Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Diclofenac is an effective oral analgesic for acute postoperative pain. In adults 25 mg is half as effective as 50 mg, but 50 mg and 100 mg are similarly effective (ceiling effect). Diclofenac has linear pharmacokinetics in this range.
Diclofenac is frequently used ‘off-label’ in children for acute pain but optimum dosing is unclear (dosing of diclofenac in clinical paediatric studies ranges from 0.5–2.5 mg kg−1). There is currently no licensed oral paediatric formulation of diclofenac.
WHAT THIS STUDY ADDS
Using a new diclofenac oral suspension, a dose of 1 mg kg−1 in children aged 1 to 12 years gives a similar exposure to 50 mg in adults; paediatric patients are unlikely to benefit from higher doses.
AIMS
To develop a population pharmacokinetic model for a new diclofenac suspension (50 mg 5 ml−1) in adult volunteers and paediatric patients, and recommend a dose for acute pain in children.
METHODS
Blood samples were drawn at the start and end of surgery, and on removal of the venous cannula from 70 children (aged 1 to 12 years, weight 9 to 37 kg) who received a preoperative oral 1 mg kg−1 dose; these were pooled with rich (14 post-dose samples) data from 30 adult volunteers. Population pharmacokinetic modelling was undertaken with NONMEM. The optimum adult dose of diclofenac for acute pain is 50 mg. Simulation from the final model was performed to predict a paediatric dose to achieve a similar AUC to 50 mg in adults.
RESULTS
A total of 558 serum diclofenac concentrations from 100 subjects was used in the pooled analysis. A single disposition compartment model with first order elimination and dual absorption compartments was used. The estimates of CL/F and VD/F were 53.98 l h−1 70 kg−1 and 4.84 l 70 kg−1 respectively. Allometric size models appeared to predict adequately changes in CL and VD with age. Of the simulated doses investigated, 1 mg kg−1 gave paediatric AUC(0, 12 h) to adult 50 mg AUC(0, 12 h) ratios of 1.00, 1.08 and 1.18 for ages 1–3, 4–6 and 7–12 years respectively.
CONCLUSIONS
This study has shown 1 mg kg−1 diclofenac to produce similar exposure in children aged 1 to 12 years as 50 mg in adults, and is acceptable for clinical practice; patients are unlikely to obtain further benefit from higher doses.
Keywords: acute pain, children, diclofenac, NONMEM, population pharmacokinetics
Introduction
The non-steroidal anti-inflammatory drug (NSAID) diclofenac is widely used to manage acute pain in children [1], but there is no licensed paediatric oral formulation in the UK, and dosing is yet to be properly established. Published clinical studies use single doses ranging from 0.5 [2] to 2.5 mg kg−1[3]. Clinical experience suggests that doses over 1 mg kg−1 may be more effective in children but the optimum dose is still a matter for debate [4].
Adult clinical studies suggest a ceiling effect in the dose–response curve with 25 mg being about half as effective as 50 mg, and 50 mg being similarly effective to 100 mg for acute postoperative pain [5]. Diclofenac has linear pharmacokinetics in this range, AUC increasing proportionally with dose [6], so this ceiling effect could be related to saturation of the target enzyme.
The main mode of analgesic action of diclofenac is through potent inhibition of cyclo-oxygenase-2 (COX-2) causing a decrease in the conversion of arachidonic acid into inflammatory prostaglandins [7]. Diclofenac displays time-dependent inhibition of COX-2 even at high concentrations of arachidonic acid [8, 9], and has central and peripheral antinociceptive activity [10]. This suggests that therapeutic effect is probably better correlated with drug exposure (AUC) rather than a target tissue concentration. If it is assumed that COX-2 expression does not differ between children and adults (no references were found to support or refute this assumption), then a paediatric dose that produces a similar AUC to 50 mg in adults should give a similar therapeutic effect. This study therefore used a surrogate for an effective dose: the AUC produced when adults are given 50 mg of diclofenac, allowing the recruitment of patients from a range of minor surgical lists and avoiding the need for pain scoring, of which the reliability and comparability in children of different ages is questionable [11].
The aim of this study was to develop a pooled population pharmacokinetic model for a new diclofenac oral suspension in paediatric patients and adult volunteers, and recommend an appropriate paediatric dose based on achieving an AUC equivalent to that attained with 50 mg in adults.
Methods
Subjects
This study included two groups of subjects who received a single oral dose of a new diclofenac 50 mg 5 ml−1 suspension (Rosemont Pharmaceuticals Ltd, UK). The first group were paediatric day surgery patients aged between 1 and 12 years, and the second were 30 healthy adult volunteers.
