (0.93, 1.19) | NS |
| Concomitant medical treatment: | ||
| β-Blocker | 1.08 (0.94, 1.24) | NS |
| ACE-i | 1.02 (0.91, 1.13) | NS |
| Statin | 1.01 (0.87, 1.18) | NS |
| Loop diuretics† | 0.95 (0.84, 1.08) | NS |
| Antidiabetic treatment‡ | 1.04 (0.85, 1.26) | NS |
| Vitamin-K antagonists | 1.24 (1.01, 1.52) | 0.04 |
| Acetylsalicylic acid | 1.02 (0.86, 1.21) | NS |
| Type of hospital: | ||
| Local hospital§ | 1.00 | |
| Main regional hospital | 1.01 (0.81, 1.26) | NS |
| Tertiary cardiac hospital | 0.97 (0.77, 1.23) | NS |
n (total number of patients 2004–2005) = 14 521. n (initiated clopidogrel treatment) = 9274, of whom 6142 were myocardial infarction (MI) patients with percutaneous coronary intervention (PCI) and 3132 where MI patients without PCI. n (breaks >30 days) = 2598.
Loop diuretics is used as a proxy for heart failure.
Antidiabetic medication used as a proxy of diabetes.
Local hospital used as reference.
A value of P > 0.05 is considered to be nonsignificant (NS).
Discussion
This study has examined initiation and persistence with clopidogrel treatment in an unselected cohort of patients surviving their first MI from 2000 to 2005. During the study period treatment with clopidogrel increased dramatically in accordance with results of large randomized trials and subsequent international guidelines [1, 15–18]. Initiation of clopidogrel in MI patients treated with PCI was high, 93.7% claiming at least one prescription within 30 days after discharge in 2004–2005. In contrast, we found that among MI patients not treated with PCI only 39.3% claimed a prescription of clopidogrel in 2004–2005. Patients with first MI undergoing PCI having heart failure were initiated on clopidogrel less often than those without heart failure. A comparable, although not statistically significant trend was seen among MI patients without PCI. Initiation rates and the increase of initiation are in agreement with results found by others [19–21].
Guidelines for duration of clopidogrel treatment have changed throughout the study period [1, 15–18]. After NSTEMI clopidogrel was recommended only for patients not tolerating ASA in 2000–2001 [22], for all patients 1–12 months in 2002–2003 [22, 23] and 9–12 months after 2004 [24]. For PCI patients treated with stents, the recommended duration of clopidogrel treatment differs according to stent type and also the time periods [4, 5]. In 2000–2001 only BMS were used, and 30 days’ clopidogrel treatment was considered sufficient [25]. From 2002–2005, 1–3 months of treatment was recommended for BMS and preferably 12 months for DES (3–6 months if the patient was at risk of bleeding) [4, 23, 26, 27].
During the study period the changes in guidelines were followed by parallel changes in clinical practice, as illustrated by Figure 2 and Table 3. Persistence rates among MI patients with PCI were remarkably high: 89.4% completed 90 days of treatment, 82.2% 180 days and 77.5% were treated throughout 9 months in 2004–2005. In contrast, MI patients without PCI had lower initiation rates followed by lower persistence with therapy, declining from 71.6% after 90 days of treatment to 53.9% after 9 months of treatment (2004–2005). Re-initiation of clopidogrel after a break of >30 days was likewise slightly less in the group of MI patients not treated with a PCI (21.1% vs. 25.8%).
As mentioned, the only indication for clopidogrel from 2000 to 2001 among MI patients without PCI was allergy towards ASA, which explains why this group had higher persistence with treatment than MI patients with PCI in the same period, since only 30 days’ treatment was recommended in 2000–2001 if treated with a PCI (Figure 2).
There are few studies devoted towards long-term clopidogrel treatment. One study has found that among PCI patients approximately 75% were treated for 12 months. The study used patients’ reports every 6 months as a source of determining persistence with clopidogrel treatment among PCI patients and did not describe breaks in therapy within the treatment period [28]. As for other cardiovascular drugs, persistence rates among Danish post-MI patients treated with β-blockers, ACE-i and statins were 58, 74 and 82%, respectively (after 5 years’ treatment) [14]. Several studies have tried to describe independent factors affecting persistence with therapy, but with conflicting results. Factors such as gender, age >80 years, diagnosis of MI or heart failure, multiple drugs therapy and consultation by a cardiologist has been associated with both good and bad persistence with ASA, β-blocker, ACE-i and statins [14, 29, 30]. The variety of results from different studies illustrates that persistence with treatment, even in comparable populations, is difficult to standardize in a multivariable model because of complexity [14, 29]. In the current study we found that male sex and concomitant treatment with vitamin-K antagonist were predictors of nonpersistence with treatment. Presumably the higher risk of nonpersistence among patients treated with concomitant vitamin-K antagonists can be explained by the higher risk of experiencing a bleeding complication. The main difference between our study and those mentioned above is that clopidogrel is given for only a limited time period and in connection with a first MI, which could explain the good persistence rates.
