Abstract
Injury to the lung parenchyma results in the acute respiratory distress syndrome (ARDS), which is a common and life-threatening cause of respiratory failure and mortality that develops after a variety of insults, including sepsis, multiple trauma, pneumonia, aspiration of gastric contents and severe burns. The pathogenesis of ARDS is complex with loss of the alveolar-capillary barrier and flooding of the airspaces with protein-rich fluid; injury to the alveolar epithelium; an influx of neutrophils and macrophages; and fibrin deposition as a result of activation of coagulation and inhibition of fibrinolysis. These changes develop over hours to a few days after the initiating event and often take days or weeks to resolve. Despite decades of research, there is only one therapy (low tidal volume ventilation) that has been shown to reduce mortality in ARDS. Further research into the pathogenesis of this devastating condition is crucial for the development of novel and specific therapies that target specific disease mechanisms. Unfortunately, no single animal model of ARDS replicates the complex pathophysiological changes seen in patients. This is a severe limitation in the study of ARDS and has impaired scientific and therapeutic progress in this field. Here, we discuss the primary features of this syndrome, highlight limitations of current animal models and suggest new approaches to investigate key components of pathogenesis. Hopefully, as new technologies and approaches emerge, barriers to scientific progress in ARDS will be overcome.
Acute respiratory distress syndrome (ARDS): clinical concepts and pathogenic mechanisms
The first published reports of ARDS appeared in 1967 when Ashbaugh and colleagues described 12 adult patients with the acute onset of respiratory distress, refractory hypoxemia and bilateral infiltrates in chest X-rays (Ashbaugh et al., 1967). This was initially called the adult respiratory distress syndrome (Ashbaugh et al., 1969) and was later modified to the acute respiratory distress syndrome (Ashbaugh and Petty, 1972) when it was recognized to occur in children. Epidemiological studies suggest that there are approximately 200,000 cases per year in the USA and that the average mortality rate approaches 40% (Rubenfeld et al., 2005). ARDS can occur in the setting of either direct (pneumonia, aspiration, contusion) or indirect (sepsis, trauma, pancreatitis) lung insults, with sepsis accounting for the majority of cases. Among patients with sepsis, 40% will develop ARDS (Hudson et al., 1995). The heterogeneity of causes of ARDS makes studying the pathogenesis of the syndrome and testing potential therapies complicated. Certain subsets of patients, for example, children and those with trauma-associated ARDS, have a much better prognosis. Unlike sepsis-associated ARDS, the development of ARDS in trauma patients is not an independent predictor of mortality (Treggiari et al., 2004). Furthermore, many of the initial clinical studies used different definitions of ARDS, making the results difficult to generalize. An advance in the field of ARDS research was the establishment of a consensus definition in 1994 (Bernard et al., 1994). ARDS is now defined by: (1) the acute onset of bilateral infiltrates on chest imaging, (2) the acute onset of hypoxemia with a partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio of <200, and (3) the absence of left heart failure. This consensus definition was the first step in planning and executing large, multicenter, randomized clinical trials. Several of these studies have now changed the way we care for patients with ARDS and have resulted in improved clinical outcomes. These proven interventions include a low tidal volume protective ventilator strategy (The Acute Respiratory Distress Syndrome Network, 2000) and conservative fluid management (Wiedemann et al., 2006). These advances in care have resulted from improved supportive care as opposed to the development of specific interventions that target the underlying etiology of the lung injury. The lack of specific pharmacological interventions is frustrating in light of several decades of research investigating the biological and molecular pathogenesis of ARDS. Numerous pharmacological interventions have proven unsuccessful in ARDS patients, even though preclinical trials in animal models looked very promising. Therefore, in ARDS, as with many diseases, there are discrepancies between the positive findings in animal studies and human clinical trials, which are probably, in part, because of limitations in the available animal models. To address the issues in modeling this syndrome, it is essential to understand the key pathogenic features of the human disorder.