Paediatric patients
Written informed consent was obtained from parents of patients aged 1 to 12 years scheduled to undergo minor day-case surgery at Great Ormond Street Hospital for Children. The study was approved by Great Ormond Street Hospital research ethics committee. The exclusion criteria were: allergy to diclofenac or other NSAID; history of hepatic disease, renal dysfunction, known coagulation defects, gastrointestinal bleeding; having received one or more doses of diclofenac within the previous 24 h.
Three blood samples following a single pre-operative dose of diclofenac were drawn from each patient. All patients had undergone an overnight fast and, once recruited, a bottle of diclofenac sodium 10 mg ml−1 (Rosemont Pharmaceuticals Ltd, UK) was dispensed from the hospital pharmacy and a digital watch (Constant CT011 quartz sport LCD watch) allocated. The watch was used to record dose and blood sampling times to the nearest minute, and presented to the child as a gift at the end of the study. A single oral dose of diclofenac 1 mg kg−1 (rounded to the nearest 5 mg) was administered by oral syringe (Baxa Ltd, UK) in the 2 h prior to surgery.
The first blood sample was obtained in the anaesthetic room on insertion of the venous cannula, the second at the end of the procedure, and the third on removal of the cannula before discharge. No further restrictions were placed on when samples were to be taken, with the aim of ensuring a spread of sampling times within the population. The digital watch that each patient was allocated with was used to measure dosing and sampling times to the nearest minute. Approximately 0.5 ml of deadspace blood was withdrawn prior to blood sampling unless a sample was drawn from a newly inserted cannula. For the diclofenac assay 0.5–1 ml of whole clotted blood was collected and centrifuged, serum extracted and frozen at −20°C. Where a blood sample could not be obtained, for example if the patient pulled out their venous cannula or no blood could be withdrawn, no provision was made to re-insert the cannula. All patients, regardless of the number of samples obtained, were included in the population pharmacokinetic analysis. Any child re-admitted for surgery during the recruitment phase was eligible for entering the study on a second occasion.
In addition to diclofenac, at the discretion of the anaesthetist, some patients were also given oral midazolam 0.5 mg kg−1 (Special Products Ltd., UK) and/or oral paracetamol 20 mg kg−1 (Pfizer Consumer Healthcare Ltd., UK) as pre-operative medication. Anaesthesia was induced with either inhalational sevofluorane (Baxter Ltd, UK) or intravenous propofol 2–4 mg kg−1 (Fresenius Kabi Ltd, UK) depending on the child's age and parental/patient preference. In the case of inhalational induction, once the patient was unconscious an intravenous cannula was inserted into a peripheral vein and the first blood sample was drawn. Where patients had intravenous anaesthesia, a blood sample was drawn before the propofol was administered if the patient was calm following cannulation, otherwise patients were anaesthetised and then a blood sample drawn.
Diclofenac assays were performed by the analytical unit at St George's Hospital, London using sequential high performance liquid chromatography followed by mass spectrometer detection (HPLC/MS). Solid phase extraction was used to clean the samples prior to HPLC using an Altima C18 column with methanol and ammonium acetate (5 mmol l−1) 50 : 50 (Rathburn Chemicals Ltd, UK) as the mobile phase. Ketoprofen (Sigma-Aldrich Ltd, UK) was used as internal standard and detection was performed by an AP1400 mass spectrometer with nitrogen as the collision gas. Diclofenac sodium (Sigma-Aldrich Ltd, UK) was used for calibration. Output was analyzed using Analyst (version 1.3.2) software that performed integration of diclofenac detection peaks. The lower limit of detection for diclofenac was 10.1 ng ml−1. The intra-assay precision, defined by the percentage coefficient of variation, ranged from 0.8 to 11.8%, the mean percentage accuracy ranged from 94.2 to 112.6%.
Adverse events were monitored for and recorded throughout the hospital admission. In addition, parents were telephoned approximately 1 week following discharge to check for any delayed adverse events. It was planned to gauge the cause of any serious adverse events using the WHO causality assessment criteria [12].