Among MI patients with PCI, patients with heart failure were less likely to initiate clopidogrel treatment. Among MI patients without PCI this was only borderline significant. However, it is a concerning trend, since these patients are at high risk, particularly if they experience a new MI [31]. Heart failure patients were likewise underrepresented in the treatment group in another clopidogrel study [19]. In addition, MI patients without PCI admitted to a tertiary cardiac hospital were more likely to initiate clopidogrel treatment than those admitted to a local hospital. This is in accordance with the findings of Tricoci [19]. Patients being treated with a PCI in Denmark are all admitted, at least for the day of the procedure, to a tertiary cardiac hospital. Usually the tertiary cardiac hospital makes a plan for the subsequent antithrombotic regime.
MI patients, independent of PCI status treated with concomitant vitamin-K antagonists were less likely to be initiated on clopidogrel after a MI. This can reasonably be explained by lack of recommendations on combinational therapy of ASA, clopidogrel and vitamin-K antagonists for patients with an indication for anticoagulant treatment (i.e. atrial fibrillation, mechanical valve) in both past and present guidelines [32, 33].
In the group of MI patients without PCI, women were less likely to initiate clopidogrel treatment during the last time period, 2004–2005. It has been documented that women more often have refractory ischaemia during admission for MI and are more often re-hospitalized for unstable angina in the following period. Guidelines recommend clopidogrel treatment to all patients regardless of gender, and treatment with clopidogrel should be considered for every individual fulfilling indications [34].
Suggestions have been made that inadequate persistence with clopidogrel therapy could explain a component of ‘hyporesponding’ to clopidogrel treatment [8, 9]. However, we find it unlikely that among the MI patients treated with PCI nonpersistence with clopidogrel treatment can explain differences in platelet inhibition, since 82.1% adhered with treatment throughout the first 180 days.
Strengths and limitations
The current study was a register-based study. The register holds information on all hospital admittances in Denmark since 1978, which allows analysis on a complete and unselected cohort of MI patients. The register has proven high sensitivity and specificity of the MI diagnosis [11]. The completeness of the register leaves out selection biases of only including certain hospitals, certain areas or certain healthcare insurance systems. The Danish healthcare system delivers healthcare services to all citizens regardless of socioeconomic status and partially reimburses drug expenses, which is typical of many Western countries. The concordance between drug dispensing and drug consumption is likely to be very high, since reimbursement of drug expenses is only partial.
We made a decision that a break of 30 days was needed to be registered as nonpersistent. This is arbitrary, since it is not known how long clopidogrel treatment can be interrupted before its beneficial effect wears off. In the literature it is stated that the effect of a single antiplatelet agent diminishes after 8–20 days, according to the turnover of platelets [24, 35]. However, most patients received dual antiplatelet treatment with a combination of ASA and clopidogrel, and the time interval between a break in dual antiplatelet treatment to disappearance of clinical effect is unknown. Further research on the vulnerable period is needed [24]. The national prescription register does not hold complete information on individuals’ ASA use, since ASA can be purchased OTC and also by prescription (in order to receive reimbursement). In our population, 83.2% of patients claimed a prescription of ASA, which is probably as underestimate, since many patients purchase ASA as an OTC drug. The proportions concur with results from two previous studies, with comparable populations, where 86.5 and 92.6% (an average of two groups) received ASA as prophylactic treatment after 6 months and 3 years post MI, respectively [36, 37].
We do not know how many patients initiated clopidogrel treatment in the hospital and did not continue treatment after discharge.
Finally, the registries do not hold information on contraindications, side-effects of the drug or allergies, which may be causes of nonpersistence.
Conclusion
Initiation and persistence with therapy was very high among MI patients treated with PCI, indicating that guidelines were followed. Nonpersistence as a cause of low response to clopidogrel treatment can therefore mostly be ruled out in this population. Only one-third of MI patients without PCI were initiated on clopidogrel treatment, and of these only two-thirds completed treatment for 6 months. Among the MI patients without PCI we found substantial underuse among patients admitted to local hospitals and women. Furthermore, patients with heart failure undergoing PCI were treated less. In the future, focus is needed on the underuse of clopidogrel in these subgroups, since treatment has been shown to improve outcome.
Competing interests
None to declare.
This study was supported by a research fellowship from the Danish Heart Foundation (Grant No. 07-4-B641-A1510-22377).
