Abstract
Bipolar disorder (BD) affects a significant portion of the population of the world, yet there has been limited success in developing novel treatments for the disorder. One of the major reasons for this dearth is the absence of suitable animal models for BD. Traditionally, animal models of human phenomena have been evaluated based on similarity to the human syndrome, response to appropriately corresponding medications, and the degree to which a model supports a common mechanistic theory between the human disorder and the model itself. The following review emphasizes the use of ‘reverse translation’, drawing on patient-based findings to develop suitable animal models for BD. We highlight some examples of this strategy, emphasizing their construct validity as a starting point. These studies have produced informative models that have altered the expression of genes/pathways implicated in BD, including the point mutation D181A of mouse mitochondrial DNA polymerase (POLG), glutamate receptor 6 (GluR6), Clock, extracellular regulated kinase 1 (ERK1), glycogen synthase kinase-3β (GSK-3β), B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated athanogene (BAG-1). These studies demonstrate that this method is useful, viable and deserves attention in new efforts to generate animal models of BD.
Introduction
Bipolar disorder (BD) is a severe psychiatric disorder that affects approximately one to two percent of the general population (Belmaker, 2004; Goodwin and Jamison, 2007). Although this disease impacts a significant number of individuals, in the last few decades no truly novel therapeutics have been introduced to treat the disorder. This is, at least in part, because of the fact that no animal models fully encompass BD. Phenotypically, BD is a complex disease in which patients alternate between episodes of mania and depression (Belmaker, 2004; Goodwin and Jamison, 2007). Although some serious attempts have been made to develop animal models that reflect the oscillating nature of BD [for instance, repeated exposure to cocaine (Antelman et al., 1998) or sleep deprivation (Gessa et al., 1995)], most relevant animal models for BD have struggled in their attempt to capture the cyclic nature of this disease. As a result, research on animal models in BD has long been constrained by the conceptual framework and validity of existing behavioral paradigms.
Although some researchers argue that it is necessary to model both the manic and depressed facets of BD (Machado-Vieira et al., 2004), others contend that endophenotype models, which only capture certain elements of the disorder, can be effective and useful in research efforts (Einat, 2007). Endophenotypes are more defined and quantifiable measures that are believed to involve fewer genes and fewer interacting levels; however, this has not been established definitively. Although the use of endophenotypes to develop preclinical animal models is in its infancy, this approach has clear parallels with the general mechanisms employed in preclinical research, where the concept of studying more than one variable simultaneously would rarely be warranted. In fact, animal modeling in psychiatry has relied almost exclusively on simpler phenotypes (Gould and Gottesman, 2006). Although we believe that novel, clinically validated endophenotype models with distinct gene-endophenotype relationships will play a major role in preclinical research in psychiatry, such models are not the focus of the current paper.
Traditionally, three criteria have been used to design and evaluate models in general: face validity, predictive validity and construct validity, and these have been applied to the specific development of animal models (McKinney and Bunney, 1969; Willner, 1986). Face validity is the phenomenological similarity of the model to the syndrome it is imitating. Predictive validity refers to the ability of the model to respond to appropriate medications; in the case of BD, this refers to established mood-stabilizing drugs or antidepressants. Construct validity is the most complex of these three terms; loosely defined, it essentially reflects the degree to which a model measures what it claims to be measuring, and it allows researchers to generate a possible common mechanistic theory that can explain both the animal model and the human disorder (Willner, 1986). Ideally, a successful model has elements of all three criteria; it can, however, still provide significant insight when it only meets some of the standards outlined by McKinney and Bunney (Willner, 1984; Willner, 1991). In this context, it is important to note that the more criteria that any proposed model meets, the more compelling it will be.
Over the years, there have been many attempts to create valid animal models of BD (summarized in Tables 1 and 2) (Gould and Einat, 2007; Kato et al., 2007; Young et al., 2007). All of the proposed animal models measure facets of BD – typically either mania or depression – and some have been extremely helpful in the study of BD; however, none of them completely satisfy all three validity criteria, a phenomenon that also applies to animal modeling of other diseases. Nevertheless, one should not underestimate the utility of these traditional models; they have helped clarify and explore the mechanisms of specific putative endophenotypes of BD [for instance, aggressive behavior (Einat, 2007)], thus shedding additional light on this disease. Furthermore, one of the main reasons supporting the usefulness of at least some of these paradigms concerns predictive validity; that is, that the outcome measures are sensitive to treatment with antidepressants or mood stabilizers such as lithium or valproate (Cryan and Holmes, 2005; Einat and Manji, 2006; O’Donnell and Gould, 2007). Conceptually, however, the terms ‘mood stabilizer-like’ or ‘antidepressant-like’ are not equivocal to mood-stabilizing or antidepressive actions.
Table 1.
Facets of depression and relevant animal models
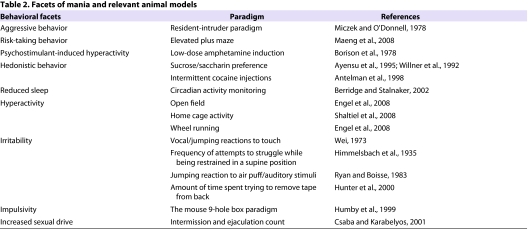
Table 2.
Facets of mania and relevant animal models
Although the last few decades have seen numerous contributions to our understanding of the pathophysiology of BD, improvements in its diagnosis and treatment have been minimal. As noted above, strategies for designing animal models of BD that use face validity as a starting point encounter severe problems owing to the complex nature of this psychiatric disease. However, as our knowledge of the disorder increases, research can be used to create strategies for designing models of BD that use construct validity, instead of face validity, as a starting point (Insel, 2007). Although there are strengths and weaknesses that are inherent in any such method (see summary), recent studies have begun to use approaches that are based on findings from human studies to design novel animal models.