Adult volunteers
Pharmacokinetic data on diclofenac sodium suspension was obtained from healthy adult volunteers during a bioequivalence study (unpublished) undertaken by the Shandon Clinic Ltd, Cork, Ireland. After fasting overnight the volunteers received a single 50 mg dose of diclofenac suspension (Rosemont Pharmaceuticals Ltd, UK) and 6 ml blood samples for diclofenac assay were drawn at 0.25, 0.5, 0.75, 1.0, 1.33, 1.67, 2.0, 2.5, 3.0, 3.5, 4.0, 6.0, 9.0 and 12.0 h post-dose. Where actual sampling times deviated from this schedule by more than 1 min, actual time was recorded. As with the paediatric samples, HPLC/MS was used although naproxen (Sigma Ltd, UK) was the internal standard and plasma rather than serum was assayed. The assay lower limit of quantification was 10 ng ml−1. The intra-assay precision, defined by the percentage coefficient of variation, ranged from 5.5 to 12.1%, the mean percentage accuracy ranged from 94.7 to 104.1%.
Pharmacokinetic model building
Pooled data from the adult volunteers and paediatric patients were analyzed with NONMEM (version 6) [13]. A Dell D600 Notebook with Intel Pentium processor (2.00 GHz) running NONMEM compiled with a Compaq Visual Fortran (version 6.1) compiler was used, and data analyzed with the first-order conditional estimation plus interaction (between inter/intra-individual and residual variability) method. All mass units were expressed as nanomoles (assuming a molecular weight of 318.13 g for diclofenac sodium and 296.15 g for diclofenac) and all volume quantities in litres.
Raw plots of serum (paediatric) or plasma (adult) diclofenac concentration vs. time were generated in Excel (Microsoft Office 2003) and inspected for possible structural models. Structural models investigated were standard one disposition compartment, two disposition compartments, one disposition compartment with dual absorption compartments and two disposition compartments with dual absorption compartments. Absorption between depot and central compartments was investigated by zero, first, mixed zero-first order rates, and by the transit compartment model [14]. The transit absorption model replaces the lag time estimated in traditional pharmacokinetic models and consists of a number of transit compartments leading to the depot compartment. In the case of the dual absorption compartment models, a full dose was administered to both compartments. Bioavailability from each compartment was estimated in NONMEM as a fixed effect, with limits forcing the combined bioavailability to equal 100%, thereby only allowing one whole dose into the central disposition compartment.
Allometric weight scaling was added to all clearance and volume fixed effects a priori and standardized to a body weight of 70 kg [15, 16] according to the following relationships:
Clearance = θCL(W/70)0.75
Volume = θV(W/70)
Where:
θCL = Population estimate of clearance term (l h−1).
θV = Population estimate of distribution volume term (l).
W = Body weight (kg).
As there were differences in assay method for the adult and paediatric data, estimation of residual variability was undertaken separately for the two groups, with proportional, additive and mixed proportional-additive error models tested.
Interindividual variability was added in a stepwise fashion, firstly to clearance and volume parameters, and then to absorption parameters. When the final structural model was stabilized (successful run in NONMEM with realistic parameter estimates and reasonable goodness-of-fit plots) between occasion variability [17] was added to clearance and volume terms. Graphical analysis of final parameter estimates for clearance and volume vs the covariates age, height, sex and ethnicity were examined for trends not explained by the allometric size models.
Pharmacokinetic model evaluation
NONMEM table files were used to generate diagnostic plots in Excel and Xpose (version 4.0 run in R version 2.4.0) for general structural model and residual error model evaluation during the model building process. Due to the risk of random effect shrinkage caused by including sparse data [18], the main form of model evaluation was performed with the following simulation-based techniques: visual predictive check, mirror plots with Xpose and comparison of calculated AUC from the raw data and model-derived simulations (posterior predictive check).
Simulations
Once the final model and population parameter values had been derived, simulated doses of 0.5, 1, 1.5 and 2 mg kg−1 were investigated with NONMEM. A new dataset containing 100 patients with the same demographics as the subjects originally studied was created. Using the final model, 100 simulations were run where subjects received doses of 0.5, 1, 1.5 and 2 mg kg−1. The resulting simulated concentration–time data for each dose concentration were used to calculate median AUC(0,12 h) values for the age ranges 1 to 3 years, 4 to 6 years and 7 to 12 years, along with the AUC(0,12 h) for 50 mg in adults. Paediatric AUC(0,12 h) values at each dose concentration were divided by this adult 50 mg AUC(0,12 h) giving a ratio. Values closest to 1 gave the most similar exposure, and so were recommended doses.
Results
Over a 10 month period 74 paediatric patients were recruited and 70 provided blood samples (see Figure 1). Seven patients were re-admitted for further surgery during the recruitment period so were re-entered on a second occasion. Demographic details for paediatric patients and 30 adult volunteers given diclofenac suspension are given in Table 1.
Figure 1.
