Abstract
The metabolic syndrome refers to the co-occurrence of several known cardiovascular risk factors, including insulin resistance, obesity, atherogenic dyslipidemia and hypertension. These conditions are interrelated and share underlying mediators, mechanisms and pathways. There has been recent controversy about its definition and its utility. In this article, I review the current definitions for the metabolic syndrome and why the concept is important. It identifies a subgroup of patients with shared pathophysiology who are at high risk of developing cardiovascular disease and type 2 diabetes. By considering the central features of the metabolic syndrome and how they are related, we may better understand the underlying pathophysiology and disease pathogenesis. A comprehensive definition for the metabolic syndrome and its key features would facilitate research into its causes and hopefully lead to new insights into pharmacologic and lifestyle treatment approaches.
Physicians and scientists have long known that certain conditions increase a person’s risk of developing atherosclerotic cardiovascular disease (CVD). These risk factors include a family history of premature coronary disease, hypertension, hyperlipidemia, diabetes and smoking. Age increases the risk of CVD, as does male gender and post-menopausal hormonal status. Of these risks, some can be modified – for example, cessation of smoking – whereas others, like genetic predisposition, cannot. The risk of CVD can be decreased by addressing these individual risk factors, both by lifestyle modifications and, if appropriate, pharmacologic treatment (National Cholesterol Education Program, 2002).
It has become increasingly clear that certain CVD risks tend to cluster, or occur together. Furthermore, the lifestyle modifications of dietary change and increased physical activity can significantly affect several risk factors simultaneously and, in so doing, reduce the risk of CVD. This clustering of some risk factors and their shared responsiveness to lifestyle modifications suggests that they are not independent of one another and that they share underlying causes, mechanisms and features (Grundy et al., 2005; Kahn et al., 2005).
The metabolic syndrome is a clustering of hyperglycemia/insulin resistance, obesity and dyslipidemia. It is important for several reasons. First, it identifies patients who are at high risk of developing atherosclerotic CVD and type 2 diabetes (T2D). Second, by considering the relationships between the components of metabolic syndrome, we may be able to better understand the pathophysiology that links them with each other and with the increased risk of CVD. Third, it facilitates epidemiological and clinical studies of pharmacological, lifestyle and preventive treatment approaches.
Current definitions of metabolic syndrome
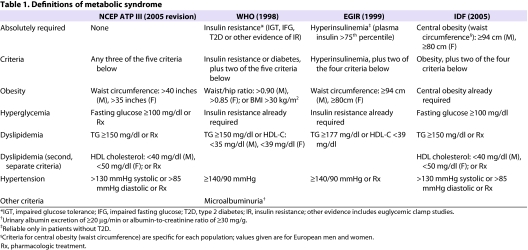
Table 1 summarizes four of the most commonly used definitions of metabolic syndrome. The World Health Organization (WHO) first developed its definition in 1998 (Alberti and Zimmet, 1998). Because insulin resistance was felt to be central to the pathophysiology of metabolic syndrome, evidence for insulin resistance is an absolute requirement in the WHO definition. This could be impaired fasting glucose [IFG, defined as a fasting glucose level above a predetermined cutoff, commonly 100 milligrams per deciliter (mg/dl)] or impaired glucose tolerance (IGT, defined as a glucose level above a predetermined cutoff, commonly 140 mg/dl, for 120 minutes after ingestion of 75 grams of glucose load during an oral glucose tolerance test). Alternatively, other measures could serve as evidence of insulin resistance, such as an elevated homeostatic model assessment of insulin resistance (HOMA-IR) value, which is proportional to the product of the fasting insulin and fasting glucose level. Finally, euglycemic hyperinsulinemic clamp studies could be used as evidence of insulin resistance. In addition to this absolute requirement for insulin resistance, two additional criteria have to be met. These include obesity, dyslipidemia, hypertension and microalbuminuria.
Table 1.
Definitions of metabolic syndrome
The WHO definition was the first to tie together the key components of insulin resistance, obesity, dyslipidemia and hypertension. The definition mandates that insulin resistance be present; without it, even if all the other criteria were met, the patient would not have metabolic syndrome. The WHO definition also allows patients with T2D to be diagnosed with metabolic syndrome if they meet the other criteria. Because some of the measurements are not performed routinely, for example, euglycemic clamp studies, this definition is not easily applied clinically and does not lend itself as well to large epidemiologic studies, where rapid and simple assessment is important.