The major features of ARDS pathogenesis
Loss of the alveolar-capillary barrier
One of the earliest abnormalities seen in injured lung is the loss of the alveolar-capillary barrier integrity. This is seen both ultrastructurally on histological analysis (Riede et al., 1978) and clinically, as vascular leak with flooding of the alveolar space with protein-rich edema fluid. As described in our case study, this is evident both radiographically with bilateral, fluffy alveolar infiltrates and clinically with tachypnea and hypoxemia. Experimental evidence has shown that the alveolar-capillary leak allows bidirectional travel of fluid and proteins. In contrast to normal lungs, the lungs of ARDS patients who are administered with an intravenous injection of hetastarch have an accumulation of this molecule in the airspaces (Wang et al., 1999). In addition, patients with ARDS have elevated plasma levels of surfactant proteins that are only produced by lung alveolar epithelial cells (Doyle et al., 1997). Although the edema caused by vascular leak is crucial to the pathogenesis of ARDS, lung edema alone does not constitute ARDS. Patients with hydrostatic pulmonary edema have flooding of the airspaces with edema fluid as a result of increased pulmonary capillary pressure; however, these patients often resolve their pulmonary edema very quickly once hydrostatic pressure is decreased. In fact, because these patients are so different from those with ARDS, exclusion of patients with hydrostatic edema is incorporated into the current definition of ARDS.
Alveolar epithelial injury
An early event in the pathogenesis of acute lung injury is injury to both type I and type II alveolar epithelial cells, which occurs in the setting of both direct and indirect injury to the lung. Patients with ARDS have elevated levels of the receptor for advanced glycation end products (RAGE), a marker of type I alveolar epithelial cell injury, in both the lung compartment and circulating in the plasma (Uchida et al., 2006). In a rat model of ARDS, the level of RAGE in the bronchoalveolar lavage fluid was shown to correlate with the histological degree of lung injury. The mechanisms of alveolar epithelial injury probably include both apoptosis and necrosis. Histological analysis of the lungs from patients with ARDS shows an extensive loss of the alveolar epithelium with exposure of the basement membrane (Bachofen and Weibel, 1982), demonstrating that loss of the epithelial barrier is an important feature of ARDS. This damage to the alveolar epithelium impairs surfactant production, which, in combination with the accumulation of pulmonary edema, significantly inhibits gas exchange and leads to persistent hypoxemia, as is seen in our case study.
Case study.
FM is a 39-year-old previously healthy man who was admitted to the emergency department (E.D.) with severe pain in his left calf and ankle, a fever to 103.1 degrees Fahrenheit, tachycardia with a heart rate of 130 beats per minute, and hypotension with a blood pressure of 92/48 mmHg. He had been well until the previous day, when he noticed a small area of redness and swelling on the lateral aspect of his left calf, in the same area where he had sustained a small abrasion a few days previously. The pain and redness continued to worsen and, in the night before admission, he was unable to sleep owing to severe pain. Approximately 12 hours before admission, he developed fever and malaise. He presented to the E.D. and was found to have a swollen, red, tender left calf and ankle, and signs of a systemic inflammatory response syndrome. His blood pressure and heart rate did not respond to initial fluid resuscitation, so he was started on broad-spectrum antibiotics and admitted to the hospital with a diagnosis of septic shock. Laboratory evaluation at admission revealed a white blood cell count of 18,000/mm3 with 90% neutrophils, a platelet count of 35,000/mm3 and a creatinine level of 1.9 mg/dl. Over the next 8 hours, he became tachypneic and hypoxemic, requiring supplemental oxygen, his blood pressure declined further, he was started on a continuous infusion of norepinephrine, and his urine output declined despite aggressive fluid resuscitation. Over the next 4 hours, his oxygenation worsened and his work of breathing increased, prompting intubation and mechanical ventilation. Following intubation, a chest X-ray revealed diffuse bilateral alveolar infiltrates. He required a high level of ventilatory support with 90% oxygen and 14 cm H2O of positive end-expiratory pressure to keep his oxygen saturation at 89%, and he had a minute ventilation of 30 liters per minute, consistent with a diagnosis of ARDS. His blood cultures grew Gram-positive cocci that were later identified as group A streptococcus. Despite appropriate antibiotics and aggressive supportive care, over the next 6 hours his urine output decreased to zero, he developed worsening hypotension that was refractory to multiple vasopressors, his lung injury worsened, and he was unable to be oxygenated with maximal ventilator settings. He died approximately 24 hours after admission. On autopsy, his lungs were boggy and edematous, and histologically there was evidence of severe diffuse alveolar damage, consistent with ARDS.
