Summary
The habenulae are evolutionarily conserved bilateral nuclei in the epithalamus that relay input from the forebrain to the ventral midbrain. In zebrafish, the habenulae display left-right (L/R) asymmetries in gene expression and axonal projections. The elaboration of habenular asymmetries requires the presence of a second asymmetric structure, the parapineal, the laterality of which is biased by unilateral Nodal signalling. Here we show that neurons are present earlier in the left habenula than in the right, but, in contrast to other habenular asymmetry phenotypes, this asymmetry in neurogenesis is not dependent on the parapineal. Embryos in which the L/R asymmetry in Nodal signalling is abolished display symmetric neurogenesis, revealing a requirement for this pathway in asymmetrically biasing neurogenesis. Our results provide evidence of a direct requirement for unilateral Nodal activity in establishing an asymmetry per se, rather than solely in biasing its laterality.
Keywords: Nodal signalling, Asymmetry, Habenular nuclei, Neurogenesis, Zebrafish
INTRODUCTION
Despite our outwardly symmetric appearance, many differences exist between our left and right sides. With respect to the nervous system, left-right (L/R) anatomical asymmetries are thought to mediate lateralised behavioural and cognitive functions (Hamada et al., 2002; Levin, 2005). For instance, in humans, aspects of language processing occur predominantly in the left hemisphere. Although brain lateralisation is particularly obvious in humans, it is not unique to us, being widespread among both vertebrates and invertebrates (Cooke, 2004; Vallortigara et al., 1999).
The molecular mechanisms that underlie the development of brain asymmetry in vertebrates are beginning to be unravelled, and in this regard the zebrafish is proving to be a powerful model. Zebrafish exhibit both neuroanatomical and behavioural asymmetries that are amenable to genetic analysis (Barth et al., 2005; Concha, 2004; Halpern et al., 2003). The zebrafish dorsal diencephalon (epithalamus) is composed of the pineal complex and the bilateral habenular nuclei that are part of a highly conserved conduction system that interconnects sites in the forebrain and ventral midbrain (Bianco and Wilson, 2009; Facchin et al., 2009). Both the pineal complex and habenulae display prominent asymmetries (Concha and Wilson, 2001; Halpern et al., 2003). The photoreceptive pineal complex consists of the medially located pineal (epiphysis) and the left-sided parapineal nucleus. During development, parapineal precursors detach from the anterior pineal anlage and migrate leftward, coming to lie adjacent to the left habenula (Concha et al., 2003). This migration is dependent upon the activity of Fgf8 expressed bilaterally in the anlagen of the habenulae (Regan et al., 2009). The left and right habenulae display differences in the proportion of neuronal subtypes with distinct patterns of gene expression, axon terminal morphology and connectivity (Aizawa et al., 2005; Aizawa et al., 2007; Bianco et al., 2008; Concha et al., 2003; Concha and Wilson, 2001; Gamse et al., 2005; Gamse et al., 2003).
The handedness (laterality) of habenular asymmetry is always concordant with that of the parapineal. Fate mapping and tissue ablation experiments indicate that the establishment of parapineal and habenulae lateralities is coordinated through a stepwise mechanism (Concha et al., 2003; Gamse et al., 2003). First, the anlage of the left habenula provides cues that bias the orientation of Fgf8-dependent parapineal migration towards the left (Concha et al., 2003; Regan et al., 2009). Subsequently, the parapineal promotes the elaboration of left-sided habenular identity; in the absence of the parapineal, much habenular asymmetry is lost and both nuclei display predominantly right-sided character (Bianco et al., 2008; Concha et al., 2003; Gamse et al., 2005; Gamse et al., 2003). However, some features of habenular neurons, including subtle aspects of axon terminal morphology, remain asymmetric following parapineal ablation, suggesting that the parapineal is not an absolute determinant of habenular asymmetry (Bianco et al., 2008).
The Nodal signalling pathway is required for asymmetric development of the heart, visceral organs and brain. In zebrafish, a Nodal protein encoded by the ndr2 (cyclops) gene and its downstream targets are transiently expressed on the left side of the dorsal diencephalon. However, in embryos in which Nodal signalling is bilaterally symmetric or absent, the epithalamus still develops asymmetrically but handedness is randomised: 50% have normal laterality and 50% are reversed, with the parapineal on the right and the habenulae showing L/R reversals in gene expression and projection patterns (Aizawa et al., 2005; Concha et al., 2000; Gamse et al., 2005; Gamse et al., 2003). Although Nodal signalling is required for specifying the laterality of epithalamic asymmetry, the manner in which Nodal imposes this bias is not known.
In this study, we have explored the involvement of Nodal signalling in the establishment of very early epithalamic asymmetry by focussing on the earliest stages of habenular neurogenesis. We describe a novel marker of habenular progenitors/neurons, C-X-C chemokine receptor 4b (cxcr4b), and show that the expression of cxcr4b appears earlier in the left habenula than in the right. This is consistent with previous results showing asymmetries in the generation of neuronal subtypes between left and right habenulae (Aizawa et al., 2007). The temporal L/R difference in neurogenesis revealed by cxcr4b expression occurs prior to the leftward migration of the parapineal and is still detected if the parapineal is ablated. By contrast, removing the L/R bias in Nodal signalling renders habenular neurogenesis symmetric. Our results provide a clear example of a role for Nodal signalling in promoting an asymmetry per se, rather than in directing laterality.
MATERIALS AND METHODS
Fish lines and maintenance
Zebrafish embryos were collected and staged using standard protocols. For later-staged embryos, PTU was used to block the formation of pigment (Westerfield, 1995). The transgenic lines Tg(huC:gfp), Tg(ngn1:gfp), Tg(flh:egfp) and Tg(foxD3:gfp) have been described previously (Blader et al., 2003; Concha et al., 2003; Gilmour et al., 2002; Park et al., 2000). Embryos were fixed overnight at 4°C in 4% paraformaldehyde, after which they were dehydrated through an ethanol series and stored at -20°C.
In situ hybridisation (ISH) and antibody labelling
Antisense probes for lov (Gamse et al., 2003), brn3a (Aizawa et al., 2005), cxcr4b (David et al., 2002), ngn1 (Blader et al., 1997), lft1 (Thisse and Thisse, 1999), ndr2 and pitx2 (Essner et al., 2000) were generated using standard procedures. Embryos were stained using BCIP and NBT (Roche) or Fast Red (Sigma) as substrate. In certain cases, embryos were further stained with rabbit anti-GFP antibody (1/500, Torrey Pines Biolabs) or anti-HuC/D antibody (16A11, Molecular Probes) in blocking buffer (1×PBS, 0.5% Triton X-100, 10% goat serum, 1% DMSO) and visualised with an Alexa Fluor 488-conjugated secondary antibody (1/500, Molecular Probes). For nuclear staining, embryos were incubated in 1×PBS, 0.5% Triton X-100, 1% bovine serum albumin containing ToPro (1/1000, Molecular Probes).
BrdU labelling
Embryos at 48 hpf were incubated in 15% DMSO and 10 mM 5-bromo 2′-deoxyuridine (BrdU) for 90 minutes at 4°C, rinsed and development allowed to continue at 28°C until 60 hpf, at which time embryos were fixed. BrdU incorporation was detected as previously described (Shepard et al., 2004) using anti-BrdU antibody (1/100, G3G4 Hybridoma Bank) and Alexa Fluor 548-conjugated secondary antibody (1/500, Molecular Probes).