Novel animal models for BD
Unlike traditional animal models, newer models are beginning to be developed based on the concept of ‘reverse translation’; that is, moving from studies in humans with BD to understanding how risk genes or abnormal proteins alter brain development and function. This method shifts the focus from creating abnormal animal behaviors that phenotypically resemble aspects of mental disorders (i.e. animal models) to using what we have learned about the mechanisms of disease in humans in order to identify and develop animals that have the molecular and cellular abnormalities found in BD (i.e. model animals). This approach has become increasingly feasible with the advent of technologies that allow for the direct study of humans. Advances in genomics, proteomics, metabolomics and non-invasive imaging have made humans more tractable for biological investigation, including in the field of psychiatry (Insel, 2007). Current studies are already altering the biological function of animals based on information gleaned from studies of BD patients and their response to therapeutic drugs, thereby using construct validity as the starting point for the model. It is important to acknowledge that, even when models are developed through reverse translation, traditional behavioral measures are still relevant to the study of these mechanisms.
Below, we highlight some examples of leading BD models that have adopted elements of this strategy. Although all of the models below have used genetic modification, it is important to note that effective model animals have been identified in strains or cohorts of species without any genetic engineering, often through observation of species-specific behavior or environmental perturbation; however, such model animals have not yet been identified for the study of BD (Einat et al., 2006; Insel, 2007).
Modeling facets of depression
Point mutation D181A of mouse mtDNA polymerase (POLG)
Construct validity
Although BD is not a traditional mitochondrial disorder, considerable data from diverse sources suggest that altered mitochondrial function may play a role in its pathophysiology and treatment (Kato and Kato, 2000; Quiroz et al., 2008; Stork and Renshaw, 2005). Thus, for example, a number of abnormal magnetic resonance spectroscopy (MRS) findings in BD [including altered N-acetylaspartate (NAA), lactate and high-energy phosphate levels] are compatible with altered mitochondrial function (Kato and Kato, 2000; Quiroz et al., 2008; Stork and Renshaw, 2005). Indeed, genetic analyses of mitochondrial DNA (mtDNA) note a significant association between several mtDNA polymorphisms or mutations and BD (Kato et al., 2001; Munakata et al., 2004). The contribution of mitochondrial protein-encoding nuclear genes to BD was revealed by genetic study (Washizuka et al., 2004) and by genome-wide gene expression analysis (Iwamoto et al., 2005; Konradi et al., 2004). In addition, deleted mtDNA is detected in the postmortem brains of patients with BD (Kato et al., 1997). Finally, lithium and valproate – mood stabilizers that are the mainstays of BD treatment – increase the levels of B-cell lymphoma 2 (Bcl-2, a major mitochondrial protein) and enhance mitochondrial function (Bachmann et al., 2009; Schloesser et al., 2008). Working with the hypothesis that mitochondrial dysfunction is key to the etiology of BD, researchers developed a transgenic (Tg) mouse carrying mtDNA deletions – specifically, POLG in the brain – to act as an animal model for BD.
Face validity
POLG Tg mice (males and females) exhibit a depression-like phenotype based on their overall reduced wheel-running activity. The Tg mice also show altered intra-day activity rhythms, which are characterized by a prolonged duration of activity such as delayed and anticipatory activity; the researchers hypothesized that this trait might echo the altered circadian rhythms that are often seen in mood disorders. In addition, female mice suffered from insomnia-like alterations at some stages of the estrous cycle. However, there were no differences between wild-type (WT) and POLG Tg mice on several behavioral paradigms, including the forced swim test (FST), the elevated plus maze test (EPM), and the open field test (OFT) (Kasahara et al., 2006). Nevertheless, the authors postulate that these Tg mice with neuron-specific mtDNA defects are particularly relevant to creating animal models of recurrent major depressive disorder (MDD) or bipolar depression (Kasahara et al., 2006).
Predictive validity
Treatment of the POLG Tg mice with tricyclic antidepressants (TCAs) significantly increases wheel-running activity, modeling a manic-like response to TCA treatment. Administration of the classic mood stabilizer lithium attenuates this cyclic activity change in female Tg mice (Kasahara et al., 2006). The researchers conclude that the responsiveness of Tg mice to a TCA and to lithium suggests that their behavioral phenotype is more similar to BD than MDD.
Modeling facets of mania
Glutamate receptor 6 (GluR6)
Construct validity
GluR6, also known as GRIK2, is a member of the kainate receptor (KAR) family. The GRIK2 gene resides in a genetic linkage region (6q21) associated with BD; genetic linkage of this region with BD has been demonstrated in several studies, and genome-wide significant linkage was established by meta-analysis (Dick et al., 2003; McQueen et al., 2005; Schulze et al., 2004). A completely independent genetic study also recently implicated the GRIK2 gene in a behavioral phenotype that may be associated with BD (Laje et al., 2007); notably, the same gene conferred sensitivity to treatment-emergent suicidal ideation in a large, collaborative study of individuals with mood disorders [Sequenced treatment alternatives to relieve depression (STAR*D)] (Laje et al., 2007). In addition, some human postmortem studies found reduced GRIK2 mRNA levels in the brains of individuals with BD (Benes et al., 2007; Beneyto et al., 2007). Collectively, these genetic and postmortem brain data raise the possibility that reduced GluR6 levels contribute to the pathophysiology of BD.
Face validity
GluR6 knockout (KO) mice are more active in multiple behavioral tests and are super-responsive to amphetamine. In a battery of specific tests, GluR6 KO mice exhibited increased activity in multiple tests; less anxious, or more risk-taking type, behavior in the OFT and EPM tests; less despair-type manifestations in the FST; more aggressive displays in both the social interaction and resident-intruder tests; and more home cage activity and exploration. These actions were consistent over two consecutive days of testing. In addition, the mice show increased locomotor response to amphetamine (Shaltiel et al., 2008). The data from these behavioral experiments imply that GluR6 may be key to the control of behaviors related to some symptoms of mania, including hyperactivity, aggravated aggression, risk taking, and sensitivity to psychostimulants.
Predictive validity
Chronic, but not short-term, treatment with lithium reduced hyperactivity, aggressive displays and risk-taking behavior in the GluR6 KO mice (Shaltiel et al., 2008). The attenuation of these behaviors is similar to the effects of lithium in BD patients. Although chronic lithium reverses many of the phenotypic abnormalities seen in the GluR6 KO mouse model, it is not yet clear if lithium acts on a pathway that is regulated directly by GluR6, or on a compensatory pathway.