Inflammatory cell influx
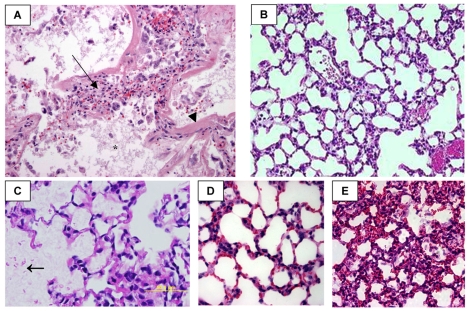
Concurrent with injury to the alveolar-capillary barrier, there is massive inflammatory cell influx into the lungs (Fig. 1A) (de Hemptinne et al., 2008). This can occur as a result of a direct injury to the lung (such as aspiration of gastric contents) or, as in our case study, as a response to systemic inflammation and cytokine production. This accumulation is reflected by the neutrophils and bronchoalveolar lavage fluid that fill the airspaces (Fowler et al., 1987). Injurious mediators, such as elastases (Fujishima et al., 2008) and oxidants (Lamb et al., 1999), are liberated from neutrophils and contribute to lung injury. Macrophages and lymphocytes are also recruited to the lung and produce large amounts of both pro- and anti-inflammatory cytokines (Park et al., 2001). Despite the predominance of inflammatory cells in the airspace in acute lung injury, anti-inflammatory therapies have not proven to be beneficial in ARDS patients to date. Furthermore, ARDS occurs clinically even in the setting of neutropenia, as seen in patients who develop ARDS following bone marrow transplant, suggesting that inflammatory cells are not absolutely required for the induction of lung injury.
Fig. 1.
Comparison of human ARDS with different mouse models. (A) Lung biopsy from a patient with ARDS, showing alveolar edema (*), inflammation (arrow) and fibrin deposition (arrowhead). Reprinted with permission from Chest (de Hemptinne et al., 2008). (B) Mouse lung after ventilation with high tidal volume (15 ml/kg) for 5 hours, showing a modest amount of inflammation and alveolar thickening, but very little edema or fibrin. Reprinted with permission from Critical Care (Wolthuis et al., 2009). (C) Mouse lung following acid aspiration showing extensive edema (arrow), but modest inflammation and septal thickening. Republished with permission from the American Society for Clinical Investigation (Zarbock et al., 2006). (D,E) Mouse lung following a single intraperitoneal dose of lipopolysaccharide (LPS) (D) compared with an intraperitoneal LPS pump (E), which has increased inflammation and edema. Reprinted with permission from The Journal of Immunology. Copyright 2006. The American Association of Immunologists, Inc. (Everhart et al., 2006).
Activation of coagulation and inhibition of fibrinolysis
Another key pathological feature of ARDS is the presence of hyaline membranes (Bachofen and Weibel, 1982), which are a direct result of intra-alveolar fibrin polymerization (Fig. 1A). For decades it has been known that the alveolar compartment in ARDS has an increase in procoagulant protein activity and a decrease in fibrinolytic therapy, favoring fibrin formation (Idell, 2003). More recently, there is emerging evidence that resident lung cells, including alveolar epithelial cells, actively modulate alveolar fibrin deposition through upregulation and activation of tissue factor (Bastarache et al., 2007), the most potent initiator of coagulation, and loss of the ability to activate protein C (Wang et al., 2006), a key anti-coagulant protein. Modulation of these pathways in patients with severe sepsis and ARDS by treatment with activated protein C reduced mortality (Bernard et al., 2001) and was associated with a more rapid recovery of lung function in those with ARDS (Vincent et al., 2003); these findings provide further evidence for the central role of coagulation in the pathogenesis of sepsis-induced ARDS. In addition to activation of coagulation in the airspace, systemic coagulation is also activated causing consumption of platelets and resulting in microvascular thrombosis that, clinically, causes an increase in the dead space in the lungs. These pathological abnormalities manifest clinically as thrombocytopenia and high minute ventilation, both of which were seen in our case study. Furthermore, activation of coagulation generates thrombin and fibrin, both of which are proinflammatory proteins that promote ongoing inflammation. Thus, coagulation and inflammation are intimately linked both in the systemic circulation and in the lung.
