Abstract
Vertebrate muscle arises sequentially from embryonic, fetal, and adult myoblasts. Although functionally distinct, it is unclear whether these myoblast classes develop from common or different progenitors. Pax3 and Pax7 are expressed by somitic myogenic progenitors and are critical myogenic determinants. To test the developmental origin of embryonic and fetal myogenic cells in the limb, we genetically labeled and ablated Pax3+ and Pax7+ cells. Pax3+Pax7− cells contribute to muscle and endothelium, establish and are required for embryonic myogenesis, and give rise to Pax7+ cells. Subsequently, Pax7+ cells give rise to and are required for fetal myogenesis. Thus, Pax3+ and Pax7+ cells contribute differentially to embryonic and fetal limb myogenesis. To investigate whether embryonic and fetal limb myogenic cells have different genetic requirements we conditionally inactivated or activated β-catenin, an important regulator of myogenesis, in Pax3- or Pax7-derived cells. β-Catenin is necessary within the somite for dermomyotome and myotome formation and delamination of limb myogenic progenitors. In the limb, β-catenin is not required for embryonic myoblast specification or myofiber differentiation but is critical for determining fetal progenitor number and myofiber number and type. Together, these studies demonstrate that limb embryonic and fetal myogenic cells develop from distinct, but related progenitors and have different cell-autonomous requirements for β-catenin.
Keywords: Pax3, Pax7, β-catenin, limb, myogenesis
Muscle development, growth, and regeneration take place throughout vertebrate life and are mediated by myogenic progenitors. As with other stem cells, myogenic progenitors are capable of self-renewal and differentiation. Therefore, a key to understanding vertebrate myogenesis is an understanding of the developmental origin of muscle progenitors and the signals that regulate their proliferation and differentiation.
Vertebrate myogenesis occurs in three successive phases, fulfilling different functional needs (for review, see Stockdale 1992; Biressi et al. 2007a). Embryonic myogenesis establishes the basic muscle pattern. Fetal myogenesis is critical for growth and maturation of the muscle. Adult myogenesis allows for postnatal growth and repair of damaged muscle. Each one of these phases involves specification of myoblasts that express the myogenic regulatory factors (MRFs) Myf5, MyoD, and/or Mrf4; differentiation of committed myocytes; fusion of myocytes into multinucleate myofibers; and maturation of myofibers with different speeds of contraction and myosin heavy chain (MHC) isoforms. During embryonic myogenesis (embryonic day 10.5–12.5 [E10.5–E12.5] mouse) (Biressi et al. 2007a) embryonic myoblasts differentiate into primary myofibers. During fetal myogenesis (E14.5–E17.5), fetal myoblasts both fuse to primary fibers and fuse to one another to make secondary myofibers. Finally, during adult myogenesis (postnatal day 0 [P0] and onward), muscle growth, fiber maturation, and regeneration are mediated by adult progenitors, satellite cells.
Embryonic, fetal, and adult myoblasts are distinct myogenic populations. These myoblast classes were initially classified based on their in vitro characteristics. They differ in their appearance, media requirements, response to extrinsic signaling molecules, drug sensitivity, and morphology of myofibers they generate (Stockdale 1992; Biressi et al. 2007a). In vivo these myoblasts generate primary, secondary, and adult myofibers that express different MHC isoforms and muscle enzymes (Gunning and Hardeman 1991; Wigmore and Evans 2002). Recent genetic analyses of Pax3/7 and Myf5/MyoD/Mrf4 families of transcription factors and microarray studies reveal that the three myoblast classes are specified by different transcription factor combinations and express different genes (Kassar-Duchossoy et al. 2004, 2005; Relaix et al. 2006; Biressi et al. 2007b). Of intense interest is whether embryonic, fetal, and adult myoblasts derive from common or different progenitor populations.
During vertebrate development all axial and limb skeletal muscle originates from progenitors in the somites, epithelial structures that arise from the presomitic mesoderm (psm) (Bryson-Richardson and Currie 2008). Muscle progenitors derive from the dorsal somite, the dermomyotome. Axial muscle forms in two waves from the dermomyotome. Initially, cells delaminate from the dermomyotomal lips to form the underlying primary myotome, and later central dermomyotomal cells translocate to the myotome to form all subsequent axial muscle. Limb muscle originates from progenitors that delaminate from the dermomyotome ventrolateral lip and migrate into the limb (Christ and Brand-Saberi 2002).
Research on muscle progenitors has concentrated on two closely related paired domain homeobox transcription factors, Pax3 and Pax7, expressed in somitic cells (for review, see Relaix et al. 2004; Buckingham 2007). Pax3 is initially expressed (E8) in the psm as somites form, but is progressively restricted, first to the dermomyotome and later to the dorsomedial and ventrolateral dermomyotomal lips. In the limb Pax3 is expressed in somitically derived cells during E10.5–E13.5. Somitic Pax7 expression initiates later (E9) and is restricted to the central region of the dermomyotome. In the mouse limb Pax7 expression begins at E11.5 in somitically derived cells. Pax7 continues to be expressed during fetal myogenesis and by adult satellite cells. Although Pax3 is not generally expressed in muscle after E13.5, a few adult satellite cells are Pax3+ (Relaix et al. 2006).
Functionally, Pax3 and Pax7 are important for myogenesis. Pax3 is required for somite segmentation and formation of the dermomyotomal lips (Schubert et al. 2001; Relaix et al. 2004). In addition, Pax3 is required for multiple aspects of limb myogenesis, and all limb muscle is lost in Pax3−/− mice (Relaix et al. 2004, and references therein). Pax3 is sufficient to induce MyoD and Myf5 in vitro (Maroto et al. 1997) and directly binds and transactivates enhancers of Myf5 (Bajard et al. 2006) and MyoD (Hu et al. 2008). Pax7−/− mice show no defects in embryonic or fetal myogenesis. However, Pax7 is required for maintenance of adult satellite cells (Seale et al. 2000; Oustanina et al. 2004; Kuang et al. 2006; Relaix et al. 2006). Pax7 is sufficient to drive myogenic specification in vitro (Seale et al. 2004) and binds a Myf5 enhancer (McKinnell et al. 2008). Pax3−/−;Pax7−/− mice have no limb muscles (recapitulating the Pax3−/− phenotype), but also have no embryonic or fetal axial muscle (although the primary myotome does initially form) (Relaix et al. 2005). Thus, expression of Pax3 or Pax7 is critical for assuring the survival of embryonic, fetal, and adult muscle progenitors.
Recent studies have identified Pax3+/Pax7+ somitic cells as the source of muscle progenitors (Kassar-Duchossoy et al. 2005; Relaix et al. 2005). Using a Pax3GFP allele (in which GFP is expressed similarly to Pax3 but perdures beyond the transient endogenous Pax3 expression) to track the fate of Pax3 cells and Pax3 and Pax7 expression and loss-of-function experiments, these studies suggest that Pax3+/Pax7+ cells are the common progenitors responsible for all embryonic, fetal, and adult myogenesis in axial and limb muscles. However, both Pax3 and Pax7 are only transiently expressed in progenitors and down-regulated during myogenic differentiation. Therefore, a full assessment of the contribution of Pax3+ and Pax7+ cells to embryonic, fetal, and adult myoblasts and myofibers requires a lineage analysis in which Pax3+ and Pax7+ cells and their progeny are genetically labeled.
Wnt/β-catenin signaling is an important regulator of muscle development, and likely to be critical for regulating muscle progenitor proliferation and differentiation. Wnts are secreted ligands for receptor-mediated signaling (Clevers 2006). Cytoplasmic β-catenin is the central mediator of canonical Wnt signaling. In the absence of Wnts, β-catenin is phosphorylated and targeted for degradation. Wnt binding to receptors leads to the formation of stabilized, dephosphorylated β-catenin that translocates to the nucleus where it binds to Tcf/Lef proteins and activates transcription of Wnt-responsive genes.
During axial myogenesis, Wnt/β-catenin signaling is important for dermomyotome and myotome formation. Dermomyotome formation requires Wnt/β-catenin signaling (Ikeya and Takada 1998; Linker et al. 2003; Schmidt et al. 2004), and Wnts also positively regulate the number of dermomyotomal Pax3+ and Pax7+ progenitors (Galli et al. 2004; Schmidt et al. 2004; Otto et al. 2006). Subsequently, Wnts are necessary and sufficient to promote normal Myf5 and MyoD expression (Munsterberg et al. 1995; Maroto et al. 1997; Tajbakhsh et al. 1998; Borello et al. 1999b; Schmidt et al. 2000; Linker et al. 2003; Galli et al. 2004; Chen et al. 2005; Brunelli et al. 2007). Myf5 is directly regulated by β-catenin, as two Tcf/Lef-binding sites in the Myf5 enhancer are essential for myotomal expression (Borello et al. 2006). In contrast, MyoD appears to be regulated predominantly via a Wnt/PKC-mediated pathway (Chen et al. 2005; Brunelli et al. 2007).
In the limb, Wnts and β-catenin have been found to play several, sometimes conflicting, roles in embryonic myogenesis. Several studies show that Wnts promote embryonic limb myogenesis (Anakwe et al. 2003; Geetha-Loganathan et al. 2005, 2006; Takata et al. 2007). However, others found that Tcf/Lef signaling inhibits differentiation of muscle progenitors (Miller et al. 2007). Wnts also regulate embryonic fiber type, via canonical and noncanonical signaling (Anakwe et al. 2003; Takata et al. 2007).
Despite this wealth of studies, several important questions remain unanswered. First, it is unresolved whether Wnt/β-catenin signaling is critical cell-autonomously within myogenic cells for skeletal myogenesis in vivo. Second, it is unclear whether β-catenin's function may differ between different phases of myogenesis.
In this study we used genetic lineage, ablation, and conditional mutagenesis in the mouse to analyze the developmental origin of limb muscle progenitors and test the role of β-catenin signaling in regulating the number and differentiation of these progenitors. We genetically labeled and ablated Pax3+ and Pax7+ cells in vivo to determine whether Pax3+ and/or Pax7+ cells are committed myogenic progenitors that give rise to and are required for limb embryonic and fetal muscle. We also tested whether embryonic and fetal limb myogenic cells in vivo have different cell-autonomous requirements for β-catenin by conditionally inactivating and activating β-catenin in Pax3+ and Pax7+ cells and their progeny. These studies demonstrate that limb embryonic and fetal myogenic cells develop from distinct, but related progenitors and have different cell-autonomous requirements for β-catenin.
