Abstract
Microarray gene expression profiling has been used to distinguish histological subtypes of renal cell carcinoma (RCC), and consequently to identify specific tumor markers. The analytical procedures currently in use find sets of genes whose average differential expression across the two categories differ significantly. In general each of the markers thus identified does not distinguish tumor from normal with 100% accuracy, although the group as a whole might be able to do so. For the purpose of developing a widely used economically viable diagnostic signature, however, large groups of genes are not likely to be useful. Here we use two different methods, one a support vector machine variant, and the other an exhaustive search, to reanalyze data previously generated in our Lab (Lenburg et al. 2003). We identify 158 genes, each having an expression level that is higher (lower) in every tumor sample than in any normal sample, and each having a minimum differential expression across the two categories at a significance of 0.01. The set is highly enriched in cancer related genes (p = 1.6 × 10−12), containing 43 genes previously associated with either RCC or other types of cancer. Many of the biomarkers appear to be associated with the central alterations known to be required for cancer transformation. These include the oncogenes JAZF1, AXL, ABL2; tumor suppressors RASD1, PTPRO, TFAP2A, CDKN1C; and genes involved in proteolysis or cell-adhesion such as WASF2, and PAPPA.
Keywords: Cancer diagnosis, biomarker identification, microarray analysis, Renal cell carcinoma
1. Introduction
Renal-cell carcinoma (RCC) is the most common kidney neoplasm, comprising 3% of all adult malignancies (Jemal et al. 2003). Its incidence has increased steadily over the past 20 years in the United States and Europe; 35,000 new cases and 12,000 deaths now occur annually in the United States alone. Histopathologically, about 60–70% of RCC is clear-cell type (cc-RCC). Small and localized tumors are generally asymptomatic; pain, flank mass, or hematuria, being generally associated with locally advanced or metastatic tumors. Diagnosis is confirmed by imaging, including X-ray and computed-tomography. The 5-year survival rate of metastatic RCC is less than 10%. Moreover, RCC is one of the most therapy-resistant carcinomas, responding very poorly or not at all to radiotherapy, hormonal therapy, and chemotherapy. All these facts emphasize the importance of developing early diagnostic markers.
Microarray gene expression profiling has been used by ourselves (Lenburg et al. 2003) and others (Young et al. 2001; Boer et al. 2001; Gieseg et al. 2002; Young et al. 2003; Yamazaki et al. 2003; Lenburg et al. 2003; Higgins et al. 2003; Takahashi et al. 2003; Sultmann et al. 2005; Jones et al. 2005) to distinguish the various histological subtypes of RCC, and consequently to identify novel tumor markers. The general procedure identifies markers in accordance with average differential expression level (fold change) and/or some level of significance as measured by the t-test. Lenburg et al. used a 3-fold difference in expression and a level of significance of 0.03.
Here, we reanalyze the data of Lenburg et al. using a rigorous exhaustive search approach (Dalgin and DeLisi, 2005), and a more general, but approximate, approach based on support vector machines. We identify, by exhaustive search, 158 genes each of which (i) is consistently over- or under-expressed in all tumors and (ii) has a minimum expression level difference at better than 99% confidence. Sixty four of these markers were not identified previously (Lenburg et al. 2003). The set is highly enriched in cancer related genes (p = 1.6 × 10−12), containing 43 previously associated with either RCC or other types of cancer.
Among the set of genes that we identify as being related to RCC, some were known from previous studies (e.g. ATP6V1B1, EGLN3, SLC25A5, TUBB, ALDOA); others had never before been associated with RCC, but have been identified with other cancers (e.g. ABL2, JAZF1, TFAP2A). We identified biological roles of marker genes, and found pathways that are dominantly up-regulated (83% of immune response genes, all amino acid transport genes) or down-regulated (all cation and anion transport genes, all OXPHOS genes) which are related to kidney function (cation/anion transport genes) and RCC physiology (OXPHOS). Finally we constructed a model for RCC through functional classification of genes related to changes in cellular processes that are critical to initiation and progression of cancer.
2. Methods
2.0. Background
Briefly, (Lenburg et al. 2003) hybridized total RNA isolated from 9 clear-cell renal tumors and adjacent normal tissue (18 samples) to Affymetrix U133A and U133B arrays containing approximately 45,000 probe-sets. Of these, 27,609, representing 20,192 unigenes, gave a signal above background. Differentially expressed genes were identified by t-test and fold change. The average fold change was calculated as log2 (C/N) where C and N represent the average of tumor and normal expression values, respectively. Some 1706 probe-sets (1471 unique genes) were more than three-fold changed in renal tumors and had a p-value <0.03. Of these, 113 had been previously identified in three or more studies (Young et al. 2001; Boer et al. 2001; Takahashi et al. 2001; Gieseg et al. 2002). An obvious limitation of drawing conclusions from such a study is the small number of samples per category with a relatively large number of potential markers. We discuss this in detail below.
2.1. Identifying single gene biomarkers by exhaustive search
Here we identify single genes that correctly classify every sample, by direct comparison of differential expression in every tumor-normal pair (92 comparisons per gene × 20,192 genes). A gene whose level of expression in every normal sample is always either greater or less than its level in every tumor sample, is ranked according to the smallest expression level distance across the two categories (Perl script is available at http://visant.bu.edu/skirca/script.pl). The smallest separation for a down (up)-regulated gene is the difference between the maximum (minimum) expression level in the tumor samples, and the minimum (maximum) expression level in the normal samples. In particular define Em,i as the expression level of the ith gene in the mth tumor sample and let En,i be similarly defined for the normal samples. The minimum distance for the ith gene is defined as
| (1) |
provided all differences have the same sign, where m and n range independently over all samples.
We identified 478 probes, corresponding to 466 unique genes, that separate all tumor from all normal tissue. Each gene was then tested for significance as follows.
For a given gene we randomly selected 9 tumor expression values from all tumor expression values (20,192 genes × 9 tumor samples = 181,728 expression values), and 9 normal values from all normal expression values, subject to the constraint that all tumor values are greater (less) than all normal values. We calculated the minimum distance and repeated the procedure 500 times (to mimic random selection of 466 genes) to obtain a distribution of minimum distances (Figure 1(a)). The procedure was repeated 200 times to estimate the dispersion in the parameters of the sampling distribution. Overall, we obtained skew minimum distance distributions with an average mean minimum distance 0.0425 (standard deviation over 200 simulations = 0.00261) and average standard deviation 0.05768 (standard deviation over 200 simulations = 0.00531) across all simulations. The p-value for each actual minimum distance (d) was calculated using the total random data (500 × 200 = 105) as probability of finding ≥d randomly (P(drandom ≥d). Of the initial set of 466 genes 158 were found to have significant minimum distances >0.28 with p-value ≤0.01 (Figure 1(b)). We refer to these as significant single gene biomarkers or simply markers.
Figure 1.
(a) The minimum distances of randomly formed expression profiles in four simulations are shown as representative of other simulations. The x-axis is the minimum distance and y-axis is the number of genes having that distance. (b) The distribution of minimum distances for 466 genes. 158 of these genes have minimum distances with p-values ≤0.01, hence identified as significant single gene biomarkers.
2.2. SVM Recursive feature elimination
An alternative strategy for selecting markers is to use statistical feature selection techniques to rank genes. This can be done in a number of ways. Here we use a support vector machine (SVM) (Vapnik, 1998). which has been used effectively in other contexts (Holloway et al. 2005; Holloway et al. 2006a; Holloway et al. 2006b), and which has a well established statistical framework. The idea is again to find genes whose distance between tumor/normal sample expression values is in some sense maximum. Guyon et al. (Guyon et al. 2002) used a similar method to identify genes that stratify leukemia (Golub et al. 1999), and for distinguishing between colon cancer and normal tissue (Alon et al. 1999), by dividing the data sets into two equal halves for training and testing. They recursively discarded the features (genes) with smallest weight (see below for detailed discussion) to select genes that separate the two classes at a specified accuracy. For leukemia they discovered 2 genes that yield zero leave-one-out error. For colon cancer, they obtained 98% accuracy using 4 genes. The main difference here is the introduction of a procedure that does not assume the highest ranked gene is necessarily more useful than genes that rank slightly lower.
An SVM is an effective method for making predictions on many types of data including handwritten text, protein sequence, DNA sequence, and microarray profiles, when the number of data attributes for each sample is very large. Briefly, the method seeks to find a maximal separation between two training sets (Sholkopf and Smola, 2002), a positive set, in this case tumor samples; and a negative set, normal samples. Each sample is labeled by a set of attributes, here gene expression levels, and hence can be represented by a vector that can have tens of thousands of components. The separation between positive and negative vectors (samples) is achieved through an optimization which finds a hyperplane bisecting the two sets. The hyperplane must be as distant as possible from the two sets thus creating a maximal-margin separator (Holloway et al. 2005).
Most of the attributes are irrelevant to separation. The SVM algorithm can be used to rank the importance of the various attributes by a method referred to as recursive feature elimination (RFE), and thus identify those genes that are most discriminatory. An important SVM output is a vector of learned weights; each component of the vector being a weight of an attribute: the higher the weight the more useful in separating positives from negatives. The original SVM-RFE algorithm trains an SVM, calculates the weight of each attribute, and discards a specified number of low weight attributes (Guyon et al. 2002). The process is repeated until the desired number of attributes remains. Typically half the attributes are removed at each iteration until some threshold is reached after which only one at a time is removed.
The procedure employed here is different in that the entire SVM-RFE ranking is performed many times within a leave-one-out cross validation. Briefly, the procedure is as follows. Prior to applying the SVM, we use a t-test with a loose p-value threshold of 0.1 to filter the large majority of statistically irrelevant genes, leaving 10,479 genes for further analysis. We then perform SVM-RFE on n-1 samples, save the results, and repeat n times. Thus for the renal cancer dataset with n = 18 samples we ranked the performance of each gene 18 times (18 cross-validations). Intuitively, genes that are repeatedly ranked near the top are robust to changes in the training set and are considered more reliable. The fluctuation of gene choice during cross validation can be seen more clearly when examining a list showing the highest ranked gene on each training set of the leave-one-out procedure. On the 18 possible training sets, 12 different genes were given a rank of 1 at least once (data not shown). Because gene rank can change markedly with dataset, choosing consistent genes increases the chances that the chosen genes are truly reliable markers. This highlights the need to choose genes that are consistent across samples, as opposed to genes whose average value differs significantly across the two categories.