ARDS: current model systems and challenges
The complex pathogenesis of ARDS makes animal models a necessity in the study of this disorder. Larger animals that more closely replicate human disease, such as primates, are prohibitively expensive for most researchers to study and require specialized research facilities. Smaller animals, such as mice, are much more widely accessible to researchers and are a very powerful research tool as they can be genetically manipulated in multiple ways to facilitate the detailed mechanistic study of complex pathways. In addition, there are multiple methods, both direct and indirect, of inducing lung injury in mice and other animals. The goal of each model system used is to mimic human disease. In humans, pneumonia and sepsis are the two most common predisposing conditions for the development of ARDS. These conditions can be modeled in mice by using the Gram-negative bacterial endotoxin LPS, which can be administered either directly to the lungs through intratracheal injection or inhalation, or given intraperitoneally or intravenously to incite a systemic inflammatory response. Mice treated with intratracheal LPS have an acute and robust inflammatory cell influx to the lung with resolution by 48 hours. Intraperitoneal LPS activates systemic inflammation and is associated with a mild lung injury. This injury can be augmented with either repeated injections of LPS or the implantation of an LPS pump in the peritoneal cavity (Fig. 1E) to continually release LPS for hours, or even days (Everhart et al., 2006; Cheng et al., 2007). Another commonly used model of lung injury is hyperoxia, where mice breathe a high partial pressure of oxygen that is highly toxic to the alveolar epithelium and causes extensive alveolar epithelial injury with only a modest amount of inflammation. However, the direct relationship to human ARDS is unclear with this model. A third commonly studied model is ventilator-induced lung injury (Fig. 1B) (Wolthuis et al., 2009), which correlates excellently to human ventilator-induced lung injury; however, in the absence of an additional stimulus or extremely high tidal volumes, this model does not induce substantial lung injury in mice. One recent study showed a modest degree of lung inflammation, vascular leak and activation of alveolar coagulation with tidal volumes of 15 ml/kg (Fig. 1B) compared with low tidal volumes of 7.5 ml/kg (Wolthuis et al., 2009). To get a more severe injury, higher tidal volumes (as high as 35 ml/kg in one study) are required (Wilson et al., 2003). Although these models induce some of the pathological changes seen in human disease, the biological relevance is uncertain given the extremely high tidal volumes that are used in these models compared with the 4–6 ml/kg tidal volume that is recommended for patients with ARDS (The Acute Respiratory Distress Syndrome Network, 2000). There have been several other animal models developed to study lung injury. A recent, comprehensive review on animal models of acute lung injury by Matute-Bello et al. discussed each model in great detail and the reader is referred to this reference for more information (Matute-Bello et al., 2008).
Common challenges to modeling ARDS in animals
Limited ability to provide critical care to small animals
Regardless of the specific model system used, one of the major limitations to research in this field is the limited ability to provide prolonged supportive care to small animals. The extent of lung injury seen in patients with ARDS is so severe that lifesaving mechanical ventilation is needed to provide adequate gas exchange (extraction of oxygen and elimination of carbon dioxide) during the period of acute illness. Since it is not possible to provide long-term mechanical ventilation in small animals, the mouse lung injury models that are available do not cause an injury that is severe enough to accurately mimic the human disease. If a murine model does cause significant impairment of gas exchange, the animals die and cannot be studied further. In addition to the challenges with providing mechanical ventilation to small animals, there are also additional challenges in providing hemodynamic support. Patients with ARDS are often in shock and require aggressive fluid resuscitation and cardiovascular support with vasopressors. Typically, these interventions are titrated to the physiological measurements acquired through invasive hemodynamic monitoring using arterial and central venous catheters, along with tools to measure intravascular pressures. In order to adequately model ARDS in mice, we would need to create a ‘mouse intensive care unit (ICU)’, in which all of these therapies could be administered adequately. Current technology makes this impossible. Larger animals, such as primates, can be maintained in an intensive care setting but, as mentioned above, primate models are available to only a very few laboratories throughout the world. One potential solution to this problem is the development of unilateral lung injury models, such as unilateral acid aspiration, which may provide the ability to create a severe lung injury in one lung, while preserving a normal lung with which the animal can survive (Amigoni et al., 2008). Although this represents a novel approach to lung injury models, it has yet to gain widespread use and has not been well studied.