Results
Pax3+Pax7− somitic cells give rise to all Pax7+ and embryonic myogenic cells, while Pax3+ and Pax7+ cells contribute to fetal myogenic cells in the limb
To test whether Pax3+ and Pax7+ somitic cells are the progenitors for embryonic and fetal myogenic cells in the limb, we conducted a lineage analysis of Pax3+ and Pax7+ cells. We genetically labeled Pax3+ or Pax7+ cells and their progeny using Pax3Cre (Engleka et al. 2005) or Pax7iCre (Keller et al. 2004) mice, in which Cre is faithfully expressed in all cells in which either Pax3 or Pax7 is normally expressed. These mice were crossed with R26RLacZ reporter mice (Soriano 1999), in which LacZ is expressed in response to Cre recombinase. In Pax3Cre/+;R26RLacZ/+ or Pax7iCre/+;R26RLacZ/+ mice Pax3+ or Pax7+ cells and their progeny are positive for β-galactosidase (β-gal) activity.
To confirm that Pax3+ and Pax7+ cells give rise to limb muscle and test the temporal–spatial relationship between the Pax3 and Pax7 lineages, we analyzed Pax3Cre/+;R26RLacZ/+ and Pax7iCre/+;R26RLacZ/+ E9–E14.5 embryos. Whole-mount analysis of Pax3Cre/+;R26RLacZ/+ embryos shows β-gal+ cells are present in somites by E9 (beginning in the newly most formed somite, somite stage I) and in limbs by E10.5 with strong expression in all limb muscles by E14.5 (Suppplmental Fig. 1A–C; data not shown). In Pax7iCre/+;R26RLacZ/+ embryos, a few β-gal+ cells are present in somites at E9.5 (beginning at somite stage X), with increasing numbers at E10.5, while β-gal+ cells are absent in the limbs at E10.5, just beginning at E11.5, and widely expressed in all limb muscles by E14.5 (Supplemental Fig. 1E–G; data not shown). Thus, both Pax3+ and Pax7+ cells contribute to all limb muscles by E14.5. However, Pax7-derived cells appear later in somites and limbs relative to Pax3-derived cells, consistent with previous Pax3 and Pax7 RNA expression studies (e.g., Relaix et al. 2004). To test whether Pax7+ cells may be derived from and a subset of Pax3-derived cells, we analyzed sections of E10.5–E14.5 Pax3Cre/+;R26RLacZ/+ embryos. In the limb all Pax7+ cells are β-gal+ and thus derived from Pax3+ cells (Fig. 1A–D; data not shown). Therefore, in the limb both Pax3+ and Pax7+ somitic cells contribute to all muscles by E14.5, and Pax7+ cells are derived from and a subset of the Pax3 lineage.
Figure 1.
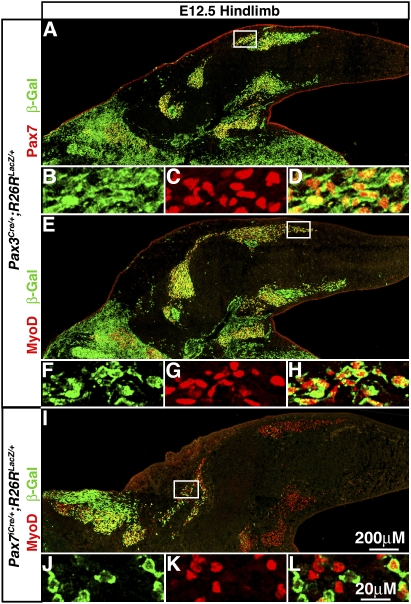
Pax3+ somitic cells give rise to all Pax7+ cells and embryonic myoblasts, while Pax7+ cells give rise to only a few myoblasts at E12.5 in the limb. (A–H) All Pax7+ cells and MyoD+ embryonic myoblasts are β-gal+ in E12.5 Pax3Cre/+;R26RLacZ/+ hindlimbs. (I–L) Only a few proximal MyoD+ myoblasts are β-gal+ in E12.5 Pax7iCre/+;R26RLacZ/+ hindlimbs.
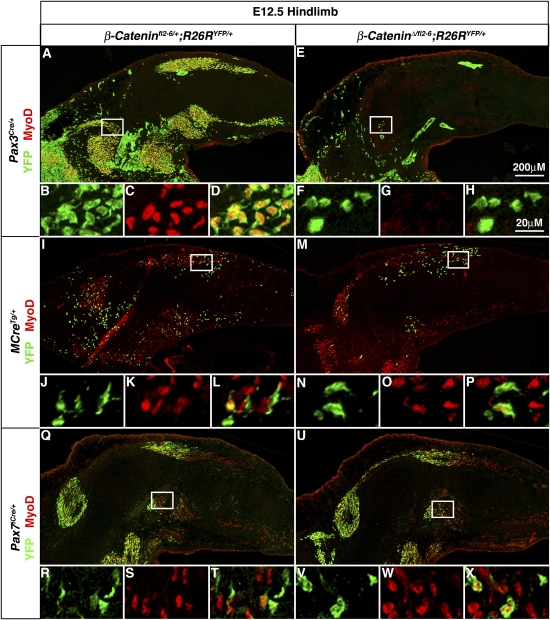
The temporal differences in Pax3 and Pax7 gene expression and lineage suggest that Pax3+ and Pax7+ cells could be differentially contributing to limb embryonic myogenic cells. Myoblasts and myofibers present in the limb between E10.5 and E12.5 have been classified as embryonic myogenic cells based on their distinctive in vitro characteristics (molecular markers distinguishing embryonic vs. fetal myogenic cells are only just starting to be identified) (Biressi et al. 2007a,b). Therefore, we examined hindlimb sections of E12.5 Pax3Cre/+;R26RLacZ/+ and Pax7iCre/+;R26RLacZ/+ embryos. In Pax3Cre/+;R26RLacZ/+ limbs all MyoD+ myoblasts (Fig. 1E–H) and myosin+ myofibers (Fig. 2A) are β-gal+ and thus derived from Pax3+ cells. In contrast, in E12.5 Pax7iCre/+;R26RLacZ/+ embryos, only a few MyoD+ myoblasts (Fig. 1I–L) and myosin+ myofibers (Fig. 2D) are β-gal+. This demonstrates that Pax3+ cells give rise to all embryonic myoblasts and myofibers, while Pax7+ cells give rise to few embryonic myogenic cells. This indicates that in the limb almost all embryonic myoblasts and myofibers have arisen directly from Pax3+ cells without ever having expressed Pax7.
Figure 2.
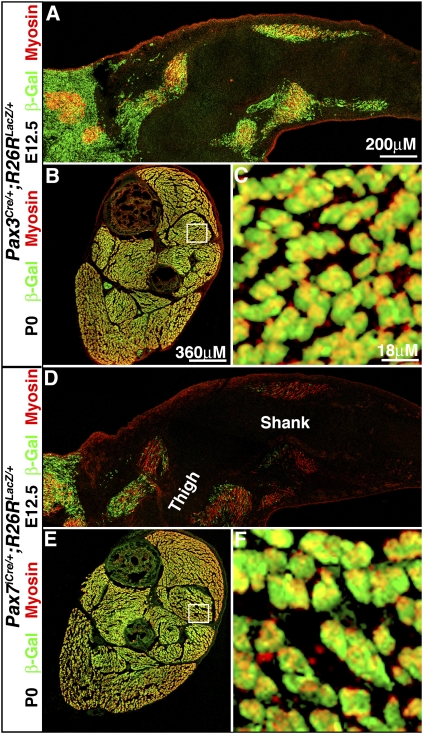
In the limb embryonic myofibers are derived from Pax3+Pax7− somitic cells, while fetal myofibers have contributions from Pax3+ and Pax7+ somitic cells. At E12.5 all embryonic myosin+ myofibers are β-gal+ in Pax3Cre/+;R26RLacZ/+ hindlimbs (A), while only a few proximal myosin+ myofibers are β-gal+ in Pax7iCre/+;R26RLacZ/+ hindlimbs (D). At P0 all fetal myosin+ myofibers are β-gal+ in Pax3Cre/+;R26RLacZ/+ (B,C) and Pax7iCre/+;R26RLacZ/+ (E,F) hindlimbs.
The whole-mount analysis of E14.5 Pax3Cre/+;R26RLacZ/+ and Pax7iCre/+;R26RLacZ/+ limbs suggests that both Pax3+ and Pax7+ cells may contribute to fetal myogenic cells. Myogenic cells present in the limb between E14.5 and E17.5 have been classified as fetal myogenic cells (Biressi et al. 2007a,b). We examined hindlimbs sections from newly born (P0) Pax3Cre/+;R26RLacZ/+ and Pax7iCre/+;R26RLacZ/+ mice, just at the end of fetal myogenesis. In both Pax3Cre/+;R26RLacZ/+ and Pax7iCre/+;R26RLacZ/+ limbs, all myosin+ myofibers are β-gal+ (Fig. 2B,C,E,F). This demonstrates that fetal myofibers have contributions from both Pax3+ and Pax7+ cells.
In summary, our lineage analysis shows that Pax3+ and Pax7+ cells contribute differentially to embryonic and fetal myogenic cells in the limb. Pax3+Pax7− cells give rise to all embryonic myoblasts and myofibers and also Pax7+ cells. Subsequently, all fetal myofibers have contributions from both Pax3+ and Pax7+ cells.
Pax3+, but not Pax7+ somitic cells are required for embryonic limb myogenesis
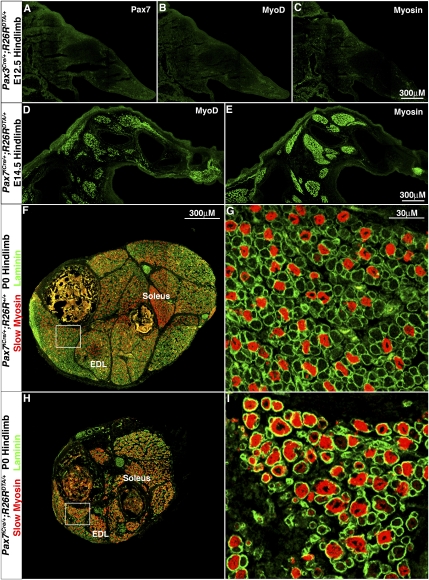
The finding that all embryonic myogenic cells in the limb are derived from Pax3+ cells, while only a few are derived from Pax7+ cells, suggests that the Pax3 lineage is required for embryonic myogenesis, while the Pax7 lineage is not. To test whether Pax3+ and/or Pax7+ cells and their progeny are required for embryonic limb myogenesis, we genetically ablated Pax3+ and Pax7+ cells. In Pax3Cre/+;R26RDTA/+ and Pax7iCre/+;R26RDTA/+ mice, Cre activates the expression of diphtheria toxin (DTA) and kills Pax3+ and Pax7+ cells (Wu et al. 2006). Pax3Cre/+;R26RDTA/+ embryos do not survive beyond E12.5. In these embryos, myosin+ myofibers are present in the head (data not shown), consistent with the lack of Pax3 expression in myogenic progenitors in head muscles (Horst et al. 2006). However, there is no Pax7, MyoD, or myosin expression in the limb or trunk (Fig. 3A–C; data not shown), indicating a complete lack of appendicular and axial myogenesis. Pax7iCre/+;R26RDTA/+ mice survive until birth. In contrast to Pax3Cre/+;R26RDTA/+, there is a normal embryonic pattern of MyoD+ myoblasts and myosin+ myofibers in E14.5 Pax7iCre/+;R26RDTA/+ hindlimbs, just at the end of embryonic myogenesis (Fig. 3D,E).