To derive our final gene ranking, we arbitrarily selected the top 20 genes from each ranking and counted the number of times each gene appears in this combined set. The maximum occurrence of 18 would indicate a very stable biomarker; i.e. one that was chosen by each training set. The final list represents genes that are stable across sample sets and can thus be considered reliable biomarkers.
3. Results
3.1. Single gene markers
Pathway/process enrichment
Of the 158 markers, 73 are annotated in a KEGG pathway and another 42 are in a GO process at level 5 or higher. The pathways/processes with the highest number of classifiers are shown in Table 1. The categories are biologically plausible, having already been implicated in cancer transformation (e.g. OXPHOS, apoptosis, cell adhesion, MAPK signaling) or being potentially important (calcium signaling pathway, fatty acid metabolism and cation transport) in transformation.
Table 1.
Top ranked pathways with percentage of significant classifier genes.
| Pathway | Number of classifier genes | Number of classifier genes/number of genes in the pathway (%) |
|---|---|---|
| Glycolysis | 5 | 12.5 |
| Antigen processing | 4 | 11.76 |
| Oxidative phosphorylation | 5 | 3.36 |
| Calcium signaling pathway | 4 | 3.13 |
| G-Protein coupled signaling | 4 | 2.99 |
| MAPK signaling pathway | 4 | 2.06 |
| Immune response | 14 | 1.71 |
| Fatty acid metabolism | 4 | 1.53 |
| Cation transport | 6 | 1.19 |
| Apoptosis | 6 | 0.98 |
| Intracellular transport | 6 | 0.95 |
| Regulation of transcription | 12 | 0.58 |
| Cell adhesion | 4 | 0.56 |
Marker Significance
We (Lenburg et al. 2003) previously identified 1471 genes that were more than three-fold changed in renal tumors with a p-value <0.03. The relationship between minimum distance determined by exhaustive search, and the average fold change, is shown in Figure 2. Of the 158 markers (indicated in red) 94 (59%) have changed at least 3 fold in tumor with a t-test p-value <0.03, and hence overlap with the 1471 genes identified by (Lenburg et al. 2003).
Figure 2.
The relationship between minimum distance and average fold change (log2(C/N)). Average fold change was previously calculated by Lenburg et al. and the significance was found by t-test. Here, C and N denotes the average expression values in tumor and normal samples, respectively. Significant markers (p-value ≤0.01, 158 genes) are indicated as red. 64 genes are shown in between the vertical arrows. These genes have an average fold change less than 3 hence were not identified as previously differentially expressed. Yet, these genes have been identified as new potential biomarkers by the current algorithm.
There are at least two reasons to expect that the entire set of markers, including the 64 not previously identified, are likely to be useful signatures. The first, as indicated above, is that they are not only uniformly over or under-expressed, but the magnitude of the minimum differential expression is statistically significant, taking into account of the small sample size (see Section 3.3. for further analysis). Second, the set (158) and subset (64) are both highly enriched in cancer associated genes. In particular, of the 158 markers, 43 (27.2%) have been previously reported to be cancer associated as indicated in the Genetic association database or OMIM. In addition, 15 of the 64 are found to be cancer associated (23.4%). In comparison with the OMIM database, which includes 1351 cancer related genes (8.1%) out of 16603 total genes, 158 classifier genes are enriched 3.3 times (Fisher’s exact test p-value = 1.6 × 10−12) with the cancer related genes; and 64 genes are enriched 2.9 times with p = 1. 6 × 10−4. Both results indicate that the enrichment of 158 genes and 64 genes with cancer genes is significant with respect to OMIM.
We also performed simulations by randomly selecting 158 genes from OMIM, and recorded the percentage of cancer associated genes. We tossed 1000 times, obtained a random distribution, and repeated the entire procedure 10 times to estimate the dispersion of the parameters of the 10 distributions. We obtained an average of 6.8% cancer related genes with a standard deviation 1.9 (The dispersion of both parameters is less than 2%). The actual percentage for 158 genes, 27.2%, is 10.7 standard deviations away from the random mean. We performed the same simulations by drawing randomly 64 genes, which yielded an average of 6.85% cancer related genes with a standard deviation 3.1. Hence, the actual percentage for 64 genes (23.2%) is 5.3 standard deviations away from the random mean. These results confirm that the percentage of cancer related marker genes are significantly different from what would be obtained randomly. Of the genes identified previously (Lenburg et al. 2003) 220 (16%) are cancer associated; i.e. the 64 new genes are enriched nearly 50% more than the original set.
3.2. Identifying biomarkers by SVM
The number of occurrences of the genes across the 18 top 20 rankings is given as Supplementary Figure 1. Each gene is shown by its rank (the highest ranked gene, i.e. gene #1, occurs in the top 20, 17 out of 18 times; the number 2 ranked genes has 16 occurrences, the genes ranked 3 and 4 have 14 occurrences etc). Examination of the genes most frequently ranked in the top 20 reveals that they can all individually distinguish tumor from normal tissue with no error. More importantly, these are the genes that are most stable over different choices of training set (18 training sets each lacks one sample), suggesting that they would be the most likely to make accurate predictions in unseen tissue samples.
Genes ranked in the top 20 (Table 2) were all identified as classifiers by the exhaustive search (Section 2.1), 17 with a p-value ≤0.01; i.e. 7/20 (85%) are in our set of 158 markers. Twelve of the genes were previously identified by Lenburg et al., two genes by four or more other RCC studies as well. HUGO (The Human Genome Organization) gene symbol (http://www.gene.ucl.ac.uk/nomenclature/) is used to represent the genes in Table 2 and throughout the paper.
Table 2.
Top 20 ranked genes by SVM and their significance as classifier by exhaustive search.
| SVM rank | Gene | p-value (min dist) | Min dist rank | Related disease |
|---|---|---|---|---|
| 1 | ALDOB** | 6.00E-05 | 23 | RCC |
| 2 | NDUFA4 | 2.00E-05 | 8 | |
| 3 | TFAP2B* | 0 | 1 | tumor suppressor candidate, melanoma |
| 4 | TCTE1L | 0.00275 | 93 | |
| 5 | LOC57821 | 2.00E-05 | 11 | |
| 6 | GABARAPL3*† | 0.02892 | 239 | |
| 7 | SLC38A1* | 7.00E-05 | 27 | |
| 8 | POLDIP2 | 0.00456 | 124 | |
| 9 | DACH1 | 0.00033 | 43 | |
| 10 | GABARAPL1** | 2.00E-05 | 9 | RCC |
| 11 | HLA-DPA1* | 5.00E-05 | 16 | Melanoma |
| 12 | ERBB4* | 0 | 2 | Breast, ovarian cancer |
| 13 | HIG1 | 0.00362 | 108 | hypoxia induced |
| 14 | EHD2* | 0.00169 | 74 | |
| 15 | CD81 | 0.00013 | 38 | Hepatoma |
| 16 | PRG-3* | 0 | 3 | |
| 17 | NPHS1* | 6.00E-05 | 19 | Non cancerous kidney diseases |
| 18 | C1QA* | 0.00379 | 112 | |
| 19 | ZNF697† | 0.01543 | 191 | |
| 20 | PIGR*† | 0.38576 | 432 |
†: minimum distance p-value >0.01
identified by four or more RCC studies (Young et al. 2001; Boer et al. 2001; Takahashi et al. 2001; Gieseg et al. 2002; Lenburg et al. 2003)
identified by Lenburg et al.
Of the 20 genes, 7 had not been previously identified as RCC related, of those 5 are identified by both methods, and 2 of the five are implicated in other cancers.
3.3. Small sample size
Although we confined ourselves to perfect separators with minimum separation distances that are highly significant, human polymorphism makes it unlikely that perfect separation will continue to hold as the population size increases. In an effort to gain some insight into this effect we analyzed a breast cancer data set (Ma et al. 2003), which includes 32 normal samples and 53 breast cancer samples (30 ductal carcinoma in situ and 23 invasive ductal carcinoma samples). Raw data is available for 1940 genes which were found to be differentially expressed between normal and cancer stages by linear discriminant analysis (Ma et al. 2003).
We drew random groups of 18 (9 normal, 9 tumor) and repeated the analysis of 2.1. The significant markers were then tested on the remaining samples. The entire procedure was repeated 100 times. Denoting tumor samples as positives and normal samples as negatives, the following performance measures were used for the classifier genes: sensitivity = TP/(TP+FN); specificity = TN/(TN+FP) and positive predictive value (PPV) = TP/(TP+FP) where TP stands for true positives, FP for false positives, TN for true negatives and FN for false negatives.
We first compared the performance of the classifier genes that have significantly high minimum distances (p-value ≤0.05) with all classifiers (Table 3). The former group performs only slightly better (The simulations performed on different initial sample sizes confirmed this conclusion, data not shown) probably because the starting set (1940 genes) in breast cancer is pre-selected by discriminant analysis. The results suggest that the markers inferred using sample numbers comparable to RCC would still provide a very high degree of separation, even when more samples are used.
Table 3.
Performance of classifiers on the test samples in breast cancer dataset with 18 initial samples.
| Classifiers with p-value ≤0.05 | All classifiers | |
|---|---|---|
| % correctly classified samples | 88% | 82% |
| % misclassified samples | 11% | 18% |
| Sensitivity | 0.87 | 0.8 |
| Specificity | 0.92 | 0.85 |
| PPV | 0.96 | 0.91 |
More direct testing was carried out by selecting different initial number of samples to compare the performance of the method with 18 samples, to other samples sizes, ranging from 8 (4 normal, 4 tumor) to 24 samples (12 normal, 12 tumor). In each case, we recorded the number of classifiers, number of significant classifiers (p-value ≤0.05) and the performance of the significant classifiers on the test samples (samples not selected initially to identify classifier genes). The number of significant genes were projected (Supplementary Figure 2(a)) based on the results of breast cancer simulations (inset). Overall, the number of classifiers and significant classifiers decreases as the number of samples increases. The performance of significant breast cancer markers (p ≤ 0.05) is shown in (b) and (c). Percentage of correctly classified samples increases with the sample size, having a plateau near 18 samples (b). The values for percentage of misclassified samples and unclassified samples obtained for 18 samples are very close to >20 samples, but much better with respect to lower sample sizes. Sensitivity increases with the sample size, but specificity and PPV of the significant classifiers are independent of the number of samples. Hence, in all cases, the significant classifiers perform well on the test samples in terms of sensitivity and PPV irrespective of how many samples were used to select those classifiers. Overall, the performance of classifiers obtained with 18 samples is better than obtained with smaller number of samples (12, 14 or 16) and almost the same with larger number of samples (≥20 samples).