Mutation of the gene encoding Clock
Construct validity
Several studies hypothesize that BD is associated with circadian abnormalities (Jones, 2001; Mansour et al., 2005). Although there are no extant data regarding alterations in the CLOCK gene in BD patients, many individuals with BD have major alterations in circadian functions including sleep, activity, hormonal secretions and appetite (Roybal et al., 2007). In addition, mood stability appears to rely heavily on establishing sleep-wake cycles and activity-dependent cues, although the mechanisms underlying this remain unknown; moreover, disrupting these patterns triggers manic episodes in susceptible individuals (Boivin, 2000). More importantly, some successful treatments for mood disorders may exert some of their therapeutic effects by altering the circadian cycle (Bunney and Bunney, 2000). For instance, lithium lengthens the circadian period in several organisms, including Drosophila, rodents and humans, and this effect may play a role in its therapeutic efficacy (Klemfuss, 1992). Furthermore, valproate alters the expression of several circadian genes in the amygdala (Ogden et al., 2004), and chronic treatment with the antidepressant fluoxetine increases the expression of Clock and Bmal1 in the hippocampus (Manev and Uz, 2006).
Face validity
Mice with a mutation in the Clock gene display an overall behavioral profile that is similar to some facets of human mania, including hyperactivity and decreased sleep, suggesting circadian rhythm dysfunction. These animals spend more time in the center area in the OFT, and also spend more time in the open arms during the EPM, suggesting reduced anxiety. In addition, they display less despair-type behavior in the FST, and increased reward-seeking behavior for cocaine, sucrose and medial forebrain bundle stimulation (Roybal et al., 2007).
Predictive validity
Chronic administration of lithium returns many of the abnormal behavioral responses to WT levels. Treating KO mice with lithium increases their immobility during the FST to levels seen with WT controls. Similar results were also observed in measures of anxiety; compared with untreated Clock mice, KO mice spend less time in the open arms during the EPM and less time in the center of the OFT. It is also important to note that these significant responses were seen in the lower therapeutic range, following 10 days of lithium treatment (Roybal et al., 2007).
Extracellular regulated kinase 1 (ERK1)
Construct validity
The ERK pathway is a major intracellular signaling cascade mediating the biological effects of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) (Huang and Reichardt, 2003). Neurotrophic factors have increasingly been implicated in the pathophysiology and treatment of mood disorders (Chen and Manji, 2006; Coyle and Duman, 2003). Furthermore, recent studies demonstrate that both valproate and lithium activate the ERK signaling cascades in discrete regions of the rodent brain and in neuronal cells in vitro (Chen and Manji, 2006). Patient-based and genetic studies have found indirect evidence that implicate the ERK pathway in mood disorders. For instance, there are significant correlations between mood disorders and known ERK-associated genes, including DISC1 (Maeda et al., 2006), BDNF (Sklar et al., 2002) and RASGRP1 (Ferreira et al., 2008). Lithium and valproate also enhance cellular functions induced by ERK activation, such as neurogenesis, neurite growth and neuronal survival (Chuang, 2004; Chuang, 2005; Hao et al., 2004).
Face validity
Mice with an ERK1 ablation are hyperactive and display certain behaviors that are typical of mania, including enhanced goal-directed and reward-seeking activity, with potentially harmful consequences. In the EPM, ERK1 KO mice enter the open arms more often, although the overall time spent in the open arms does not differ from WT mice. These mice also show less immobility in the FST, a paradigm that echoes the despair/depressive stages of BD. Compared with WT mice, the ERK1 KO mice are also hypersensitive to the psychostimulant amphetamine, showing increased locomotor activity post-injection in the OFT (Engel et al., 2008). Taken together, these findings suggest that ERK1 KO may be an effective model for facets of mania and hyperactivity.
Predictive validity
In ERK KO mice, lithium treatment reduces activity following amphetamine injection. Treatment with valproate, as well as the atypical antipsychotic olanzapine, reduces late-phase baseline motor activity in the KO mice, although lithium had no effect on the same measures (Engel et al., 2008). This difference suggests that lithium and valproate have different therapeutic mechanisms; subsequent work should attempt to elucidate whether there is any convergence on the ERK signaling cascade at a molecular level.
Glycogen synthase kinase-3β (GSK-3β)
Construct validity
GSK-3 is a serine/threonine kinase. In vertebrates, it is found as two isoforms: GSK-3α and GSK-3β. The pathways through which GSK-3 acts as a key regulator have been implicated in the development of a wide variety of psychiatric diseases, including BD (Manji et al., 2003). Although the mechanisms of its therapeutic effect remain unknown, lithium was found to selectively inhibit a limited number of enzymes, including GSK-3 (Gould et al., 2004a; Klein and Melton, 1996). In addition, pharmacologic inhibition of GSK-3 activity produces antidepressant-like effects in rodents (Gould et al., 2004b; Kaidanovich-Beilin et al., 2004). Although present data about alterations in the genes encoding GSK-3 in BD patients are very limited, developing an animal model with alterations in at least one of the GSK-3 isoforms may prove to be a relevant way to model facets of BD.
Face validity
Tg mice overexpressing the GSK-3β protein showed hypophagia, increased general locomotor activity, decreased habituation (as assessed by the OFT), increased acoustic startle response, and decreased habituation to the acoustic startle response. The Tg mice also display reduced immobility in the FST; however, this was probably confounded by their overall heightened activity (Prickaerts et al., 2006). These behavioral observations suggest that the model may mimic some of the hyperactivity that is typical of the manic phase of BD.
Predictive validity
No use of mood stabilizers or antidepressants in this model has yet been described in the literature.