Figure 3.
In the limb Pax3+ somitic cells, but not Pax7+ somitic cells are required for embryonic myogenesis, while Pax7+ cells are required for fetal myogenesis. (A–C) E12.5 Pax3Cre/+;R26RDTA/+ hindlimb sections show a complete loss of Pax7+ progenitors (A), MyoD+ myoblasts (B), and myosin+ myofibers (C), indicating a complete loss of embryonic limb myogenesis when Pax3+ cells are ablated. (D,E) E14.5 Pax7iCre/+;R26RDTA/+ hindlimb sections show a normal pattern of embryonic MyoD+ myoblasts (D) and myosin+ myofibers (E) when Pax7+ cells are ablated. P0 Pax7iCre/+;R26RDTA/+ shank cross-section shows a basic muscle pattern (H,I), but muscles contain fewer laminin+ myofibers with a higher proportion of slow myosin+ myofibers compared with control Pax7iCre/+;R26R+/+ sections (F,G).
To rule out that the normal muscle pattern in Pax7iCre/+;R26RDTA/+ mice is due to the presence of surviving Pax7+ progenitors, we investigated the efficiency of ablation of Pax7+ cells. Previous studies (Wu et al. 2006; Haldar et al. 2008) demonstrated that the DTA in the R26RDTA/+ mice specifically kills all Cre+ cells within 24 h of Cre expression. In addition, Cre appears to be expressed in nearly all Pax7+ cells since in Pax7iCre/+;R26RLacZ/+ nearly all Pax7+ cells are β-gal+ (Supplemental Fig. 2A,B); the few cells that are Pax7+β-gal− are likely ones in which the reporter is not yet expressed. Likewise, in Pax7iCre/+;R26RDTA/+ there is almost a complete loss of Pax7+ cells (Supplemental Fig. 2C,D); the few Pax7+ are likely cells that have not yet been killed by the DTA. Thus, in the Pax7iCre/+;R26RDTA/+ mice, it is probable that all Pax7+ cells are being killed and the remaining embryonic myoblasts and myofibers are derived from Pax3+Pax7− progenitors.
These data demonstrate that the Pax3 lineage, but not the Pax7 lineage, is required to give rise to limb (and axial) embryonic myogenic cells. Therefore, during normal development Pax3+Pax7− somitic progenitors are essential for embryonic myogenesis. The Pax3+Pax7− progenitors are also required to give rise to Pax7+ muscle progenitors.
Limb fetal myogenesis is compromised in the absence of Pax7+ somitic cells
Our lineage analysis demonstrated that all fetal myofibers are derived from Pax3 and Pax7 progenitors, suggesting these progenitors are essential for fetal myogenesis. We tested whether Pax7+ progenitors are required for fetal myogenesis by examining P0 Pax7iCre/+;R26RDTA/+ mice, at the end of fetal myogenesis and just prior to their death (examination of the requirement for Pax3+ progenitors was precluded by the E12.5 death of Pax3Cre/+;R26RDTA/+ mice). The basic pattern of muscles is present in P0 Pax7iCre/+;R26RDTA/+ mice, but each muscle is much smaller and contains fewer myofibers (Fig. 3H vs. F; Supplemental Fig. 3). Also, a higher proportion of myofibers express slow MHC in most muscles (with the exception of the normally slow-enriched soleus) in the Pax7iCre/+;R26RDTA/+ compared with Pax7iCre/+;R26R+/+ (Fig. 3F–H; Supplemental Fig. 3). The proportion of slow myofibers in P0 limbs is determined by maturation of both embryonic and fetal muscle. Previous studies showed that all embryonic myofibers express slow myosin and then fibers destined to become fast gradually lose slow myosin (Condon et al. 1990; Gunning and Hardeman 1991). In contrast, most fetal myofibers never express slow (Kelly and Rubinstein 1980; Condon et al. 1990; Gunning and Hardeman 1991; Biressi et al. 2007a). Therefore, the increased proportion of slow myofibers in Pax7iCre/+;R26RDTA/+ mice likely results from a retention of slow-expressing embryonic myofibers and a decreased formation of fetal myofibers, which mainly do not express slow myosin. Taken together, the overall decreased number of myofibers and the increased proportion of slow myofibers in the Pax7iCre/+;R26RDTA/+ mice suggest that fetal myofibers have not differentiated and that Pax7+ progenitors are required for their differentiation.
Pax3+, but not Pax7+, somitic cells give rise to limb endothelial cells
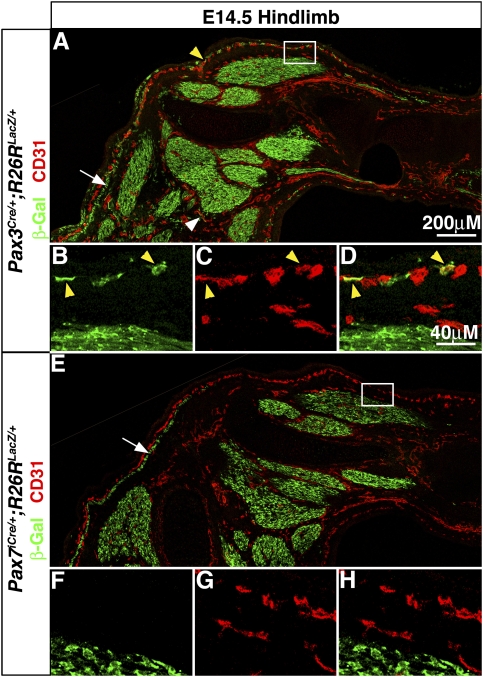
Our whole-mount analysis of Pax3Cre/+;R26RLacZ/+ limbs revealed obvious nonmyogenic β-gal+ cells that appeared to be associated with vessels in the shank and foot (Supplemental Fig. 1C,D). Previous studies have shown that somitic cells contribute to the endothelial cells of the limb vasculature (Pardanaud et al. 1996; He et al. 2003; Huang et al. 2003). Therefore, Pax3+ and/or Pax7+ somitic cells could give rise to limb endothelial cells. To test whether Pax3+ cells contribute to endothelial cells, we immunolabeled E14.5 sections of Pax3Cre/+;R26RLacZ/+ limbs with an endothelial marker, CD31. We found β-gal+CD31+ cells, indicating that Pax3+ cells give rise to endothelial cells (Fig. 4A–D). Most Pax3-derived CD31+ cells were found in superficial vessels directly below the epidermis and a few in deeper vessels. Overall, only a small proportion of limb endothelial cells were β-gal+ and therefore derived from Pax3+ somitic cells. The majority of limb endothelial cells appear to be derived from nonsomitic sources (Pardanaud et al. 1996). Nevertheless, the finding of Pax3-derived endothelial cells demonstrates that Pax3+ somitic cells are not committed to a myogenic fate in the limb, but are able to differentiate into either myogenic or endothelial cells. In contrast to Pax3, neither whole-mount nor section analysis of Pax7iCre/+;R26RLacZ/+ limbs (Fig. 4E–H; Supplemental Fig. 1G,H) revealed β-gal+ endothelial cells and indicates that Pax7+ somitic cells never differentiate into endothelial cells. This suggests that Pax7+ somitic cells may be restricted, and potentially committed, to a myogenic fate in the limb.
Figure 4.
Pax3+, but not Pax7+, somitic cells give rise to limb endothelial cells. (A–D) CD31+ endothelial cells superficial (yellow arrowheads) and adjacent (white arrowhead) to muscle are β-gal+ in E14.5 Pax3Cre/+;R26RLacZ/+ hindlimbs. (E–H) CD31+ endothelial cells are not β-gal+ in E14.5 Pax7iCre/+;R26RLacZ/+ hindlimbs. Arrows point to subcutaneous trunci muscle near superficial endothelial cells.
Wnt/β-catenin signaling is active in myogenic precursors and myoblasts
Wnt/β-catenin signaling is an important regulator of muscle development and is likely to be critical for embryonic and fetal limb myogenesis. To first determine whether canonical β-catenin signaling is active within myogenic cells, we examined BAT-gal mice, in which seven TCF/LEF-binding sites drive nuclear LacZ expression when bound by transcriptionally active TCF/LEFs (Maretto et al. 2003). At E12.5 Pax7+β-gal+ and MyoD+β-gal+ cells are found in the limb and somites (Supplemental Fig. 4; see also Borello et al. 2006), indicating that Wnt/β-catenin signaling is active in myogenic progenitors and myoblasts. Because not all Pax7+ and MyoD+ cells were β-gal+ and also BAT-gal mice are inefficient in reporting all cells with active Wnt/β-catenin signaling (Barolo 2006), it is unclear whether all or just some myogenic progenitors and myoblasts are active for Wnt/β-catenin signaling.
β-catenin is required in Pax3-derived somitic cells for dermomyotome and myotome formation, but not subsequently in Pax7-derived cells for embryonic axial myogenesis
As Pax3 is initially expressed in the somites and Wnt/β-catenin signaling is active there, we first tested whether β-catenin is required cell-autonomously within the Pax3 lineage for somite development. We generated Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice in which both alleles of β-catenin have been inactivated, via excision of exons 2–6 (Brault et al. 2001), in all Pax3+ cells and their progeny (the Δ allele is constitutively inactivated, while the fl2–6 allele is inactivated in response to Cre). These mice also contained the Cre-responsive R26RYFP/+ reporter (Srinivas et al. 2001) and allowed us to visualize cells, via expression of YFP, in which Cre has been expressed and β-catenin inactivated. Pax3Cre/+;β-cateninfl2–6/+;R26RYFP/+ mice, in which only one allele of β-catenin is excised in response to Cre, serve as heterozygous controls and are indistinguishable from wild type. Cre-mediated reporter expression is present and β-catenin is inactivated in the somites by E9. Sections through E10.5 somites of Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice reveal that the somites are disorganized with poorly formed dermomyotomes and fewer Pax7+ progenitors (Supplemental Fig. 5A–H). Since Pax3 and Paraxis are required for formation of epithelial somites and proper dermomyotomes (Burgess et al. 1996; Relaix et al. 2004), we analyzed by whole-mount in situ hybridization expression of Pax3 (Supplemental Fig. 5I) and Paraxis (data not shown). Expression of both genes initiates in the psm and early somites (prior to Cre-mediated inactivation β-catenin), but is not maintained in mature somites (after β-catenin inactivation) in Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice. In addition, analysis of MyoD and Myf5 (Supplemental Fig. 5K,L) and myosin (data not shown) of Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice show a complete absence of the myotome with no Myf5+ or MyoD+ myoblasts or myosin+ myofibers. We did not see a significant increase in cell death or change in proliferation (as assayed by TUNEL and phospho-histone H3) (data not shown) in the somites of these mice. These data demonstrate that β-catenin is required within Pax3-derived cells for maintenance of Pax3 and Paraxis somitic expression, proper dermomyotome formation, and the subsequent differentiation of myoblasts and myofibers within the myotome.
