Abstract
Objective
Left ventricular (LV) end-systolic volume indexed to body surface area (ESVI) is a simple yet powerful echocardiographic marker of LV remodeling that can be measured easily. The prognostic value of ESVI and its merit relative to other markers of LV remodeling in patients with coronary heart disease are unknown.
Methods
We examined the association of ESVI with hospitalization for heart failure (HF) and mortality in a prospective study of patients with coronary heart disease.
Results
Of the 989 participants, 110 (11%) were hospitalized for HF during 3.6 ± 1.1 years of follow-up. Among participants in the highest ESVI quartile (>25 mL/m2), 67 of 248 (27%) developed HF compared with 8 of 248 (3%) among those in the lowest quartile. The association between ESVI and HF hospitalization persisted after adjustment for potential confounders (hazard ratio 5.0, 95% confidence interval, 1.5–16.9; P = .01).
Conclusion
ESVI >25 mL/m2 is an independent predictor of hospitalization for HF in patients with stable coronary heart disease.
Keywords: Coronary artery disease, End-systolic volume index, Heart failure hospitalization, Left ventricular remodeling
Coronary heart disease (CHD) is the strongest risk factor for heart failure (HF), and when CHD coexists with HF, patients have increased morbidity and mortality compared with those with either disease alone.1–4 Therefore, early identification of patients with CHD who are at increased risk for HF is of great importance because it might enable earlier treatment, allow for closer monitoring, and potentially reduce considerable morbidity and mortality.
Left ventricular (LV) end-systolic volume (ESV) has been shown to be an important determinant of survival after myocardial infarction (MI).5,6 A decrease in ESV with angiotensin-converting enzyme inhibitor therapy has been associated with a reduction in cardiac events in patients with moderately decreased LV systolic function.7 By using left ventriculography, ESV has been shown to be an important predictor of both postoperative LV function and survival after coronary artery bypass grafting in patients with LV systolic dysfunction.8,9 The aforementioned studies have consistently shown that large increases in ESV predict adverse cardiovascular outcomes in participants with LV systolic dysfunction.
With advances in the treatment of CHD, most patients are living longer, and the number of patients at risk of and dying of HF continues to grow.2,4,10 Current HF guidelines11 emphasize early identification of patients who are at risk for morbidity and mortality from HF, which is important not only for the prevention of HF and early initiation of therapies for HF but also for the containment of health care costs associated with treating those with advanced HF. Echocardiography has become one of the most commonly used noninvasive modalities for assessment of ventricular volumes and function,12 and can provide prognostic information for the prediction of future HF events.13 Although many sophisticated echocardiographic markers exist, ESV is a simple parameter that can be measured easily in clinical practice.14 We sought to study ESV indexed to body surface area (ESVI) in patients with stable coronary disease to define its prognostic value. We hypothesized that ESVI, measured by echocardiography, is an important predictor of hospitalization for HF, even in patients with normal ejection fraction (EF). We also hypothesized that ESVI is a better predictor than other echocardiographic measures of LV systolic and diastolic enlargement, and that even subtle increases in ESVI would have prognostic importance. We therefore studied the relationship between ESVI and adverse cardiovascular outcomes, including HF hospitalization, in a cohort of 989 ambulatory patients with CHD.
MATERIALS AND METHODS
Participants
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients with CHD. Details regarding recruitment procedures and inclusion and exclusion criteria have been published.15 The study involved 3 medical centers and 9 public health clinics in the San Francisco Bay Area. Patients were eligible to participate if they met one of the following inclusion criteria: history of MI, angiographic evidence of more than 50% stenosis in 1 or more coronary vessels, evidence of exercise-induced ischemia by treadmill testing, or history of coronary revascularization.
Between September 2000 and December 2002, a total of 1024 participants enrolled. Participants completed a baseline study appointment that included a medical history interview, a physical examination, an exercise treadmill test with a baseline and stress echocardiogram, and a comprehensive health status questionnaire. We excluded 35 participants for whom the LVESV could not be accurately determined for technical reasons (poor acoustic windows). The remaining 989 participants constituted the analytic sample for our analysis. This protocol was approved by the appropriate institutional review boards, and all participants provided written, informed consent.