In 1999, the European Group for the Study of Insulin Resistance (EGIR) proposed a modification to the WHO definition (Balkau and Charles, 1999). Like the WHO, the EGIR felt that insulin resistance is central to the pathophysiology of the metabolic syndrome, so it also requires it for the definition. In this case, insulin resistance is defined by a fasting plasma insulin value that is greater than the 75th percentile. The use of elevated fasting insulin alone as a reflection of insulin resistance simplifies the definition, but it also means that patients with T2D cannot be diagnosed as having metabolic syndrome, since fasting insulin may not be a useful measure of insulin resistance in such patients. Also, similar to the WHO definition, the EGIR definition requires two additional criteria, which can be selected from obesity, hypertension and dyslipidemia. The obesity criteria were simplified to waist circumference, whereas the WHO definition used a choice of waist-to-hip ratio or body-mass index. Microalbuminuria was eliminated as a diagnostic criterion.
In 2001, the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) devised a definition for the metabolic syndrome (National Cholesterol Education Program, 2002), which was updated by the American Heart Association and the National Heart Lung and Blood Institute in 2005 (Grundy et al., 2005). According to the NCEP ATP III definition, metabolic syndrome is present if three or more of the following five criteria are met: waist circumference over 40 inches (men) or 35 inches (women), blood pressure over 130/85 mmHg, fasting triglyceride (TG) level over 150 mg/dl, fasting high-density lipoprotein (HDL) cholesterol level less than 40 mg/dl (men) or 50 mg/dl (women) and fasting blood sugar over 100 mg/dl.
The NCEP ATP III definition is one of the most widely used criteria of metabolic syndrome. It incorporates the key features of hyperglycemia/insulin resistance, visceral obesity, atherogenic dyslipidemia and hypertension. It uses measurements and laboratory results that are readily available to physicians, facilitating its clinical and epidemiological application. It is also simple and easy to remember. Importantly, it does not require that any specific criterion be met; only that at least three of five criteria are met. Thus, the definition does not build in any preconceived notion of the underlying cause of metabolic syndrome, whether it is insulin resistance or obesity.
In 2005, the International Diabetes Foundation (IDF) published new criteria for metabolic syndrome (Zimmet et al., 2005). Although it includes the same general criteria as the other definitions, it requires that obesity, but not necessarily insulin resistance, be present. The obesity requirement is met by population-specific cutpoints. This accounts for the fact that different populations, ethnicities and nationalities have different distributions of norms for body weight and waist circumference. It also recognizes that the relationship between these values and the risk for T2D or CVD differs in different populations. For example, South Asian populations have an increased risk for T2D and CVD at smaller waist circumferences that would not be considered to meet the criteria in a Western population. Although visceral obesity is now recognized as an important factor, the IDF definition has been criticized for its emphasis on obesity, rather than insulin resistance, in the pathophysiology (Reaven, 2006).
Utility of the concept of metabolic syndrome
Patients at risk of CVD and T2D
The concept of metabolic syndrome has several practical uses. One important use is in the everyday clinical assessment of patients, to identify patients at higher risk of T2D or CVD. However, the metabolic syndrome should not be considered only as a way to identify patients at increased risk, as other established risk assessment methods take other important factors into consideration (Meigs, 2004). For example, none of the definitions of metabolic syndrome take into account family history of diabetes, which is one of the most potent known T2D risk factors. Thus, determination of metabolic syndrome would be inferior to the use of a specific risk assessment method such as the diabetes predicting model, which takes family history into account. Similarly, the metabolic syndrome definitions do not consider age, gender (although some of the cutpoints are gender specific), smoking, low-density lipoprotein (LDL) or total cholesterol levels, all known to be important CVD risk factors. Thus, metabolic syndrome would be inferior to a risk assessment tool, such as the Framingham risk score, for the prediction of CVD risk. The major use of metabolic syndrome is not so much in identifying patients at general risk of CVD and T2D, but that it identifies a specific subgroup of patients with a shared pathophysiology. Thus, the term serves as shorthand for clinicians for the common underlying biological processes.