We also tested whether β-catenin may be required subsequently for formation of embryonic axial muscles. To test this we generated Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice in which the later expression of Pax7 drives Cre recombination and widespread inactivation of β-catenin at E10.5 in the somites. Surprisingly, at E14.5 (at the end of embryonic myogenesis) axial myogenesis appears unperturbed, with a normal pattern of MyoD+ myoblasts (Supplemental Fig. 5M–T) and myosin+ myofibers (data not shown). Because these myoblasts and myofibers are YFP+, these myogenic cells are Pax7-derived and have Cre-mediated inactivation of β-catenin. The presence of myoblasts and myofibers despite their cell-autonomous inactivation of β-catenin demonstrates that β-catenin, although required for dermomyotome and myotome formation, is not required subsequently for embryonic axial myogenesis.
β-catenin is required in the somite for delamination of limb myogenic progenitors, but not required for embryonic limb myoblasts and myofibers
The disorganization of the dermomyotome in the Pax3Cre/+;β-cateninΔ/fl2–6/+;R26RYFP/+ mice suggests that β-catenin may be required for formation of the ventrolateral dermomyotome and delamination of limb myogenic progenitors. Indeed in E12.5–E14.5 Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice inactivation of β-catenin in Pax3-derived cells results in the complete absence of Pax7+ myogenic progenitors, MyoD+ myoblasts, or myosin+ myofibers in the limb (Fig. 5A–H; Supplemental Fig. 6A–P). The lack of any myogenic cells in the limb suggests that Pax3-derived myogenic progenitors did not delaminate from the dermomyotome ventrolateral lip. This delamination process requires the ligand Hgf, expressed in the limb, to signal to its receptor Met, expressed in ventrolateral somitic cells (Dietrich et al. 1999). In the absence of either Hgf or Met, somitic cells fail to delaminate from the ventrolateral dermomyotome and no limb myogenesis occurs (Bladt et al. 1995; Maina et al. 1996). In Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice expression of Met initiates in the most newly formed somites (prior to complete inactivation of β-catenin) (arrow in Supplemental Fig. 5J), but fails to be maintained in more mature somites and limb myogenic progenitors (after complete inactivation of β-catenin). This loss of Met expression is likely to result from the loss of Pax3 in the somites, as it has been previously shown that Met expression is dependent on activation by Pax3 (Epstein et al. 1996). Therefore, β-catenin is required in the somites to maintain Pax3 expression and Met+ ventrolateral dermomyotomal cells that delaminate and migrate into the limb.
Figure 5.
β-Catenin is required early in Pax3-derived somitic cells for generation of limb myogenic cells, but is not required in Pax3-derived migrating progenitors for specification of embryonic myoblasts. Loss of β-catenin in Pax7-derived cells does not affect myoblast specification. During embryonic myogenesis at E12.5 mutant Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ hindlimbs (E–H) contain many fewer YFP+ cells in the limb and none give rise to MyoD+ myoblasts as compared with heterozygous control Pax3Cre/+;β-cateninfl2−6/+;R26RYFP/+ hindlimbs in which many YFP+ cells are present that give rise to YFP+MyoD+ myoblasts (A–D). In E12.5 mutant MCreTg/+;β-cateninΔ/fl2–6;R26RYFP/+ hindlimbs (M–P) in which β-catenin is inactivated in Pax3-derived cells after delamination from the somite, YFP+ MyoD+ myoblasts are present similar to the heterozygous control MCreTg/+;β-cateninfl2–6/+;R26RYFP/+ hindlimbs (I–L). In E12.5 mutant Pax7iCre/+;β-cateninΔ/fl2–-6;R26RYFP/+ hindlimbs (U–X) YFP+MyoD+ myoblasts are present in similar numbers as found in heterozygous Pax7iCre/+;β-cateninfl2–6/+;R26RYFP/+ hindlimbs (Q–T).
Analysis of the BAT-gal reporter mice suggested canonical β-catenin signaling is active within myogenic cells in the limb. However, the requirement for β-catenin early in the somites precluded analysis of later roles for β-catenin in limb myogenesis using Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice. Therefore we analyzed MCreTg/+;β-cateninΔ/fl2–6;R26RYFP/+ mice. MCre mice have a transgene containing a Pax3 enhancer element and proximal promoter expressed only in the ventrolateral lip of the dermomyotome, the body wall, and limb myogenic cells (Brown et al. 2005) and resulting in Cre-mediated recombination in newly delaminated myogenic cells and their progeny (data not shown). In MCreTg/+;β-cateninfl2–6/+;R26RYFP/+ mice, in which one allele of β-catenin is inactivated, YFP+MyoD+ myoblasts (as well as YFP+Pax7+ muscle progenitors and YFP+ myofibers) (data not shown) are present (Fig. 5I–L). The efficiency of labeling limb myogenic cells in these mice is considerably lower and more variable than Pax3Cre/+;R26RYFP/+ mice perhaps because not all elements necessary for Pax3 expression in the hypaxial somitic cells are present in the transgene (Schienda et al. 2006). Nevertheless, in MCreTg/+;β-cateninΔ/fl2–6;R26RYFP/+ mice, in which both β-catenin alleles are inactivated, YFP+MyoD+ myoblasts (as well as YFP+Pax7+ muscle progenitors and YFP+ myofibers) (data not shown) are present (Fig. 5M–P). This indicates that once in the limb β-catenin is not required within Pax3-derived cells for specification of embryonic myoblasts and differentiation of myofibers. Whether the total number of myoblasts and myofibers differs from wild-type levels is difficult to evaluate because of the inefficiency of the MCre driver.
β-catenin is not necessary for Pax3-derived limb endothelial cells, nor is it sufficient to drive Pax3+ somitic cells to a myogenic fate
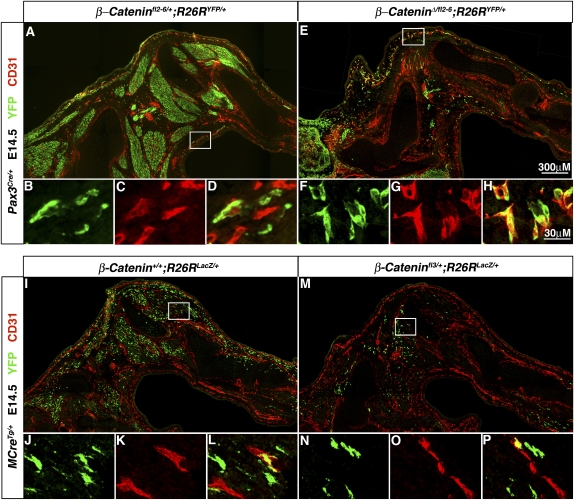
Our lineage analysis revealed that Pax3+ somitic cells give rise to limb myogenic and endothelial cells. Potentially, β-catenin is required in Pax3+ somitic cells for generation of both limb myogenic progenitors and endothelial progenitors. However, in Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice YFP+CD31+ endothelial cells are present in the limb (Fig. 6A–H). This indicates that, unlike myogenic progenitors, Pax3-derived endothelial progenitors do not cell-autonomously require β-catenin when residing within the somites or subsequently when in the limb for delamination, migration, or differentiation into endothelium. Furthermore, because Met is absent in the Pax3Cre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice, this also indicates that, unlike myogenic progenitors, limb endothelial progenitors do not require Met for delamination.
Figure 6.
β-Catenin is not necessary for Pax3-derived limb endothelial cells, nor is it sufficient to drive Pax3+ somitic cells to a myogenic fate. (A–D) In E14.5 Pax3Cre/+;β-cateninfl2–6/+;R26RYFP/+ hindlimbs YFP+CD31+ endothelial cells are present. (E–H) In Pax3Cre/+;β-cateninΔ/fl6;R26RYFP/+ hindlimbs many fewer YFP+ cells are present in the limb but these still give rise to YFP+CD31+ endothelial cells. (I–L) In E14.5 MCreTg/+;β-catenin+/+;R26RLacZ/+ hindlimbs Pax3-derived cells expressing Cre after delamination from the somite give rise to YFP+CD31+ endothelial cells. (M–P) In MCreTg/+;β-cateninfl3/+;R26RLacZ/+ hindlimbs Pax3-derived cells still give rise YFP+CD31+.
Because β-catenin is required for generation of limb myogenic but not endothelial cells from the somite, we hypothesized that β-catenin signaling may act in Pax3+ somitic cells as a molecular switch between myogenic and endothelial cell fates. To test this we conducted a gain-of-function experiment in which β-catenin is conditionally, constitutively activated via a β-cateninfl3/+ allele (Harada et al. 1999). In the β-cateninfl3/+ allele, the presence of Cre causes deletion of exon 3 and the formation of a stabilized, constitutively activated form of β-catenin. Pax3Cre/+;β-cateninfl3/+;R26RYFP/+ mice die at E11.5 (prior to the presence of Pax3-derived CD31+ cells in the limb at E12.5), and so we analyzed MCreTg/+;β-cateninfl3/+;R26RYFP/+ mice. If β-catenin indeed mediates between a myogenic versus an endothelial fate, we anticipated that activation of β-catenin would drive all somitic progenitors in the limb to a myogenic fate at the expense of an endothelial fate. In contrast, in both E14.5 MCreTg/+;β-catenin+/+;R26RYFP/+ and MCreTg/+;β-cateninfl3/+;R26RYFP/+ mice we found YFP+CD31+ endothelial cells (Fig. 6I–P). The presence of MCre-derived endothelial cells even in the presence of constitutively active β-catenin demonstrates that β-catenin is not sufficient to drive somitic cells in the limb to a myogenic fate.