3.4. RCC substructure
We organized the markers into genetic networks using hierarchical clustering (HCL) and principal component analysis (PCA). HCL gives the distances between nodes (samples or genes) and reveals the substructure within nodes; PCA identifies the primary axes upon which the samples vary and how the samples are distributed along these axes based on the similarity between their expression profiles.
3.4.1. HCL
Markers and samples were clustered by average-linkage hierarchical clustering (Figure 3(a)). Each marker is represented as a vector of normalized expression values across all samples and Euclidean distance between vectors was computed. An analogous procedure was used for samples. The distance between two clusters is defined as the distance between pairs of nodes, averaged over all pairs, each pair consisting of one node from each group. At each stage of clustering, the two clusters for which the distance is minimum, are merged. In the resulting dendrograms for samples (top) and genes (left), the height of vertical/horizontal lines are proportional to the degree of similarity between samples/genes.
Figure 3.
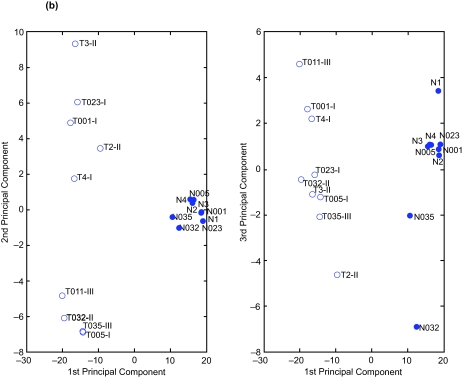
(a) Clustering of samples and 158 significant markers using hierarchical clustering. The expression values are normalized for each gene by dividing every expression value to the mean of normal values for that gene and then transforming those values to logarithmic values (log2) to emphasize up or down-regulation with respect to normal expression values. Black represents the mean of normal values, green represents down-regulation and red represents up-regulation with respect to the mean. Clustering of genes reveals two big clusters: down-regulated genes in RCC (upper half) and up-regulated in RCC (bottom half) with respect to the normal samples, together with subclusters within these groups. The markers cluster the samples perfectly well into two major groups (Fig 3a, upper dendrogram). It is clear that tumor samples are separated into two major sub-clusters according to the grade. The only exception is T005 sample (grade I) which clusters with high grade samples. Within the normal samples, N032 and N035 have expression profiles most similar to tumor samples. (b) The projection of the samples onto the first three principal components (PC). The eigenvectors of the first three eigenvalues accounted for 86.9% of the variation (81.5, 5.4 and 2.2, respectively) in the data. Tumor samples are represented by open circles; normal samples are shown by filled circles. First principle component separates normal samples from tumor samples while second principle component separates tumor samples with low grade (T3, T023, T001, T2 and T4) from high grade (T011, T032, T035) again with the exception of T005 sample. Third principal component separates normal samples N032 and N035 from the rest of the normal samples as was observed with HCL.
The expression values are normalized for each gene by dividing every expression value to the mean of normal values for that gene and then transforming those values to logarithmic values (log2 transformation) to emphasize up or down-regulation with respect to normal expression values. The expression values are color coded such that red denotes up-regulation with respect to the mean of normal values, green denotes down-regulation and black denotes the mean of normal values. Two big clusters of genes are revealed: down-regulated genes in RCC (upper half) and up-regulated in RCC (bottom half) with respect to the normal samples, together with subclusters within these groups. We didn’t examine these subclusters, but instead analyzed the expression profiles of groups of genes that are in the same pathway in Section 4.1. The markers cluster the samples well into two major groups (Fig. 3a, upper dendrogram). It is clear that tumor samples are separated into two major sub-clusters according to the grade. The only exception is T005 sample (grade I) which clusters with high grade samples for which we cannot provide a satisfactory biological explanation. Within the normal samples, N032 and N035 have expression profiles most similar to tumor samples. Clustering the data with PCA supports these observations as we now explain.
3.4.2. PCA
PCA was applied to the 18 row × 158 column matrix of expression values, normalized as explained above. The eigenvectors of the first three eigenvalues accounted for 86.9% of the variation (81.5, 5.4 and 2.2, respectively) in the data. Figure 3 (b) shows the projection of the samples onto these first three principal components (PCs). The first PC separates normal samples from tumor samples while second PC separates tumor samples with low grade (T3, T023, T001, T2 and T4) from those of high grade (T011, T032, T035) with the exception of T005 sample, as observed with HCL. The third principal component separates normal samples N032 and N035 from the other normal samples. Two of the tumor samples, T3 and T2, are separated from other tumors along the second and third axes, respectively. Both samples are grade II tumors. As expected, PCA suggests that there is more variation within tumor samples than within normal samples, which points to distinct tumor subgroups, reflecting the heterogeneity in cancer phenotype and may have implications for disease progression and response to different therapies.
The separation of N032 and N035 from other normal samples was noted previously (Lenburg et al. 2003) by clustering samples using all 20,192 genes. They observed that N035 clustered with tumor samples; however, we find that it clusters with normal samples as it should. The difference in results reflects our use of markers for clustering. The N032 and N035 profiles are more similar, than other normals, to tumor sample profiles (Figure 3(a)), but still they are more similar to normals than to tumor. Both samples are from RCC patients with grade III tumors, hence there is a possibility that these samples, which were classified as normal by standard histology, are actually a mixture of normal and cancerous tissue.
4. Discussion
4.1. Pathway associations of markers
Biological roles of 158 markers (Supplementary Table 1) were determined using DAVID (Dennis et al. 2003) and MatchMiner (Bussey et al. 2003) databases. 115 (73%) of the markers (Table 4) are annotated in a KEGG pathway or a GO process at level 5 or higher. Genes that have been identified as having noteworthy differential gene expression in four or more RCC studies (Young et al. 2001; Boer et al. 2001; Takahashi et al. 2001; Gieseg et al. 2002; Lenburg et al. 2003) are indicated with **. The 64 genes implicated in RCC in this study that were not reported by Lenburg et al. are indicated in italic.
Table 4.
Biological roles (from KEGG and GO databases) and disease associations of 115 annotated gene markers.
| Down-regulated genes | |||
|---|---|---|---|
| Gene | Related disease | Weinberg category | Pathway |
| DUSP9 | MAPK Signaling/JNK cascade | ||
| FGF9 | Prostate, ovarian cancer | Self-sufficiency in growth signals | MAPK Signaling/Regulation of actin cytoskeleton/growth factor |
| ITPR2 | Tissue invasion and metastasis | Calcium Signaling Pathway/Gap junction | |
| ERBB4 | Breast, ovarian cancer | Insensitivity to anti-growth signals | Calcium Signaling Pathway |
| SLC25A5 | RCC | Self-sufficiency (Loss of cancer cell dependence on OXPHOS) | Calcium Signaling Pathway/Intracellular transport |
| DSCR1 | Calcium Signaling Pathway | ||
| PTHR1 | Chronic kidney failure | G-Protein coupled signaling | |
| RASD1 | Suppresses cell growth in human breast cancer and lung cancer cell lines | Insensitivity to anti-growth signals | G-Protein coupled signaling |
| SFRP1** | RCC, bladder cancer, cervical cancer | Evasion of apoptosis | Wnt Signaling Pathway/Apoptosis |
| CHGB | Neuroendocrine tumors | Signaling/hormone | |
| PTPRO | Lung cancer | Insensitivity to anti-growth signals | Signaling/tumor suppressor candidate |
| GABARAPL1** | RCC | Signaling | |
| ATP6V1G3 | Self-sufficiency (Loss of cancer cell dependence on OXPHOS) | Oxidative phosphorylation | |
| ATP6V0A4 | Self-sufficiency (Loss of cancer cell dependence on OXPHOS) | Oxidative phosphorylation | |
| ATP6V1B1** | RCC/renal tubular acidosis | Self-sufficiency (Loss of cancer cell dependence on OXPHOS) | Oxidative phosphorylation |
| KCTD1 | Cation transport | ||
| SCNN1B | Cation transport | ||
| SLC12A3 | Cation transport | ||
| SCN3A | Cation transport | ||
| EHO1 | Cation transport | ||
| RHCG | Cation transport | ||
| SLC4A1 | Anion transport | ||
| SLC12A3 | Anion transport | ||
| SLC22A6 | Anion transport | ||
| COLEC11 | Anion transport/Immune response | ||
| CLIC5 | Anion transport | ||
| MTP | Intracellular transport | ||
| DACH1 | Transcription regulation | ||
| TFAP2B | Char syndrome | Transcription regulation | |
| TFAP2A | Melanoma | Insensitivity to anti-growth signals | Transcription regulation/tumor suppressor candidate |
| VDR | RCC | Insensitivity to anti-growth signals | Transcription regulation |
| EHF | Prostate, breast, and lung carcinomas | Insensitivity to anti-growth signals | Transcriptional repressor |
| LSM3 | RNA splicing | ||
| RCQ5 | DNA repair | ||
| HELAD1 | Up in colorectal cancer | Limitless replicative potential | DNA replication |
| PAPPA | Tissue invasion and metastasis | Proteolysis | |
| DNAJC12 | Protein folding | ||
| CNGLN** | RCC | Tissue invasion and metastasis | Regulation of actin cytoskeleton |
| AIM1 | Melanoma | Insensitivity to anti-growth signals+Tissue invasion and metastases | Cell adhesion/ tumor suppressor candidate |
| NPHS1 | Kidney diseases | Tissue invasion and metastasis | Cell adhesion |
| SPP1 | down-regulated in RCC and intrahepatic cholangiocarcinoma; up-regulated in breast, prostate, colon (and others) carcinomas | Insensitivity to anti-growth signals | Cell adhesion/Apoptosis/Immune response |
| CDKN1C | Breast, pancreatic, thyroid cancer | Insensitivity to anti-growth signals | Cell cycle/tumor suppressor gene |
| UMOD | Immune response | ||
| ADH6** | RCC | Glycolysis/Fatty acid metabolism | |
| ALDOB** | RCC, hepatocellular carcinoma | Glycolysis | |
| G3P2 | Glycolysis | ||
| CYP2B6 | Breast cancer | Fatty acid metabolism | |
| GCDH | Fatty acid metabolism | ||
| CROT | Fatty acid metabolism | ||
| MGC11324 | Membrane lipid metabolism | ||