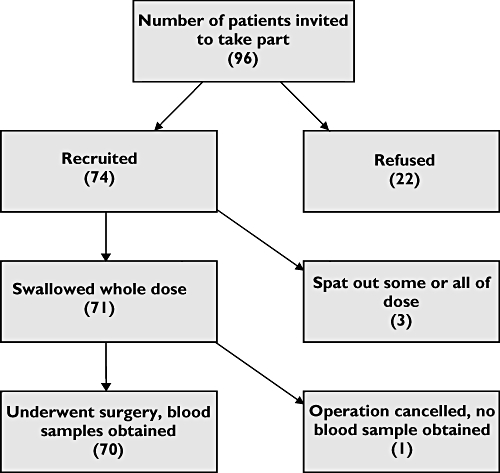
Paediatric patient recruitment
Table 1.
Demographic details of paediatric patients and adult volunteers included in the pooled population pharmacokinetic analysis
| Frequency given as mean (range) or number (percentage) as appropriate | |||
|---|---|---|---|
| Children n = 70 | Adults n = 30 | Pooled n = 100 | |
| Age (years) | 3 (1–12) | 21 (18–28) | 9 (1–28) |
| Weight (kg) | 17 (9–37) | 72 (48–94) | 34 (9–94) |
| Height (cm) | 101 (69–146) | 170 (158–187) | 122 (69–187) |
| Male | 41 (59%) | 14 (47%) | 55 (55%) |
| Female | 29 (41%) | 16 (53%) | 45 (45%) |
| Surgery type: | |||
| Dermatology | 54 (77%) | – | – |
| General* | 12 (17%) | – | – |
| Plastic* | 4 (6%) | – | – |
Excision of lesions undertaken by general and plastic surgeons, classification made by surgeon specialty.
A total of 558 (206 paediatric, 352 adult) diclofenac concentrations from 100 subjects (70 paediatric and 30 adult) were used in the pooled pharmacokinetic analysis. Sampling times for the paediatric patients ranged from 0.2 to 6.47 h post dosing, with at least two samples per dose being drawn from all but one patient, where only one sample was obtained. Reasons for not obtaining the full three samples were: patients or parents refused to allow the third sample (when the child would be awake); no blood could be withdrawn from the cannula; patient pulled out the cannula in the recovery area. No paediatric sample was below the limit of quantification (BLQ), and the values of BLQ samples in the adult data set (mainly post 6 h) were not reported by the laboratories, and so were discarded. No paediatric patient suffered a serious adverse event.
Inspection of the raw data revealed most variability occurred in the absorption phase. The elimination phase generally showed mono-exponential decrease with time. In the individual concentration vs time plots it was noted that 11 out of 30 adult profiles showed double or multiple peaks. At least four of the paediatric patients appeared to have similar atypical absorption profiles, although sparse sampling meant identifying double peaks was not possible.
Best fit was obtained using dual absorption compartments with a single disposition compartment, and transit absorption. Residual error was estimated separately for adult and paediatric data and a proportional error model was chosen for both groups. A schematic diagram of the final structural model and list of fixed effects estimated in NONMEM are given in Figure 2.
Figure 2.
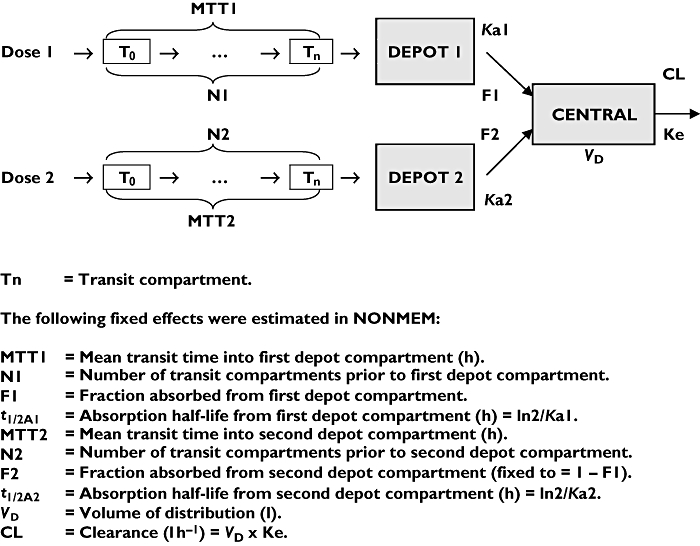
Schematic diagram of final model and overview of fixed-effects estimated in NONMEM
A plot of weighted residual error vs time is given in Figure 3. This lacked trend, indicating the structural model adequately described the data at all time points. Population and individual predicted concentrations vs observed concentrations are given in Figure 4. The visual predictive check given in Figure 5 shows that the final model was able to simulate data with a similar distribution to the observed data. Mirror plots from Xpose (not shown) showed raw simulated data to be similar to the original data set, and standard diagnostic plots were also similar. The final predictive check was to investigate how well the model predicted AUC(0,12 h). Calculation of the raw AUC(0,12 h) of the adult data using WinNonlin [19] gave a mean (SD) value of: 3368 (879) nmol l−1 h. AUC(0,12 h) calculated in WinNonlin from the mean serum concentrations of 3000 simulated adults was 2806 nmol l−1 h, which is within 1 SD of the raw value.