β-catenin is critical for fetal myogenesis in the limb
Although β-catenin is not required for embryonic myogenesis once somitic progenitors have entered the limb, β-catenin may be critical subsequently for fetal myogenesis. To test this, we analyzed Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ and Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ mice at P0 (both genotypes die at birth) in which β-catenin has been either inactivated or constitutively activated in Pax7-derived cells. In Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ and Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ limbs all myofibers are YFP+ (data not shown), indicating that Pax7-derived cells contributed to fetal myofibers, despite the inactivation or activation of β-catenin (Supplemental Fig. 2E–I). In Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice the overall pattern of muscles in the limb is unaffected (Supplemental Fig. 7A). However, individual muscles contain significantly fewer myofibers and in most muscles significantly fewer of the myofibers are slow (Fig. 7A; Supplemental Fig. 7D, 8, 9). In contrast, in Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ mice the pattern of muscles was highly perturbed (Fig. 7C; Supplemental Fig. 7C) with many of the muscle boundaries obscured and myofibers misoriented. There are also fewer myofibers but almost all myofibers are slow, although the irregular fiber orientations make this difficult to quantify (Fig. 7C; Supplemental Fig. 7F). In the Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ and Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ mice defects in muscle pattern, myofiber number, and/or type arose during fetal myogenesis. Muscle pattern is normal in E14.5 Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ mice (data not shown), and a normal pattern of myoblasts (Fig. 5U–X) and myofibers (Supplemental Fig. 6U–X) is present at E12.5–E14.5 in Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice. It is likely that embryonic muscle is not affected at E12 because Cre-mediated deletion of β-catenin is just beginning and at E14.5 multinucleate myofibers contain myonuclei derived from Pax7+ precursors in which β-catenin has been deleted as well as Pax3+Pax7− precursors in which β-catenin is still expressed. Overall, our data show that during fetal myogenesis β-catenin affects myofiber number and positively regulates the number of slow fibers.
Figure 7.
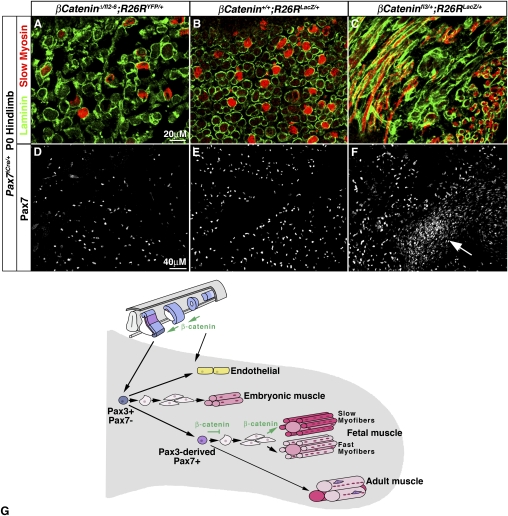
β-Catenin regulates progenitor number and myofiber number and type during fetal myogenesis in the limb. In P0 Pax7iCre/+;β-cateninΔ/fl6;R26RYFP/+ hindlimbs inactivation of β-catenin in Pax7-derived fetal muscle leads to fewer overall laminin+ myofibers with a lower proportion of slow myosin+ myofibers (A vs. B) but relatively the same number of Pax7+ progenitors (D vs. E) as compared with heterozygous Pax7iCre/+;β-cateninfl2–6/+;R26RYFP/+. In P0 Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ constitutive activation of β-catenin leads to a disorganized pattern of myofibers, fewer overall laminin+ myofibers with a higher proportion of slow myosin+ myofibers (C vs. B), and more Pax7+ progenitors (F vs. E) as compared with Pax7iCre/+;β-cateninfl2–6/+;R26RYFP/+. (Arrow in F) Dense concentrations of Pax7+ progenitors are frequently found in Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ limbs. (G) Model of embryonic and fetal limb myogenesis.
Examination of Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ and Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ P0 mice also demonstrates that β-catenin positively regulates the number of Pax7+ myogenic progenitors during fetal myogenesis. Significantly more Pax7+ cells are present in Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ versus Pax7iCre/+;β-catenin+/+;R26RYFP/+ mice, while the number of Pax7+ cells is not detectably changed in Pax7iCre/+;β-cateninΔ/fl2–6;R26RYFP/+ mice (Fig. 7D–F; Supplemental Figs. 7G–I, 10). Interestingly, in the Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ mice there were regions of dense concentrations of Pax7+ cells (arrows in Fig. 7F; Supplemental Fig. 7I). An analysis of Pax7+ cell proliferation (PHH3+Pax7+ cells/Pax7+ cells) was inconclusive, as we found high variability both within and between individuals of all genotypes. The dense concentrations of Pax7+ cells in Pax7iCre/+;β-cateninfl3/+;R26RYFP/+ mice did not have high levels of PHH3+ cells (data not shown). Also, an analysis of apoptosis (via TUNEL) did not reveal significant levels of cell death in any of the three genotypes (data not shown). Overall, our data show that β-catenin cell-autonomously positively regulates the number of Pax7+ muscle progenitors during fetal myogenesis. In particular, activated β-catenin is sufficient to increase the number of progenitors, but β-catenin itself is not required for maintenance of Pax7+ progenitors.
Discussion
Pax3+ somitic cells give rise to muscle and endothelium, while Pax7+ somitic cells are committed to myogenesis
Pax3 and Pax7 are important regulators of myogenesis and mark myogenic progenitors. To determine the relationship between the Pax3 and Pax7 lineages in the limb and test whether Pax3+ and Pax7+ cells are committed myogenic progenitors we genetically labeled and ablated Pax3+ and Pax7+ cells. Our lineage analysis reveals that Pax3+ somitic cells in the limb contribute to both muscle and endothelial cell lineages. Transplantation studies had established that somitic cells contribute to both limb muscle and endothelial cells (Pardanaud et al. 1996; He et al. 2003; Huang et al. 2003), and chick lineage studies showed that even single somitic cells are bipotential, contributing to muscle and endothelium (Kardon et al. 2002). Here we show that Pax3+ somitic cells are bipotential in the limb. Thus, even though Pax3 is a member of the genetic cascade committing cells to myogenesis (Maroto et al. 1997; Tajbakhsh et al. 1997; Bajard et al. 2006; Buchberger et al. 2007), in vivo expression of Pax3 in somitic cells is not sufficient to commit these cells to a myogenic fate.
We also found that Pax7+ cells are a subset of Pax3+ somitic cells and only give rise to myogenic, and not endothelial cells. Our ablation studies demonstrate that the Pax3 lineage is required for the emergence of Pax7+ cells, even though Pax3 function is not required for the specification of Pax7+ cells (Pax7+ cells are present in axial muscles of Pax3−/− mice) (Kassar-Duchossoy et al. 2005). Subsequently, Pax7 somitic derivatives appear to be restricted, and therefore potentially committed, to the muscle lineage. Whether the expression of Pax7 itself commits Pax3+ cells to myogenesis or simply marks committed cells is unclear. Pax7 regulates Myf5 expression (McKinnell et al. 2008), and myogenesis (with the exception of the primary myotome) requires either Pax3 or Pax7 expression (Relaix et al. 2005). Recently Pax7 has been shown to be more stable than Pax3, which is subject to monoubiquitination and proteasomal degradation (Boutet et al. 2007). An attractive hypothesis is that Pax3 expression initially establishes intermediate, bipotential precursors (see Lang et al. 2005; Boutet et al. 2007). Given the right extrinsic cues, some of these Pax3+ cells differentiate into muscle (or endothelium). However, Pax3+ cells that do not differentiate subsequently express the more stable Pax7, committing these cells to a myogenic fate via Pax7 activation of Myf5 expression.
Pax3+Pax7− somitic cells are required for embryonic myogenesis, while Pax3-derived Pax7+ somitic cells are required for fetal myogenesis in the limb
Recent studies have argued that Pax3+/Pax7+ cells are common muscle progenitors for embryonic and fetal myoblasts and muscle (Kassar-Duchossoy et al. 2005; Relaix et al. 2005). We used genetic lineage and ablation studies to fully assess the contribution of Pax3+ and Pax7+ cells to limb muscle and find that these two lineages contribute differentially to embryonic and fetal limb myogenesis.
Our lineage analysis of Pax3+ and Pax7+ cells found that all limb embryonic muscle is derived from Pax3+ cells. Moreover, the loss of all embryonic myofibers when Pax3+ cells are ablated demonstrates that these cells are required for limb embryonic myogenesis. Ablation of Pax3+ cells also leads to loss of all axial muscles. Thus, although Pax3 function is only required for limb muscle development (all limb muscle is lost in Pax3−/− mice) (Relaix et al. 2004 and references therein), Pax3+ cells are required for both limb and trunk muscle development. We also find that Pax7+ cells contribute little to and are not required for embryonic muscle in the limb. This shows that, although Pax7+ cells later contribute to all myofibers during fetal myogenesis, Pax3+Pax7− cells set up the embryonic muscle pattern. Similar to the founder model of Drosophila muscle development (Maqbool and Jagla 2007), the Pax3+Pax7− cells act as founder cells, establishing an initial muscle template on which Pax7+ cells subsequently fuse.
Pax7+ cells give rise to and are required for fetal myogenic cells. Pax7+ cells are Pax3-derived and do not necessarily express Pax3, as Pax3 is generally no longer expressed after E13.5. The loss of fetal myofibers when the Pax7 lineage is ablated highlights the important role of Pax7+ cells for fetal myogenesis. This role is consistent with expression and microarray studies showing that Pax7 is expressed in fetal myoblasts (Horst et al. 2006; Biressi et al. 2007b). However, this role has not been revealed by analysis of Pax7−/− mice, in which fetal myogenesis appears normal although adult satellite cells are not maintained and adult myogenesis and regeneration are severely compromised (Seale et al. 2000; Oustanina et al. 2004; Kuang et al. 2006; Relaix et al. 2006). During fetal myogenesis either Pax7's function is not essential or is compensated by other proteins. Nevertheless, clearly, Pax7+ cells are required for fetal growth and fiber-type maturation.
β-catenin is required for dermomytome and myotome formation, but not subsequently for embryonic axial myogenesis
Multiple studies have demonstrated that Wnt/β-catenin signaling is important for axial and limb myogenesis. Nevertheless, still unanswered is whether β-catenin signaling is required cell-autonomously in vivo for embryonic and fetal myogenesis. To address these questions, we first demonstrated that Wnt/β-catenin signaling is active in myogenic progenitors and myoblasts. We then tested genetically whether β-catenin is critical for myogenesis by conditional inactivation or constitutive activation of β-catenin within the Pax3 and Pax7 lineages.