Parapineal ablation
Laser ablation of parapineal precursors was performed at 24-28 hpf in Tg(flh:egfp); Tg(foxD3:gfp) double transgenic embryos as described previously (Concha et al., 2003). Larvae were subsequently fixed at 36-38 hpf and immunostained with anti-HuC/D antibody in order to count habenular neurons.
Morpholino injections and temperature shifts
Morpholino oligonucleotides (MOs) were dissolved in water at 1 mM (ntl MO) (Nasevicius and Ekker, 2000) or 3 mM (spaw MO) (Long et al., 2003). The resulting stock solution was diluted to working concentrations (0.12 mM and 1.2 mM, respectively) in water and Phenol Red before injection (0.5-2 nl) into eggs at the 1 cell stage. For temperature shifts, wild-type embryos were placed at 22°C 2 hours after laying. Embryos were subsequently fixed and processed for ISH or antibody labelling.
SB431542 treatment
For inhibition of the Nodal signalling pathway, Tg(huC:gfp) or wild-type embryos were treated with SB431542, a selective inhibitor of the Tgfβ type I receptor (Ho et al., 2006; Inman et al., 2002). SB431542 (Tocris Bioscience) was dissolved at 50 mM in DMSO and aliquots stored at -20°C. Embryos were dechorionated at 10 hpf or 16 hpf and incubated in 100 μM SB431542 or in DMSO alone at 28°C until fixation. Treated embryos were fixed at 21 hpf or 23 hpf and processed for ISH to reveal Nodal pathway gene expression or at 34-38 hpf to determine the L/R ratio of HuC:GFP+ or HuC/D+ cells.
Cell counting and statistical analysis
To detect habenular neurons, we used immunostaining with either an anti-GFP antibody in Tg(huC:gfp) embryos or an anti-HuC/D antibody and counterstained cell nuclei with ToPro to facilitate counting. Left and right habenular neurons were counted using ImageJ software (NIH) from confocal stacks acquired every 1 μm. For each embryo, we plotted the numbers of left versus right HuC:GFP+ or HuC/D+ neurons and linear regressions were performed using Prism 4 software (GraphPad Software). To test whether individual datasets were asymmetric, we performed a Wilcoxon signed-rank test (http://faculty.vassar.edu/lowry/wilcoxon.html). To determine the asymmetry index (AI) for individual embryos, the number of left neurons (L) minus the number of right neurons (R) was divided by the sum of both left and right neurons, i.e. (L-R)/(L+R). To compare AI values between two datasets, we performed a Kolmogorov-Smirnov (KS) statistical test (www.physics.csbsju.edu/stats/KS-test.n.plot_form.html), except for the spaw MO experiment for which we used a Student's t-test (two-tailed) owing to the small sample size.
Imaging
For differential interference contrast pictures, embryos were mounted in glycerol and analysed on a Zeiss Axiophot microscope using a Nikon digital camera. For all fluorescent labelling, embryos were mounted in glycerol and habenular nuclei were imaged from a dorsal view using a Leica SP2 laser-scanning confocal microscope with a ×63 oil-immersion objective. Transverse views were generated using Leica software from z-stacks acquired at 0.5 μm intervals.
RESULTS
cxcr4b is expressed in habenular precursors/newly generated neurons
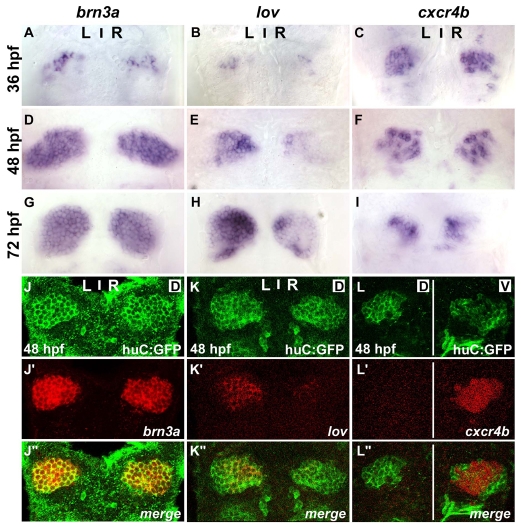
Two general categories of expression patterns have been described for habenular markers in the zebrafish. The first comprises genes such as brn3a and er81 (pou4f1 and etv1 - ZFIN) for which expression begins ∼36 hours post-fertilisation (hpf), becomes broader at 48 and 72 hpf, and is largely symmetrical (Fig. 1A,D,G; data not shown) (Aizawa et al., 2005; Gamse et al., 2003; Roussigne and Blader, 2006). The second category comprises genes such as leftover (lov), right on (ron) and dexter (dex) (kctd12.1, kctd12.2 and kctd8 - ZFIN) that are expressed from 36 hpf or later in a strongly asymmetric manner (Fig. 1B,E,H; data not shown) (Gamse et al., 2005; Gamse et al., 2003). Many habenular neurons are born before 36 hpf (Aizawa et al., 2007), so in order to find earlier markers for these neurons we searched the expression pattern database on ZFIN (http://zfin.org) for genes expressed in the habenulae. This directed our focus to cxcr4b. The spatiotemporal dynamics of cxcr4b expression do not fit into either of the two categories mentioned above because expression is already robust at 36 hpf, peaks at 48 hpf and then decreases, becoming restricted to a small medial domain at 3 days of development (Fig. 1C,F,I). These results suggest that cxcr4b expression defines a different cell population within the habenulae than those identified by the markers described to date.
Fig. 1.
cxcr4b is expressed in the habenulae. (A-I) Expression of brn3a (A,D,G), lov (B,E,H) and cxcr4b (C,F,I) in habenulae of wild-type zebrafish embryos at 36 (A-C), 48 (D-F) and 72 (G-I) hpf. (J-L″) Single confocal images of 48 hpf Tg(huC:gfp) embryos showing immunolabelled GFP protein (J-L) and the localisation of mRNA encoding brn3a (J′), lov (K′) or cxcr4b (L′). (J″-L″) Merged images. Dorsal views of embryos with anterior at the top. Single confocal sections correspond to dorsal (D) or ventral (V) z-sections through the habenular nuclei.
To better appreciate the expression domains of the various markers within the developing habenulae, we mapped their expression relative to that of the transgene Tg(huC:gfp). huC is a marker of post-mitotic neurons and the transgene recapitulates the expression of the endogenous gene (Park et al., 2000). At 48 hpf, transcripts of brn3a and lov were found to completely overlap with the domain of GFP transgene expression, confirming that these genes are expressed in differentiated habenular neurons (Fig. 1J-J″,K-K″). By contrast, cxcr4b was expressed in cells located more ventrally, closer to the ventricular surface of the neuroepithelium, than those expressing the transgene (Fig. 1L-L″, Fig. 2C).
Fig. 2.