Modeling vulnerability to states that trigger either depression or mania
B-cell lymphoma 2 (Bcl-2)
Construct validity
The neurotrophic protein Bcl-2 plays a crucial role in brain function and is a well-known major modulator of cell growth and survival. Bcl-2 has been shown to promote cell survival in a diverse array of cell types through the inhibition of programmed cell death (Chuang, 2005). More recently, Bcl-2 has been implicated in the regulation of a number of interrelated central nervous system (CNS) functions, including brain development (Shacka and Roth, 2005); neuronal process growth and regeneration (Chen et al., 1997); neuronal survival after a variety of insults (Chuang, 2005); and adult hippocampal neurogenesis (Kuhn et al., 2005). Promoting Bcl-2 function has been proposed for the treatment of neurodegenerative diseases, CNS trauma and acute neurological disorders such as stroke (Chuang, 2005). Multiple studies have also demonstrated that treatment with the mood stabilizers lithium and valproate (Chen et al., 1999; Manji et al., 2000); the antidepressants amitriptyline, desipramine and venlafaxine (Huang et al., 2007; Xu et al., 2003); and the atypical antipsychotic drugs olanzapine and clozapine (Bai et al., 2004; Luo et al., 2004) increase Bcl-2 levels in the rodent brain.
In addition, genetic variants on chromosome 18 have long been suspected of contributing to the biological risk of BD (Hayden and Nurnberger, 2006). A chromosome-wide, open-ended scan of chromosome 18 found that the gene encoding Bcl-2 contained one of two single nucleotide polymorphisms (SNPs) that are significantly associated with an increased risk for BD.
Face validity
In a series of experiments, Bcl2 heterozygous knockout (Bcl2+/−) mice show behaviors modeling two facets of mania: increased reward-seeking and amphetamine sensitization (Lien et al., 2008); specifically, these mice display an aggravated behavioral response to amphetamine. The mice also display enhanced helplessness after severe and uncontrollable stress, and slow spontaneous recovery from helplessness (G.C., A. Baum, G. Moore, H.K.M. et al., unpublished). Although some studies found that Bcl2+/− mice displayed anxiety-like behaviors (Einat et al., 2005), this has not been replicated in all studies (Lien et al., 2008). These results suggest that Bcl2+/− mice exhibit a vulnerability-like phenotype to states that trigger either depression or mania, rather than intrinsically express any manic-like or depression-like phenotypes.
Predictive validity
Chronic pretreatment with lithium attenuates amphetamine sensitization in Bcl2+/− mice (Lien et al., 2008). The findings suggest that the heterozygous animals were sensitive to an amphetamine dose that had no sensitization effect in the WT mice, and that lithium treatment attenuates this difference in behavior. The results of the study offer some additional support for a possible relationship between the observed behavioral changes and mania, because chronic treatment of the mutant mice with lithium ameliorates the increased sensitivity to amphetamine.
Bcl-2-associated athanogene (BAG-1)
Construct validity
Interest in BAG-1 as a putative mediator of affective resilience initially arose out of microarray studies which demonstrated that two agents that are structurally highly dissimilar – lithium and valproate – increased levels of BAG-1 mRNA, protein and function (Zhou et al., 2005). BAG-1 exerts three functions that make it potentially noteworthy in the pathophysiology and treatment of BD: (1) BAG-1 potentiates the antiapoptotic activity of Bcl-2; (2) BAG-1 activates the ERK cascade; and (3) BAG-1 regulates glucocorticoid receptor (GR) trafficking and function. Notably, considerable clinical data have found that glucocorticoids are one of the few agents capable of triggering both depressive and manic episodes in BD patients (Goodwin and Jamison, 2007). Furthermore, mice that overexpress GR in their forebrain display affective lability (Wei et al., 2004). Consistent with the known actions of BAG-1 on GR trafficking and function, previous studies have noted that lithium-or valproate-induced upregulation of BAG-1 attenuates both GR nuclear translocation and the activity of a reporter gene driven by GRs (Zhou et al., 2005). GRs are known to interact with several chaperone proteins, including BAG-1, Hsp70 and FKBP-5 (Schneikert et al., 1999; Westberry et al., 2006). Notably, recent human genetic studies have demonstrated that variants in the FKBP5 gene may be associated with BD (Willour et al., 2009), responsiveness to antidepressants (Binder et al., 2004; Lekman et al., 2008), and the likelihood of developing post-traumatic stress disorder (PTSD) (Binder et al., 2008).
Face validity
An extensive behavioral battery conducted on neuron-selective BAG-1 Tg mice, as well as Bag1 heterozygous knockout (Bag1+/−) mice, reveals intriguing results. BAG-1 Tg mice recover much faster than WT mice in the amphetamine-induced hyperlocomotion test and display a clear resistance to cocaine-induced behavioral sensitization. In contrast, Bag1+/− mice display an enhanced response to cocaine-induced behavior sensitization. The BAG-1 Tg mice showed less anxious-like behavior in the EPM and had higher spontaneous recovery rates from helplessness behavior compared with WT mice. In contrast, fewer Bag1+/− mice recover from helplessness behavior compared with their WT controls (Maeng et al., 2008).
Collectively, Bag1+/− mice do not intrinsically express a manic-like or depression-like phenotype; rather, they appear to have a vulnerability-like phenotype to states triggering depression and mania. These extensive data suggest that BAG-1 plays a key role in affective resilience and in regulating recovery from both manic-like and depression-like behavioral impairments.
Predictive validity
No use of mood stabilizers or antidepressants in this model has yet been described in the literature.
Conclusion
Given the recent advent of technologies that aid in elucidating the neurobiology of complex neuropsychiatric illnesses, it is now possible to identify and generate animals that reflect the biological changes observed in studies of individuals with BD. Thus, we have focused on a few leading models and have emphasized their construct validity as a starting point for generating improved animal models for BD. Here, we present models that implicate a variety of molecular signaling cascades, and acknowledge that other regulators in each implicated pathway are valid targets for animal models (for example, β-catenin, which is a downstream target of GSK-3). Additionally, we focus predominantly on rodent models that highlight facets of manic behavior, as well as a vulnerability phenotype [for example, we did not include the vesicular monoamine transporter 1 (VMAT1) model which used Drosophila as the model organism].