During axial muscle development, β-catenin is required within the Pax3 lineage for dermomyotome formation. Inactivation of β-catenin in the most newly formed somites in Pax3Cre/+;β-cateninΔ/fl2–6 mice resulted in disorganized somites with poorly formed dermomyotomes. The poor formation of epithelial dermomyotomes suggests that β-catenin's cell adhesion function may be critical. Alternatively, β-catenin's function in Wnt signaling may be critical. Similar dermomyotome defects have been found in Wnt1−/−;Wnt3a−/− mice (Ikeya and Takada 1998) as well as embryos in which Wnt/β-catenin/Lef1 signaling is blocked (Schmidt et al. 2004; Linker et al. 2005). The dermomyotomal defects in Pax3Cre/+;β-cateninΔ/fl2–6 mice may be mediated by Pax3 and Paraxis. Previous studies showed that these genes are critical for proper formation of epithelial somites and dermomyotomes (Burgess et al. 1996; Relaix et al. 2004), and Pax3 and Paraxis expression is not maintained in somites of Pax3Cre/+;β-cateninΔ/fl2–6 mice. These data, combined with expression studies (e.g., Tajbakhsh et al. 1998; Borello et al. 1999a, 2006), suggest that Wnts, produced in the neural tube and ectoderm, activate β-catenin/Lef1 signaling within newly formed somites, which regulates Paraxis and Pax3 expression and is critical for proper dermomyotome formation.
Inactivation of β-catenin in the most newly formed somites also ablated the myotome, MyoD and Myf5 expression, and subsequently all axial muscle. The loss of MyoD and Myf5 is consistent with Myf5 and MyoD down-regulation in the myotome of Wnt1−/−;Wnt3a−/− mice and in psm explants in which Wnt/β-catenin signaling has been blocked (Borello et al. 1999b; Linker et al. 2003; Brunelli et al. 2007). However, the complete loss of MyoD is unexpected given recent explant data suggesting that MyoD expression is predominantly regulated by a Wnt/PKC pathway (Chen et al. 2005; Brunelli et al. 2007). Multiple mechanisms may explain why β-catenin is required for both myotomal Myf5 and MyoD. Two Tcf/Lef-binding sites in the early epaxial enhancer of Myf5 are critical for myotomal Myf5 expression (Borello et al. 2006). However, the incomplete loss of Myf5 after deletion of these binding sites, as compared with the complete loss of Myf5 in the Pax3Cre/+;β-cateninΔ/fl2–6 mice, suggests that β-catenin may also be regulating Myf5 via other mechanisms. MyoD is not regulated by direct β-catenin/Tcf/Lef transcriptional activation as no Tcf/Lef-binding sites have been found in the core enhancer controlling MyoD expression (Kucharczuk et al. 1999). Paraxis may be an important mediator between β-catenin signaling and Myf5 and MyoD expression; Paraxis is down-regulated in Pax3Cre/+;β-cateninΔ/fl2–6 mice and in Paraxis−/− mice Myf5 and MyoD are down-regulated (Burgess et al. 1996; Wilson-Rawls et al. 1999). Nevertheless, the incomplete loss of Myf5 and MyoD in Paraxis−/− mice suggests that loss of Paraxis cannot explain the complete loss of Myf5 and MyoD in Pax3Cre/+;β-cateninΔ/fl2–6 mice. Finally, Pax3 may also mediate the loss of Myf5 and MyoD expression; Pax3 expression is not maintained in Pax3Cre/+;β-cateninΔ/fl2–6 mice, and Pax3 has been shown to bind and activate Myf5 and MyoD enhancers (Bajard et al. 2006; Hu et al. 2008). Therefore, β-catenin is likely playing multiple roles in myotome formation, and it is loss of all these functions that results in the complete loss of myotomal Myf5 and MyoD. The consequence of both the loss of the dermomyotome, which gives rise to later axial muscle progenitors (Gros et al. 2005; Relaix et al. 2005), and the myotome, which serves as a scaffold for these progenitors, is the subsequent loss of all axial muscles in Pax3Cre/+;β-cateninΔ/fl2–6 mice.
After dermomyotome and myotome formation, surprisingly, β-catenin is not required within myogenic cells for embryonic axial myogenesis. In Pax7iCre/+;β-cateninΔ/fl2–6 mice, β-catenin is deleted in Pax7+ progenitors and their derivatives in stage X somites, in which the dermomyotome and myotome have already formed. Despite the loss of β-catenin in the Pax7 lineage, embryonic axial myogenesis is normal, indicating that β-catenin is not required at this later stage of myogenesis. Thus, although many experiments demonstrate that Wnt/β-catenin signaling is important for axial myogenesis (for review, see Cossu and Borello 1999; Borycki and Emerson 2000), we find that β-catenin is essential for dermomyotome and myotome development but not critical for embryonic axial myogenesis. Previous explant and in vivo chick experiments all manipulate Wnt/β-catenin signaling in the psm and early somites, thus affecting dermomyotome, myotome, and subsequent axial myogenesis. Our results emphasize the difference between the developmental requirements for the myotome versus those for later embryonic axial myogenesis. Such differences are also evident in the Myf5 enhancer where the early epaxial enhancer only drives expression in the myotome (Teboul et al. 2002); in Pax3−/−;Pax7−/− mutants where the primary myotome is formed, but subsequent axial myogenesis is ablated (Relaix et al. 2005); and in Myf5−/−;Mrf4−/− mice where the myotome fails to form, but subsequent myogenesis is relatively normal (Kassar-Duchossoy et al. 2005).
Pax3-derived limb endothelial cells do not require β-catenin
We found that Pax3-derived limb endothelial cells migrate and differentiate independent of β-catenin. In Pax3Cre/+;β-cateninΔ/fl2–6 mice, despite the absence of a dermomyotome and Met expression, endothelial progenitors migrate from the somites into the limb. Similarly, Geetha-Loganathan et al. (2006) have found in chick endothelial cells do not, while myogenic cells do, require Wnt6 for their emigration from the somites into the limb. Pax3-derived endothelial progenitors may be intrinsically different from myogenic progenitors within the somite and thus not require the dermomyotomal lip and Met expression for their migration. However, the finding of individual somitic cells that give rise to endothelial and myogenic cells argues against this interpretation (Kardon et al. 2002). Alternatively, Pax3+ cells migrating (perhaps earlier) from the somites independent of the dermomyotomal lip and Met enter a limb environment that promotes an endothelial fate. Subsequently, once in the limb Pax3-derived endothelial cells do not require β-catenin for their differentiation, nor does β-catenin promote a myogenic versus an endothelial cell fate.
Once in the limb, myogenic cells do not require β-catenin for embryonic myogenesis but require β-catenin for fetal myogenesis
β-catenin plays several cell-autonomous roles during limb myogenesis. First within the somite β-catenin is required for formation of the dermomyotome and maintenance of Pax3 and Met expression, which are prerequisites for delamination of limb myogenic cells (Bladt et al. 1995; Maina et al. 1996; Dietrich et al. 1999; Relaix et al. 2003, 2004). Subsequently, once in the limb embryonic and fetal myogenic cells have different requirements for β-catenin.
Limb myogenic cells do not require β-catenin for specification of embryonic myoblasts and differentiation of myofibers. We found, via analysis of BAT-gal embryos, that canonical β-catenin signaling is active in limb myogenic progenitors and myoblasts. Yet, deletion of β-catenin cell-autonomously within limb myogenic cells had no obvious effect on embryonic myoblast specification and myofiber differentiation. This indicates that, despite the presence of active β-catenin signaling within some myogenic cells, during normal limb development β-catenin is not critical for embryonic myogenesis. It is possible that β-catenin regulates the overall number of limb embryonic myoblasts and myofibers, but this possibility could only be evaluated with a more robust Cre driver (e.g., a tamoxifen-inducible Pax3CreERT2).
During fetal myogenesis, β-catenin is critical for determining the number of Pax7+ progenitors and myofibers. β-Catenin positively regulates the overall number of Pax7+ progenitors, but did not detectably or consistently regulate their rate of proliferation or cell death. Recent in vitro studies on adult satellite cells show that nuclear, dephosphorylated β-catenin is present in activated satellite cells and Wnts and β-catenin promote self-renewal of Pax7+ satellite cells (Otto et al. 2008; Perez-Ruiz et al. 2008; but see Brack et al. 2008). Because β-catenin either does not consistently affect (our finding) or slows (Perez-Ruiz et al. 2008) Pax7+ cell proliferation and does not alter apoptosis (our finding; Perez-Ruiz et al. 2008), this suggests that β-catenin may expand the number of Pax7+ fetal myoblasts or satellite cells by preventing their differentiation. The frequent appearance of dense clusters of Pax7+ cells that are not highly proliferative in Pax7iCre/+;β-cateninfl3/+ mice supports this notion. We also found that the overall myofiber number decreased when either β-catenin was deleted or constitutively activated in Pax7-derived cells. Myofiber number is determined by several factors: progenitor number, rate of proliferation, and rate of differentiation and fusion. Fewer myofibers may be present in Pax7iCre/+;β-cateninΔ/fl2–6 mice because progenitors prematurely differentiate (as found in vitro by Gavard et al. 2004; Perez-Ruiz et al. 2008), ultimately resulting in the formation of fewer myofibers. In Pax7iCre/+;β-cateninfl3/+ mice, reduction in myofibers is likely due to a block in progenitor differentiation and fusion, as constitutive activation of β-catenin has been shown in vitro to block myogenic differentiation and fusion (Goichberg et al. 2001; Gavard et al. 2004; Perez-Ruiz et al. 2008). Mechanistically how β-catenin is functioning is unclear. Currently, there are multiple proposals for how β-catenin regulates adult myogenesis that may be applicable to fetal myogenesis. β-Catenin can function via canonical Wnt/β-catenin signaling (Brack et al. 2008; Otto et al. 2008; Perez-Ruiz et al. 2008), but can also regulate myogenesis by direct modification of MRF transcriptional activity (Pan et al. 2005; Kim et al. 2008) and by modification of adherens junctions and cell adhesion (Goichberg et al. 2001; Gavard et al. 2004; Nastasi et al. 2004; Kramerova et al. 2006).
Finally, we also found that β-catenin positively modulates the number of slow myofibers during fetal myogenesis. How β-catenin regulates the expression of slow myosin is potentially complex. β-Catenin may be negatively regulating the maturation of embryonic myofibers so that more retain slow myosin, positively regulating fetal myofibers so that more acquire slow myosin, and/or increasing the relative proportion of slow myosin+ embryonic myofibers versus slow myosin− fetal myofibers.
