Solifenacin, an antimuscarinic drug, has been widely used for the treatment of overactive bladder (OAB), but severe cardiac adverse effects have not been reported. An 81-year-old woman was prescribed solifenacin 5 mg day−1 for OAB. Two weeks later she developed the first syncope. Nine days after the first syncope she developed recurrent syncope, and electrocardiogram monitoring showed marked QT prolongation and torsade de pointes (TdP). Concomitant drugs remained unchanged. Serum electrolytes and hepatic function were normal. Calculated creatinine clearance was mildly decreased. We considered that the loss of consciousness was associated with solifenacin. Although intravenous magnesium sulphate could not terminate the recurrence of TdP, increasing the pacing rate prevented further attacks of TdP. Solifenacin might be a possible cause of QT prolongation and TdP.
Solifenacin is a newer bladder-selective antimuscarinic drug for the treatment of OAB [1]. Although terodiline, an older antimuscarinic drug for OAB, has been reported to predispose to QT prolongation and TdP and was withdrawn from the market in the early 1990s [2–4], solifenacin has not been reported to have severe cardiac adverse effects [1].
An 81-year-old woman was admitted to the division of orthopaedics with dislocation of hip joint and infection of decubitus. One year before admission she had undergone total hip arthroplasty. Her past medical history included hypertension and pacemaker implantation for sick sinus syndrome. Her medication included amlodipine, but not any over-the-counter drugs or herbals. Electrocardiogram (ECG) on admission showed sinus rhythm with normal QT interval (QT400 ms/QTc360 ms) (Figure 1a). Solifenacin 5 mg day−1 was started for OAB after admission. Her infection was initially treated with intravenous cefazolin, but was changed to intravenous teicoplanin because of methicillin-resistant Staphylococcus aureus infection. Two weeks later she suddenly developed loss of consciousness, but she spontaneously regained full consciousness.
Figure 1.
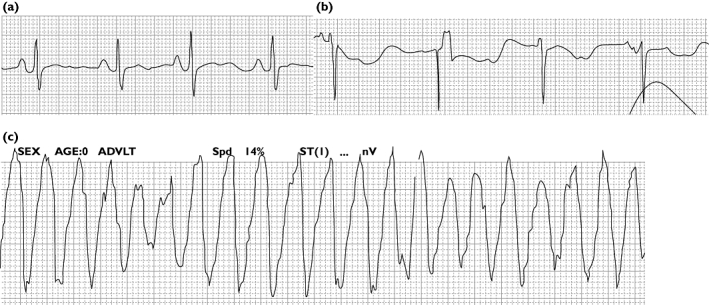
ECG monitoring. (a) Sinus rhythm with normal QT interval on admission. (b) Marked QT prolongation after the first syncope. (c) Typical episode of torsade de pointes 9 days after the first syncope
Although ECG was not recorded during the episode, ECG monitoring showed QT prolongation (QT600 ms/QTc581 ms) after regaining consciousness (Figure 1b). Nine days after the first syncope she developed recurrent loss of consciousness and ECG monitoring showed TdP (Figure 1c). She was resuscitated with a single DC shock. She was immediately transferred to the division of cardiology. ECG monitoring showed QT prolongation (QT600 ms) and recurrent TdP. Serum electrolytes and hepatic function were normal. Calculated creatinine clearance was 60 ml min−1. We considered that solifenacin was closely related to syncope and TdP. We stopped solifenacin. Intravenous magnesium sulphate could not terminate recurrent episodes of TdP. The pacing rate was increased 60 to 90 beats min−1 to shorten the QT interval, resulting in a QT interval of 600 to 500 ms (QTc 408 ms). There were no further attacks of TdP. Electrocardiographic abnormalities resolved a few days after solifenacin was discontinued.
The high bladder selectivity of solifenacin might be expected to reduce adverse effects [1], which include dry mouth, constipation and blurred vision, but severe cardiac adverse effects have not been reported [1]. Known risk factors for drug-induced TdP are female sex, hypokalaemia, hypomagnesaemia, bradycardia, simultaneous administration of inhibitor compounds, and genetic factors [4–6]. Serum electrolytes, especially potassium and magnesium, were normal. Age and mild renal dysfunction had no clinically relevant effects on the pharmacokinetics of solifenacin [1]. The main metabolic pathway of solifenacin is CYP3A4 [1]. Although hepatic dysfunction decreased clearance of solifenacin [1], hepatic function was also normal. Anti-infective agents that were administered intravenously did not include CYP3A4 inhibitors such as erythromycin [4–6]. She had no other structural heart diseases, nor any family history of sudden death. Concomitant drugs remained unchanged. Amlodipine would not have interacted with solifenacin because of little influence of first-pass effect by CYP3A4 at therapeutic dose [7]. Marked QT prolongation and TdP were considered to be related to solifenacin. Intravenous magnesium sulphate, the first-choice treatment of drug-induced TdP [6], was not effective. Recurrence of TdP was prevented after the QT interval was shortened by increasing the pacing rate. We did not investigate her genetic factors and plasma drug concentration. Although the mechanism is not fully understood, the start of solifenacin might have influenced the outcome in our elderly female patient. To our knowledge, this is the first reported case of QT prolongation and TdP associated with solifenacin.
REFERENCES
- 1.Payne CK. Solifenacin in overactive bladder syndrome. Drugs. 2006;66:175–90. doi: 10.2165/00003495-200666020-00004. [DOI] [PubMed] [Google Scholar]
- 2.Connolly MJ, Astridge PS, White EG, Morley CA, Cowan JC. Torsades de pointes ventricular tachycardia and terodiline. Lancet. 1991;33:344–5. doi: 10.1016/0140-6736(91)90481-4. [DOI] [PubMed] [Google Scholar]
- 3.McLeod AA, Thorogood S, Barnett S. Torsades de pointes complicating treatment with terodiline. BMJ. 1991;302:1469. doi: 10.1136/bmj.302.6790.1469-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dmochowski R, Staskin DR. The QT interval and antimuscarinic drugs. Curr Urol Rep. 2005;6:405–9. doi: 10.1007/s11934-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 5.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Lawrence AT, Krisnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:819–99. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 7.Meredith PA, Elliott HL. Clinical pharmacokinetics of amlodipine. Clin Pharmacokinet. 1992;22:22–31. doi: 10.2165/00003088-199222010-00003. [DOI] [PubMed] [Google Scholar]