| BDH | Synthesis and degradation of ketone bodies | ||
| NDUFA4 | Self-sufficiency (Loss of cancer cell dependence on OXPHOS) | Oxidative phosphorylation | |
| NPHS2 | Kidney diseases | Energy metabolism | |
| GCSH | Amino Acid metabolism | ||
| GCH1 | Amino Acid metabolism | ||
| ACPP | Reduces cell growth in prostate cancer upon induction by Vitamin D receptor agonists | Insensitivity to anti-growth signals | Metabolism |
| DHRS6 | Metabolism | ||
| HIG1 | Hypoxia induced gene | ||
| MT1G** | RCC, papillary thyroid carcinoma, prostate cancer | Insensitivity to anti-growth signals | Metallothionein gene/tumor suppressor candidate |
| MT1F | Breast, liver cancer. Suppresses growth of liver cell line HepG2 | Insensitivity to anti-growth signals | Metallothionein gene |
| Up-regulated genes | |||
|---|---|---|---|
| Gene | Related disease | Weinberg category | Pathway |
| CD81 | Hepatoma | Tissue invasion and metastasis | MAPK Signaling/Immune response/Membrane lipid metabolism/Cell adhesion |
| ZAK | Tissue invasion and metastasis | MAPK Signaling/Tight junction/Cell cycle | |
| ADORA3 | ADORA3 agonists inhibit growth in leukemia and breast cancer cell lines | Insensitivity to anti-growth signals | G-Protein coupled signaling/Immune response/Hypoxia induced gene |
| WASF2 | Wiscott-Aldrich syndrome | Tissue invasion and metastasis | G-Protein coupled signaling/Adherens junction |
| NCOR2 | Suppresses antiproliferative targets of VDR in prostate cancer | Insensitivity to anti-growth signals | Notch Signaling Pathway/transcriptional repressor |
| CSH2 | JAK-STAT Signaling Pathway | ||
| LCP2 | Wiscott-Aldrich syndrome | Receptor protein Tyr-kinase Signaling | |
| INHBB** | RCC | Self-sufficiency in growth signals | TGF-β Signaling Pathway /Immune response/growth factor |
| STC2 | Breast cancer | Signaling/hormone/renal and intestinal calcium and phosphate transport | |
| PRDX4 | NF-κB cascade | ||
| PLXND1 | Signaling | ||
| C1QA | Immune response | ||
| C1QG | Immune response | ||
| C1QB** | RCC | Immune response | |
| SLC38A1 | Amino Acid Transport | ||
| SLC1A4 | Amino Acid Transport | ||
| TUBB | Ovarian, lung cancer | Evasion of apoptosis | Intracellular transport/Apoptosis |
| TAP2 | Type 1 diabetes | Intracellular transport/Antigen processing | |
| FYB | Wiscott-Aldrich syndrome | Intracellular transport | |
| RAB4A | Intracellular transport | ||
| YEATS2 | Transcription regulation | ||
| PTRF | Transcription regulation | ||
| SP100 | Liver diseases | Transcription regulation | |
| JAZF1 | Endometrial stromal tumors | Self-sufficiency in growth signals | Transcription regulation/oncogene |
| IFI16 | Head and neck squamous cell carcinomas. Up-regulation inhibits tumor growth | Insensitivity to anti-growth signals | Transcription regulation/ Antigen processing |
| HLX1 | Transcription regulation | ||
| ZNF395 | Transcription regulation | ||
| RPS5 | Protein biosynthesis | ||
| FUT11 | Protein biosynthesis | ||
| U5-116KD | RNA splicing | ||
| NOL3 | Breast cancer | Evasion of apoptosis | RNA splicing/anti-apoptotic |
| SART3 | Colorectal cancer | RNA processing | |
| PSMB9 | RCC | Tissue invasion and metastasis | Proteolysis |
| MARCH-I | Tissue invasion and metastasis | Ubiquitin cycle | |
| ITGB2 | Tissue invasion and metastasis | Cell adhesion/Immune response | |
| ABL2 | Gastric adenocarcinoma | Self-sufficiency in growth signals + Tissue invasion and metastases | Focal adhesion/oncogene |
| CLD1 | Tissue invasion and metastasis | Tight junction | |
| AXL | RCC | Self-sufficiency in growth signals + Evasion of apoptosis | Apoptosis/oncogene |
| EGLN3** | RCC | Evasion of apoptosis | Apoptosis |
| CLECSF6 | Immune response | ||
| TNIP1 | Immune response | ||
| HLA-DMA | Antigen processing | ||
| HLA-DMB | Antigen processing | ||
| HLA-DPA1 | Melanoma | Antigen processing | |
| SAMHD1 | Interferon induction | ||
| PDK1 | Glycolysis/Gluconeogenesis | ||
| ALDOA | RCC, HIF1 activated gene, also activates HIF1 | Increased glycolysis (Warburg effect) + Angiogenesis | Glycolysis/Gluconeogenesis |
| GM2A | Membrane lipid metabolism | ||
| VKORC1 | Biosynthesis of steroids | ||
| NUOMS | Oxidative phosphorylation | ||
| CA9 | RCC | Nitrogen metabolism | |
| SQRDL | Energy metabolism | ||
| APOC1** | RCC | Lipoprotein metabolism | |
| P4HB | May be HIF1 related | Metabolism | |
| VGF | Self-sufficiency in growth signals | Growth factor | |
Genes in italic: 64 genes previously not reported by Lenburg et al.
genes previously reported by 4 or more RCC studies
Disease association was obtained from OMIM and the Genetic association databases. As summarized in Table 4, 43 are cancer related. The relation between the cancer associated markers previously identified—as well as those identified by this study—and critical physiological changes associated with tumor development (Hanahan and Weinberg, 2000) is also shown in Table 4 (third column, also see Figure 5). In particular column 3 includes (1) genes that were previously implicated in cancer e.g. tumor suppressors and oncogenes and (2) genes that were not previously found to be associated and whose role in transformative processes has not been established. As an example of the latter, we annotated proteolysis genes and genes involved in cell-adhesion and/or regulation of actin cytoskeleton as potentially involved in “tissue invasion and metastasis.”
Figure 5.
Genes related to critical processes underlying kidney cell transformation. Marker genes were replaced into six Weinberg categories which are essential for tumor development. Genes previously found by at least four other RCC studies are indicated with **, genes implicated in other cancers with *, and markers not identified previously by Lenburg et al. are given in red. Since the exact order of these steps is not known, the processes are given in here with no particular order.
As summarized in Table 4, the markers include several signaling proteins, some of them previously implicated in cancer transformation (Notch signaling, Wnt signaling, TGF-β signaling, NF-κB cascade and MAPK signaling cascades). The heatmap of signaling pathway genes is shown in Figure 4(a). Interestingly, all markers involved in calcium signaling pathways are down-regulated in RCC. Another interesting group of genes are immune system related genes, 83% of which are upregulated (Figure 4(b)). Considering that RCC is resistant to chemo, radio and hormonal therapy while immunotherapy (cytokines IL-2 and interferon-alpha) appears to be effective for RCC treatment, these immune response related biomarkers could be potentially important for therapeutics. Our results also indicate that all cation and anion transport genes identified by our analysis—which encode mainly ion channels—are down-regulated (Figure 4(c)). Conversely, genes involved in intracellular transport and amino acid transport are mostly up-regulated. Since the kidney is at the junction of circulatory and urological processes both of which involve transport of different body fluids as well as ions; down-regulation of ion channels may be the result of a nonfunctioning kidney in RCC. Increase in intracellular transport point to increased communication of cancerous cells with ECM, with normal cells as well as with other cancer cells. Up-regulated amino acid transport shows that cancer cells need more building blocks to make proteins for different biological processes as they grow.
Figure 4. (a-f).
Heatmaps of some of the important pathways that 158 markers are involved in. The expression values for each gene are transformed (Section 3.4.1) and color coded as in Figure 3. Black represents the mean of normal values, green represents down-regulation and red represents up-regulation with respect to the mean.
Other groups of genes potentially important for tumor progression include those for proteolysis, and cell-adhesion and/or regulation of actin cytoskeleton (Figure 4(d)). These genes are likely to be involved in tissue invasion and metastasis.
The apoptotic genes (Figure 4(e)) SPP1 and SFRP1 are candidate tumor suppressors for various cancers; AXL gene is a proto-oncogene, and NOL3 (ARC, apoptosis repressor with CARD domain) is induced in human breast cancer and confers chemo- and radiation-resistance (Mercier et al. 2005). These genes may all contribute to evasion of apoptosis during tumor development.
Finally, a number of markers are involved in metabolism; some are shown in Figure 4(f). It is clear that the processes shown are all linked to energy generation in the cell and that most of these genes are suppressed in RCC. Studies by Warburg (Warburg, 1956) indicate that the vast majority of human and animal tumors display a high rate of glycolysis under aerobic conditions. Human solid tumors endure profound hypoxia; hence adaptation to hypoxic conditions is a crucial step in tumor progression. The anaerobic use of glucose as an energy source through glycolysis is therefore a feature common to solid tumors, in turn leading to a lesser dependence on the mitochondria for oxidative phosphorylation. This loss of cell dependence on oxidative metabolism is an important factor in the development of tumors. In accordance with that, it was shown that expression levels of OXPHOS genes were down-regulated in RCC (Simonnet et al. 2002; Meierhofer et al. 2004). Hence, overall suppression of these genes can be due to loss of tumor dependence on normal energy generating pathways in hypoxic conditions.
4.2. Disease associations
Eleven of the 158 biomarkers are especially definitive, both because of their biology, and because they have been identified as RCC related in four or more studies (Young et al. 2001; Boer et al. 2001; Takahashi et al 2001; Gieseg et al. 2002; Lenburg et al. 2003). These genes are GABARAPL1, EGLN3, MT1G, SFRP1, INHBB, ATP6V1B1, APOC1, ADH6, C1QB, ALDOB and CNGLN. SFRP1 and EGLN3 are involved in apoptosis, which is a critical process as evasion of apoptosis is one of the key steps in tumorigenesis. CNGLN is involved in regulation of actin cytoskeleton, which may have a role in tissue invasion and metastasis. Four other genes have a role in metabolism, specifically glycolysis (ADH6, ALDOB), lipoprotein metabolism (APOC1), and oxidative phosphorylation (ATP6V1B1). INHBB and C1QB are immune response related genes. INHBB is a growth factor, hence its up-regulation may cause uncontrolled activation of downstream targets.