Animal models do not replicate key pathogenic features of the human disease
As is the case with most human diseases, the pathogenesis of ARDS is complex and involves many interrelated biological pathways that lead to the loss of the alveolar-capillary barrier, epithelial cell injury, inflammatory cell influx and fibrin deposition (as discussed above). Many commonly used mouse lung injury models do not replicate all of these key pathogenic abnormalities. For example, murine acid aspiration, which is used to model the aspiration of gastric contents in humans (Zarbock et al., 2006), results in a significant amount of pulmonary edema (Fig. 1C) but only has modest inflammation. Conversely, some models that cause an extensive inflammatory cell influx in the lung (such as intratracheal LPS) involve very little alveolar epithelial injury or vascular leak. Furthermore, systemic administration of LPS, with either a single intravenous or intraperitoneal LPS injection (Fig. 1D), results in systemic inflammation and high levels of circulating cytokines, but little inflammatory cell influx into the alveolar space and modest impairment of the alveolar-capillary barrier. How relevant, then, are these models to human disease given that they model some but not all of the pathological aspects of the disease? The answer is not clear, but the long list of ineffective anti-inflammatory therapies that have been tried in ARDS indicate that effectiveness in these models does not easily translate into successful human trials. In an attempt to better model sepsis-induced ARDS, we have recently developed a model using sustained delivery of LPS to the peritoneal cavity using an osmotic pump that models ARDS secondary to abdominal infection (Fig. 1D,E). In this model, a sustained systemic inflammatory response results in prolonged activation of the nuclear factor-κB (NF-κB) pathway in the lungs, epithelial cell injury, inflammatory cell influx and loss of the alveolar-capillary barrier (Everhart et al., 2006). The cecal ligation and puncture model, a more commonly used model of sepsis-induced ARDS, results in a similar lung injury pattern but is limited by difficulties in reproducibility and by potential confounding effects if the interventions affect bacterial proliferation.
In addition to the considerations discussed above, we are in need of models that replicate the coagulation and fibrinolytic abnormalities that are key to ARDS pathogenesis, but that are not currently modeled well in animals. Clinical studies from decades ago identified abnormalities in coagulation and fibrinolysis in the lungs of patients with ARDS (Idell et al., 1987; Idell et al., 1989; Idell et al., 1991). The lung compartment becomes procoagulant and anti-fibrinolytic, favoring fibrin deposition, in the setting of ARDS (Fig. 1A). More recently, evidence suggests that there may be a local source of specific coagulation and fibrinolytic proteases in the lung. In patients with ARDS, plasminogen activator inhibitor type 1 (PAI-1) levels were significantly higher in undiluted pulmonary edema fluid samples compared with simultaneous plasma samples, suggesting local formation in the airspace (Prabhakaran et al., 2003). The alveolar epithelium may be one source of these proteins, as it has been shown to upregulate tissue factor in the setting of inflammation and injury (Bastarache et al., 2007) and to modulate protein C activation (Wang et al., 2006), which is attenuated in ARDS. There is mounting evidence that coagulation and fibrinolysis are modulated locally in the airspace by resident lung cells and that this is important to the pathogenesis of ARDS in humans; however, this has not been well studied in animals. The inability to robustly model these coagulation abnormalities in mouse models of lung injury is a major limitation to advances in the field.
Clinical terms.
Fibrinolysis – a normal physiological process in which fibrin is broken down to dissolve small blood clots
Hyaline membranes – transparent glassy protein-containing film composed primarily of polymerized fibrin that settles in the lung and blocks air exchange in several respiratory diseases
Hydrostatic pulmonary edema – an abnormal accumulation of fluid in the lung owing to increased capillary pressure
Hypoxemia – insufficiency of blood oxygen levels
Lung parenchyma – lung tissues engaged in gas exchange
Neutropenia – an abnormally low number of neutrophils (a type of white blood cell)
Sepsis – evidence of systemic inflammation (fever, tachycardia, tachypnea, elevated white blood cell count) in the setting of a known or suspected infection
Tachypnea – an abnormally rapid respiratory rate
Thrombocytopenia – an abnormally low blood platelet count
Lack of good surrogate endpoints