Specifically, the results discussed above deal with animal models that are based on a small group of molecules that are known to contribute significantly to the behavioral control mechanisms related to different facets of symptoms associated with BD (Beneyto et al., 2007; Kato et al., 2007). These include: POLG (Kasahara et al., 2006), GluR6 (Shaltiel et al., 2008), Clock (Roybal et al., 2007), ERK1 (Engel et al., 2008), GSK-3β (Prickaerts et al., 2006), Bcl-2 (Einat et al., 2005; Lien et al., 2008) and BAG-1 (Maeng et al., 2008), in addition to some other molecules that are not reviewed above (e.g. VMAT1) (Chang et al., 2006), DISC1 (Ishizuka et al., 2006) and β-catenin (Gould et al., 2007) (see below). Animals with genetic alterations of these molecules display some common phenotypes, including hyperactivity (Ishizuka et al., 2006; Chang, 2006), social impairment (Ishizuka et al., 2006) and less sensitization to the effects of cocaine (Chang et al., 2006).
One relevant issue is that other models have used reverse-translational strategies to investigate animal models of MDD, which may ultimately be useful when studying the depressive phases of BD. For instance, mice with reduced levels of VMAT2, a CNS-enriched protein involved in loading monoamines into vesicles, exhibit depression-like behavioral states in certain paradigms [e.g. sucrose preference, FST, tail suspension test (TST)] (Fukui et al., 2007). In addition, mice with altered levels of p11, which interacts with the serotonin receptor 5-HT1B, exhibit significant changes in anxiety-like measures (thigmotaxis) and display a depression-like state during the TST (Svenningsson et al., 2006). Furthermore, although many of the chosen models reviewed in this paper were selected because of their relevance to BD research, several of them may not exclusively model BD and could provide information for the study of other mood disorders, even in the context of non-disease adaptive changes (e.g. motor deficits).
It is important to acknowledge that, as with traditional animal models, none of the models highlighted in the current review completely satisfy all three validity criteria, nor do they fully encompass the underlying mechanisms of BD or overcome the difficulty that is inherent in reflecting the oscillating nature of BD. In addition, no one gene or one system highlighted in this review reflects either the core pathology of BD or the core target of its effective medications; thus, it would be difficult to assess how any specific mutation in one or two genes – as is typical of the models reviewed above – could result in a model that truly encompasses the many complex facets of BD.
Clearly, the reverse translation approach highlighted in this paper has both advantages and disadvantages. As is the case with traditional models, using preclinical model animals to investigate how risk genes or abnormal proteins alter brain development and function might not suffice to adequately explore this issue. Furthermore, the notion that models developed in this manner would be animal models for BD but not for more general ‘abnormal animal behavior’ that might be interpreted as an animal model for BD is a subtle distinction that is one of the greatest strengths and weaknesses of this approach, given that it impacts both the specificity and the generalization of any findings. Nevertheless, data obtained from these models can contribute directly to our understanding of the mechanisms of this disease, rather than just imply such knowledge (in direct contrast to traditional animal models of BD). Indeed, these models have already contributed substantially toward our understanding of the molecular and cellular basis of the abnormalities associated with this disease. Although any animal model has well-known limitations in its ability to mirror human mood states, developing models that use the strategies outlined here will help to support valid hypotheses regarding the mechanisms of BD. This approach has enormous potential to eventually yield therapeutics that are more effective in treating individuals afflicted with this devastating disorder.
Acknowledgments
Work in the authors’ lab was supported by the Intramural Research Program at the National Institute of Mental Health (NIMH-NIH). Ioline Henter provided invaluable editorial assistance.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing financial interests. Dr Manji is now at Johnson and Johnson Pharmaceutical Research and Development.
AUTHOR CONTRIBUTIONS
O.M. analyzed the data and wrote the review; D.A. assisted in data collection, analysis and revised the manuscript; G.C. helped define the core concept and framework of the review, and revised the manuscript; H.K.M. helped define the core concept and framework of the review, and was the manuscript’s final editor.
REFERENCES
- Antelman S.M., Caggiula A.R., Kucinski B.J., Fowler H., Gershon S., Edwards D.J., Austin M.C., Stiller R., Kiss S., Kocan D. (1998). The effects of lithium on a potential cycling model of bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 22, 495–510 [DOI] [PubMed] [Google Scholar]
- Ayensu W.K., Pucilowski O., Mason G.A., Overstreet D.H., Rezvani A.H., Janowsky D.S. (1995). Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol. Behav. 57, 165–169 [DOI] [PubMed] [Google Scholar]
- Bachmann R.F., Wang Y., Yuan P., Zhou R., Li X., Alesci S., Du J., Manji H.K. (2009). Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage. Int. J. Neuropsychopharmacol. January 19, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai O., Zhang H., Li X.M. (2004). Antipsychotic drugs clozapine and olanzapine upregulate bcl-2 mRNA and protein in rat frontal cortex and hippocampus. Brain Res. 1010, 81–86 [DOI] [PubMed] [Google Scholar]
- Belke T.W. (1997). Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J Exp Anal Behav. 67, 337–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker R.H. (2004). Bipolar disorder. N. Engl. J. Med. 351, 476–486 [DOI] [PubMed] [Google Scholar]
- Benes F.M., Lim B., Matzilevich D., Walsh J.P., Subburaju S., Minns M. (2007). Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc. Natl. Acad. Sci. USA 104, 10164–10169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M., Kristiansen L.V., Oni-Orisan A., McCullumsmith R.E., Meador-Woodruff J.H. (2007). Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32, 1888–1902 [DOI] [PubMed] [Google Scholar]