The NCEP ATP III definition is applied easily in the clinical setting. Physicians can easily score patients (and, indeed, motivated patients can score themselves) on the five criteria using easily measured end points and come up with a ‘yes’ or ‘no’ answer as to whether metabolic syndrome is present. This differs from some of the more complicated risk calculation methods, which may require complicated algorithms or computation to come up with an answer. Although it has not been proven, the hope is that realization of a diagnosis of metabolic syndrome will motivate people and their physicians to take appropriate steps to reduce their risk of CVD and T2D. This may involve lifestyle modifications such as improved food choices and increased physical activities, and appropriate pharmacological management for the component criteria.
Understanding common pathophysiological processes
The metabolic syndrome ties together insulin resistance, visceral adiposity, dyslipidemia and hypertension, which are known to be interrelated. In so doing, the concept may help us to better understand the common pathophysiological processes; to develop useful animal models for the disorder; and to devise and test new therapies.
The metabolic syndrome has been assigned its own ICD-9 diagnostic code: 277.7. Despite this, there is ongoing controversy about whether metabolic syndrome is a homogeneous disorder or disease, and whether it merits recognition as a syndrome (Meigs, 2004; Grundy et al., 2005; Kahn et al., 2005; Reaven, 2006; Grundy, 2007). When considering the pathophysiology, it is important to recognize that people with isolated components, but who do not fit the definition of metabolic syndrome, are not at as high a risk for T2D or CVD. For example, people with isolated hypertension or isolated hyperlipidemia are at risk of CVD, but less so than people who meet multiple criteria. People with isolated obesity are at risk for T2D, but less so than people with metabolic syndrome. Although diabetes is considered by NCEP ATP III to be a CVD risk equivalent, additional risk factors that lead to the diagnosis of metabolic syndrome further increase the risk of CVD in these patients. The argument has been made that hypothetical patients with some, but not other, features may be miscategorized by one or another definition (Reaven, 2006). However, as discussed below, the structure of the definitions make this unlikely, and patients who truly reflect the common pathophysiological processes that underlie metabolic syndrome should, in fact, be captured by most of the definitions.
Epidemiological studies
There have been many epidemiological studies on metabolic syndrome, focusing on the prevalence of metabolic syndrome in various populations and the magnitude of risks for T2D, CVD and other related medical problems, including fatty liver, cholesterol gallstones, polycystic ovary syndrome, obstructive sleep apnea and gout. Such epidemiologic studies require a simple, readily applied definition. These studies may add to our understanding not only of the pathophysiology of the condition, but also its genetic basis, using genome-wide association approaches. They may also lead to the development of treatment approaches that target the composite physiological abnormalities, rather than the individual component criteria.
Central features
The expression, ‘make it as simple as possible, but not simpler’ has been attributed to Albert Einstein. Following this principle, the current definitions of metabolic syndrome may be distilled into four central features: insulin resistance, visceral obesity, atherogenic dyslipidemia and endothelial dysfunction. Of these, the first two appear to be absolutely required for metabolic syndrome. In patients with metabolic syndrome, weight loss can lead to improvements in multiple features at the same time, so a certain degree of adiposity appears to be required to manifest the abnormal pathophysiology. Conversely, there are patients who are obese but who do not manifest any of the other components of metabolic syndrome, so both metabolic predisposition to insulin resistance and obesity appear to be necessary for expression of the metabolic syndrome phenotype. Atherogenic dyslipidemia follows from insulin resistance and visceral obesity, and can be captured in the definition by including separate criteria for high serum TG levels and low HDL levels. Endothelial dysfunction also follows from insulin resistance and from adipokines and free fatty acids (FFAs) that are released from visceral adipose tissue. Endothelial dysfunction is captured by the requirement for hypertension in the definition. Both atherogenic dyslipidemia and endothelial dysfunction contribute mechanistically to the development of atherosclerosis and CVD.