In addition to these eleven genes, 83 others were identified by Lenburg et al. as differentially expressed in RCC. They include carbonic anyhdrase IX, which is the RCC associated antigen G250 and is induced in many cancer types, hypoxia induced gene ADORA3, potentially oncogenic AXL gene which causes transformation when overexpressed in NIH 3T3 cells, and vitamin D receptor (VDR, up-regulated), which was found to be over-expressed in pancreatic cell lines (Albrechtsson et al. 2003) and is down-regulated by resveratrol compound (Shi et al. 2004) in RCC cell lines, which acts as a chemopreventive agent for RCC and other types of cancers.
Another 64 genes were not identified in our previous study. Of these genes 25 were found to be significantly up-/down-regulated (in the same direction with this study) by one or more other RCC studies as summarized in Table 5. The overall summary of the 7 RCC studies and the overlap between their gene sets and our genes are given in Supplementary Table 2.
Table 5.
25 genes that were not identified by Lenburg et al but identified by other RCC studies including this study.
TUBB, PRDX4 and ZNF395 genes are potentially important genes since they have been identified by four or more RCC studies including us. Some of the genes were already shown to be important in RCC by additional studies other than listed in Table 5. These genes include SLC25A5 (ANT2, down), which catalyzes the exchange of ATP for ADP across the mitochondrial membrane, thus playing an important role in oxidative phosphorylation. Renal carcinomas were found to have reduced levels of ANT2 and other oxidative phosphorylation genes (Heddi et al. 1996) in line with the argument that the loss of cell dependence on oxidative metabolism is an important factor in the development of tumors under hypoxic conditions (Chevrollier et al. 2005). ALDOA enzyme (up), originally found to be up-regulated in lung cancer (Ojika et al. 1991), was determined to be an indicator of poor prognosis in RCC patients in combination with gamma-enolase (Takashi et al. 1993).
We further classified 64 markers into three groups (Table 6) based on their disease associations: (I) previously reported RCC related genes (Table 5), (II) genes related to cancers other than RCC, and (III) genes related to diseases other than cancer.
Table 6.
Disease related 64 markers not identified by Lenburg et al.
| Genes related to cancers other than RCC | ||
|---|---|---|
| NCOR2 | Up | Suppresses target genes for the vitamin D receptor (VDR) in prostate cancer cells resulting in hormonal insensitivity (Khanim et al. 2004) |
| ABL2 | Up | Related to proto-oncogene ABL. Implicated in hematologic neoplasms (Yagasaki et al. 2001) and gastric adenocarcinoma (Wu et al. 2003) |
| CD81 | Up | The loss of CD81 was found to be associated with differentiation and metastasis of HCC (Inoue et al. 2001) |
| TUBB | Up | Implicated in many cancers including ovarian (Mozzetti et al. 2005) and lung cancer (de Castro et al. 2003) |
| SART3 | Up | Induces HLA-A24-restricted and tumor-specific cytotoxic T lymphocytes in colorectal cancer patients (Sasatomi et al. 2002) |
| HELAD1 | Down | Up-regulated in colorectal carcinomas (Ishiguro et al. 2002) |
| CHGB | Down | Up-regulated in neuroendocrine tumors (Kimura et al. 2000) |
| JAZF1 | Up | Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors (Huang et al. 2004) |
| PRKCDBP | Up | epigenetic or mutational inactivation contribute to the pathogenesis of breast and lung cancers (Xu et al. 2001) |
| TFAP2A | Down | Loss of AP-2 results in metastasis of melanoma cells (Jean et al. 1998) |
| Genes related to diseases other than cancer | ||
|---|---|---|
| WASF2 | Up | Wiscott-Aldrich syndrome |
| LCP2 | Up | Wiscott-Aldrich syndrome |
| FYB | Up | Wiscott-Aldrich syndrome |
| TAP2 | Up | Type 1 diabetes |
(II) These genes include NCOR2 (SMRT, up), which forms a large co-repressor complex that contains SIN3A/B and histone deacetylases HDAC1 and HDAC2. This complex associates with the thyroid (TR) and the retinoid acid receptors (RAR) in the absence of ligand, and may stabilize their interactions with TFIIB. Recently, it has been shown that elevated SMRT levels result in suppression of target genes for the vitamin D receptor (VDR) in prostate cancer cells and in apparent hormonal insensitivity (Khanim et al. 2004). ABL2 (up) tyrosine kinase is related to proto-oncogene ABL, and is implicated in hematologic neoplasms (Yagasaki et al. 2001) and gastric adenocarcinoma (Wu et al. 2003).
CD81 antigen (up) is reported to influence adhesion, morphology, activation, proliferation, and differentiation of B, T, and other cells. Antibodies against CD81 induce homotypic aggregation of cells and can inhibit their growth. The loss of CD81 was found to be associated with differentiation and metastasis of HCC (Inoue et al. 2001). Betta-tubulin, TUBB, gene is implicated in many cancers including ovarian and lung cancer. Non-small cell lung cancers have a high incidence of somatic mutations of the beta-tubulin (class I) gene, which may cause paclitaxel resistance (de Castro et al. 2003). Moreover, recently, class III beta-tubulin overexpression was found to be a prominent mechanism of paclitaxel resistance in ovarian cancer patients (Mozzetti et al. 2005).
SART3 (squamous cell carcinoma antigen recognized by T cells 3), is an RNA-binding nuclear protein that is a tumor-rejection antigen. This antigen possesses tumor epitopes capable of inducing HLA-A24-restricted and tumor-specific cytotoxic T lymphocytes in colorectal cancer patients and may be useful for specific immunotherapy (Sasatomi et al. 2002). TFAP2 is known to suppress a number of genes including MCAM/MUC18, C/EBP alpha and MYC. The loss of this gene was shown to be to be associated with malignant transformation and tumor progression in malignant melanoma (Karjalainen et al. 1998; Jean et al. 1998). This gene may be a potential tumor suppressor protein for RCC as well.
(III) Markers related to diseases other than cancer include WASF2, LCP2 and FYB (Wiscott-Aldrich syndrome, all up) and TAP2 (up, polymorphisms in this gene are implicated in type 1 diabetes (Lotfi et al. 1994; Penfornis et al. 2002). The relationship between Wiscott-Aldrich syndrome and diabetes should be elaborated in the future.
Other genes which we’ve identified as RCC related, but which have not previously been associated with any disease include ITPR2, which has roles in the calcium and phosphatidylinositol signaling pathways; glutaryl-Coenzyme A dehydrogenase (GCDH), which takes part in fatty acid metabolism,; 40S ribosomal protein S5 (RPS5), HIG1 (likely ortholog of hypoxia induced gene 1) and P4HB (proline 4-hydroxylase).
4.3. Genes related to critical processes underlying kidney cell transformation
The development of RCC, as with other cancers, is accompanied by alterations in cell physiology, which collectively dictate malignant growth (Hanahan and Weinberg, 2000). Briefly these changes include environment independent growth; insensitivity to antigrowth factors (loss of tumor suppressor genes); evasion of apoptosis (producing survival factors); limitless replicative potential (turning on telomerase); sustained angiogenesis (producing VEGF inducer) and tissue invasion and metastasis (inactivation of E-cadherin). The order in which these capabilities are acquired is likely to be variable across different cancer types and subtypes. In this section we discuss RCC markers in the context of these alterations.
The main associations for RCC are summarized in (Figure 5 and Table 4). We include (1) genes that were previously implicated in cancer e.g. tumor suppressors and oncogenes and (2) genes that were not previously found to be associated with cancer but which have a function critical to tumor development. We did not include the genes whose expression is not correlated with the associated category e.g. NCOR2 gene (Table 4) suppresses tumor growth in prostate cancer cells but is up-regulated in RCC, and hence it is not included. Our results suggest the following for RCC (1) Self-sufficiency in growth signals is achieved via activation of oncogenes JAZF1, AXL, ABL2; and growth factors INHBB and VGF. Further, loss of OXPHOS genes SLC25A5, ATP6V1B1, B3, V0A4, and NDUFA4 may contribute to the self-sufficiency of the cancer cells with the ability to be less dependent on OXPHOS (2) The loss of tumor suppressor genes PTPRO, TFAP2A, CDKN1C, AIM1 and MT1G as well as other genes that were shown to suppress tumor growth in cancer cell lines but not yet identified as tumor suppressor candidates (RASD1, VDR, EHF, SPP1, ACPP, MT1F and ERBB4) contributes to insensitivity to antigrowth signals; (3) Evasion of apoptosis is mediated through loss of SPP1 and SFRP1, and activation of TUBB, NOL3 and EGLN3. (4) Two groups of genes are likely to be involved in tissue invasion and metastasis: proteolysis genes (PAPPA, PSMB9 and MARCH-1) and genes involved in cell-adhesion and/or regulation of actin cytoskeleton (CNGLN, ITPR2, NPHS1, ITGB2, CLD1, ZAK, WASF2, CD81) and (5) Angiogenesis may be mediated through ALDOA enzyme which is shown to be activated by HIF1 under hypoxic conditions and by increased glycolytic activity (Warburg effect), and which in a feedback loop activates HIF1 (Lu et al. 2002) which then activates several angiogenic factors including VEGF.
The identification of these genes opens up many paths for investigation that would not otherwise have been apparent. For example down regulation is often the result of epigenetic modification of upstream regions; especially methylation. The identification of CpG islands in or around binding sites and their analysis by RT-PCR or MALDI-TOF would be an obvious route to take, and if significant methylation difference are found, it would suggest a simple and sensitive assay for potentially significant markers.
Supplementary
Supplementary Table 1.