- Berridge C.W., Stalnaker T.A. (2002). Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse 46, 140–149 [DOI] [PubMed] [Google Scholar]
- Binder E.B., Salyakina D., Lichtner P., Wochnik G.M., Ising M., Putz B., Papiol S., Seaman S., Lucae S., Kohli M.A., et al. (2004). Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36, 1319–1325 [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradle R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B., et al. (2008). Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin D.B. (2000). Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J. Psychiatry Neurosci. 25, 446–458 [PMC free article] [PubMed] [Google Scholar]
- Borison R.L., Sabelli H.C., Maple P.J., Havdala H.S., Diamond B.I. (1978). Lithium prevention of amphetamine-induced ‘manic’ excitement and of reserpine-induced ‘depression’ in mice: possible role of 2-phenylethylamine. Psychopharmacology (Berl.) 59, 259–262 [DOI] [PubMed] [Google Scholar]
- Bunney W.E., Bunney B.G. (2000). Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology 22, 335–345 [DOI] [PubMed] [Google Scholar]
- Chang H.Y., Grygoruk A., Brooks E.S., Ackerson L.C., Maidment N.T., Bainton R.J., Krantz D.E. (2006). Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol. Psychiatry 11, 99–113 [DOI] [PubMed] [Google Scholar]
- Chen D.F., Schneider G.E., Martinou J.C., Tonegawa S. (1997). Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature 385, 434–439 [DOI] [PubMed] [Google Scholar]
- Chen G., Manji H.K. (2006). The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr. Opin. Psychiatry 19, 313–323 [DOI] [PubMed] [Google Scholar]
- Chen G., Zeng W.Z., Yuan P.X., Huang L.D., Jiang Y.M., Zhao Z.H., Manji H.K. (1999). The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J. Neurochem. 72, 879–882 [DOI] [PubMed] [Google Scholar]
- Chuang D.M. (2004). Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit. Rev. Neurobiol. 16, 83–90 [DOI] [PubMed] [Google Scholar]
- Chuang D.M. (2005). The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann. NY Acad. Sci. 1053, 195–204 [DOI] [PubMed] [Google Scholar]
- Coyle J.T., Duman R.S. (2003). Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron 38, 157–160 [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Holmes A. (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 4, 775–790 [DOI] [PubMed] [Google Scholar]
- Csaba G., Karabelyos C. (2001). The effect of a single neonatal treatment (hormonal imprinting) with the antihormones, tamoxifen and mifepristone on the sexual behavior of adult rats. Pharmacol. Res. 43, 531–534 [DOI] [PubMed] [Google Scholar]
- Dick D.M., Foroud T., Flury L., Bowman E.S., Miller M.J., Rau N.L., Moe P.R., Samavedy N., El-Mallakh R., Manji H., et al. (2003). Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am J Hum Genet. 73, 107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H. (2007). Different behaviors and different strains: potential new ways to model bipolar disorder. Neurosci. Biobehav. Rev. 31, 850–857 [DOI] [PubMed] [Google Scholar]
- Einat H., Manji H.K. (2006). Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol. Psychiatry 59, 1160–1171 [DOI] [PubMed] [Google Scholar]
- Einat H., Yuan P., Manji H.K. (2005). Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav. Brain Res. 165, 172–180 [DOI] [PubMed] [Google Scholar]
- Einat H., Kronfeld-Schor N., Eilam D. (2006). Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behav. Brain Res. 173, 153–157 [DOI] [PubMed] [Google Scholar]
- Engel S.R., Creson T.K., Hao Y., Shen Y., Maeng S., Nekrasova T., Landreth G.E., Manji H.K., Chen G. (2008). The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol. Psychiatry 14, 448–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M.A., O’Donovan M.C., Meng Y.A., Jones I.R., Ruderfer D.M., Jones L., Fan J., Kirov G., Perlis R.H., Green E.K., et al. (2008). Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 40, 1056–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M., Rodriguiz R.M., Zhou J., Jiang S.X., Phillips L.E., Caron M.G., Wetsel W.C. (2007). Vmat2 heterozygous mutant mice display a depressive-like phenotype. J. Neurosci. 27, 10520–10529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa G.L., Pani L., Fadda P., Fratta W. (1995). Sleep deprivation in the rat: an animal model of mania. Eur. Neuropsychopharmacol.5 Suppl, 89–93 [DOI] [PubMed] [Google Scholar]
- Goodwin F.K., Jamison K.R. (2007). Manic-Depressive Illness. New York: Oxford University Press [Google Scholar]
- Gould T.D., Gottesman I.I. (2006). Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 5, 113–119 [DOI] [PubMed] [Google Scholar]
- Gould T.D., Einat H. (2007). Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci. Biobehav. Rev. 31, 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould T.D., Chen G., Manji H.K. (2004a). In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology 29, 32–38 [DOI] [PubMed] [Google Scholar]
- Gould T.D., Einat H., Bhat R., Manji H.K. (2004b). AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int. J. Neuropsychopharmacol. 7, 387–390 [DOI] [PubMed] [Google Scholar]
- Gould T.D., Einat H., O’Donnell K.C., Picchini A.M., Schloesser R.J., Manji H.K. (2007). Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology 32, 2173–2183 [DOI] [PubMed] [Google Scholar]
- Hao Y., Creson T., Zhang L., Li P., Du F., Yuan P., Gould T.D., Manji H.K., Chen G. (2004). Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 24, 6590–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden E.P., Nurnberger J.I., Jr (2006). Molecular genetics of bipolar disorder. Genes Brain Behav. 5, 85–95 [DOI] [PubMed] [Google Scholar]
- Himmelsbach C.K., Gerlach G.H., Stanton E.J. (1935). A method for testing addiction, tolerance and abstinence in the rat: results of its application to several morphine alkaloids. J. Phamacol. Exp. Ther. 53, 179–188 [Google Scholar]