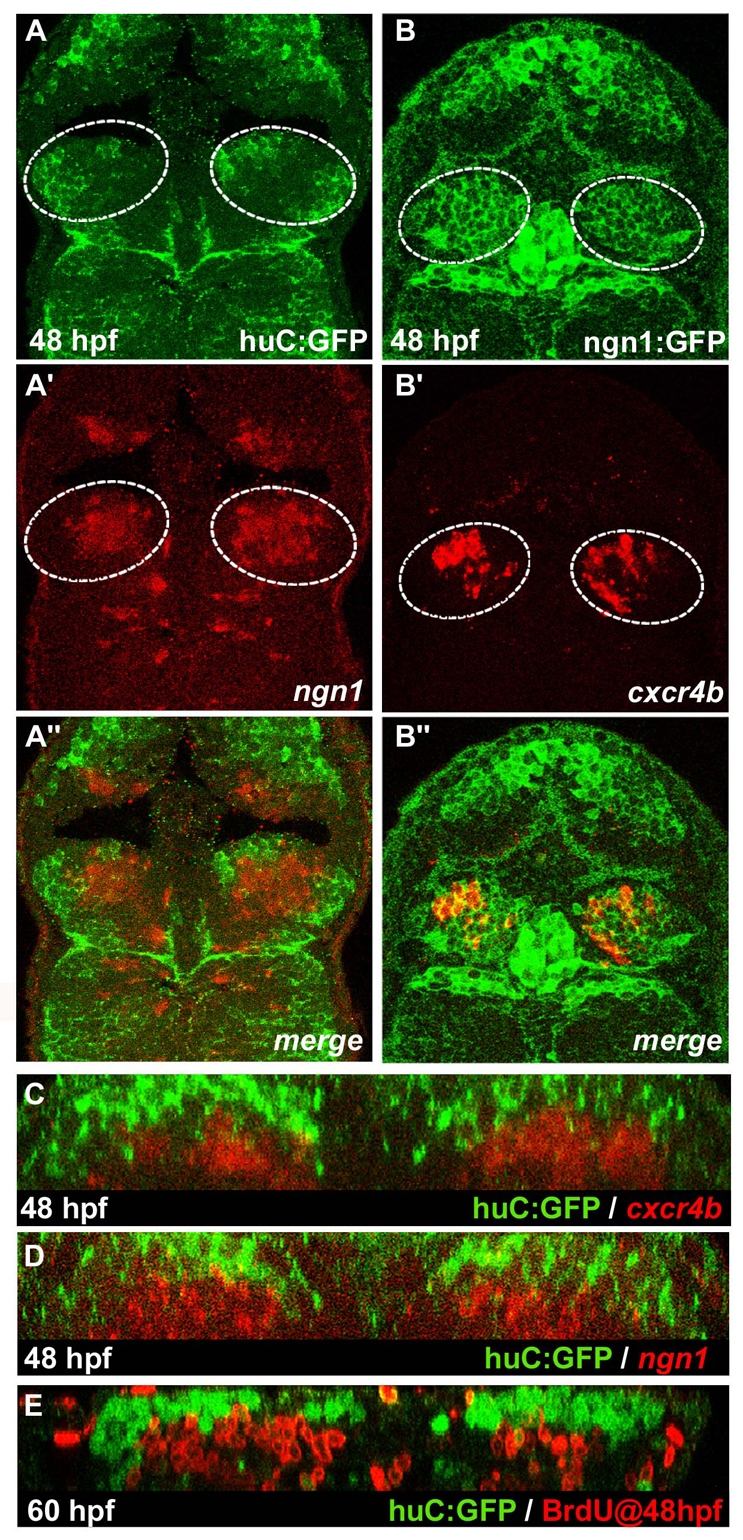
cxcr4b is a marker of habenular progenitors/early-born neurons. (A-B″) Single confocal images (dorsal views) of a 48 hpf Tg(huC:gfp) zebrafish embryo (A-A″) with in situ labelling of ngn1 and a 48 hpf Tg(ngn1:gfp) embryo (B-B″) with in situ labelling of cxcr4b. (A″-B″) Merged images. The habenulae are indicated by a dashed white line. (C-E) Cross-sections of double-labelled habenulae generated from confocal z-series. (C) Immuno-detection of GFP protein (green) and localisation of cxcr4b transcripts (red) in a 48 hpf Tg(huC:gfp) embryo. (D) Immuno-detection of GFP protein (green) and localisation of ngn1 mRNA (red) in a 48 hpf Tg(huC:gfp) transgenic embryo. (E) Immuno-detection of BrdU (red) and GFP protein (green) in a 60 hpf Tg(huC:gfp) embryo pulsed with BrdU at 48 hpf.
The location and dynamic nature of cxcr4b expression, broad early and decaying with time, suggests that cxcr4b could be a transient early marker of nascent habenular neurons or neuronal progenitors. Neuronal progenitors are characterised by the expression of proneural genes that encode members of the basic helix-loop-helix (bHLH) family of transcriptional regulators (Bertrand et al., 2002; Chapouton and Bally-Cuif, 2004). The proneural genes neurogenin 1 (ngn1; neurog1) and achaete-scute complex-like 1b (ascl1b) are expressed in the habenulae (Mueller and Wullimann, 2003), and we compared their expression with that of the huC:gfp transgene. As with cxcr4b, the ngn1 and ascl1b transcripts were located ventral to GFP+ neurons, suggesting that these proneural genes are coexpressed with cxcr4b (Fig. 2A-A″,D; data not shown). In support of this conclusion, expression of cxcr4b partially overlaps with the expression of GFP driven by ngn1 regulatory sequences (Fig. 2B-B″) (Blader et al., 2003; Mueller and Wullimann, 2003). As cells located ventral to the huC:GFP+ neurons incorporate BrdU (Fig. 2E), we suggest that cxcr4b-expressing cells are either neural progenitors or newly born neurons that have only recently finished their last S phase.
cxcr4b expression reveals an early asymmetry in habenular neurogenesis
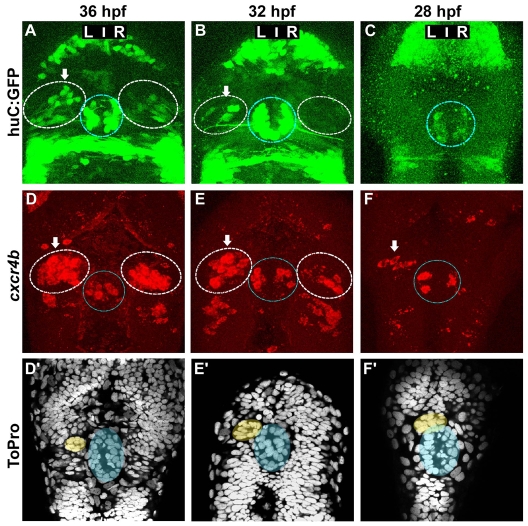
Supporting previous observations of an asymmetry in the pattern of habenular neurogenesis (Aizawa et al., 2007), we observed that at 48 hpf there are more huC:GFP+ neurons in the left compared with the right habenula (data not shown). To address whether this asymmetry arises from accelerated neurogenesis in the left habenula, we determined the earliest time at which this L/R difference could be detected. An asymmetry in the pool of Tg(huC:gfp)+ neurons is present as early as 36 hpf (Fig. 3A) (Aizawa et al., 2007). At 32 hpf, GFP+ habenular neurons were often only detected on the left (Fig. 3B), and prior to 32 hpf no GFP+ neurons were detected in either the left or right habenula (Fig. 3C). We also observed asymmetry in the expression of cxcr4b at 36 and 32 hpf (Fig. 3D,E). However, compared with huC:GFP, the asymmetric expression of cxcr4b extended to earlier stages of habenular development: whereas no Tg(huC:gfp)-expressing cells were detected prior to 32 hpf, cxcr4b-expressing cells were consistently found in the left habenula at 28 hpf (Fig. 3C versus 3F). Thus, cxcr4b mRNA reveals an asymmetry in habenular neurogenesis at least 4 hours earlier than Tg(huC:gfp) or the endogenous HuC protein (Fig. 3; data not shown).