158 significant (p-value ≤0.01) markers.
| GenBank Accession | Symbol | p-value |
|---|---|---|
| NM_003221 | TFAP2B | 0 |
| NM_005235 | ERBB4 | 0 |
| NM_017753 | PRG-3 | 0 |
| NM_003714 | STC2 | 1x10−5 |
| AI655467 | 2x10−5 | |
| BF478120 | 2x10−5 | |
| NM_001692 | ATP6V1B1 | 2x10−5 |
| NM_002489 | NDUFA4 | 2x10−5 |
| NM_012232 | PTRF | 2x10−5 |
| NM_021179 | LOC57821 | 2x10−5 |
| NM_031412 | GABARAPL1 | 2x10−5 |
| AI733359 | 3x10−5 | |
| NM_005950 | MT1G | 3x10−5 |
| NM_006990 | WASF2 | 3x10−5 |
| NM_172369 | C1QG | 3x10−5 |
| BF541967 | 5x10−5 | |
| NM_002010 | FGF9 | 5x10−5 |
| NM_033554 | HLA-DPA1 | 5x10−5 |
| AI589190 | 6x10−5 | |
| BC005314.1 | 6x10−5 | |
| NM_004646 | NPHS1 | 6x10−5 |
| NM_133262 | ATP6V1G3 | 6x10−5 |
| NM_174896 | MGC24133 | 6x10−5 |
| NM_002848 | PTPRO | 7x10−5 |
| NM_003113 | SP100 | 7x10−5 |
| NM_014625 | NPHS2 | 7x10−5 |
| NM_000339 | SLC12A3 | 8x10−5 |
| NM_000491 | C1QB | 9x10−5 |
| NM_001009 | RPS5 | 9x10−5 |
| NM_000767 | CYP2B6 | 0.00011 |
| NM_003012 | SFRP1 | 0.00011 |
| NM_004894 | C14orf2 | 0.00011 |
| NM_016929 | CLIC5 | 0.00011 |
| NM_022073 | EGLN3 | 0.00011 |
| NM_033201 | BC008967 | 0.00011 |
| NM_004356 | CD81 | 0.00013 |
| NM_138799 | OACT2 | 0.00013 |
| NM_000211 | ITGB2 | 0.00014 |
| AK026764.1 | 0.00015 | |
| NM_152522 | MGC33864 | 0.0003 |
| AV691491 | 0.00033 | |
| NM_004392 | DACH1 | 0.00033 |
| NM_005565 | LCP2 | 0.00033 |
| NM_014463 | LSM3 | 0.00033 |
| NM_015474 | SAMHD1 | 0.00036 |
| BG251556 | 0.00037 | |
| NM_018162 | HELAD1 | 0.0004 |
| NM_000376 | VDR | 0.00043 |
| NM_001819 | CHGB | 0.00047 |
| NM_020142 | NUOMS | 0.00047 |
| NM_004578 | RAB4A | 0.00055 |
| AI962367 | 0.00058 | |
| NM_021800 | DNAJC12 | 0.0006 |
| NM_021199 | SQRDL | 0.00061 |
| NM_153233 | FLJ36445 | 0.00073 |
| NM_017606 | NM_017606 | 0.0008 |
| NM_015488 | MR-1 | 0.00084 |
| BF439449 | 0.00093 | |
| NM_000342 | SLC4A1 | 0.00093 |
| NM_006120 | HLA-DMA | 0.00098 |
| NM_000918 | P4HB | 0.00108 |
| NM_001099 | ACPP | 0.00113 |
| NM_021151 | CROT | 0.00113 |
| BG434272 | 0.00121 | |
| NM_001216 | CA9 | 0.00122 |
| NM_198991 | KCTD1 | 0.00125 |
| NM_006312 | NCOR2 | 0.00126 |
| NM_016582 | SLC15A3 | 0.00142 |
| NM_020632 | ATP6V0A4 | 0.00142 |
| NM_003220 | TFAP2A | 0.00166 |
| NM_005158 | ABL2 | 0.00169 |
| NM_014601 | EHD2 | 0.00169 |
| NM_003116 | SPAG4 | 0.00185 |
| AW771565 | AIM1 | 0.00187 |
| NM_003946 | NOL3 | 0.00192 |
| NM_000076 | CDKN1C | 0.00195 |
| NM_006058 | TNIP1 | 0.00197 |
| NM_000336 | SCNN1B | 0.00198 |
| NM_000035 | ALDOB | 0.00202 |
| NM_015103 | PLXND1 | 0.00206 |
| BF130943 | 0.00217 | |
| BE552097 | 0.00222 | |
| NM_000672 | ADH6 | 0.00248 |
| BE739519 | 0.00259 | |
| NM_198446 | FLJ45459 | 0.00259 |
| NM_021958 | HLX1 | 0.0026 |
| NM_001395 | DUSP9 | 0.00262 |
| NM_018023 | YEATS2 | 0.00267 |
| NM_001004196 | CD200 | 0.00269 |
| NM_006520 | TCTE1L | 0.00275 |
| NM_001152 | SLC25A5 | 0.00276 |
| NM_002193 | INHBB | 0.00277 |
| NM_006922 | SCN3A | 0.00277 |
| NM_000159 | GCDH | 0.0029 |
| NM_002800 | PSMB9 | 0.00314 |
| NM_004051 | BDH | 0.00314 |
| NM_145040 | PRKCDBP | 0.0032 |
| N58278 | 0.00325 | |
| NM_024006 | VKORC1 | 0.00335 |
| NM_004710 | SYNGR2 | 0.00339 |
| AI796222 | 0.00342 | |
| NM_000161 | GCH1 | 0.00348 |
| NM_000544 | TAP2 | 0.00357 |
| NM_014706 | SART3 | 0.00357 |
| NM_014056 | HIG1 | 0.00362 |
| NM_001645 | APOC1 | 0.00364 |
| NM_012153 | EHF | 0.00364 |
| NM_175061 | JAZF1 | 0.00368 |
| NM_015991 | C1QA | 0.00379 |
| NM_145648 | SLC15A4 | 0.00384 |
| NM_178014 | TUBB | 0.00391 |
| NM_000405 | GM2A | 0.00392 |
| AW242899 | 0.00407 | |
| NM_000582 | SPP1 | 0.00408 |
| NM_002610 | PDK1 | 0.00412 |
| NM_007021 | C10orf10 | 0.00413 |
| NM_016084 | RASD1 | 0.00423 |
| NM_016184 | CLECSF6 | 0.00433 |
| NM_017923 | MARCH-I | 0.00438 |
| NM_015584 | POLDIP2 | 0.00456 |
| NM_006406 | PRDX4 | 0.00468 |
| NM_020991 | CSH2 | 0.0047 |
| NM_000677 | ADORA3 | 0.00478 |
| NM_002223 | ITPR2 | 0.00483 |
| BF590528 | 0.00485 | |
| NM_005949 | MT1F | 0.00489 |
| NM_003038 | SLC1A4 | 0.0049 |
| NM_001465 | FYB | 0.00503 |
| NM_004790 | SLC22A6 | 0.00514 |
| NM_024027 | COLEC11 | 0.00514 |
| AI769774 | 0.00525 | |
| NM_016653 | ZAK | 0.00525 |
| NM_014629 | ARHGEF10 | 0.00527 |
| NM_000253 | MTP | 0.00571 |
| NM_003361 | UMOD | 0.00576 |
| BF510426 | 0.00583 | |
| NM_005531 | IFI16 | 0.006 |
| AI282982 | LOC120224 | 0.00629 |
| NM_004247 | U5-116KD | 0.00634 |
| NM_032118 | FLJ12953 | 0.00641 |
| NM_004414 | DSCR1 | 0.00655 |
| NM_032866 | CNGLN | 0.00665 |
| NM_002118 | HLA-DMB | 0.00719 |
| NM_004483 | GCSH | 0.00742 |
| NM_000316 | PTHR1 | 0.00743 |
| T90295 | 0.0076 | |
| NM_030674 | SLC38A1 | 0.00765 |
| NM_001699 | AXL | 0.00773 |
| AW242836 | LOC120224 | 0.0078 |
| NM_205848 | SYT6 | 0.00895 |
| NM_000034 | ALDOA | 0.00896 |
| NM_032717 | MGC11324 | 0.00942 |
| NM_020139 | DHRS6 | 0.00945 |
| AA148534 | PAPPA | 0.00951 |
| NM_016321 | RHCG | 0.00956 |
| H99792 | 0.00983 | |
| NM_053000 | TIGA1 | 0.00983 |
| NM_018660 | ZNF395 | 0.00994 |
Supplementary Table 2.
Comparison with other RCC studies.
| Method | number of samples/microarray platform | Final number of genes | Overlap with 158 marker genes | Overlap with 64* marker-genes | |
|---|---|---|---|---|---|
| Young et al. 2001 | More than two-fold changed in two or more tumor samples | 7 tumor (4 cc-RCC), 7 normal/cDNA 7,075 genes | 189 | 8 | 1 |
| Takahashi et al. 2001 | Three-fold or more changed in 75% or more of the tumor samples | 29 cc-RCC and 29 normal/cDNA 21,632 genes | 109 | 7 | - |
| Gieseg et al. 2002 | Changed genes in cc-RCC with Wilcoxon test p-value ≤0.001 and fold change ≥1.1 | 13 RCC (9cc-RCC), 9 normal/ Affymetrix 5600 genes | 355 genes, 85 reported | 4 | 1 |
| Boer et al. 2001 | adapted sign test by counting for each gene the number of times that its measured intensity in the set of repeated pair-wise comparisons is higher in T and N | 37 cc-RCC, 37 normal | 1738 cDNAs | 45 | 11 |
| Higgins et al. 2003 | No reported gene set; selected genes with avg fold change >3 and t-test p-value >0.03. | 41 RCC (23 cc-RCC), 3 normal/cDNA 22,648 genes | 182 genes | 8 | 1 |
| Jones et al. 2005 | 90% lower confidence boung of the fold change was >2 and t-test p-value <0.001 | 8 clear cell stage I, 23 normal, Affymetrix 22,283 genes | 1359 up-regulated, 493 down-regulated | 37 up, 19 down-regulated | 15 up-regulated |
| Sultmann et al. 2005 | t-test with estimated false discovery rate <0.23 | 25 ccRCC, 25 normal/RCC-specific cDNA microarrays with 4207 genes | 620 up-regulated; 561 down-regulated genes | 13 up, 11 down-regulated | 5 up, 2 down-regulated |
genes not identified by Lenburg et al.