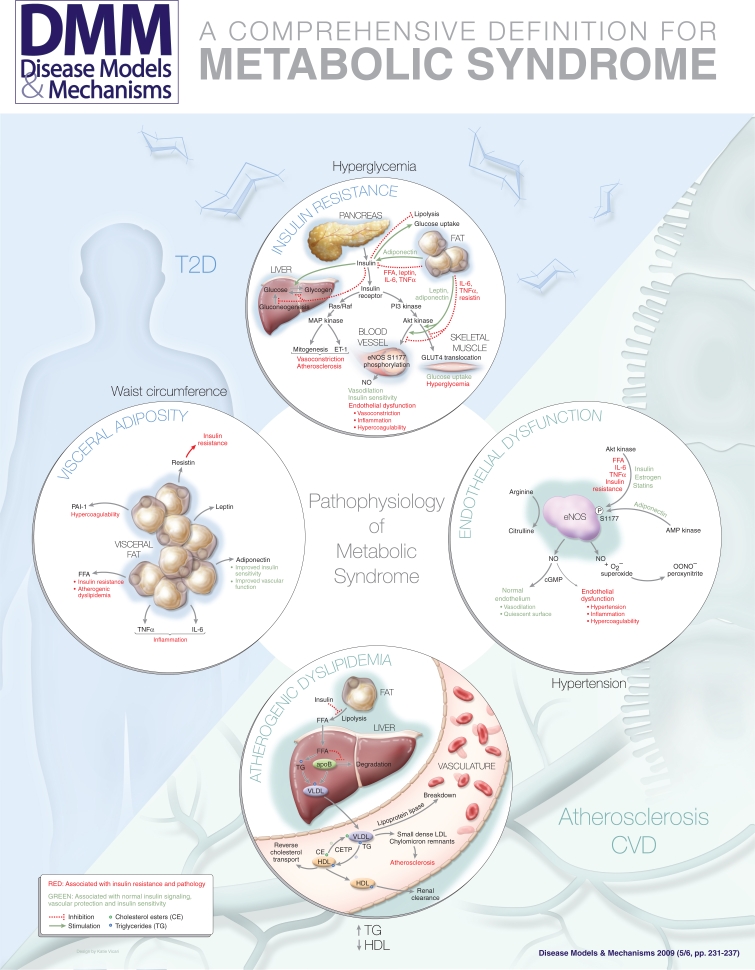
Thus, the four central features – insulin resistance, visceral adiposity, atherogenic dyslipidemia and endothelial dysfunction –would make up the simplest comprehensive definition for the metabolic syndrome, which cannot be simplified further. Even if other associated findings such as systemic inflammation, hypercoagulability or microalbuminuria are important to the pathophysiology, they would not be necessary as part of the definition because these findings would not be required independently. We will discuss each of these central features in the following section. Their interrelationships are shown in the accompanying poster.
Insulin resistance
Insulin is produced by the pancreas in response to hyperglycemia and stimulates glucose use differently in various tissues. The tissues that remove glucose from the circulation and impact glucose use the most are skeletal muscle, liver and adipose tissue. In the skeletal muscle and adipose tissue, insulin stimulates glucose uptake by translocation of the GLUT4 glucose transporter to the cell surface. In the skeletal muscle and liver, insulin stimulates the synthesis of glycogen from glucose and inhibits glycogenolysis. In the liver, insulin also decreases hepatic gluconeogenesis, preventing an influx of more glucose into the bloodstream. In adipose tissue, insulin inhibits fat breakdown, or lipolysis, and stimulates glucose uptake. The net effect of all of these changes is to increase glucose uptake, reduce circulating glucose levels and increase the conversion of glucose into the storage molecules, glycogen or fat (Kim et al., 2006). In insulin resistance, adipose, muscle and liver cells do not respond appropriately to insulin, and circulating glucose levels remain high, which leads to pathology. This is exacerbated by the deregulation of feedback mechanisms.
Insulin-mediated glucose disposal rates vary in the population by over six-fold. Some of this variation is because of adiposity and fitness, and some is the result of genetic origin. Insulin resistance occurs when there is a decrease in the responsiveness of peripheral tissues (skeletal muscle, fat and liver) to the effects of insulin. Insulin resistance is a powerful predictor of T2D, and hyperinsulinemia is a surrogate marker for insulin resistance.
Physiological insulin signaling occurs following the binding of insulin to the insulin receptor, a ligand-activated tyrosine kinase. Binding of insulin results in tyrosine phosphorylation of downstream substrates and activation of two parallel pathways: the phosphoinositide 3-kinase (PI3K) pathway and the mitogen-activated protein (MAP) kinase pathway. Tyrosine phosphorylation of insulin receptor substrates (IRS) activates PI3K, leading to activation of the 3-phosphoinositide-dependent protein kinase 1 (PDK1) kinase and Akt kinase. The PI3K-Akt pathway is responsible for many of the downstream metabolic effects of insulin. In vascular endothelial cells, Akt kinase phosphorylates and activates endothelial nitric oxide synthase (eNOS). In skeletal muscle and adipose tissue, Akt kinase stimulates translocation of the insulin-responsive glucose transporter GLUT4 to the cell surface, leading to increased glucose uptake.