Fig. 3.
Asymmetric cxcr4b expression precedes the migration of the parapineal and the appearance of HuC+ habenular neurons. (A-C) Confocal projections of GFP fluorescence in Tg(huC:gfp) zebrafish embryos at 36 (A), 32 (B) and 28 (C) hpf. (D-F′) Confocal projections of fluorescent in situ hybridisations showing cxcr4b transcripts (D-F) and ToPro nuclear staining in the corresponding embryos (D′-F′) at 36 (D,D′), 32 (E,E′) or 28 (F,F′) hpf. Nuclear staining permits detection of the parapineal (yellow) and pineal (blue). In A-F, the habenulae are indicated by a dashed white line, the epiphysis by a blue line. Arrows indicate the habenula with the higher number of huC:GFP-positive habenular neurons or cxcr4b-expressing cells.
The parapineal does not determine early habenular asymmetry
The parapineal plays an instructive role in the elaboration of asymmetries between the left and right habenula (Halpern et al., 2003). To address whether the parapineal is involved in the generation of the asymmetry in early habenular neurogenesis, we first assessed the position of the migrating parapineal relative to the onset of lateralised cxcr4b expression in the left habenula. Parapineal cells display a rosette organisation from the onset of their migration that is readily visible in fixed embryos after staining nuclei (Fig. 3D′,E′,F′). In 28 hpf embryos, in which left-sided expression of cxcr4b is already detected, the parapineal is either at the midline or just beginning to orient to the left (Fig. 3F versus 3F′) (Concha et al., 2003). These results indicate that left-sided neurogenesis begins before, or is concomitant with, left-sided parapineal migration, raising the possibility that this asymmetry might not be dependent upon the parapineal.
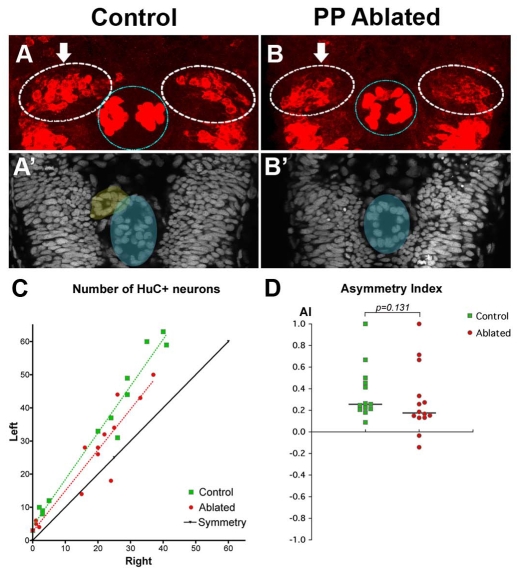
To test whether the parapineal plays a role in the establishment of this asymmetry, we ablated parapineal progenitors with a laser prior to their migration, as previously described (Concha et al., 2003). Subsequently, early habenular neurogenesis was analysed by counting the number of HuC/D+ (Elav3/4+) neurons in the left and right habenulae and nuclear staining was performed to confirm that the parapineal had been successfully ablated (Fig. 4A-B′).
Fig. 4.
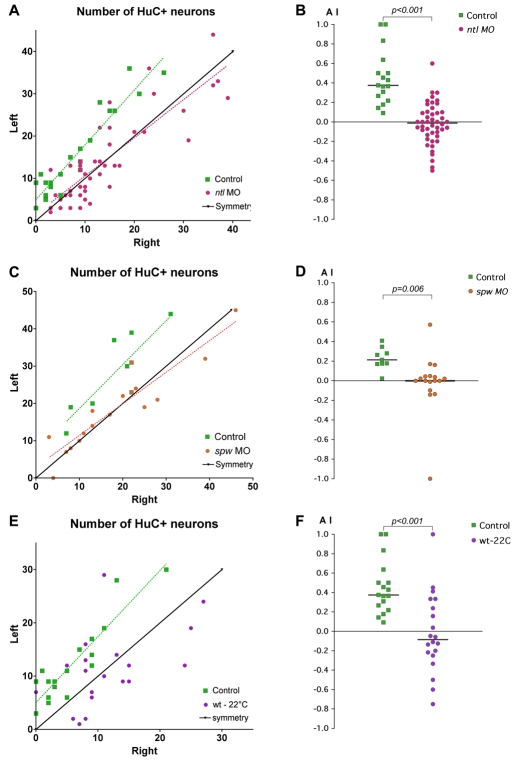
Asymmetric habenular neurogenesis is independent of the parapineal. (A,B) Confocal projection of immuno-detected HuC/D proteins in 36-38 hpf control (A) and parapineal-ablated (B) zebrafish embryos. Arrows indicate the habenula with the higher number of HuC/D+ neurons. The habenulae are indicated by a dashed white line, the epiphysis by a blue line. (A′,B′) ToPro nuclear staining of the embryos in A,B reveals the parapineal in the control (yellow) but not in the parapineal-ablated embryo; the epiphysis is shaded blue. (C) Numbers of left versus right HuC+ habenular neurons with each point representing one embryo. A symmetry line (black) is included as a reference. The dotted lines represent the best-fit linear regression for controls and ablated embryos. (D) Asymmetry index (AI, see Materials and methods) plotted for each control or parapineal-ablated embryo. The horizontal black lines indicate the median AI value for the group of like-treated embryos.
In all control embryos, a greater number of HuC/D-expressing neurons was detected in the left as compared with the right habenula (P=0.002, Wilcoxon signed-rank sum test) (Fig. 4C). An overall left bias was retained after ablation of parapineal progenitor cells (P=0.005, Wilcoxon signed-rank sum test) (Fig. 4C). This leftwards asymmetry, in both control and ablated embryos, can also be quantitatively expressed as an asymmetry index (AI), defined as the difference between the number of neurons on the left versus the right side, normalised to the total number of neurons [AI=(L-R)/(L+R)] (Fig. 4D). Our data do not demonstrate a significant difference in the degree of asymmetry between control and ablated embryos [median AI for parapineal ablated=0.18, n=14; median AI for controls=0.26, n=13; P=0.131, Kolmogorov-Smirnov (KS) test]. Thus, in contrast to its role in later habenular gene expression and axonal targeting asymmetries (Bianco et al., 2008; Concha et al., 2003; Gamse et al., 2005; Gamse et al., 2003), our results suggest that the parapineal is dispensable for the establishment of early asymmetry in the timing of neurogenesis between the left and right habenulae.