Footnotes
Please note that this article may not be used for commercial purposes. For further information please refer to the copyright statement at http://www.la-press.com/copyright.htm
References
- Albrechtsson E, Jonsson T, Moller S, et al. Vitamin d receptor is expressed in pancreatic cancer cells and a vitamin d3 analogue decreases cell number. Pancreatology. 2003;3:41–6. doi: 10.1159/000069149. [DOI] [PubMed] [Google Scholar]
- Alon U, Barkai N, Notterman DA, et al. Broad patterns of gene expression revealed by clustering analysis of tumor and normal colon tissues probed by oligonucleotide arrays. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6745–50. doi: 10.1073/pnas.96.12.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer JM, Huber WK, Sultmann H, et al. Identification and classification of differentially expressed genes in renal cell carcinoma by expression profiling on a global human 31,500-element cdna array. Genome Res. 2001;11:1861–170. doi: 10.1101/gr.184501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey KJ, Kane D, Sunshine M, et al. Matchminer: A tool for batch navigation among gene and gene product identifiers. Genome Biol. 2003;4:R27. doi: 10.1186/gb-2003-4-4-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrollier A, Loiseau D, Gautier F, et al. Ant2 expression under hypoxic conditions produces opposite cell-cycle behavior in 143b and hepg2 cancer cells. Mol. Carcinog. 2005;42:1–8. doi: 10.1002/mc.20059. [DOI] [PubMed] [Google Scholar]
- Dalgin GS, DeLisi C. Simple discriminant functions identify small sets of genes that distinguish cancer phenotype from normal. Genome Inform Ser. Workshop Genome Inform. 2005;16:245–53. [PubMed] [Google Scholar]
- de Castro J, Belda-Iniesta C, Cejas P, et al. New insights in beta-tubulin sequence analysis in non-small cell lung cancer. Lung Cancer. 2003;41:41–8. doi: 10.1016/s0169-5002(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Dennis G, Sherman BT, Hosack DA, et al. David: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Gieseg MA, Cody T, Man MZ, et al. Expression profiling of human renal carcinomas with functional taxonomic analysis. BMC Bioinformatics. 2002;3:26. doi: 10.1186/1471-2105-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub TR, Slonim DK, Tamayo P, et al. Molecular classification of cancer: Class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–7. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- Guyon I, Weston J, Barnhill S, et al. Gene selection for cancer classification using support vector machines. Machine Learning. 2002;46:389–422. [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Heddi A, Faure-Vigny H, Wallace DC, et al. Coordinate expression of nuclear and mitochondrial genes involved in energy production in carcinoma and oncocytoma. Biochim. Biophys. Acta. 1996;1316:203–9. doi: 10.1016/0925-4439(96)00026-9. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Shinghal R, Gill H, et al. Gene expression patterns in renal cell carcinoma assessed by complementary DNA microarray. Am. J. Pathol. 2003;162:925–32. doi: 10.1016/S0002-9440(10)63887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway D, Kon M, DeLisi C. Integrating genomic data to predict transcription factor binding. Genome Inform Ser. Workshop Genome Inform. 2005;16:83–94. [PubMed] [Google Scholar]
- Holloway D, Kon M, DeLisi C.2006aMachine learning for predicting targets of transcription factors in yeast Synthetic and Systems Biologyin press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway D, Kon M, DeLisi C.2006bMachine learning for predicting transcription data integration IBM Journal of Research and Development on Systems Biologyin press [Google Scholar]
- Huang HY, Ladanyi M, Soslow RA. Molecular detection of jazf1-jjaz1 gene fusion in endometrial stromal neoplasms with classic and variant histology: Evidence for genetic heterogeneity. Am. J. Surg. Pathol. 2004;28:224–32. doi: 10.1097/00000478-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Inoue G, Horiike N, Onji M. The cd81 expression in liver in hepatocellular carcinoma. Int. J. Mol. Med. 2001;7:67–71. doi: 10.3892/ijmm.7.1.67. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Shimokawa T, Tsunoda T, et al. Isolation of helad1, a novel human helicase gene up-regulated in colorectal carcinomas. Oncogene. 2002;21:6387–94. doi: 10.1038/sj.onc.1205751. [DOI] [PubMed] [Google Scholar]
- Jean D, Gershenwald JE, Huang S, et al. Loss of ap-2 results in up-regulation of mcam/muc18 and an increase in tumor growth and metastasis of human melanoma cells. J. Biol. Chem. 1998;173:16501–8. doi: 10.1074/jbc.273.26.16501. [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Samuels A, et al. Cancer statistics. CA Cancer. J. Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- Jones J, Otu H, Spentzos D, et al. Gene signatures of progression and metastasis in renal cell cancer. Clin. Cancer Res. 2005;11:5730–9. doi: 10.1158/1078-0432.CCR-04-2225. [DOI] [PubMed] [Google Scholar]
- Karjalainen JM, Kellokoski JK, Eskelinen MJ, et al. Down-regulation of transcription factor ap-2 predicts poor survival in stage i cutaneous malignant melanoma. J. Clin. Oncol. 1998;16:3584–91. doi: 10.1200/JCO.1998.16.11.3584. [DOI] [PubMed] [Google Scholar]
- Khanim FL, Gommersall LM, Wood VH, et al. Altered smrt levels disrupt vitamin d3 receptor signalling in prostate cancer cells. Oncogene. 2004;23:6712–25. doi: 10.1038/sj.onc.1207772. [DOI] [PubMed] [Google Scholar]
- Kimura N, Pilichowska M, Okamoto H, et al. Immunohistochemical expression of chromogranins a and b, prohormone convertases 2 and 3, and amidating enzyme in carcinoid tumors and pancreatic endocrine tumors. Mod. Pathol. 2000;13:140–6. doi: 10.1038/modpathol.3880026. [DOI] [PubMed] [Google Scholar]
- Lenburg ME, Liou LS, Gerry NP, et al. Previously unidentified changes in renal cell carcinomas gene expression identified by parametric analysis of microarray data. BMC Cancer. 2003;3:31. doi: 10.1186/1471-2407-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi M, Sastry A, Ye M, et al. Hla-dq and tap2 genes in patients with insulin-dependent diabetes mellitus. Immunol. Lett. 1994;41:201–4. doi: 10.1016/0165-2478(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the warburg effect in carcinogenesis. J. Biol. Chem. 2002;277:23111–5. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- Ma XJ, Salunga R, Tuggle JT, et al. Gene expression profiles of human breast cancer progression. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5974–9. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierhofer D, Mayr JA, Foetschl U, et al. Decrease of mitochondrial DNA content and energy metabolism in renal cell carcinoma. Carcinogenesis. 2004;25:1005–10. doi: 10.1093/carcin/bgh104. [DOI] [PubMed] [Google Scholar]
- Mercier I, Vuolo M, Madan R, et al. Arc, an apoptosis suppressor limited to terminally differentiated cells, is induced in human breast cancer and confers chemo- and radiation-resistance. Cell. Death. Differ. 2005;12:682–6. doi: 10.1038/sj.cdd.4401631. [DOI] [PubMed] [Google Scholar]
- Mozzetti S, Ferlini C, Concolino P, et al. Class iii beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin. Cancer. Res. 2005;11:298–305. [PubMed] [Google Scholar]
- Ojika T, Imaizumi M, Abe T, et al. Immunochemical and immunohistochemical studies on three aldolase isozymes in human lung cancer. Cancer Res. 1991;67:2153–8. doi: 10.1002/1097-0142(19910415)67:8<2153::aid-cncr2820670825>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Penfornis A, Tuomilehto-Wolf E, Faustman DL, et al. Analysis of tap2 polymorphisms in finnish individuals with type i diabetes. Hum. Immunol. 2002;63:61–70. doi: 10.1016/s0198-8859(01)00365-2. [DOI] [PubMed] [Google Scholar]
- Reddy TE, DeLisi C, Shakhnovich BE. Assessing transcription factor motif drift from noisy decoy sequences. Genome Inform. 2005;16:59–67. [PubMed] [Google Scholar]
- Sasatomi T, Suefuji Y, Matsunaga K, et al. Expression of tumor rejection antigens in colorectal carcinomas. Cancer. 2002;94:1636–41. doi: 10.1002/cncr.10421. [DOI] [PubMed] [Google Scholar]
- Shi T, Liou LS, Sadhukhan P, et al. Effects of resveratrol on gene expression in renal cell carcinoma. Cancer Biol. Ther. 2004;3:882–8. doi: 10.4161/cbt.3.9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholkopf B, Smola AJ. Learning with kernels. The MIT Press; Cambridge, M.A: 2002. [Google Scholar]
- Simonnet H, Alazard N, Pfeiffer K, et al. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23:759–68. doi: 10.1093/carcin/23.5.759. [DOI] [PubMed] [Google Scholar]
- Sultmann H, von Heydebreck A, Huber W, et al. Gene expression in kidney cancer is associated with cytogenetic abnormalities, metastasis formation, and patient survival. Clin Cancer Res. 2005;11:646–55. [PubMed] [Google Scholar]
- Takahashi M, Rhodes DR, Furge KA, et al. Gene expression profiling of clear cell carcinoma: Gene identification and prognostic classification. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9754–9. doi: 10.1073/pnas.171209998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Yang XJ, Sugimura J, et al. Molecular subclassification of kidney tumors and the discovery of new diagnostic markers. Oncogene. 2003;22:6810–8. doi: 10.1038/sj.onc.1206869. [DOI] [PubMed] [Google Scholar]
- Takashi M, Sakata T, Kato K. Use of serum gamma-enolase and aldolase a in combination as markers for renal cell carcinoma. Jpn. J. Cancer Res. 1993;84:304–9. doi: 10.1111/j.1349-7006.1993.tb02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnik V. Statistical learning theory. John Wiley & Sons; New York: 1998. [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wu CW, Li AF, Chi CW, et al. Arg tyrosine kinase expression in human gastric adenocarcinoma is associated with vessel invasion. Anticancer Res. 2003;23:205–10. [PubMed] [Google Scholar]
- Xu XL, Wu LC, Du F, et al. Inactivation of human srbc, located within the 11p15.5–p15.4 tumor suppressor region, in breast and lung cancers. Cancer Res. 2001;61:7943–9. [PubMed] [Google Scholar]
- Yagasaki F, Wakao D, Yokoyama Y, et al. Fusion of etv6 to fibroblast growth factor receptor 3 in peripheral t-cell lymphoma with at (4; 12)(p16; p13) chromosomal translocation. Cancer Res. 2001;61:8371–4. [PubMed] [Google Scholar]
- Yamazaki K, Sakamoto M, Ohta T, et al. Overexpression of kit in chromophobe renal cell carcinoma. Oncogene. 2003;22:847–52. doi: 10.1038/sj.onc.1206153. [DOI] [PubMed] [Google Scholar]
- Young AN, Amin MB, Moreno CS, et al. Expression profiling of renal epithelial neoplasms: A method for tumor classification and discovery of diagnostic molecular markers. Am. J. Pathol. 2001;158:1639–51. doi: 10.1016/S0002-9440(10)64120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AN, de Oliveira Salles PG, Lim SD, et al. Beta defensin-1, parvalbumin, and vimentin: A panel of diagnostic immunohistochemical markers for renal tumors derived from gene expression profiling studies using cdna microarrays. Am. J. Surg. Pathol. 2003;27:199–205. doi: 10.1097/00000478-200302000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
158 significant (p-value ≤0.01) markers.