REFERENCES
- 1.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 2.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Kristensen SD, Widimsky P, McGregor K, Sechtem U, Tendera M, Hellemans I, Gomez JL, Silber S, Funck-Brentano C, Kristensen SD, Andreotti F, Benzer W, Bertrand M, Betriu A, De Caterina R, Desutter J, Falk V, Ortiz AF, Gitt A, Hasin Y, Huber K, Kornowski R, Lopez-Sendon J, Morais J, Nordrehaug JE, Silber S, Steg PG, Thygesen K, Tubaro M, Turpie AG, Verheugt F, Windecker S. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: the Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J. 2007;28:1598–660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE., Jr Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, editors. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction-executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:652–726. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Silber S, Albertsson P, Aviles FF, Camici PG, Colombo A, Hamm C, Jorgensen E, Marco J, Nordrehaug JE, Ruzyllo W, Urban P, Stone GW, Wijns W. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804–47. doi: 10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, III, Morrison DA, O’Neil WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 6.Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol. 2005;45:1392–6. doi: 10.1016/j.jacc.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Michelson AD, Linden MD, Furman MI, Li Y, Barnard MR, Fox ML, Lau WC, McLaughlin TJ, Frelinger AL. Evidence that pre-existent variability in platelet response to ADP accounts for ‘clopidogrel resistance. J Thromb Haemost. 2007;5:75–81. doi: 10.1111/j.1538-7836.2006.02234.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Donoghue M, Wiviott SD. Clopidogrel response variability and future therapies: clopidogrel: does one size fit all? Circulation. 2006;114:e600–6. doi: 10.1161/CIRCULATIONAHA.106.643171. [DOI] [PubMed] [Google Scholar]
- 9.Fefer P, Hod H, Matetzky S. Clopidogrel resistance – the cardiologist's perspective. Platelets. 2007;18:175–81. doi: 10.1080/09537100601039747. [DOI] [PubMed] [Google Scholar]
- 10.Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44:445–8. [PubMed] [Google Scholar]
- 11.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003;56:124–30. doi: 10.1016/s0895-4356(02)00591-7. [DOI] [PubMed] [Google Scholar]
- 12.Tu JV, Austin PC, Walld R, Roos L, Agras J, McDonald KM. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37:992–7. doi: 10.1016/s0735-1097(01)01109-3. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen S, Zwisler AD, Abildstrom SZ, Madsen JK, Madsen M. Hospital variation in mortality after first acute myocardial infarction in Denmark from 1995 to 2002: lower short-term and 1-year mortality in high-volume and specialized hospitals. Med Care. 2005;43:970–8. doi: 10.1097/01.mlr.0000178195.07110.d3. [DOI] [PubMed] [Google Scholar]
- 14.Gislason GH, Rasmussen JN, Abildstrom SZ, Gadsboll N, Buch P, Friberg J, Rasmussen S, Kober L, Stender S, Madsen M, Torp-Pedersen C. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153–8. doi: 10.1093/eurheartj/ehi705. [DOI] [PubMed] [Google Scholar]
- 15.Steinhubl SR, Berger PB, Mann JT, III, Fry ET, DeLago A, Wilmer C, Topol EJ. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–20. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–33. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 17.Holmes DR, Jr, Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, Brown C, Fischell T, Wong SC, Midei M, Snead D, Kuntz RE. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109:634–40. doi: 10.1161/01.CIR.0000112572.57794.22. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–7. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 19.Tricoci P, Roe MT, Mulgund J, Newby LK, Smith SC., Jr Pollack CV Jr, Fintel DJ, Cannon CP, Bhatt DL, Gibler WB, Ohman EM, Peterson ED, Harrington RA, editors. Clopidogrel to treat patients with non-ST-segment elevation acute coronary syndromes after hospital discharge. Arch Intern Med. 2006;166:806–11. doi: 10.1001/archinte.166.7.806. [DOI] [PubMed] [Google Scholar]
- 20.Vikman S, Airaksinen KE, Tierala I, Peuhkurinen K, Majamaa-Voltti K, Niemela M, Tuunanen H, Nieminen MS, Niemela K. Improved adherence to practice guidelines yields better outcome in high-risk patients with acute coronary syndrome without ST elevation: findings from nationwide FINACS studies. J Intern Med. 2004;256:316–23. doi: 10.1111/j.1365-2796.2004.01374.x. [DOI] [PubMed] [Google Scholar]
- 21.Carlhed R, Bojestig M, Wallentin L, Lindstrom G, Peterson A, Aberg C, Lindahl B. Improved adherence to Swedish national guidelines for acute myocardial infarction: the Quality Improvement in Coronary Care (QUICC) study. Am Heart J. 2006;152:1175–81. doi: 10.1016/j.ahj.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand ME, Simoons ML, Fox KA, Wallentin LC, Hamm CW, McFadden E, de Feyter PJ, Specchia G, Ruzyllo W. Management of acute coronary syndromes: acute coronary syndromes without persistent ST segment elevation; recommendations of the Task Force of the European Society of Cardiology. Eur Heart J. 2000;21:1406–32. doi: 10.1053/euhj.2000.2301. [DOI] [PubMed] [Google Scholar]