Perturbations in Nodal signalling correlate with disruptions in asymmetric habenular neurogenesis
Asymmetric epithalamic Nodal signalling determines the laterality of epithalamic asymmetries but is not thought to be required for the elaboration of asymmetry per se (Concha et al., 2000). Accordingly, genetic or other manipulations that remove the asymmetric bias in Nodal signalling (such that signalling is either bilateral or absent) randomise epithalamic laterality, whereas L/R reversal in biased Nodal signalling reverses laterality (Aizawa et al., 2005; Carl et al., 2007). To address whether Nodal signalling is involved in determining either the asymmetry of early neurogenesis or its laterality, we assessed neurogenesis in situations in which epithalamic Nodal signalling is disrupted.
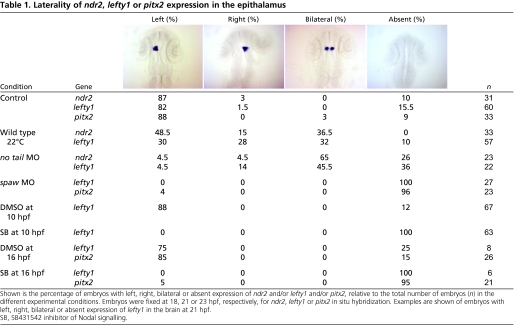
Abrogation of no tail (ntl) function results in either bilateral or absent epithalamic Nodal pathway gene expression and randomised epithalamic laterality (Concha et al., 2000) (Table 1). In ntl morpholino (MO)-injected embryos, pools of left- and right-sided habenular huC+ neurons were significantly more symmetric than in control embryos (Fig. 5A,B) (median AI for ntl morphants=0.00, n=46; median AI for controls=0.38, n=17; P<0.001, KS test). For example, ∼60% (n=27/46) of injected embryos had a small AI of between 0.15 and -0.15 versus 14% in control embryos (n=3/21) (Fig. 5B).
Table 1.
Laterality of ndr2, lefty1 or pitx2 expression in the epithalamus
Fig. 5.
Lateralised habenular neurogenesis is compromised by treatments that disrupt Nodal signalling. Plots of the numbers of HuC+ neurons in the left and right habenulae and corresponding asymmetry indices (AI) in ntl morphants (A,B), spaw morphants (C,D) and cold-treated zebrafish embryos (E,F). (A,C,E) Habenular neurons were detected in Tg(huC:gfp) transgenic embryos using either anti-GFP (A,E) or HuC/D (C) antibody. A symmetry line (black) is included as a reference. Dotted lines represent the best-fit linear regression for each experimental condition. (B,D,F) AI values plotted for all embryos. The same control dataset is plotted for the ntl MO and cold-treatment experiments. Applying the Bonferroni correction for multiple comparisons shows that both the cold-treated and ntl MO datasets are significantly different from the control at the 5% confidence level. Horizontal black lines indicate the median AI value for the group of like-treated embryos.
Abrogation of the lateral plate mesoderm (LPM)-expressed Nodal ligand Southpaw (Spaw) leads to a loss of epithalamic Nodal signalling (Long et al., 2003) and again to randomised epithalamic laterality (Gamse et al., 2005). In spaw morphants, we observed a significant reduction in the asymmetry of early neurogenesis between the left and right habenulae (Fig. 5C,D) (median AI for spaw morphants=0.00, n=17; median AI for controls=0.21, n=9; P=0.006, Student's t-test; see Materials and methods). Thus, although neuroanatomical asymmetry is eventually established in ntl and spaw morphants, the loss of unilateral Nodal signalling correlates with a loss of the initial asymmetry in neurogenesis.
Embryos raised at low temperature (22°C instead of 28°C) also show randomised parapineal/habenular laterality, but in contrast to ntl and spaw morphants, epithalamic Nodal signalling is randomised (Table 1) (Liang et al., 2000). The distribution of AI values for cold-treated embryos was statistically different from controls (P<0.001, KS test). Interestingly, similar proportions of cold-treated embryos displayed leftwards asymmetry (35%), rightwards asymmetry (35%), and symmetric pools of neurons (30%) (where we classified -0.15<AI<0.15 as symmetric, AI>0.15 as left-sided and AI<-0.15 as right-sided; n=20) (Fig. 5E,F). Thus, whereas ntl and spaw morphants display symmetric Nodal signalling and symmetric neurogenesis, the majority of temperature-shifted embryos display randomised unilateral Nodal signalling and asymmetric neurogenesis with randomised laterality (Fig. 5, Table 1).
Altogether, these results strongly suggest that although Nodal signalling is not required for the eventual elaboration of neuroanatomical asymmetries, it is required for the initial asymmetry in habenular neurogenesis.
Nodal signalling is required for asymmetric habenular neurogenesis
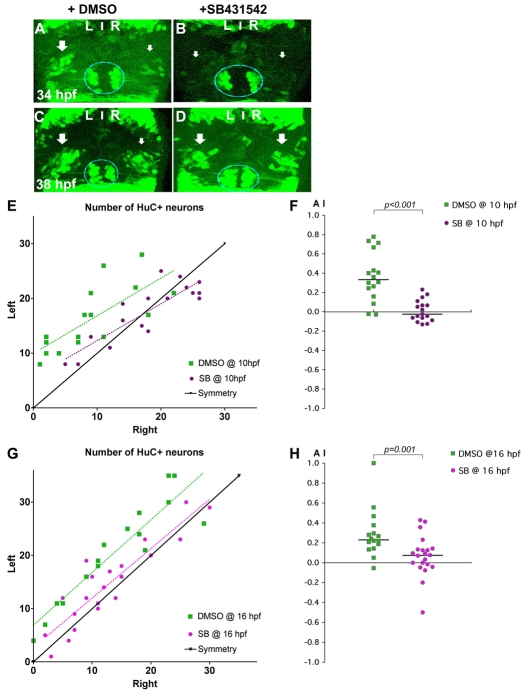
To test the hypothesis that lateralised Nodal signalling leads to asymmetric neurogenesis, we analysed embryos in which Nodal signalling was compromised pharmacologically using the small-molecule inhibitor SB431542 (Inman et al., 2002). Zebrafish embryos grown from early stages in SB431542 display a phenotype identical to that of embryos genetically deficient for Nodal signalling (Ho et al., 2006). Embryos treated with SB431542 from 10 hpf, after the early requirement for Nodal signalling in gastrulation, developed normally but lefty1 (lft1) expression was no longer detected in the epithalamus (Table 1), consistent with the drug blocking Nodal signalling in the LPM and brain. In embryos treated with SB431542 from 10 hpf until 34-38 hpf, pools of early-born huC:GFP+ habenular neurons were symmetric (Wilcoxon sign-rank sum test for SB431542, P=0.749, n=17; for DMSO controls, P=0.001, n=16) (Fig. 6).
Fig. 6.