| GenBank Accession | Symbol | p-value |
|---|---|---|
| NM_003221 | TFAP2B | 0 |
| NM_005235 | ERBB4 | 0 |
| NM_017753 | PRG-3 | 0 |
| NM_003714 | STC2 | 1x10−5 |
| AI655467 | 2x10−5 | |
| BF478120 | 2x10−5 | |
| NM_001692 | ATP6V1B1 | 2x10−5 |
| NM_002489 | NDUFA4 | 2x10−5 |
| NM_012232 | PTRF | 2x10−5 |
| NM_021179 | LOC57821 | 2x10−5 |
| NM_031412 | GABARAPL1 | 2x10−5 |
| AI733359 | 3x10−5 | |
| NM_005950 | MT1G | 3x10−5 |
| NM_006990 | WASF2 | 3x10−5 |
| NM_172369 | C1QG | 3x10−5 |
| BF541967 | 5x10−5 | |
| NM_002010 | FGF9 | 5x10−5 |
| NM_033554 | HLA-DPA1 | 5x10−5 |
| AI589190 | 6x10−5 | |
| BC005314.1 | 6x10−5 | |
| NM_004646 | NPHS1 | 6x10−5 |
| NM_133262 | ATP6V1G3 | 6x10−5 |
| NM_174896 | MGC24133 | 6x10−5 |
| NM_002848 | PTPRO | 7x10−5 |
| NM_003113 | SP100 | 7x10−5 |
| NM_014625 | NPHS2 | 7x10−5 |
| NM_000339 | SLC12A3 | 8x10−5 |
| NM_000491 | C1QB | 9x10−5 |
| NM_001009 | RPS5 | 9x10−5 |
| NM_000767 | CYP2B6 | 0.00011 |
| NM_003012 | SFRP1 | 0.00011 |
| NM_004894 | C14orf2 | 0.00011 |
| NM_016929 | CLIC5 | 0.00011 |
| NM_022073 | EGLN3 | 0.00011 |
| NM_033201 | BC008967 | 0.00011 |
| NM_004356 | CD81 | 0.00013 |
| NM_138799 | OACT2 | 0.00013 |
| NM_000211 | ITGB2 | 0.00014 |
| AK026764.1 | 0.00015 | |
| NM_152522 | MGC33864 | 0.0003 |
| AV691491 | 0.00033 | |
| NM_004392 | DACH1 | 0.00033 |
| NM_005565 | LCP2 | 0.00033 |
| NM_014463 | LSM3 | 0.00033 |
| NM_015474 | SAMHD1 | 0.00036 |
| BG251556 | 0.00037 | |
| NM_018162 | HELAD1 | 0.0004 |
| NM_000376 | VDR | 0.00043 |
| NM_001819 | CHGB | 0.00047 |
| NM_020142 | NUOMS | 0.00047 |
| NM_004578 | RAB4A | 0.00055 |
| AI962367 | 0.00058 | |
| NM_021800 | DNAJC12 | 0.0006 |
| NM_021199 | SQRDL | 0.00061 |
| NM_153233 | FLJ36445 | 0.00073 |
| NM_017606 | NM_017606 | 0.0008 |
| NM_015488 | MR-1 | 0.00084 |
| BF439449 | 0.00093 | |
| NM_000342 | SLC4A1 | 0.00093 |
| NM_006120 | HLA-DMA | 0.00098 |
| NM_000918 | P4HB | 0.00108 |
| NM_001099 | ACPP | 0.00113 |
| NM_021151 | CROT | 0.00113 |
| BG434272 | 0.00121 | |
| NM_001216 | CA9 | 0.00122 |
| NM_198991 | KCTD1 | 0.00125 |
| NM_006312 | NCOR2 | 0.00126 |
| NM_016582 | SLC15A3 | 0.00142 |
| NM_020632 | ATP6V0A4 | 0.00142 |
| NM_003220 | TFAP2A | 0.00166 |
| NM_005158 | ABL2 | 0.00169 |
| NM_014601 | EHD2 | 0.00169 |
| NM_003116 | SPAG4 | 0.00185 |
| AW771565 | AIM1 | 0.00187 |
| NM_003946 | NOL3 | 0.00192 |
| NM_000076 | CDKN1C | 0.00195 |
| NM_006058 | TNIP1 | 0.00197 |
| NM_000336 | SCNN1B | 0.00198 |
| NM_000035 | ALDOB | 0.00202 |
| NM_015103 | PLXND1 | 0.00206 |
| BF130943 | 0.00217 | |
| BE552097 | 0.00222 | |
| NM_000672 | ADH6 | 0.00248 |
| BE739519 | 0.00259 | |
| NM_198446 | FLJ45459 | 0.00259 |
| NM_021958 | HLX1 | 0.0026 |
| NM_001395 | DUSP9 | 0.00262 |
| NM_018023 | YEATS2 | 0.00267 |
| NM_001004196 | CD200 | 0.00269 |
| NM_006520 | TCTE1L | 0.00275 |
| NM_001152 | SLC25A5 | 0.00276 |
| NM_002193 | INHBB | 0.00277 |
| NM_006922 | SCN3A | 0.00277 |
| NM_000159 | GCDH | 0.0029 |
| NM_002800 | PSMB9 | 0.00314 |
| NM_004051 | BDH | 0.00314 |
| NM_145040 | PRKCDBP | 0.0032 |
| N58278 | 0.00325 | |
| NM_024006 | VKORC1 | 0.00335 |
| NM_004710 | SYNGR2 | 0.00339 |
| AI796222 | 0.00342 | |
| NM_000161 | GCH1 | 0.00348 |
| NM_000544 | TAP2 | 0.00357 |
| NM_014706 | SART3 | 0.00357 |
| NM_014056 | HIG1 | 0.00362 |
| NM_001645 | APOC1 | 0.00364 |
| NM_012153 | EHF | 0.00364 |
| NM_175061 | JAZF1 | 0.00368 |
| NM_015991 | C1QA | 0.00379 |
| NM_145648 | SLC15A4 | 0.00384 |
| NM_178014 | TUBB | 0.00391 |
| NM_000405 | GM2A | 0.00392 |
| AW242899 | 0.00407 | |
| NM_000582 | SPP1 | 0.00408 |
| NM_002610 | PDK1 | 0.00412 |
| NM_007021 | C10orf10 | 0.00413 |
| NM_016084 | RASD1 | 0.00423 |
| NM_016184 | CLECSF6 | 0.00433 |
| NM_017923 | MARCH-I | 0.00438 |
| NM_015584 | POLDIP2 | 0.00456 |
| NM_006406 | PRDX4 | 0.00468 |
| NM_020991 | CSH2 | 0.0047 |
| NM_000677 | ADORA3 | 0.00478 |
| NM_002223 | ITPR2 | 0.00483 |
| BF590528 | 0.00485 | |
| NM_005949 | MT1F | 0.00489 |
| NM_003038 | SLC1A4 | 0.0049 |
| NM_001465 | FYB | 0.00503 |
| NM_004790 | SLC22A6 | 0.00514 |
| NM_024027 | COLEC11 | 0.00514 |
| AI769774 | 0.00525 | |
| NM_016653 | ZAK | 0.00525 |
| NM_014629 | ARHGEF10 | 0.00527 |
| NM_000253 | MTP | 0.00571 |
| NM_003361 | UMOD | 0.00576 |
| BF510426 | 0.00583 | |
| NM_005531 | IFI16 | 0.006 |
| AI282982 | LOC120224 | 0.00629 |
| NM_004247 | U5-116KD | 0.00634 |
| NM_032118 | FLJ12953 | 0.00641 |
| NM_004414 | DSCR1 | 0.00655 |
| NM_032866 | CNGLN | 0.00665 |
| NM_002118 | HLA-DMB | 0.00719 |
| NM_004483 | GCSH | 0.00742 |
| NM_000316 | PTHR1 | 0.00743 |
| T90295 | 0.0076 | |
| NM_030674 | SLC38A1 | 0.00765 |
| NM_001699 | AXL | 0.00773 |
| AW242836 | LOC120224 | 0.0078 |
| NM_205848 | SYT6 | 0.00895 |
| NM_000034 | ALDOA | 0.00896 |
| NM_032717 | MGC11324 | 0.00942 |
| NM_020139 | DHRS6 | 0.00945 |
| AA148534 | PAPPA | 0.00951 |
| NM_016321 | RHCG | 0.00956 |
| H99792 | 0.00983 | |
| NM_053000 | TIGA1 | 0.00983 |
| NM_018660 | ZNF395 | 0.00994 |
Supplementary Table 2.
Comparison with other RCC studies.
| Method | number of samples/microarray platform | Final number of genes | Overlap with 158 marker genes | Overlap with 64* marker-genes | |
|---|---|---|---|---|---|
| Young et al. 2001 | More than two-fold changed in two or more tumor samples | 7 tumor (4 cc-RCC), 7 normal/cDNA 7,075 genes | 189 | 8 | 1 |
| Takahashi et al. 2001 | Three-fold or more changed in 75% or more of the tumor samples | 29 cc-RCC and 29 normal/cDNA 21,632 genes | 109 | 7 | - |
| Gieseg et al. 2002 | Changed genes in cc-RCC with Wilcoxon test p-value ≤0.001 and fold change ≥1.1 | 13 RCC (9cc-RCC), 9 normal/ Affymetrix 5600 genes | 355 genes, 85 reported | 4 | 1 |
| Boer et al. 2001 | adapted sign test by counting for each gene the number of times that its measured intensity in the set of repeated pair-wise comparisons is higher in T and N | 37 cc-RCC, 37 normal | 1738 cDNAs | 45 | 11 |
| Higgins et al. 2003 | No reported gene set; selected genes with avg fold change >3 and t-test p-value >0.03. | 41 RCC (23 cc-RCC), 3 normal/cDNA 22,648 genes | 182 genes | 8 | 1 |
| Jones et al. 2005 | 90% lower confidence boung of the fold change was >2 and t-test p-value <0.001 | 8 clear cell stage I, 23 normal, Affymetrix 22,283 genes | 1359 up-regulated, 493 down-regulated | 37 up, 19 down-regulated | 15 up-regulated |
| Sultmann et al. 2005 | t-test with estimated false discovery rate <0.23 | 25 ccRCC, 25 normal/RCC-specific cDNA microarrays with 4207 genes | 620 up-regulated; 561 down-regulated genes | 13 up, 11 down-regulated | 5 up, 2 down-regulated |
genes not identified by Lenburg et al.