Figure 3.
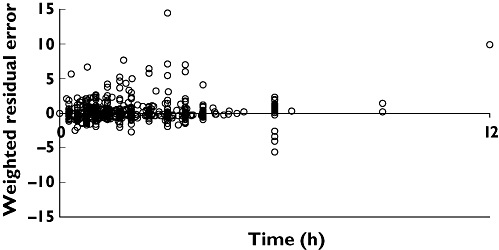
Scatter plot of weighted residual error vs time for final model
Figure 4.
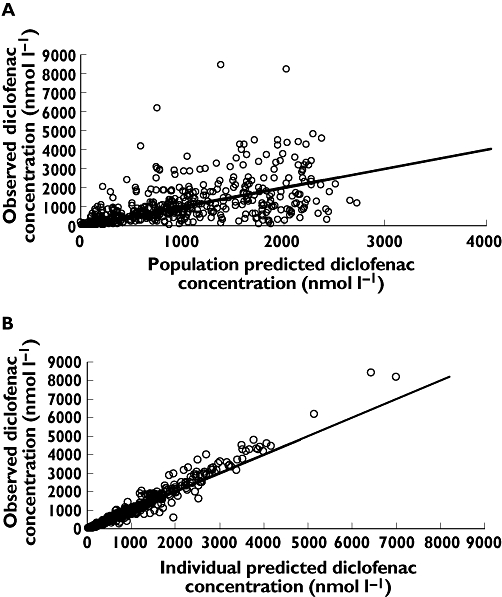
Scatter plots of observed diclofenac concentrations vs A) population and B) individual model predictions. Line of unity added for reference
Figure 5.
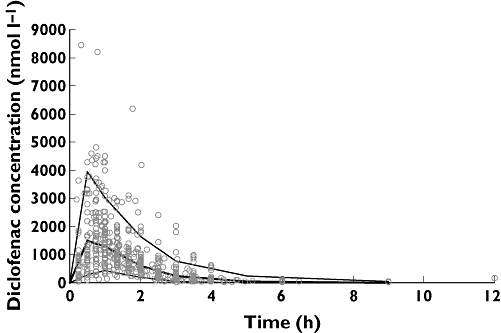
Visual predictive check of final model: raw data superimposed on median, 5th and 95th percentiles of data simulated from model. Median ( ); 95th percentile (
); 95th percentile ( ); 5th percentile (—); Raw data (
); 5th percentile (—); Raw data ( )
)
Standardized clearance (CLSTD/F) and volume (VDSTD/F), which were centred on 70 kg according to the allometric size model were plotted against age, weight, height and sex, with no obvious relationships seen. Geometric mean standardized CL/F of 50.6, 48.1 and 48.9 l h−1 70 kg−1 were estimated for patients aged 1–3, 4–12 years and adults respectively. The final parameter estimates are given in Table 2. The results of simulated population AUC(0,12 h) values at different dosing concentrations compared with the adult 50 mg value are given in Table 3.
Table 2.
NONMEM parameter estimates from final model
| Fixed effects (θ) | Random effects (η) | ||
|---|---|---|---|
| Parameter | Estimate (RSE) | Inter-individual variability (%) (RSE) | Between occasion variability (%) (RSE) |
| MTT1 (h) | 0.68 (11.8%) | 82 (149%) | – |
| N1 | 1.03 (28.6%) | 102 (35.6%) | – |
| F1 | 0.70 (7.6%) | 24 (19.9%) | – |
| t1/2A1 (h) | 0.09 (50.1%) | 31 (58.8%) | – |
| MTT2 (h) | 1.37 (6.97%) | 117 (59.4%) | – |
| N2 | 41.60 (73.6%) | 147 (83.9%) | – |
| t1/2A2 (h) | 1.06 (12.2%) | 49 (21.3%) | – |
| VD/F (l 70 kg−1) | 4.84 (86.2%) | 54 (254%) | 93 (280%) |
| CL/F (l h−1 70 kg−1) | 53.98 (4.8%) | 26 (18.2%) | 20 (89.6%) |
| Residual variability (ε) (%) | |||
| Adult data | 29 (5.9%) | ||
| Paediatric data | 18 (19.9%) | ||
RSE, Relative standard error from the S-matrix of the NONMEM covariance step.