In parallel, tyrosine phosphorylation of the Shc protein activates the GTP exchange factor Sos. This results in activation of the MAP kinase pathway involving Ras, Raf, MAP kinase kinase (MEK) and extracellular regulated kinase (ERK). The MAP kinase pathway mediates endothelin-1 (ET-1) production, leading to vasoconstriction; expression of the vascular cell adhesion molecules VCAM-1 and E-selectin, leading to more leukocyte-endothelial interactions; and growth and mitogenesis effects on vascular smooth muscle cells.
In insulin resistance, the PI3K-Akt pathway is affected, whereas the MAP kinase pathway is not. This leads to a change in the balance between these two parallel pathways. Inhibition of the PI3K-Akt pathway leads to a reduction in endothelial nitric oxide (NO) production, resulting in endothelial dysfunction, and a reduction in GLUT4 translocation, leading to decreased skeletal muscle and fat glucose uptake. By contrast, the MAP kinase pathway is unaffected, so there is continued ET-1 production, expression of vascular cell adhesion molecules and mitogenic stimulus to vascular smooth muscle cells. In these ways, insulin resistance leads to vascular abnormalities that predispose to atherosclerosis.
Insulin increases local blood flow in tissues through the activation of eNOS, leading to two separable effects (Kim et al., 2006; Jonk et al., 2007). Capillary recruitment occurs within minutes, whereas dilation of the larger-resistance vessels increases overall perfusion between 30 minutes and 2 hours. Both of these effects contribute to vasodilation and increased delivery of glucose and insulin to tissues. The vascular effects of insulin couple glucose homeostasis with blood flow and contribute to glucose metabolism at physiological concentrations of insulin. Pharmacologic inhibition of NO production reduces glucose disposal by 40%.
Thus, insulin signaling coordinately affects peripheral glucose use, vascular tone and blood flow. Common mechanisms that contribute to insulin resistance can, therefore, also affect vascular function, including hyperglycemia, advanced glycation products, toxicity from FFAs, obesity, dyslipidemia and other proinflammatory conditions.
Visceral adiposity
Visceral obesity causes a decrease in insulin-mediated glucose uptake, and is clearly related to insulin resistance. The mechanisms for this probably involve adipokines, which are made by adipose tissue, that modulate crosstalk between metabolism and vascular function (Kershaw and Flier, 2004). These include tumor necrosis factor α (TNFα) and interleukin-6 (IL-6), which are proinflammatory and contribute to insulin resistance and vascular dysfunction. The renin angiotensin system is also activated in adipose tissue, leading to hypertension and insulin resistance. By contrast, adiponectin is a protective adipokine that couples insulin sensitivity with energy metabolism. Adiponectin levels are decreased in obesity, T2D and metabolic syndrome. In addition to these adipokines, FFAs, which are released from visceral fat, and bioactive lipid intermediates act together to impair the PI3K-Akt pathway and increase oxidative stress.
Atherogenic dyslipidemia
The key features of atherogenic dyslipidemia are high plasma TG levels, low HDL cholesterol levels and an increase in small dense LDL. Insulin resistance and visceral obesity are associated with atherogenic dyslipidemia (Semenkovich, 2006).
Insulin resistance leads to atherogenic dyslipidemia in several ways. First, insulin normally suppresses lipolysis in adipocytes, so impaired insulin signaling increases lipolysis, resulting in increased FFA levels. In the liver, FFAs serve as a substrate for synthesis of TGs. FFAs also stabilize the production of apoB, the major lipoprotein of very-low-density lipoprotein (VLDL) particles, resulting in more VLDL production. Second, insulin normally degrades apoB through PI3K-dependent pathways, so insulin resistance directly increases VLDL production. Third, insulin regulates the activity of lipoprotein lipase, the rate-limiting and major mediator of VLDL clearance.