The gold standard for defining the clinical success of a new treatment for ARDS is a reduction in disease-related mortality. However, the limitations of time, personnel, money or other resources often make this an impractical endpoint in clinical studies, thus necessitating surrogate endpoints. A major problem in ARDS research is the lack of good surrogate endpoints in patients with the disorder, which makes it very difficult to determine appropriate endpoints in preclinical studies. For example, a common surrogate endpoint for clinical success of a therapeutic intervention in ARDS is improvement in blood oxygenation. Although it makes sense that improving the amount of oxygen that is carried by the blood, and potentially delivered to tissues, would be a desirable goal in patients, there are major problems in using this as a surrogate endpoint. Multiple clinical studies have shown an improvement in oxygenation without a mortality benefit. Furthermore, the patients in the low tidal volume group in the ARDSNet lung protective ventilator strategy study had lower mortality and worse oxygenation than patients in the control arm (The Acute Respiratory Distress Syndrome Network, 2000). In small animals, it is not feasible to use oxygenation as an endpoint since the degree of hypoxia required to meet the definition of ARDS is not sustainable in these animals while they are breathing room air. An example of a surrogate endpoint in animal models that has not translated into successful therapies for ARDS is lung inflammatory cell influx. This is a major feature of human ARDS (Fowler et al., 1987) and is also seen in many animal models of lung injury, including intratracheal LPS. The presumption is that lung inflammation is detrimental and that reducing lung inflammation will improve outcomes in ARDS. In one study, a single dose of dexamethasone in a mouse model of LPS inhalation significantly reduced lung neutrophil accumulation as well as the levels of lung pro-inflammatory cytokines (Rocksen et al., 2000); both would seem to be beneficial in ARDS. However, steroids given to patients with ARDS in a randomized, blinded trial had no beneficial effects (Bernard et al., 1987). In the animal model of lung injury, lung inflammatory cell influx and cytokine production was used as a surrogate for clinical improvement in lung injury, but this proved to be an endpoint that may or may not be important in human disease. How do we fix this problem? How can we develop sound animal models with robust outcome measures when we do not even know the factors that are important in the human disease? The answer, in part, may be to develop better biomarkers for development and outcomes from ARDS in humans that can then be translated back to animal models. To date, no biomarkers of inflammation or injury to the lungs are used as diagnostic tests in ARDS, but several have been shown to correlate with outcomes in ARDS (Calfee et al., 2008b; Calfee et al., 2008a; McClintock et al., 2008). Perhaps some of the difficulty with appropriate outcome measures in animal models of ARDS will be solved, in part, by addressing the other major limitations. If we have more ideal lung injury models that accurately recapitulate all aspects of the human disorder, then perhaps we can use the same endpoints that are of interest in people: mortality, time to extubation and prevention of other organ failures.
Clinical and basic research opportunities.
Creating a small animal ICU-type condition to provide prolonged respiratory and hemodynamic support
Creating ARDS models that replicate coagulation and fibrinolysis abnormalities
Defining surrogate endpoints that are predictive of ARDS outcomes
Identifying biomarkers for ARDS
ARDS: future research directions
Despite the challenges posed in the study of ARDS, there have been advances in the understanding of lung injury pathogenesis and in the treatment of ARDS patients. To continue to improve the outlook for patients with ARDS, advancement in disease modeling is a necessity. Improvements in disease modeling should take into account the key pathophysiological features described here and may require the use of multiple modalities (for example, LPS in addition to mechanical ventilation) in an effort to recapitulate multiple features of acute lung injury. Better supportive care for animals during the course of lung injury would be an important advance, particularly in the development of these complex, combined injury models. In addition, focusing on previously underappreciated biological pathways, such as coagulation and fibrinolysis, may aid in modeling this complex syndrome.
The successful identification of surrogate endpoints or biomarkers that are predictive of outcome and that can be used in human and animal studies of ARDS will probably require increased collaboration between clinical and basic researchers. There is no question that a crucial factor in scientific advancement is the development of robust animal models that can help to define disease states and aid in the development of successful therapies. Optimal use of these models, however, requires active and ongoing communication between basic and clinical researchers. ARDS animal models pose unique challenges, as discussed above, but as new tools become available and novel concepts are incorporated into these models, real progress can be made, making this an extremely exciting area of scientific inquiry.