- 23.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, III, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC., Jr ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction – summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–74. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 24.Patrono C, Bachmann F, Baigent C, Bode C, De Caterina R, Charbonnier B, Fitzgerald D, Hirsh J, Husted S, Kvasnicka J, Montalescot G, Garcia Rodriguez LA, Verheugt F, Vermylen J, Wallentin L, Priori SG, Alonso Garcia MA, Blanc JJ, Budaj A, Cowie M, Dean V, Deckers J, Fernandez Burgos E, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Morais J, Deckers J, Ferreira R, Mazzotta G, Steg PG, Teixeira F, Wilcox R. Expert consensus document on the use of antiplatelet agents. The task force on the use of antiplatelet agents in patients with atherosclerotic cardiovascular disease of the European society of cardiology. Eur Heart J. 2004;25:166–81. doi: 10.1016/j.ehj.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Smith SC, Jr, Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ, Kuntz RE, Popma JJ, Schaff HV, Williams DO, Gibbons RJ, Alpert JP, Eagle KA, Faxon DP, Fuster V, Gardner TJ, Gregoratos G, Russell RO, Smith SC., Jr. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines) – executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation. 2001;103:3019–41. doi: 10.1161/01.cir.103.24.3019. [DOI] [PubMed] [Google Scholar]
- 26.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Ornato JP. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand ME, Simoons ML, Fox KA, Wallentin LC, Hamm CW, McFadden E, De Feyter PJ, Specchia G, Ruzyllo W. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2002;23:1809–40. doi: 10.1053/euhj.2002.3385. [DOI] [PubMed] [Google Scholar]
- 28.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE, Peterson ED, Schulman KA, Califf RM. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–68. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 29.Eagle KA, Kline-Rogers E, Goodman SG, Gurfinkel EP, Avezum A, Flather MD, Granger CB, Erickson S, White K, Steg PG. Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med. 2004;117:73–81. doi: 10.1016/j.amjmed.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Kopjar B, Sales AE, Pineros SL, Sun H, Li YF, Hedeen AN. Adherence with statin therapy in secondary prevention of coronary heart disease in veterans administration male population. Am J Cardiol. 2003;92:1106–8. doi: 10.1016/j.amjcard.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Capewell S, Murphy NF, MacIntyre K, Frame S, Stewart S, Chalmers JW, Boyd J, Finlayson A, Redpath A, McMurray JJ. Short-term and long-term outcomes in 133 429 emergency patients admitted with angina or myocardial infarction in Scotland, 1990–2000: population-based cohort study. Heart. 2006;92:1563–70. doi: 10.1136/hrt.2005.085399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE., Jr Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, editors. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 33.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Kristensen SD, Widimsky P, McGregor K, Sechtem U, Tendera M, Hellemans I, Gomez JL, Silber S, Funck-Brentano C, Kristensen SD, Andreotti F, Benzer W, Bertrand M, Betriu A, De Caterina R, DeSutter J, Falk V, Ortiz AF, Gitt A, Hasin Y, Huber K, Kornowski R, Lopez-Sendon J, Morais J, Nordrehaug JE, Silber S, Steg PG, Thygesen K, Tubaro M, Turpie AG, Verheugt F, Windecker S. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J. 2007;28:1598–660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 34.Anand SS, Xie CC, Mehta S, Franzosi MG, Joyner C, Chrolavicius S, Fox KA, Yusuf S. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J Am Coll Cardiol. 2005;46:1845–51. doi: 10.1016/j.jacc.2005.05.091. [DOI] [PubMed] [Google Scholar]
- 35.Burger W, Chemnitius JM, Kneissl GD, Rucker G. Low-dose aspirin for secondary cardiovascular prevention – cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation – review and meta-analysis. J Intern Med. 2005;257:399–414. doi: 10.1111/j.1365-2796.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 36.Busk M, Maeng M, Rasmussen K, Kelbaek H, Thayssen P, Abildgaard U, Vigholt E, Mortensen LS, Thuesen L, Kristensen SD, Nielsen TT, Andersen HR. The Danish multicentre randomized study of fibrinolytic therapy vs. primary angioplasty in acute myocardial infarction (the DANAMI-2 trial): outcome after 3 years follow-up. Eur Heart J. 2008;29:1259–66. doi: 10.1093/eurheartj/ehm392. [DOI] [PubMed] [Google Scholar]
- 37.EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J. 2001;22:554–72. doi: 10.1053/euhj.2001.2610. [DOI] [PubMed] [Google Scholar]