Model of embryonic and fetal myogenesis in the limb
Vertebrate myogenesis occurs in three successive phases: embryonic, fetal, and adult. The embryonic, fetal, and adult myoblasts responsible for these phases of myogenesis are distinct. However, it has been unclear whether these classes of myoblasts arise from common or different progenitors. From our lineage and ablation studies a model of the developmental origin of embryonic and fetal myogenic cells in the limb emerges (Fig. 7G). Pax3+Pax7− somitic cells initially entering the limb are bipotential progenitors and contribute to limb muscle and endothelium. These cells give rise to and are required for embryonic myogenesis. Pax3+ cells also give rise to Pax7+ cells. These Pax3-derived, Pax7+ cells give rise to and are required for fetal myogenesis. Subsequently, some of these Pax3-derived, Pax7+ cells also give rise to adult satellite cells, critical for adult myogenesis (Gros et al. 2005; Kassar-Duchossoy et al. 2005; Relaix et al. 2005; Schienda et al. 2006). Thus, in the limb embryonic and fetal myoblasts arise from developmentally distinct, although related, progenitors. Future in vitro and expression studies using recently identified genes expressed in embryonic versus fetal myoblasts (Biressi et al. 2007b) will further test whether Pax3+Pax7− cells are embryonic myogenic progenitors, while Pax3-derived Pax7+ cells are fetal myogenic progenitors.
During development, functional contractile, post-mitiotic myofibers must differentiate to allow for movement, while proliferating progenitors must be maintained for growth. Therefore, in the same environment some myogenic progenitors must differentiate, while others must maintain their proliferative status. It has been hypothesized that embryonic, fetal and adult progenitors and/or myoblasts are intrinsically different so that these cells will respond differently to similar environmental signals (Biressi et al. 2007a,b). For instance, in vitro TGFβ inhibits differentiation of fetal but not embryonic myoblasts (Cusella-De Angelis et al. 1994). Here we show in vivo that the different progenitor populations have different cell-autonomous requirements for β-catenin (Fig. 7G). In the somite β-catenin is required in Pax3-derived cells for dermomyotome and myotome formation and delamination of myogenic, but not endothelial, progenitors. Once in the limb Pax3+Pax7− cells and their progeny do not require β-catenin for embryonic myoblast specification and myofiber differentiation. During fetal myogenesis β-catenin is critical within the Pax7 lineage for determining progenitor number and myofiber number and type. If β-catenin is functioning via canonical Wnt/β-catenin signaling this suggests that the fetal Pax7 lineage is sensitive to Wnts. In contrast, the embryonic Pax3+Pax7− lineage appears to be insensitive to Wnt signals. Thus differential sensitivity to Wnt signaling may be a mechanism to allow embryonic progenitors to differentiate, but maintain a fetal progenitor population.
Materials and methods
Mice
All lines of mice were generated previously and reported. Cre drivers included Pax3Cre (Engleka et al. 2005), Pax7iCre (Keller et al. 2004), and MCre (Brown et al. 2005); reporters included R26RLacZ (Soriano 1999), R26RYFP (Srinivas et al. 2001), and BAT-gal (Maretto et al. 2003); and conditional alleles included β-catenin loss-of-function β-cateninΔ/fl2–6 (Brault et al. 2001), constitutive gain-of-function β-cateninfl3/+ (Harada et al. 1999), and cell ablation R26RDTA (Wu et al. 2006).
β-gal staining, immunofluorescence, microscopy, and in situ hybridization
Whole-mount β-gal staining protocol is online at http://genetics.med.harvard.edu/∼cepko/protocol/xgalplap-stain.htm. For section immunofluorescence, 12-μm cryosections were washed in PBS and then, if needed (see Supplemental Table 1), subjected to antigen retrieval, consisting of heating slides in citrate buffer (1.8 mM citric acid, 8.2 mM sodium citrate in H2O) in a 2100 PickCell Retriever. Slides were incubated 1 h in 5% goat serum in PBS, overnight at 4°C in 1° antibody, washed in PBS, for 2 h at room temperature in 2° antibody, washed in PBS, and stained 5 min Hoechst. Primary antibodies are listed in Supplemental Table 1 and secondary antibodies consisted of Alexa 488 or 594 goat anti-mouse IgG1 (Invitrogen) or Cy2 or Cy3 goat anti-rabbit, anti-rat, or anti-chick (Jackson ImmunoResearch). All sections were imaged on a Zeiss TCS SP5 confocal microscope. Each image is a composite of maximum projections derived from stacks of optical sections. Whole-mount in situ hybridization protocol is online at http://genetics.med.harvard.edu/∼cepko/protocol/ctlab/ish.ct.htm.
Cell counts and statistics
For counts of laminin+ myofibers, MHCslow+ myofibers, and Pax7+ cells the three sections in the center of each muscle or limb were identified. Total and slow myofibers were counted by hand and Pax7+ and PH3+Pax7+ cells were counted using ImageJ software. Counts for three sections of three individuals for each genotype were analyzed using a repeated-measures ANOVA with JMP7 software.
Acknowledgments
We thank Cori Compton for initial help with this project, and A. Letsou, L.C. Murtaugh, F. Relaix, and members of the Kardon laborartory for helpful comments. NIH R01 HD053728 to G.K. funded this work.
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1769009.
Supplemental material is available at http://www.genesdev.org.
References
- Anakwe K., Robson L., Hadley J., Buxton P., Church V., Allen S., Hartmann C., Harfe B., Nohno T., Brown A.M., et al. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 2003;130:3503–3514. doi: 10.1242/dev.00538. [DOI] [PubMed] [Google Scholar]
- Bajard L., Relaix F., Lagha M., Rocancourt D., Daubas P., Buckingham M.E. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes & Dev. 2006;20:2450–2464. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barolo S. Transgenic Wnt/TCF pathway reporters: All you need is Lef? Oncogene. 2006;25:7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- Biressi S., Molinaro M., Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev. Biol. 2007a;308:281–293. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Biressi S., Tagliafico E., Lamorte G., Monteverde S., Tenedini E., Roncaglia E., Ferrari S., Cusella-De Angelis M.G., Tajbakhsh S., Cossu G. Intrinsic phenotypic diversity of embryonic and fetal myoblasts is revealed by genome-wide gene expression analysis on purified cells. Dev. Biol. 2007b;304:633–651. doi: 10.1016/j.ydbio.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Borello U., Buffa V., Sonnino C., Melchionna R., Vivarelli E., Cossu G. Differential expression of the Wnt putative receptors Frizzled during mouse somitogenesis. Mech. Dev. 1999a;89:173–177. doi: 10.1016/s0925-4773(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Borello U., Coletta M., Tajbakhsh S., Leyns L., De Robertis E.M., Buckingham M., Cossu G. Transplacental delivery of the Wnt antagonist Frzb1 inhibits development of caudal paraxial mesoderm and skeletal myogenesis in mouse embryos. Development. 1999b;126:4247–4255. doi: 10.1242/dev.126.19.4247. [DOI] [PubMed] [Google Scholar]
- Borello U., Berarducci B., Murphy P., Bajard L., Buffa V., Piccolo S., Buckingham M., Cossu G. The Wnt/β-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development. 2006;133:3723–3732. doi: 10.1242/dev.02517. [DOI] [PubMed] [Google Scholar]
- Borycki A.G., Emerson C.P., Jr Multiple tissue interactions and signal transduction pathways control somite myogenesis. Curr. Top. Dev. Biol. 2000;48:165–224. doi: 10.1016/s0070-2153(08)60757-7. [DOI] [PubMed] [Google Scholar]
- Boutet S.C., Disatnik M.H., Chan L.S., Iori K., Rando T.A. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 2007;130:349–362. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Brack A.S., Conboy I.M., Conboy M.J., Shen J., Rando T.A. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D.H., McMahon A.P., Sommer L., Boussadia O., Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brown C.B., Engleka K.A., Wenning J., Min Lu M., Epstein J.A. Identification of a hypaxial somite enhancer element regulating Pax3 expression in migrating myoblasts and characterization of hypaxial muscle Cre transgenic mice. Genesis. 2005;41:202–209. doi: 10.1002/gene.20116. [DOI] [PubMed] [Google Scholar]
- Brunelli S., Relaix F., Baesso S., Buckingham M., Cossu G. β Catenin-independent activation of MyoD in presomitic mesoderm requires PKC and depends on Pax3 transcriptional activity. Dev. Biol. 2007;304:604–614. doi: 10.1016/j.ydbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Bryson-Richardson R.J., Currie P.D. The genetics of vertebrate myogenesis. Nat. Rev. Genet. 2008;9:632–646. doi: 10.1038/nrg2369. [DOI] [PubMed] [Google Scholar]
- Buchberger A., Freitag D., Arnold H.H. A homeo-paired domain-binding motif directs Myf5 expression in progenitor cells of limb muscle. Development. 2007;134:1171–1180. doi: 10.1242/dev.02798. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C. R. Biol. 2007;330:530–533. doi: 10.1016/j.crvi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Burgess R., Rawls A., Brown D., Bradley A., Olson E.N. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature. 1996;384:570–573. doi: 10.1038/384570a0. [DOI] [PubMed] [Google Scholar]
- Chen A.E., Ginty D.D., Fan C.M. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433:317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- Christ B., Brand-Saberi B. Limb muscle development. Int. J. Dev. Biol. 2002;46:905–914. [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Condon K., Silberstein L., Blau H.M., Thompson W.J. Development of muscle fiber types in the prenatal rat hindlimb. Dev. Biol. 1990;138:256–274. doi: 10.1016/0012-1606(90)90196-p. [DOI] [PubMed] [Google Scholar]
- Cossu G., Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusella-De Angelis M.G., Molinari S., Le Donne A., Coletta M., Vivarelli E., Bouche M., Molinaro M., Ferrari S., Cossu G. Differential response of embryonic and fetal myoblasts to TGF β: A possible regulatory mechanism of skeletal muscle histogenesis. Development. 1994;120:925–933. doi: 10.1242/dev.120.4.925. [DOI] [PubMed] [Google Scholar]
- Dietrich S., Abou-Rebyeh F., Brohmann H., Bladt F., Sonnenberg-Riethmacher E., Yamaai T., Lumsden A., Brand-Saberi B., Birchmeier C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–1629. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
- Engleka K.A., Gitler A.D., Zhang M., Zhou D.D., High F.A., Epstein J.A. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev. Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Epstein J.A., Shapiro D.N., Cheng J., Lam P.Y., Maas R.L. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc. Natl. Acad. Sci. 1996;93:4213–4218. doi: 10.1073/pnas.93.9.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli L.M., Willert K., Nusse R., Yablonka-Reuveni Z., Nohno T., Denetclaw W., Burrus L.W. A proliferative role for Wnt-3a in chick somites. Dev. Biol. 2004;269:489–504. doi: 10.1016/j.ydbio.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Gavard J., Marthiens V., Monnet C., Lambert M., Mege R.M. N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: Involvement of p120 and β-catenin. J. Biol. Chem. 2004;279:36795–36802. doi: 10.1074/jbc.M401705200. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P., Nimmagadda S., Prols F., Patel K., Scaal M., Huang R., Christ B. Ectodermal Wnt-6 promotes Myf5-dependent avian limb myogenesis. Dev. Biol. 2005;288:221–233. doi: 10.1016/j.ydbio.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P., Nimmagadda S., Huang R., Scaal M., Christ B. Role of Wnt-6 in limb myogenesis. Anat. Embryol. (Berl.) 2006;211:183–188. doi: 10.1007/s00429-005-0069-6. [DOI] [PubMed] [Google Scholar]