- Huang E.J., Reichardt L.F. (2003). Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- Huang Y.Y., Peng C.H., Yang Y.P., Wu C.C., Hsu W.M., Wang H.J., Chan K.H., Chou Y.P., Chen S.J., Chang Y.L. (2007). Desipramine activated Bcl-2 expression and inhibited lipopolysaccharide-induced apoptosis in hippocampus-derived adult neural stem cells. J. Pharmacol. Sci. 104, 61–72 [DOI] [PubMed] [Google Scholar]
- Humby T., Laird F.M., Davies W., Wilkinson L.S. (1999). Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur. J. Neurosci. 11, 2813–2823 [DOI] [PubMed] [Google Scholar]
- Hunter A.J., Hatcher J., Virley D., Nelson P., Irving E., Hadingham S.J., Parsons A.A. (2000). Functional assessments in mice and rats after focal stroke. Neuropharmacology 39, 806–816 [DOI] [PubMed] [Google Scholar]
- Insel T.R. (2007). From animal models to model animals. Biol Psychiatry 62, 1337–1339 [DOI] [PubMed] [Google Scholar]
- Ishizuka K., Paek M., Kamiya A., Sawa A. (2006). A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol. Psychiatry 59, 1189–1197 [DOI] [PubMed] [Google Scholar]
- Iwamoto K., Bundo M., Kato T. (2005). Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum. Mol. Genet. 14, 241–253 [DOI] [PubMed] [Google Scholar]
- Jones S.H. (2001). Circadian rhythms, multilevel models of emotion and bipolar disorder-an initial step towards integration? Clin. Psychol. Rev. 21, 1193–1209 [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O., Milman A., Weizman A., Pick C.G., Eldar-Finkelman H. (2004). Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol. Psychiatry 55, 781–784 [DOI] [PubMed] [Google Scholar]
- Kasahara T., Kubota M., Miyauchi T., Noda Y., Mouri A., Nabeshima T., Kato T. (2006). Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol. Psychiatry 11, 577–593, 523 [DOI] [PubMed] [Google Scholar]
- Kato T., Kato N. (2000). Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2, 180–190 [DOI] [PubMed] [Google Scholar]
- Kato T., Stine O.C., McMahon F.J., Crowe R.R. (1997). Increased levels of a mitochondrial DNA deletion in the brain of patients with bipolar disorder. Biol. Psychiatry 42, 871–875 [DOI] [PubMed] [Google Scholar]
- Kato T., Kunugi H., Nanko S., Kato N. (2001). Mitochondrial DNA polymorphisms in bipolar disorder. J. Affect. Disord. 62, 151–164 [DOI] [PubMed] [Google Scholar]
- Kato T., Kubota M., Kasahara T. (2007). Animal models of bipolar disorder. Neurosci. Biobehav. Rev. 31, 832–842 [DOI] [PubMed] [Google Scholar]
- Klein P.S., Melton D.A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemfuss H. (1992). Rhythms and the pharmacology of lithium. Pharmacol. Theory 56, 53–78 [DOI] [PubMed] [Google Scholar]
- Konradi C., Eaton M., MacDonald M.L., Walsh J., Benes F.M., Heckers S. (2004). Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch. Gen. Psychiatry 61, 300–308 [DOI] [PubMed] [Google Scholar]
- Kuhn H.G., Biebl M., Wilhelm D., Li M., Friedlander R.M., Winkler J. (2005). Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur. J. Neurosci. 22, 1907–1915 [DOI] [PubMed] [Google Scholar]
- Laje G., Paddock S., Manji H., Rush A.J., Wilson A.F., Charney D., McMahon F.J. (2007). Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am. J. Psychiatry 164, 1530–1538 [DOI] [PubMed] [Google Scholar]
- Lekman M., Laje G., Charney D., Rush A.J., Wilson A.F., Sorant A.J., Lipsky R., Wisniewski S.R., Manji H., McMahon F.J., et al. (2008). The FKBP5-gene in depression and treatment response-an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort. Biol. Psychiatry 63, 1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien R., Flaisher-Grinberg S., Cleary C., Hejny M., Einat H. (2008). Behavioral effects of Bcl-2 deficiency: implications for affective disorders. Pharmacol. Rep. 60, 490–498 [PubMed] [Google Scholar]
- Luo C., Xu H., Li X.M. (2004). Post-stress changes in BDNF and Bcl-2 immunoreactivities in hippocampal neurons: effect of chronic administration of olanzapine. Brain Res. 1025, 194–202 [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R., Kapczinski F., Soares J.C. (2004). Perspectives for the development of animal models of bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 209–224 [DOI] [PubMed] [Google Scholar]
- Maeda K., Nwulia E., Chang J., Balkissoon R., Ishizuka K., Chen H., Zandi P., McInnis M.G., Sawa A. (2006). Differential expression of disrupted-in-schizophrenia (DISC1) in bipolar disorder. Biol. Psychiatry 60, 929–935 [DOI] [PubMed] [Google Scholar]
- Maeng S., Hunsberger J.G., Pearson B., Yuan P., Wang Y., Wei Y., McCammon J., Schloesser R.J., Zhou R., Du J., et al. (2008). BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc. Natl. Acad. Sci. USA 105, 8766–8771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.F. (1984). Learned helplessness and animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 8, 435–446 [PubMed] [Google Scholar]
- Malatynska E., Goldenberg R., Shuck L., Haque A., Zamecki P., Crites G., Schindler N., Knapp R.J. (2002). Reduction of submissive behavior in rats: a test for antidepressant drug activity. Pharmacology 64, 8–17 [DOI] [PubMed] [Google Scholar]
- Manev H., Uz T. (2006). Clock genes: influencing and being influenced by psychoactive drugs. Trends Pharmacol Sci. 27, 186–189 [DOI] [PubMed] [Google Scholar]
- Manji H.K., Moore G.J., Chen G. (2000). Lithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: a role for neurotrophic and neuroprotective effects in manic depressive illness. J. Clin. Psychiatry 61 Suppl. 9, 82–96 [PubMed] [Google Scholar]
- Manji H.K., Gottesman I.I., Gould T.D. (2003). Signal transduction and genes-to-behaviors pathways in psychiatric diseases. Sci. STKE 2003, pe49. [DOI] [PubMed] [Google Scholar]
- Mansour H.A., Monk T.H., Nimgaonkar V.L. (2005). Circadian genes and bipolar disorder. Ann. Med. 37, 196–205 [DOI] [PubMed] [Google Scholar]
- McKinney W.T., Jr, Bunney W.E., Jr (1969). Animal model of depression. I. Review of evidence: implications for research. Arch. Gen. Psychiatry 21, 240–248 [DOI] [PubMed] [Google Scholar]
- McQueen M.B., Devlin B., Faraone S.V., Nimgaonkar V.L., Sklar P., Smoller J.W., Abou Jamra R., Albus M., Bacanu S.A., Baron M., et al. (2005). Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am. J. Hum. Genet. 77, 582–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek K.A., O’Donnell J.M. (1978). Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl.) 57, 47–55 [DOI] [PubMed] [Google Scholar]