Acknowledgments
Work in the authors’ lab was supported by BIRCWH and NIH/NHLBI grants to J.A.B.; and by RO1 grants, and the Department of Veterans Affairs, to T.S.B. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Amigoni M., Bellani G., Scanziani M., Masson S., Bertoli E., Radaelli E., Patroniti N., Di Lelio A., Pesenti A., Latini R. (2008). Lung injury and recovery in a murine model of unilateral acid aspiration: functional, biochemical, and morphologic characterization. Anesthesiology 108, 1037–1046 [DOI] [PubMed] [Google Scholar]
- Ashbaugh D.G., Petty T.L. (1972). Sepsis complicating the acute respiratory distress syndrome. Surg. Gynecol. Obstet. 135, 865–869 [PubMed] [Google Scholar]
- Ashbaugh D.G., Bigelow D.B., Petty T.L., Levine B.E. (1967). Acute respiratory distress in adults. Lancet 2, 319–323 [DOI] [PubMed] [Google Scholar]
- Ashbaugh D.G., Petty T.L., Bigelow D.B., Harris T.M. (1969). Continuous positive-pressure breathing (CPPB) in adult respiratory distress syndrome. J. Thorac. Cardiovasc. Surg. 57, 31–41 [PubMed] [Google Scholar]
- Bachofen M., Weibel E.R. (1982). Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin. Chest Med. 3, 35–56 [PubMed] [Google Scholar]
- Bastarache J., Wang L., Geiser T., Wang Z., Albertine K., Matthay M., Ware L. (2007). The Alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax 62, 608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard G.R., Luce J.M., Sprung C.L., Rinaldo J.E., Tate R.M., Sibbald W.J., Kariman K., Higgins S., Bradley R., Metz C.A., et al. (1987). High-dose corticosteroids in patients with the adult respiratory distress syndrome. N. Engl. J. Med. 317, 1565–1570 [DOI] [PubMed] [Google Scholar]
- Bernard G.R., Artigas A., Brigham K.L., Carlet J., Falke K., Hudson L., Lamy M., Legall J.R., Morris A., Spragg R. (1994). The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 149, 818–824 [DOI] [PubMed] [Google Scholar]
- Bernard G.R., Vincent J.L., Laterre P.F., LaRosa S.P., Dhainaut J.F., Lopez-Rodriguez A., Steingrub J.S., Garber G.E., Helterbrand J.D., Ely E.W., et al. (2001). Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344, 699–709 [DOI] [PubMed] [Google Scholar]
- Calfee C.S., Ware L.B., Eisner M.D., Parsons P.E., Thompson B.T., Wickersham N., Matthay M.A. (2008a). Plasma receptor for advanced glycation end-products and clinical outcomes in acute lung injury. Thorax 63, 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee C.S., Eisner M.D., Parsons P.E., Thompson B.T., Conner E.R., Jr, Matthay M.A., Ware L.B. (2008b). Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 35, 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D.S., Han W., Chen S.M., Sherrill T.P., Chont M., Park G.Y., Sheller J.R., Polosukhin V.V., Christman J.W., Yull F.E., et al. (2007). Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J. Immunol. 178, 6504–6513 [DOI] [PubMed] [Google Scholar]
- de Hemptinne Q., Remmelink M., Brimioulle S., Salmon I., Vincent J.L. (2008). ARDS: a Clinicopathologic Confrontation. Chest December 31 [Epub ahead of print] [doi:10.1378/chest.08-1741]. [DOI] [PubMed] [Google Scholar]
- Doyle I.R., Bersten A.D., Nicholas T.E. (1997). Surfactant proteins-A and -B are elevated in plasma of patients with acute respiratory failure. Am. J. Respir. Crit. Care Med. 156, 1217–1229 [DOI] [PubMed] [Google Scholar]
- Everhart M.B., Han W., Sherrill T.P., Arutiunov M., Polosukhin V.V., Burke J.R., Sadikot R.T., Christman J.W., Yull F.E., Blackwell T.S. (2006). Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J. Immunol. 176, 4995–5005 [DOI] [PubMed] [Google Scholar]
- Fowler A.A., Hyers T.M., Fisher B.J., Bechard D.E., Centor R.M., Webster R.O. (1987). The adult respiratory distress syndrome. Cell populations and soluble mediators in the air spaces of patients at high risk. Am. Rev. Respir. Dis. 136, 1225–1231 [DOI] [PubMed] [Google Scholar]
- Fujishima S., Morisaki H., Ishizaka A., Kotake Y., Miyaki M., Yoh K., Sekine K., Sasaki J., Tasaka S., Hasegawa N., et al. (2008). Neutrophil elastase and systemic inflammatory response syndrome in the initiation and development of acute lung injury among critically ill patients. Biomed. Pharmacother. 62, 333–338 [DOI] [PubMed] [Google Scholar]
- Hudson L.D., Milberg J.A., Anardi D., Maunder R.J. (1995). Clinical risks for development of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 151, 293–301 [DOI] [PubMed] [Google Scholar]