- Goichberg P., Shtutman M., Ben-Ze'ev A., Geiger B. Recruitment of β-catenin to cadherin-mediated intercellular adhesions is involved in myogenic induction. J. Cell Sci. 2001;114:1309–1319. doi: 10.1242/jcs.114.7.1309. [DOI] [PubMed] [Google Scholar]
- Gros J., Manceau M., Thome V., Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Gunning P., Hardeman E. Multiple mechanisms regulate muscle fiber diversity. FASEB J. 1991;5:3064–3070. doi: 10.1096/fasebj.5.15.1835946. [DOI] [PubMed] [Google Scholar]
- Haldar M., Karan G., Tvrdik P., Capecchi M.R. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev. Cell. 2008;14:437–445. doi: 10.1016/j.devcel.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M.M. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Papoutsi M., Huang R., Tomarev S.I., Christ B., Kurz H., Wilting J. Three different fates of cells migrating from somites into the limb bud. Anat. Embryol. (Berl.) 2003;207:29–34. doi: 10.1007/s00429-003-0327-4. [DOI] [PubMed] [Google Scholar]
- Horst D., Ustanina S., Sergi C., Mikuz G., Juergens H., Braun T., Vorobyov E. Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int. J. Dev. Biol. 2006;50:47–54. doi: 10.1387/ijdb.052111dh. [DOI] [PubMed] [Google Scholar]
- Hu P., Geles K.G., Paik J.H., DePinho R.A., Tjian R. Codependent activators direct myoblast-specific MyoD transcription. Dev. Cell. 2008;15:534–546. doi: 10.1016/j.devcel.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Zhi Q., Christ B. The relationship between limb muscle and endothelial cells migrating from single somite. Anat. Embryol. (Berl.) 2003;206:283–289. doi: 10.1007/s00429-002-0289-y. [DOI] [PubMed] [Google Scholar]
- Ikeya M., Takada S. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development. 1998;125:4969–4976. doi: 10.1242/dev.125.24.4969. [DOI] [PubMed] [Google Scholar]
- Kardon G., Campbell J.K., Tabin C.J. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev. Cell. 2002;3:533–545. doi: 10.1016/s1534-5807(02)00291-5. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L., Gayraud-Morel B., Gomes D., Rocancourt D., Buckingham M., Shinin V., Tajbakhsh S. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature. 2004;431:466–471. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L., Giacone E., Gayraud-Morel B., Jory A., Gomes D., Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes & Dev. 2005;19:1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C., Hansen M.S., Coffin C.M., Capecchi M.R. Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: Implications for alveolar rhabdomyosarcoma cell of origin. Genes & Dev. 2004;18:2608–2613. doi: 10.1101/gad.1243904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M., Rubinstein N.A. Why are fetal muscles slow? Nature. 1980;288:266–269. doi: 10.1038/288266a0. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Neiswender H., Baik E.J., Xiong W.C., Mei L. β-Catenin interacts with MyoD and regulates its transcription activity. Mol. Cell. Biol. 2008;28:2941–2951. doi: 10.1128/MCB.01682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerova I., Kudryashova E., Wu B., Spencer M.J. Regulation of the M-cadherin–β-catenin complex by calpain 3 during terminal stages of myogenic differentiation. Mol. Cell. Biol. 2006;26:8437–8447. doi: 10.1128/MCB.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S., Charge S.B., Seale P., Huh M., Rudnicki M.A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczuk K.L., Love C.M., Dougherty N.M., Goldhamer D.J. Fine-scale transgenic mapping of the MyoD core enhancer: MyoD is regulated by distinct but overlapping mechanisms in myotomal and non-myotomal muscle lineages. Development. 1999;126:1957–1965. doi: 10.1242/dev.126.9.1957. [DOI] [PubMed] [Google Scholar]
- Lang D., Lu M.M., Huang L., Engleka K.A., Zhang M., Chu E.Y., Lipner S., Skoultchi A., Millar S.E., Epstein J.A. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Linker C., Lesbros C., Stark M.R., Marcelle C. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 2003;130:4797–4807. doi: 10.1242/dev.00688. [DOI] [PubMed] [Google Scholar]
- Linker C., Lesbros C., Gros J., Burrus L.W., Rawls A., Marcelle C. β-Catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development. 2005;132:3895–3905. doi: 10.1242/dev.01961. [DOI] [PubMed] [Google Scholar]
- Maina F., Casagranda F., Audero E., Simeone A., Comoglio P.M., Klein R., Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- Maqbool T., Jagla K. Genetic control of muscle development: Learning from Drosophila. J. Muscle Res. Cell Motil. 2007;28:397–407. doi: 10.1007/s10974-008-9133-1. [DOI] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A.B., Volpin D., Bressan G.M., Piccolo S. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto M., Reshef R., Munsterberg A.E., Koester S., Goulding M., Lassar A.B. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- McKinnell I.W., Ishibashi J., Le Grand F., Punch V.G., Addicks G.C., Greenblatt J.F., Dilworth F.J., Rudnicki M.A. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat. Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.A., Barrow J., Collinson J.M., Davidson S., Lear M., Hill R.E., Mackenzie A. A highly conserved Wnt-dependent TCF4 binding site within the proximal enhancer of the anti-myogenic Msx1 gene supports expression within Pax3-expressing limb bud muscle precursor cells. Dev. Biol. 2007;311:665–678. doi: 10.1016/j.ydbio.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Munsterberg A.E., Kitajewski J., Bumcrot D.A., McMahon A.P., Lassar A.B. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes & Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Nastasi T., Bongiovanni A., Campos Y., Mann L., Toy J.N., Bostrom J., Rottier R., Hahn C., Conaway J.W., Harris A.J., et al. Ozz-E3, a muscle-specific ubiquitin ligase, regulates β-catenin degradation during myogenesis. Dev. Cell. 2004;6:269–282. doi: 10.1016/s1534-5807(04)00020-6. [DOI] [PubMed] [Google Scholar]
- Otto A., Schmidt C., Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat. Embryol. (Berl.) 2006;211:293–310. doi: 10.1007/s00429-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Otto A., Schmidt C., Luke G., Allen S., Valasek P., Muntoni F., Lawrence-Watt D., Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J. Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- Oustanina S., Hause G., Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Jia Y., Wang J., Tao D., Gan X., Tsiokas L., Jing N., Wu D., Li L. β-Catenin regulates myogenesis by relieving I-mfa-mediated suppression of myogenic regulatory factors in P19 cells. Proc. Natl. Acad. Sci. 2005;102:17378–17383. doi: 10.1073/pnas.0505922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanaud L., Luton D., Prigent M., Bourcheix L.M., Catala M., Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz A., Ono Y., Gnocchi V.F., Zammit P.S. β-Catenin promotes self-renewal of skeletal-muscle satellite cells. J. Cell Sci. 2008;121:1373–1382. doi: 10.1242/jcs.024885. [DOI] [PubMed] [Google Scholar]
- Relaix F., Polimeni M., Rocancourt D., Ponzetto C., Schafer B.W., Buckingham M. The transcriptional activator PAX3–FKHR rescues the defects of Pax3 mutant mice but induces a myogenic gain-of-function phenotype with ligand-independent activation of Met signaling in vivo. Genes & Dev. 2003;17:2950–2965. doi: 10.1101/gad.281203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F., Rocancourt D., Mansouri A., Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes & Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F., Rocancourt D., Mansouri A., Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Relaix F., Montarras D., Zaffran S., Gayraud-Morel B., Rocancourt D., Tajbakhsh S., Mansouri A., Cumano A., Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienda J., Engleka K.A., Jun S., Hansen M.S., Epstein J.A., Tabin C.J., Kunkel L.M., Kardon G. Somitic origin of limb muscle satellite and side population cells. Proc. Natl. Acad. Sci. 2006;103:945–950. doi: 10.1073/pnas.0510164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Tanaka M., Munsterberg A. Expression of β-catenin in the developing chick myotome is regulated by myogenic signals. Development. 2000;127:4105–4113. doi: 10.1242/dev.127.19.4105. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Stoeckelhuber M., McKinnell I., Putz R., Christ B., Patel K. Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation. Dev. Biol. 2004;271:198–209. doi: 10.1016/j.ydbio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Schubert F.R., Tremblay P., Mansouri A., Faisst A.M., Kammandel B., Lumsden A., Gruss P., Dietrich S. Early mesodermal phenotypes in splotch suggest a role for Pax3 in the formation of epithelial somites. Dev. Dyn. 2001;222:506–521. doi: 10.1002/dvdy.1211. [DOI] [PubMed] [Google Scholar]
- Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Seale P., Ishibashi J., Scime A., Rudnicki M.A. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2:E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale F.E. Myogenic cell lineages. Dev. Biol. 1992;154:284–298. doi: 10.1016/0012-1606(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Rocancourt D., Cossu G., Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Borello U., Vivarelli E., Kelly R., Papkoff J., Duprez D., Buckingham M., Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- Takata H., Terada K., Oka H., Sunada Y., Moriguchi T., Nohno T. Involvement of Wnt4 signaling during myogenic proliferation and differentiation of skeletal muscle. Dev. Dyn. 2007;236:2800–2807. doi: 10.1002/dvdy.21327. [DOI] [PubMed] [Google Scholar]
- Teboul L., Hadchouel J., Daubas P., Summerbell D., Buckingham M., Rigby P.W. The early epaxial enhancer is essential for the initial expression of the skeletal muscle determination gene Myf5 but not for subsequent, multiple phases of somitic myogenesis. Development. 2002;129:4571–4580. doi: 10.1242/dev.129.19.4571. [DOI] [PubMed] [Google Scholar]
- Wigmore P.M., Evans D.J. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 2002;216:175–232. doi: 10.1016/s0074-7696(02)16006-2. [DOI] [PubMed] [Google Scholar]
- Wilson-Rawls J., Hurt C.R., Parsons S.M., Rawls A. Differential regulation of epaxial and hypaxial muscle development by paraxis. Development. 1999;126:5217–5229. doi: 10.1242/dev.126.23.5217. [DOI] [PubMed] [Google Scholar]
- Wu S., Wu Y., Capecchi M.R. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–590. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]