Nodal signalling promotes early asymmetric neurogenesis. (A-D) Confocal projections of Tg(huC:gfp) transgenic zebrafish embryos incubated with DMSO (A,C) or with SB431542 (B,D) from 10 hpf and fixed at 34 (A,B) or 38 (C,D) hpf. Arrows indicate that the left pools of HuC+ neurons are bigger than those on the right in DMSO-treated embryos (A,C), whereas the left and right pools are symmetric in embryos incubated with SB431542 (B,D). The epiphysis is indicated by a blue line. Habenular neurogenesis is also delayed in SB431542-treated embryos. (E-H) Numbers of left and right HuC+ habenular neurons (E,G) and corresponding AI (F,H) in embryos treated with DMSO or SB431542 at 10 (E,F) or 16 (G,H) hpf. Habenular neurons were detected in Tg(huC:gfp) transgenic embryos using an anti-GFP (E) or HuC/D (G) antibody. A symmetry line (black) is included as a reference in E and G. Dotted lines represent best-fit linear regressions. Horizontal black lines in F and H indicate the median AI value for the group of like-treated embryos.
Supporting the conclusions from the analysis of spaw morphants, this indicates that Nodal signalling is required for asymmetric neurogenesis but does resolve whether it is Nodal signalling from the LPM or within the epithalamus that is required. To address this point, we treated embryos with SB431542 from 16 hpf, just prior to the initiation of expression of ndr2 in the dorsal diencephalon, but after spaw has been expressed asymmetrically in the LPM. As for early treatment, addition of SB431542 at later stages also abolished the epithalamic expression of Nodal targets. Although habenular neurogenesis continued to display mild asymmetry in SB431542-treated embryos (Wilcoxon sign-rank test for SB431542, P=0.024, n=22; for DMSO controls, P=0.001, n=16) (Table 1, Fig. 6K,L), the specific inhibition of epithalamic Nodal signalling caused a significant reduction in the degree of asymmetric neurogenesis (SB431542 median AI=0.07 and DMSO median AI=0.23, P=0.001, KS test).
Taken together, our data indicate that Nodal signalling, acting locally in the epithalamus, is required to establish asymmetric habenular neurogenesis.
DISCUSSION
Although we have a growing understanding of the mechanisms underlying asymmetric development of the epithalamus, many issues are still unresolved. For instance, although unilateral Nodal signalling specifies epithalamic laterality, it is not known how this happens. Previous studies have shown that Nodal signalling biases the direction of migration of the parapineal, a structure that subsequently influences the elaboration of habenular asymmetries. However, in this study, we show that Nodal signalling imposes an asymmetry upon early habenular neurogenesis and that this asymmetry is independent of the parapineal. We discuss our results with respect to current models concerning the establishment of asymmetries in the epithalamus.
Nodal signalling asymmetrically biases neurogenesis
Our results indicate that the Nodal pathway is required for the L/R asymmetry in the generation of early habenular neurons. However, the production of neurons continues in the absence of Nodal signalling, indicating that the signalling pathway asymmetrically biases a generic programme of habenular neurogenesis and is not required for neurogenesis per se. How might Nodal signalling drive asymmetry in habenular progenitors and/or early post-mitotic habenular neurons? One possibility is that Nodal signals might mediate L/R differences in proliferation, as has been found in the visceral endoderm (Yamamoto et al., 2004). However, no significant L/R differences in the mitotic index have been detected in the dorsal diencephalon (Aizawa et al., 2007). Alternatively, early Nodal signalling could influence the size of the neurogenic territory that gives rise to left habenular neurons by modulating the expression or activity of proneural factors or components of the Notch signalling pathway. Although Notch signalling has been examined in the epithalamus at stages after the onset of early asymmetric neurogenesis (Aizawa et al., 2007), it remains unclear whether the pathway is asymmetrically activated earlier. Further investigation of the basic programme of early neurogenesis in this system should help address these questions. Finally, the generation of chimaeric embryos in which habenular cells lack the ability to respond to Nodal signals will allow us to determine whether Nodal acts directly or indirectly upon habenular precursors.
The expression of cxcr4b, which encodes a chemokine receptor, in habenular neuronal progenitors/newly born neurons raises the intriguing question of the role of chemokine signalling in the development of the epithalamus. Cxcr4b and its ligand, Cxcl12 (Sdf1), are implicated in various aspects of brain development including migration, axon guidance, proliferation and neuronal survival (Klein and Rubin, 2004; Lazarini et al., 2003). The zebrafish cxcr4b mutant odysseus (ody) (Knaut et al., 2003) displays no obvious phenotype with respect to markers of L/R epithalamic asymmetry such as lov (our unpublished observations); whether ody mutants display subtle changes in the number, organisation or connectivity of different subtypes of habenular neurons remains to be investigated.
The Nodal pathway asymmetrically biases both habenular neurogenesis and parapineal migration
If asymmetric Nodal signalling is removed, the parapineal migrates with equal frequency to the left and right sides of the brain (Concha and Wilson, 2001). Therefore, either Nodal signalling directly influences migrating parapineal cells or the pathway indirectly influences migration by introducing an asymmetry elsewhere in the epithalamus. Supporting an indirect role for the Nodal pathway, parapineal laterality is compromised by partial ablation of the prospective left habenula shortly after unilateral Nodal signalling (Concha et al., 2003). Thus, an early L/R asymmetry exists in the prospective habenulae and this asymmetry appears to bias the orientation of parapineal migration towards the left.
Our studies and those of others (Aizawa et al., 2007) show an early asymmetry in the timing of neurogenesis between left and right habenulae prior to parapineal migration. Furthermore, the stage at which asymmetric neurogenesis is first detected (from 24 hpf) correlates well with that at which ablation experiments reveal an asymmetric activity that biases the orientation of parapineal migration (Concha et al., 2003). Here, we show that Nodal signalling is required for this early asymmetric neurogenesis. The role of Nodal in biasing parapineal laterality might, therefore, be an indirect consequence of its effect on habenular neurogenesis.
How might an early excess of precursors/neurons in the left habenula influence the orientation of migration of the parapineal? One possibility is that the precocious progenitors in the left habenula express parapineal guidance cues. Alternatively, the guidance cue might be expressed by all habenular precursors and it is the early asymmetry in the numbers of such cells that provides a left-biased difference in the overall level of the cue. We have recently shown that fgf8 is required for parapineal migration and that L/R differences in the levels of Fgf8 can bias the direction of migration (Regan et al., 2009). fgf8 is expressed bilaterally, but there appear to be subtle asymmetries between left and right habenulae. It will therefore be important to determine whether Nodal signalling biases the levels of Fgf8 activity, for instance through promoting a L/R asymmetry in the number of fgf8-expressing habenular precursors. An alternative possibility is that asymmetric neurogenesis and directed parapineal migration are independent consequences of a Nodal-dependent asymmetry in Fgf8 activity.
The parapineal is not the sole determinant of habenular asymmetry
The habenulae contain medial and lateral subnuclei that are L/R asymmetric with respect to the expression of differentiation markers and targeting of projections within the interpeduncular nucleus (IPN); the left habenula contains predominantly neurons of lateral subnucleus identity that project to the dorsal IPN, whereas the right habenula is mostly composed of neurons of medial subnucleus identity that project to the ventral IPN (Aizawa et al., 2005; Bianco et al., 2008) [see Gamse et al. for slightly different conclusions (Gamse et al., 2005)]. The elaboration of habenular asymmetries requires instructive input from the parapineal, as ablation of the parapineal anlage results in both habenulae displaying an overtly right-sided phenotype with respect to gene expression and connectivity (Bianco et al., 2008; Concha et al., 2003; Gamse et al., 2005; Gamse et al., 2003). However, some subtle differences remain between the left and right habenulae of parapineal-ablated embryos with respect to molecular markers, neuropil organisation (Concha et al., 2003; Gamse et al., 2005) and early neurogenesis (this study). Moreover, although left habenula neurons project to the ventral IPN in parapineal-ablated embryos, their axons retain distinct lateralised morphologies (Bianco et al., 2008). Thus, although the parapineal is important for the lateralisation of habenular circuitry and marker expression, it is not the sole determinant of L/R differences between the habenulae.