Thus, hypertriglyceridemia in insulin resistance is the result of both an increase in VLDL production and a decrease in VLDL clearance. VLDL is metabolized to remnant lipoproteins and small dense LDL, both of which can promote atheroma formation. The TGs in VLDL are transferred to HDL by the cholesterol ester transport protein (CETP) in exchange for cholesteryl esters, resulting in TG-enriched HDL and cholesteryl ester-enriched VLDL particles. The TG-enriched HDL is a better substrate for hepatic lipase, so it is cleared rapidly from the circulation, leaving fewer HDL particles to participate in reverse cholesterol transport from the vasculature.
Endothelial dysfunction
Endothelial dysfunction is the final common pathway between many cardiovascular risk factors and the development of atherosclerosis (Gimbrone et al., 2000; Huang, 2005; Kim et al., 2006). Endothelial cells line the inner surface of blood vessels and serve important mechanical, as well as biological, functions. The endothelium senses and responds to physiological and pathological stimuli, and produces vasoactive substances, including NO, prostacyclin and endothelins. Endothelial expression of cell adhesion molecules governs interactions with circulating leukocytes and monocytes, affecting inflammation, and with circulating platelets, affecting hemostasis and thrombosis. The endothelium also modulates the response of the vascular smooth muscle layer, which may contribute to intimal formation during the development of atherosclerotic plaques. Normal endothelial function protects against these processes, and endothelial dysfunction is central to the pathogenesis of atherosclerotic lesion development.
Endothelial dysfunction, broadly defined, occurs when the endothelium fails to serve its normal physiological and protective mechanisms. This might be because the endothelium is damaged or missing, as in the case of denuded endothelium in coronary arteries that have been subjected to angioplasty. It may occur when the normal responses of the endothelium are affected, for example by oxidative stress, hyperglycemia, advanced glycation products, FFAs, inflammatory cytokines or adipokines. A common feature of endothelial dysfunction is the reduced bioavailability of NO in the vasculature.
There are several mechanisms for endothelial dysfunction (Huang, 2005). The most important ones are a reduction in eNOS phosphorylation at S1177 (Dimmeler et al., 1999; Fulton et al., 1999) and the rapid reaction of NO with superoxide to form peroxynitrite anion (Beckman and Koppenol, 1996). In addition, asymmetric dimethylarginine (ADMA) may compete with arginine to reduce endothelial NO production. eNOS requires enzymatic cofactors, including flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), NADPH and tetrahydrobiopterin (BH4). In the absence of BH4, electron transport through eNOS can become ‘uncoupled’, resulting in the generation of superoxide by eNOS. Superoxide, whether formed by NADPH oxidase or by uncoupled eNOS, reacts with NO in an extremely rapid, diffusion-limited reaction to form peroxynitrite anion, which has its own toxic effects.
eNOS phosphorylation at S1177 appears to be a crucial regulator of its enzymatic activity. S1177 phosphorylation results in an increased electron flux through the reductase domain and reduced calmodulin dissociation. As a result, eNOS becomes more active and produces more NO, even at resting levels of intracellular calcium. eNOS phosphorylation is diminished in diabetes, hypercholesterolemia and atherosclerosis. Physiological insulin signaling increases eNOS phosphorylation through the PI3K-Akt pathway. Estrogens, statins, VEGF and leptin all increase eNOS phosphorylation by Akt kinase. Adiponectin, the protective adipokine, increases eNOS phosphorylation by AMP kinase. The fact that diverse signaling pathways affect multiple kinases that converge to modulate eNOS activity by phosphorylation suggests that this is a common integration point that underlies endothelial dysfunction from various causes. Thus, the phosphorylation of eNOS at S1177 appears to be a crucial step in the regulation of eNOS activity and an important target for intervention to treat endothelial dysfunction (Huang, 2005; Atochin et al., 2007).
Insulin resistance causes endothelial dysfunction by decreasing Akt kinase activity, resulting in diminished eNOS phosphorylation and activity. Because the phosphorylation of eNOS at S1177 is required for the hemodynamic actions of insulin, this results in diminished blood flow to skeletal muscle, creating a vicious cycle where endothelial dysfunction then worsens insulin resistance. In addition, insulin-mediated ET-1 expression and vascular smooth muscle mitogenic effects are not affected by insulin resistance, further contributing to endothelial dysfunction.