Table 3.
Simulated AUC values for dosing between 0.5 mg kg−1 and 2 mg kg−1
| Patient age group | Dose | AUC(0, 12 h) : F (nmol. h l−1) | Ratio (Paediatric AUC(0, 12 h) : Adult 50 mg AUC(0, 12 h) |
|---|---|---|---|
| Adult | 50 mg | 2793.06 | – |
| Child 1–3 years | 0.5 mg kg−1 | 1391.58 | 0.50 |
| Child 1–3 years | 1 mg kg−1 | 2788.05 | 1.00 |
| Child 1–3 years | 1.5 mg kg−1 | 4174.76 | 1.50 |
| Child 1–3 years | 2 mg kg−1 | 5566.32 | 1.99 |
| Child 4–6 years | 0.5 mg kg−1 | 1545.82 | 0.55 |
| Child 4–6 years | 1 mg kg−1 | 3022.02 | 1.08 |
| Child 4–6 years | 1.5 mg kg−1 | 4637.45 | 1.66 |
| Child 4–6 years | 2 mg kg−1 | 6183.30 | 2.21 |
| Child 7–12 years | 0.5 mg kg−1 | 1633.65 | 0.59 |
| Child 7–12 years | 1 mg kg−1 | 3301.75 | 1.18 |
| Child 7–12 years | 1.5 mg kg−1 | 4900.95 | 1.76 |
| Child 7–12 years | 2 mg kg−1 | 6534.59 | 2.34 |
Discussion
This study has shown that single doses of diclofenac 1 mg kg−1 in children aged 1 to 12 years produced similar exposure to 50 mg in adults, suggesting that the recommended single dose for children should be 1 mg kg−1 (maximum 50 mg). The final population parameter estimate for CL was similar to a previous of diclofenac suppositories in children [20]; that study estimated a CLSTD/F of 44.82 l h−1 70 kg−1 compared with 53.98 l h−1 70 kg−1 estimated for the present study, with the possibility of slightly lower oral bioavailability accounting for the difference.
A meta-analysis of 10 studies on postoperative pain in adults has provided strong evidence that diclofenac 50 mg is as effective as 100 mg [5], despite linear pharmacokinetics in this range [6], making 50 mg the optimum dose. Little or no information on developmental differences in arachidonic acid release, prostaglandin formation and COX-2 expression is available, so based on current knowledge of NSAID pharmacology and adult pharmacodynamics, we assumed that attaining a similar adult diclofenac exposure to 50 mg in children should give similar efficacy. To assess adequately and predict exposure (AUC) it was important to develop a model which described CL well. Our final model estimated CL with a relative standard error of 4.8%, indicating that it was able to describe exposure well.
The compartmental modelling of immediate-release diclofenac required a relatively complex absorption model due to the presence of double peaks in the concentration–time curve, which were not unexpected [21]. A previous attempt to fit a simple first-order absorption to model the pharmacokinetics of a diclofenac formulation containing double peaks failed to produce an adequate description of the data [22], and the dual absorption model has been implemented in modelling diclofenac data containing double peaks in the past [23]. Diclofenac has a pKa of around 4.2 [24], meaning its aqueous solubility is low in acidic environments. Although diclofenac may undergo a small degree of enterohepatic recirculation [25], pharmacokinetic studies on intravenous [26] and enteric-coated tablet [22] formulations show a single peak. The cause of the double peaks is therefore probably due to variability in pH-dependent dissolution in the upper gastrointestinal tract.
Figures 4 and 5 show that two subjects (both children) had much higher diclofenac concentrations than the others. One explanation for this could be that the paediatric patients received a slightly higher dose than the adults: 1 mg kg−1 vs approximately 0.7 mg kg−1 (= 50 mg/mean adult weight). This 30% difference in dose, however, would not account for such large differences in concentration; from dose escalation studies in adults Cmax would be expected to increase by 30% with a 30% dose elevation [6]. It may be the case that paediatric patients have a higher gastrointestinal pH than adults, causing more rapid dissolution, increasing absorption rate and causing higher peak concentrations. Another possible explanation for the seemingly more erratic absorption in the paediatric patients is that they underwent a surgical procedure, whereas the adult volunteers did not, although as the dose was given before surgery no major systematic influence on the absorption profile would be expected. Whatever the cause of these occasional higher peak concentrations, those seen here are approximately half that attained with intravenous diclofenac 0.5 mg kg−1 in children [26], and so are unlikely to pose any increased risk of toxicity.