- Idell S. (2003). Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit. Care Med. 31, S213–S220 [DOI] [PubMed] [Google Scholar]
- Idell S., Gonzalez K., Bradford H., MacArthur C.K., Fein A.M., Maunder R.J., Garcia J.G., Griffith D.E., Weiland J., Martin T.R., et al. (1987). Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Contribution of tissue factor associated with factor VII. Am. Rev. Respir. Dis. 136, 1466–1474 [DOI] [PubMed] [Google Scholar]
- Idell S., James K.K., Levin E.G., Schwartz B.S., Manchanda N., Maunder R.J., Martin T.R., McLarty J., Fair D.S. (1989). Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J. Clin. Invest. 84, 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S., Koenig K.B., Fair D.S., Martin T.R., McLarty J., Maunder R.J. (1991). Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am. J. Physiol. 261, L240–L248 [DOI] [PubMed] [Google Scholar]
- Lamb N.J., Gutteridge J.M., Baker C., Evans T.W., Quinlan G.J. (1999). Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit. Care Med. 27, 1738–1744 [DOI] [PubMed] [Google Scholar]
- Matute-Bello G., Frevert C.W., Martin T.R. (2008). Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L379–L399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D., Zhuo H., Wickersham N., Matthay M.A., Ware L.B. (2008). Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit. Care 12, R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W.Y., Goodman R.B., Steinberg K.P., Ruzinski J.T., Radella F., 2nd, Park D.R., Pugin J., Skerrett S.J., Hudson L.D., Martin T.R. (2001). Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am. J Respir Crit Care Med. 164, 1896–1903 [DOI] [PubMed] [Google Scholar]
- Prabhakaran P., Ware L.B., White K.E., Cross M.T., Matthay M.A., Olman M.A. (2003). Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 285, L20–L28 [DOI] [PubMed] [Google Scholar]
- Riede U.N., Joachim H., Hassenstein J., Costabel U., Sandritter W., Augustin P., Mittermayer C. (1978). The pulmonary air-blood barrier of human shock lungs: a clinical, ultrastructural and morphometric study. Pathol Res Pract. 162, 41–72 [DOI] [PubMed] [Google Scholar]
- Rocksen D., Lilliehook B., Larsson R., Johansson T., Bucht A. (2000). Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 122, 249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. (2005). Incidence and outcomes of acute lung injury. N Engl J Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrome Network (2000). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 342, 1301–1308 [DOI] [PubMed] [Google Scholar]
- Treggiari M.M., Hudson L.D., Martin D.P., Weiss N.S., Caldwell E., Rubenfeld G. (2004). Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med. 32, 327–331 [DOI] [PubMed] [Google Scholar]
- Uchida T., Shirasawa M., Ware L.B., Kojima K., Hata Y., Makita K., Mednick G., Matthay Z.A., Matthay M.A. (2006). Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 173, 1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Angus D.C., Artigas A., Kalil A., Basson B.R., Jamal H.H., Johnson G., 3rd, Bernard G.R. (2003). Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med. 31, 834–840 [DOI] [PubMed] [Google Scholar]
- Wang J., Oppenheimer L., Fata P., Pintin J., Stimpson R., Mantsch H.H. (1999). Spectroscopic approach to capillary-alveolar membrane damage induced acute lung injury. Can Respir J. 6, 499–506 [DOI] [PubMed] [Google Scholar]
- Wang L., Bastarache J.A., Wickersham N., Fang X., Matthay M.A., Ware L.B. (2006). Novel role of the human alveolar epithelium in regulating intra-alveolar coagulation. Am J Respir Cell Mol Biol. 36, 497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann H.P., Wheeler A.P., Bernard G.R., Thompson B.T., Hayden D., deBoisblanc B., Connors A.F., Jr, Hite R.D., Harabin A.L. (2006). Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 354, 2564–2575 [DOI] [PubMed] [Google Scholar]
- Wilson M.R., Choudhury S., Goddard M.E., O’Dea K.P., Nicholson A.G., Takata M. (2003). High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol. 95, 1385–1393 [DOI] [PubMed] [Google Scholar]
- Wolthuis E.K., Vlaar A.P., Choi G., Roelofs J.J., Juffermans N.P., Schultz M.J. (2009). Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care Med. 13, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Singbartl K., Ley K. (2006). Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 116, 3211–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]