Visceral adiposity causes endothelial dysfunction through the effects of resistin, IL-6 and TNFα on eNOS phosphorylation. In addition to blocking IRS-1 activation, TNFα directly activates NADPH oxidase, increasing superoxide generation; TNFα also stimulates lipolysis, resulting in FFA release. By contrast, adiponectin, which stimulates eNOS phosphorylation, is diminished in metabolic syndrome. In visceral fat, leptin resistance also increases the generation of reactive oxygen species. FFAs contribute to endothelial dysfunction by a combination of diminished PI3K-Akt signaling, increased reactive oxygen species and increased ET-1 production.
Conclusions
In summary, the central features of the metabolic syndrome are insulin resistance, visceral adiposity, atherogenic dyslipidemia and endothelial dysfunction. These conditions are interrelated and share common mediators, pathways and pathophysiological mechanisms. A comprehensive definition of the metabolic syndrome, expressed as simply as possible, would contain only these features. The requirement of multiple criteria would ensure the exclusion of people with individual components (e.g. isolated hypertension or isolated hyperlipidemia), as opposed to the composite pathophysiology discussed above. Inclusion of both TG and HDL criteria increases the specificity for atherogenic dyslipidemia, and inclusion of the blood pressure criterion ensures that the physiologic derangements are severe enough to have resulted in endothelial dysfunction.
Of the various definitions for the metabolic syndrome, the NCEP ATP III definition is the easiest to apply clinically and epidemiologically, because it uses straightforward criteria that are measured readily. Despite the ongoing controversy about whether the concept of metabolic syndrome is useful, it clearly defines specific pathophysiological mechanisms that link the central features. Consideration of metabolic syndrome as a specific entity allows for research on the genetic basis for susceptibility to this syndrome, a better understanding of its underlying pathophysiology and the development of treatment approaches.
Supplementary Material
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.001180/-/DC1
REFERENCES
- Alberti K.G., Zimmet P.Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 15, 539–553 [DOI] [PubMed] [Google Scholar]
- Atochin D.N., Wang A., Liu V.W., Critchlow J.D., Dantas A.P., Looft-Wilson R., Murata T., Salomone S., Shin H.K., Ayata C., et al. (2007). The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J. Clin. Invest. 117, 1961–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkau B., Charles M.A. (1999). Comment on the provisional report from the WHO consultation: European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 16, 442–44310342346 [Google Scholar]
- Beckman J.S., Koppenol W.H. (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 271, C1424–C1437 [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A.M. (1999). Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 [DOI] [PubMed] [Google Scholar]
- Fulton D., Gratton J.P., McCabe T.J., Fontana J., Fujio Y., Walsh K., Franke T.F., Papapetropoulos A., Sessa W.C. (1999). Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M.A., Jr, Topper J.N., Nagel T., Anderson K.R., Garcia-Cardena G. (2000). Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci. 902, 230–239; discussion 239–240. [DOI] [PubMed] [Google Scholar]
- Grundy S.M. (2007). Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 92, 399–404 [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Jr, et al. (2005). Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung and Blood Institute scientific statement. Circulation 112, 2735–2752 [DOI] [PubMed] [Google Scholar]
- Huang P.L. (2005). Unraveling the links between diabetes, obesity, and cardiovascular disease. Circ Res. 96, 1129–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonk A.M., Houben A.J., de Jongh R.T., Serne E.H., Schaper N.C., Stehouwer C.D. (2007). Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 22, 252–260 [DOI] [PubMed] [Google Scholar]
- Kahn R., Buse J., Ferrannini E., Stern M. (2005). The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 48, 1684–1699 [DOI] [PubMed] [Google Scholar]
- Kershaw E.E., Flier J.S. (2004). Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 89, 2548–2556 [DOI] [PubMed] [Google Scholar]
- Kim J.A., Montagnani M., Koh K.K., Quon M.J. (2006). Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113, 1888–1904 [DOI] [PubMed] [Google Scholar]
- Meigs J.B. (2004). Metabolic syndrome: in search of a clinical role. Diabetes Care 27, 2761–2763 [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP): Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults (2002). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 [PubMed] [Google Scholar]
- Reaven G.M. (2006). The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 83, 1237–1247 [DOI] [PubMed] [Google Scholar]
- Semenkovich C.F. (2006). Insulin resistance and atherosclerosis. J Clin Invest. 116, 1813–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P., Magliano D., Matsuzawa Y., Alberti G., Shaw J. (2005). The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 12, 295–300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.