How the parapineal drives habenular lateralisation is unclear, but it has been proposed that the birthdate of habenular neurons influences their lateral/medial subnuclear character (Aizawa et al., 2007). Early neurogenesis occurs predominantly in the left habenula and gives rise to lov+ neurons that will populate the lateral subnucleus, whereas later neurogenesis is L/R symmetric and produces neurons of both lateral and medial subnuclear character. Thus, one hypothesis is that the parapineal directs habenular lateralisation by regulating the timing of neurogenesis. Our study argues against such a simple mechanism as we show that the L/R asymmetry in early-born neurons is not dependent on the parapineal. Indeed, we show that this early asymmetry in neurogenesis is dependent on Nodal signalling. However, if this Nodal-dependent asymmetry were the sole mechanism generating the difference in size of the left and right lateral subnuclei, one would expect that blocking Nodal signalling would result in symmetric lateral subnuclei, which is not the case (Concha et al., 2003; Gamse et al., 2003). Thus, the Nodal-dependent L/R asymmetry of early-born habenular neurons is not the primary determinant of later left and right habenular asymmetries. It could, nonetheless, still be responsible for the subtle differences that remain between the left and right habenula in parapineal-ablated embryos (Bianco et al., 2008; Concha et al., 2003).
How can our data concerning Nodal signalling be integrated into a model that includes roles for both the parapineal and the timing of neurogenesis in the elaboration of habenular asymmetries? In our analysis, we assessed the earliest HuC-expressing neurons that appear in both habenulae between 32 and 36 hpf and which probably correspond to those that would be BrdU labelled between 24 and 28 hpf (Aizawa et al., 2007). However, a left bias in BrdU-pulse-labelled habenular neurons is detected until 32-36 hpf, suggesting that an asymmetry in the pool of HuC+ neurons lasts for at least 4 hours after the stage at which we analysed neuronal number; this asymmetry could be parapineal dependent. Thus, the parapineal might be responsible for maintaining an asymmetry in habenular neurogenesis that is initiated by Nodal signalling. Although this possibility is attractive, it is unlikely, however, that the primary role for the parapineal in promoting habenular asymmetry is simply to regulate the timing of neurogenesis. Although early and late waves of neurogenesis, as described in Aizawa's study (Aizawa et al., 2007), appear to produce different ratios of neurons destined for the lateral and medial subnuclei, these two waves overlap extensively, resulting in both lov+ and ron+ neurons being generated contemporaneously for many hours. It is hard to reconcile this with a model in which timing of neurogenesis alone (driven by Nodal early and by the parapineal later) regulates the subnuclear identity of neurons. It is probable, therefore, that the generation of different ratios of specific neurons involves additional mechanisms, such as a direct influence of the parapineal in the specification of neuronal identity.
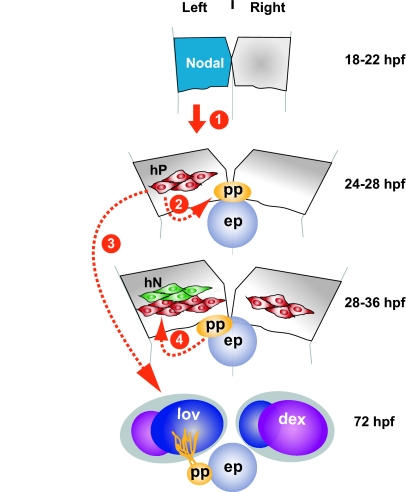
From our data and previous studies, we propose a model (Fig. 7) in which asymmetric Nodal signalling acts upon prospective habenular cells to promote left-sided neurogenesis. Early, Nodal-dependent asymmetric neurogenesis may bias parapineal migration towards the left and also be responsible for the subtle parapineal-independent differences between the left and right habenulae. Once migration is initiated, the parapineal may maintain the left bias in habenular neurogenesis and/or directly influence the specification of lateral versus medial habenular neural subtypes.
Fig. 7.
A model for the development of epithalamic asymmetry in zebrafish. Habenular neurons (hN, green) are detected earlier in the left habenula than in the right, reflecting an earlier L/R asymmetry in the pool of habenular progenitors (hP, red). The asymmetry in habenular neurogenesis occurs before parapineal migration and is independent of the parapineal. Nodal signalling in the left epithalamus drives this early asymmetry in neurogenesis (1) and either directly (not indicated) or more likely indirectly via the left habenula, biases parapineal migration to the left (2). As this migration is dependent on Fgf8 (not shown), it is possible that Nodal biases lateralisation by influencing the activity of the Fgf pathway. The early Nodal-dependent L/R asymmetry in habenular neurogenesis influences the later identity of habenular neurons (3), and is potentially responsible for subtle aspects of the axon terminal morphology of habenular neurons that remain asymmetric after parapineal ablation. Upon migration, the parapineal sends signals that promote the elaboration of habenular asymmetry (4), perhaps by maintaining a quantitative left bias in neurogenesis and/or by directly specifying the identity of the neurons that are produced. Developmental stages are indicated to the right. ep, epiphysis; pp, parapineal; lov, left over expression domain; dex, dexter expression domain. For citations to published work, see Discussion.
Acknowledgments
We thank Julie Batut, Joshua Gamse, Tae-Lin Huh, Hitoshi Okamoto, Frédéric Rosa and Lucas Waltzer for advice and/or reagents; Fernando Roch, Alain Vincent, Serge Plaza and members of the Blader and Wilson laboratories for discussions and critical reading of the manuscript; the Toulouse RIO Imaging Platform and UCL Imaging Facility personnel for help with confocal microscopy; and David Villa for artwork.
Financial support to M.R. was from the Human Frontiers Science Programme via a Young Investigators Grant (P.B.) and from a FEBS Personal Fellowship, and from the Université de Toulouse, UPS, CNRS, INSERM, FRM, FRC and the Ministère de la Recherche to P.B. and from the Wellcome Trust and EU to S.W.W. Deposited in PMC for release after 6 months.