- Munakata K., Tanaka M., Mori K., Washizuka S., Yoneda M., Tajima O., Akiyama T., Nanko S., Kunugi H., Tadokoro K., et al. (2004). Mitochondrial DNA 3644T->C mutation associated with bipolar disorder. Genomics 84, 1041–1050 [DOI] [PubMed] [Google Scholar]
- O’Donnell K.C., Gould T.D. (2007). The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci. Biobehav. Rev. 31, 932–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C.A., Rich M.E., Schork N.J., Paulus M.P., Geyer M.A., Lohr J.B., Kuczenski R., Niculescu A.B. (2004). Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol. Psychiatry 9, 1007–1029 [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Chermat R., Lenegre A., Avril I., Janvier S., Steru L. (1987). Use of the automated tail suspension test for the primary screening of psychotropic agents. Arch. Int. Pharmacodyn. Ther. 288, 11–30 [PubMed] [Google Scholar]
- Prickaerts J., Moechars D., Cryns K., Lenaerts I., van Craenendonck H., Goris I., Daneels G., Bouwknecht J.A., Steckler T. (2006). Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J. Neurosci. 26, 9022–9029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz J.A., Gray N.A., Kato T., Manji H.K. (2008). Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33, 2551–2565 [DOI] [PubMed] [Google Scholar]
- Roybal K., Theobold D., Graham A., DiNieri J.A., Russo S.J., Krishnan V., Chakravarty S., Peevey J., Oehrlein N., Birnbaum S., et al. (2007). Mania-like behavior induced by disruption of CLOCK. Proc. Natl. Acad. Sci. USA 104, 6406–6411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G.P., Boisse N.R. (1983). Experimental induction of benzodiazepine tolerance and physical dependence. J. Pharmacol. Exp. Ther. 226, 100–107 [PubMed] [Google Scholar]
- Schloesser R.J., Song J., Klein P.S., Manji H.K. (2008). Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33, 110–133 [DOI] [PubMed] [Google Scholar]
- Schneikert J., Hubner S., Martin E., Cato A.C. (1999). A nuclear action of the eukaryotic cochaperone RAP46 in downregulation of glucocorticoid receptor activity. J. Cell Biol. 146, 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze T.G., Buervenich S., Badner J.A., Steele C.J., Detera-Wadleigh S.D., Dick D., Foroud T., Cox N.J., MacKinnon D.F., Potash J.B., et al. (2004). Loci on chromosomes 6q and 6p interact to increase susceptibility to bipolar affective disorder in the national institute of mental health genetics initiative pedigrees. Biol Psychiatry 56, 18–23 [DOI] [PubMed] [Google Scholar]
- Shacka J.J., Roth K.A. (2005). Regulation of neuronal cell death and neurodegeneration by members of the Bcl-2 family: therapeutic implications. Curr. Drug Targets CNS Neurol. Disord. 4, 25–39 [DOI] [PubMed] [Google Scholar]
- Shaltiel G., Maeng S., Malkesman O., Pearson B., Schloesser R.J., Tragon T., Rogawski M., Gasior M., Luckenbaugh D., Chen G., et al. (2008). Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol. Psychiatry 13, 858–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin C.M. (1998). Voluntary wheel running: a review and novel interpretation. Anim. Behav. 56, 11–27 [DOI] [PubMed] [Google Scholar]
- Sklar P., Gabriel S.B., McInnis M.G., Bennett P., Lim Y.M., Tsan G., Schaffner S., Kirov G., Jones I., Owen M., et al. (2002). Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol. Psychiatry 7, 579–593 [DOI] [PubMed] [Google Scholar]
- Stork C., Renshaw P.E. (2005). Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 10, 900–919 [DOI] [PubMed] [Google Scholar]
- Svenningsson P., Chergui K., Rachleff I., Flajolet M., Zhang X., El Yacoubi M., Vaugeois J.M., Nomikos G.G., Greengard P. (2006). Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311, 77–80 [DOI] [PubMed] [Google Scholar]
- Washizuka S., Iwamoto K., Kazuno A.A., Kakiuchi C., Mori K., Kametani M., Yamada K., Kunugi H., Tajima O., Akiyama T., et al. (2004). Association of mitochondrial complex I subunit gene NDUFV2 at 18p11 with bipolar disorder in Japanese and the National Institute of Mental Health pedigrees. Biol Psychiatry 56, 483–489 [DOI] [PubMed] [Google Scholar]
- Wei E. (1973). Assessment of precipitated abstinence in morphine- dependent rats. Psychopharmacologia 28, 35–44 [DOI] [PubMed] [Google Scholar]
- Wei Q., Lu X.Y., Liu L., Schafer G., Shieh K.R., Burke S., Robinson T.E., Watson S.J., Seasholtz A.F., Akil H. (2004). Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc. Natl. Acad. Sci. USA 101, 11851–11856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry J.M., Sadosky P.W., Hubler T.R., Gross K.L., Scammell J.G. (2006). Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J. Steroid Biochem. Mol. Biol. 100, 34–41 [DOI] [PubMed] [Google Scholar]
- Willner P. (1984). The validity of animal models of depression. Psychopharmacol. Bull. 83, 1–16 [DOI] [PubMed] [Google Scholar]
- Willner P. (1986). Validation criteria for animal models of human mental disorders: learned helplessness as a paradigm case. Prog. Neuropsychopharmacol. Biol. Psychiatry 10, 677–690 [DOI] [PubMed] [Google Scholar]
- Willner P. (1991). Animal models as simulations of depression. Trends Pharmacol. Sci. 12, 131–136 [DOI] [PubMed] [Google Scholar]
- Willner P., Muscat R., Papp M. (1992). An animal model of anhedonia. Clin. Neuropharmacol. 15 Suppl 1 Pt A, 550A–551A [DOI] [PubMed] [Google Scholar]
- Willour V.L., Chen H., Toolan J., Belmonte P., Cutler D.J., Goes F.S., Zandi P., Lee R.S., MacKinnon D.F., Mondimore F.M., et al. (2009). Family-based association of FKBP5 in bipolar disorder. Mol. Psychiatry 14, 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Steven Richardson J., Li X.M. (2003). Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology 28, 53–62 [DOI] [PubMed] [Google Scholar]
- Young J.W., Minassian A., Paulus M.P., Geyer M.A., Perry W. (2007). A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci. Biobehav. Rev. 31, 882–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Gray N.A., Yuan P., Li X., Chen J., Chen G., Damschroder-Williams P., Du J., Zhang L., Manji H.K. (2005). The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 25, 4493–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]