The presence of sparse data in this study led to the risk of shrinkage of the random effects. Shrinkage is a term used to describe the phenomenon of the distribution of random effects shrinking towards the observed value, and occurs where there are few samples per individual; such individuals provide relatively little information to the population estimate meaning that the individual predictions (model predictions based on both the population parameter and the estimate of variability) tend to be similar to the observed value [18]. This means that the plots of individual Bayesian prediction vs observation can look falsely good, and should not necessarily be relied upon to determine the usefulness of the model for predicting future data.
Further model evaluation prior to dose predictions therefore mainly centred on simulation-based diagnostics. These techniques compare data simulated from the final model with the original data; how similar they are is a good measure of model performance, and as they do not rely on the empirical Bayesian estimates from the NONMEM posthoc step, are not prone to bias induced by shrinkage. The simulation-based evaluations showed that the model was reasonably good at predicting the original data. Figure 5 shows the visual predictive check of the final model, with most raw data points falling within the boundaries of the simulated data. Mirror plots from Xpose showed the model was able to simulate similar data with a similar distribution to the raw data, and that standard diagnostic plots for the simulated data were also similar to those derived from the original data (data not shown).
The most important evaluation was the finding that model simulated AUC(0,12 h) values were within 1 SD of the actual mean value calculated by non-compartmental analysis. As this study used AUC(0,12 h) as a surrogate marker of efficacy, this check gave confidence that the model performed well enough to predict AUC(0,12 h) values at different doses.
Plots of standardized CL and VD did not vary with age and suggest that allometric weight scaling adequately explains the changes in CL and VD during development. The allometric size model of CL varying with W3/4 was originally based on the observation that basal metabolic rate scales with W3/4 across species [27], but another potential justification is that liver size also appears to change with W3/4[28]. Diclofenac is extensively metabolized in the liver, and this study found that diclofenac CL could adequately be described by scaling estimates to W3/4 over a 10-fold weight range.
Once the final model had been evaluated and shown to predict both the original data and AUC(0, 12 h), it was possible to simulate new data where patients were given different doses, to investigate the most suitable paediatric dose. Table 3 shows that 1 mg kg−1 gave a similar AUC(0,12 h) to 50 mg in adults. Although linear (mg kg−1) dose schedules are convenient for clinical practice, an increased AUC(0,12 h) is seen in older (heavier) children (see Table 3) as diclofenac CL was described using the allometric W3/4 model. The 18% difference in exposure between infants and children aged 7 to 12 years is unlikely to be of clinical significance for diclofenac, but such differences could be important for optimizing therapy for narrow therapeutic-index treatments. The mg kg−1 system may not be ideal in that CL change in a linear fashion with weight, but it does provide a simple, easy-to-remember formula by which paediatric health professionals can calculate the dose. Furthermore, the suspension formulation gives the flexibility to administer doses based on this system to children of different ages.
Acknowledgments
Competing interests: JFS received a PhD studentship sponsored by Rosemont Pharmaceuticals Ltd.
This study was sponsored by Rosemont Pharmaceuticals Ltd as part of a PhD grant for JS. The authors would like to acknowledge the assistance Jeff Rothwell of Rosemont Pharmaceuticals Ltd for monitoring the study as sponsor and providing the adult pharmacokinetic data; Hussain Mulla and Brian Anderson who provided advice on pharmacokinetic modelling; the staff and anaesthetists responsible for patients on Island Day Unit at Great Ormond Street Hospital; and most of all to thank the patients who took part.