References
- Aizawa, H., Bianco, I. H., Hamaoka, T., Miyashita, T., Uemura, O., Concha, M. L., Russell, C., Wilson, S. W. and Okamoto, H. (2005). Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr. Biol. 15, 238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa, H., Goto, M., Sato, T. and Okamoto, H. (2007). Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev. Cell 12, 87-98. [DOI] [PubMed] [Google Scholar]
- Barth, K. A., Miklosi, A., Watkins, J., Bianco, I. H., Wilson, S. W. and Andrew, R. J. (2005). fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 15, 844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand, N., Castro, D. S. and Guillemot, F. (2002). Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517-530. [DOI] [PubMed] [Google Scholar]
- Bianco, I. H. and Wilson, S. W. (2009). The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1005-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco, I. H., Carl, M., Russell, C., Clarke, J. D. and Wilson, S. W. (2008). Brain asymmetry is encoded at the level of axon terminal morphology. Neural Dev. 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader, P., Fischer, N., Gradwohl, G., Guillemot, F. and Strahle, U. (1997). The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development 124, 4557-4569. [DOI] [PubMed] [Google Scholar]
- Blader, P., Plessy, C. and Strahle, U. (2003). Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech. Dev. 120, 211-218. [DOI] [PubMed] [Google Scholar]
- Carl, M., Bianco, I. H., Bajoghli, B., Aghaallaei, N., Czerny, T. and Wilson, S. W. (2007). Wnt/Axin1/beta-catenin signaling regulates asymmetric nodal activation, elaboration, and concordance of CNS asymmetries. Neuron 55, 393-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton, P. and Bally-Cuif, L. (2004). Neurogenesis. Methods Cell Biol. 76, 163-206. [PubMed] [Google Scholar]
- Concha, M. L. (2004). The dorsal diencephalic conduction system of zebrafish as a model of vertebrate brain lateralisation. NeuroReport 15, 1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha, M. L. and Wilson, S. W. (2001). Asymmetry in the epithalamus of vertebrates. J. Anat. 199, 63-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha, M. L., Burdine, R. D., Russell, C., Schier, A. F. and Wilson, S. W. (2000). A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron 28, 399-409. [DOI] [PubMed] [Google Scholar]
- Concha, M. L., Russell, C., Regan, J. C., Tawk, M., Sidi, S., Gilmour, D. T., Kapsimali, M., Sumoy, L., Goldstone, K., Amaya, E. et al. (2003). Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron 39, 423-438. [DOI] [PubMed] [Google Scholar]
- Cooke, J. (2004). Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol. Rev. Camb. Philos. Soc. 79, 377-407. [DOI] [PubMed] [Google Scholar]
- David, N. B., Sapede, D., Saint-Etienne, L., Thisse, C., Thisse, B., Dambly-Chaudiere, C., Rosa, F. M. and Ghysen, A. (2002). Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc. Natl. Acad. Sci. USA 99, 16297-16302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner, J. J., Branford, W. W., Zhang, J. and Yost, H. J. (2000). Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development 127, 1081-1093. [DOI] [PubMed] [Google Scholar]
- Facchin, L., Burgess, H. A., Siddiqi, M., Granato, M. and Halpern, M. E. (2009). Determining the function of zebrafish epithalamic asymmetry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1021-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse, J. T., Thisse, C., Thisse, B. and Halpern, M. E. (2003). The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development 130, 1059-1068. [DOI] [PubMed] [Google Scholar]
- Gamse, J. T., Kuan, Y. S., Macurak, M., Brosamle, C., Thisse, B., Thisse, C. and Halpern, M. E. (2005). Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development 132, 4869-4881. [DOI] [PubMed] [Google Scholar]
- Gilmour, D. T., Maischein, H. M. and Nusslein-Volhard, C. (2002). Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron 34, 577-588. [DOI] [PubMed] [Google Scholar]
- Halpern, M. E., Liang, J. O. and Gamse, J. T. (2003). Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci. 26, 308-313. [DOI] [PubMed] [Google Scholar]
- Hamada, H., Meno, C., Watanabe, D. and Saijoh, Y. (2002). Establishment of vertebrate left-right asymmetry. Nat. Rev. Genet. 3, 103-113. [DOI] [PubMed] [Google Scholar]
- Ho, D. M., Chan, J., Bayliss, P. and Whitman, M. (2006). Inhibitor-resistant type I receptors reveal specific requirements for TGF-beta signaling in vivo. Dev. Biol. 295, 730-742. [DOI] [PubMed] [Google Scholar]
- Inman, G. J., Nicolas, F. J., Callahan, J. F., Harling, J. D., Gaster, L. M., Reith, A. D., Laping, N. J. and Hill, C. S. (2002). SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62, 65-74. [DOI] [PubMed] [Google Scholar]
- Klein, R. S. and Rubin, J. B. (2004). Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol. 25, 306-314. [DOI] [PubMed] [Google Scholar]
- Knaut, H., Werz, C., Geisler, R. and Nusslein-Volhard, C. (2003). A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature 421, 279-282. [DOI] [PubMed] [Google Scholar]
- Lazarini, F., Tham, T. N., Casanova, P., Arenzana-Seisdedos, F. and Dubois- Dalcq, M. (2003). Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia 42, 139-148. [DOI] [PubMed] [Google Scholar]
- Levin, M. (2005). Left-right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 122, 3-25. [DOI] [PubMed] [Google Scholar]
- Liang, J. O., Etheridge, A., Hantsoo, L., Rubinstein, A. L., Nowak, S. J., Izpisua Belmonte, J. C. and Halpern, M. E. (2000). Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development 127, 5101-5112. [DOI] [PubMed] [Google Scholar]
- Long, S., Ahmad, N. and Rebagliati, M. (2003). The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development 130, 2303-2316. [DOI] [PubMed] [Google Scholar]
- Mueller, T. and Wullimann, M. F. (2003). Anatomy of neurogenesis in the early zebrafish brain. Brain. Res. Dev. Brain Res. 140, 137-155. [DOI] [PubMed] [Google Scholar]
- Nasevicius, A. and Ekker, S. C. (2000). Effective targeted gene `knockdown' in zebrafish. Nat. Genet. 26, 216-220. [DOI] [PubMed] [Google Scholar]
- Park, H. C., Kim, C. H., Bae, Y. K., Yeo, S. Y., Kim, S. H., Hong, S. K., Shin, J., Yoo, K. W., Hibi, M., Hirano, T. et al. (2000). Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev. Biol. 227, 279-293. [DOI] [PubMed] [Google Scholar]
- Regan, J. C., Concha, M. L., Roussigne, M., Russell, C. and Wilson, S. W. (2009). An Fgf8-dependent bistable cell migratory event establishes CNS asymmetry. Neuron 61, 27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussigne, M. and Blader, P. (2006). Divergence in regulation of the PEA3 family of ETS transcription factors. Gene Expr. Patterns 6, 777-782. [DOI] [PubMed] [Google Scholar]
- Shepard, J. L., Stern, H. M., Pfaff, K. L. and Amatruda, J. F. (2004). Analysis of the cell cycle in zebrafish embryos. Methods Cell Biol. 76, 109-125. [DOI] [PubMed] [Google Scholar]
- Thisse, C. and Thisse, B. (1999). Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development 126, 229-240. [DOI] [PubMed] [Google Scholar]
- Vallortigara, G., Rogers, L. J. and Bisazza, A. (1999). Possible evolutionary origins of cognitive brain lateralization. Brain Res. Brain Res. Rev. 30, 164-175. [DOI] [PubMed] [Google Scholar]
- Westerfield, M. (1995). The Zebrafish Book: A Guide For The Laboratory Use of Zebrafish (Brachydanio rerio): Eugene, OR: University of Oregon Press.
- Yamamoto, M., Saijoh, Y., Perea-Gomez, A., Shawlot, W., Behringer, R. R., Ang, S. L., Hamada, H. and Meno, C. (2004). Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428, 387-392. [DOI] [PubMed] [Google Scholar]