REFERENCES
- 1.Turner S, Longworth A, Nunn AJ, Choonara I. Unlicensed and off label drug use in paediatric wards: prospective study. BMJ. 1998;316:343–5. doi: 10.1136/bmj.316.7128.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay CLM, Tan S. Diclofenac or paracetamol for analgesia in paediatric myringotomy outpatients. Anaesth Intensive Care. 2002;30:55–9. doi: 10.1177/0310057X0203000110. [DOI] [PubMed] [Google Scholar]
- 3.McGowan PR, May H, Molnar Z, Cunliffe M. A comparison of three methods of analgesia in children having day case circumscision. Paediatr Anaesth. 1998;8:403–7. doi: 10.1046/j.1460-9592.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.Eustace N, O'Hare B. Use of non-steroidal anti-inflammatory drugs in infants. A survey of members of the Association of Paediatric Anaesthetists of Great Britain and Ireland. Paediatr Anaesth. 2007;17:464–9. doi: 10.1111/j.1460-9592.2007.02135.x. [DOI] [PubMed] [Google Scholar]
- 5.McQuay HJ, Moore RA. Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review. [last accessed 1 August 2008];Health Technol Assess. 1998 2:1–236. Winchester, UK. Available at http://www.jr2.ox.ac.uk/bandolier/painres/download/acuteHTA.pdf. [PubMed]
- 6.Lau HSH, Chan K, Shum L, Adair S, Ross H, Eyring H, Gause D, John V. Dose proportionality of diclofenac sodium (Voltaren) in man. Pharmaceutical Res. 1989;6:S194. conference abstract. [Google Scholar]
- 7.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68(69):165–75. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 8.Blobaum AL, Marnett LJ. Molecular determinants for the selective inhibition of cyclo-oxygenase-2 by lumiracoxib. J Biol Chem. 2007;282:16379–90. doi: 10.1074/jbc.M609883200. [DOI] [PubMed] [Google Scholar]
- 9.Rowlinson SW, Kiefer JR, Prusakiewicz JJ, Pawlitz JL, Kozak KR, Kalgutkar AS, Stallings WC, Kurumbail RG, Marnett LJ. A novel mechanism of cyclo-oxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J Biol Chem. 2003;46:45763–9. doi: 10.1074/jbc.M305481200. [DOI] [PubMed] [Google Scholar]
- 10.Burian M, Tegeder I, Seegel M, Geisslinger G. Peripheral and central antihyperalgesic effects of diclofenac in a model of human inflammatory pain. Clin Pharmacol Ther. 2003;74:113–20. doi: 10.1016/S0009-9236(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 11.von Bayer C, Spagrud L. Systematic review of observational (behavioural) measures of pain in children and adolescents aged 3 to 18 years. Pain. 2007;127:140–50. doi: 10.1016/j.pain.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 13.Beal SL, Sheiner LB, Boeckmann AJ, editors. Ellicott City, MD: Icon Development Solutions; 2006. NONMEM User Guides. [Google Scholar]
- 14.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 15.Meibohm B, Laer S, Pancetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7:E475–87. doi: 10.1208/aapsj070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:12.1–12.30. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson MO, Sheiner LB. The importance of modelling interocassion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735–50. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82:17–20. doi: 10.1038/sj.clpt.6100241. [DOI] [PubMed] [Google Scholar]
- 19.Pharsight. Mountain View, CA: Pharsight; 2005. WinNonlin Reference Guide. [Google Scholar]
- 20.van der Marel CD, Anderson BJ, Romsing J, Jacqz-Aigrain E, Tibboel D. Diclofenac and metabolite pharmacokinetics in children. Paediatr Anaesth. 2004;14:443–51. doi: 10.1111/j.1460-9592.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- 21.Macia MA, Frias J, Carcas AJ, Guerra P, Valiente R, Lucero ML. Comparative bioavailability of a dispersible formulation of diclofenac and finding of double plasma peaks. Int J Clin Pharmacol Ther. 1995;33:333–9. [PubMed] [Google Scholar]
- 22.Lotsch J, Kettenmann B, Renner B, Drover D, Brune K, Geisslinger G, Kobal G. Population pharmacokinetics of fast release oral diclofenac in healthy volunteers: relation to pharmacodynamics in an experimental pain model. Pharmaceutical Res. 2000;17:77–84. doi: 10.1023/a:1007574710140. [DOI] [PubMed] [Google Scholar]
- 23.Idkaidek NM, Amidon GL, Smith DE, Najib NM, Hassan MM. Determination of the population pharmacokinetic parameters of sustained-release and enteric-coated oral formulations, and the suppository formulation of diclofenac sodium by simultaneous data fitting using NONMEM. Biopharm Drug Dispos. 1998;19:169–74. doi: 10.1002/(sici)1099-081x(199804)19:3<169::aid-bdd83>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 24.Lund W, editor. 12th edn. London: Royal Pharmaceutical Society of Great Britain; 1993. The Pharmaceutical Codex: Principles and Practice of Pharmaceutics. [Google Scholar]
- 25.Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Clin Pharmacokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 26.Korpela R, Olkkola KT. Pharmacokinetics of intravenous diclofenac sodium in children. Eur J Clin Pharmacol. 1990;38:293–5. doi: 10.1007/BF00315034. [DOI] [PubMed] [Google Scholar]
- 27.Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27:511–41. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- 28.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;12:1481–93. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]