End-systolic Volume Index
A complete resting 2-dimensional (2D) echocardiogram and Doppler ultrasound examination, including all standard views and subcostal imaging of the inferior vena cava, was performed with a 3.5-MHz transducer (Acuson Sequoia Ultrasound System, Mountain View, CA). A single experienced reader (N.B.S.), blinded to the clinical history, physical examination, laboratory data, and outcome variables, interpreted all echocardiograms and verified LV volumetric analyses completed by 2 research sonographers. LVESV was calculated using the biplane method of discs (modified Simpson’s rule) in the apical 4- and 2-chamber views at end systole, as recommended by the American Society of Echocardiography12 and as has been validated elsewhere.16–22 ESVI was then calculated as ESV divided by body surface area.
Outcome Variables
We conducted annual telephone follow-up interviews with participants (or their proxy) to inquire about death or hospitalization for “heart trouble.” For any reported event, medical records, electrocardiograms, death certificates, and coroner’s reports were retrieved and reviewed by 2 independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. If they disagreed, they conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator as necessary.
HF was defined using the Framingham criteria.23,24 In brief, an HF outcome required that a participant be hospitalized for a clinical syndrome involving at least 2 of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, cardiomegaly on chest radiography, or pulmonary edema on chest radiograph. These clinical signs and symptoms must have represented a clear change from the normal clinical state of the patient and have been accompanied by either failing cardiac output as determined by peripheral hypoperfusion (in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary edema requiring intravenous diuretics, inotropic medications, or vasodilators. Supportive documentation of reduced cardiac index, increased pulmonary capillary wedge pressure, decreased oxygen saturation, and end-organ hypoperfusion, if available, were considered in the analysis.23,24
Other Measurements
Age, sex, medical history, and smoking status were determined by patient questionnaire. A trained research assistant reviewed each patient’s baseline medications at the initial study visit. Systolic and diastolic blood pressures were measured with participants lying supine after 5 minutes of rest using a calibrated sphygmomanometer by trained study personnel. Pulse pressure was calculated as systolic minus diastolic blood pressure. We measured weight and height and calculated body mass index (kilograms/meters squared). Laboratory measurements, including serum creatinine, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and N-terminal pro-B-type natriuretic peptide (NT-proBNP), were measured from sera after overnight fast. We measured creatinine clearance from 24-hour urine collections. During echocardiography, we obtained standard views and measured left atrial volume (biplane method of discs), LV dimensions (2D-guided M-mode), LV volumes (biplane method of discs), LV mass index (truncated ellipsoid method), and calculated EF ([end-diastolic volume {EDV}-ESV]/EDV) according to published guidelines.12 We defined 5 categories of diastolic function (normal, impaired relaxation, pseudonormal, restrictive, and indeterminate) according to published criteria24 by using early (E) and late (A) mitral inflow velocities, E deceleration time, pulmonary vein systolic/diastolic flow ratio, and the difference between pulmonary vein A-wave reversal and mitral inflow A duration. We also evaluated for valvular abnormalities, and for the purposes of our analyses, aortic and mitral regurgitation were considered present if moderate or greater in severity. Regurgitation severity was determined using published guidelines.25 All subjects also underwent exercise echocardiography (as described in detail previously26) for the detection of exercise-induced ischemia.
Statistical Analysis
Differences in baseline characteristics in participants between ESVI quartiles were compared using analysis of variance (or nonparametric equivalent) for continuous variables and chi-square tests for categoric variables. To examine the relative utility of ESVI as a diagnostic test to predict HF, we generated receiver operating characteristic (ROC) curves for LV end-systolic dimension index, ESVI, end-diastolic volume index (EDVI), and EF, and ROC curves were compared using a standard nonparametric test described by DeLong et al.27
We used a Cox proportional hazards multivariable analysis with ESVI as the predictor variable and HF, death, and a combined end point (HF or death) as the outcome variables. We then conducted a similar analysis with ESVI as the predictor and incident HF as the outcome variable. The Cox proportional hazard models were adjusted for the following variables on the basis of an association with ESVI on univariate analysis at a significance level of P less than .05: sex; body mass index; systolic and diastolic blood pressure; log NT-proBNP (because NT-proBNP levels were right-skewed, we log-transformed this variable to normalize its distribution); measured creatinine clearance; history of HF; history of MI; use of beta-blockers, diuretics, or renin-angiotensin system antagonists; LV mass index; diastolic function; left atrial volume index; mitral regurgitation; inducible ischemia; EF; and EDVI. Cumulative incidence curves were then generated to illustrate differences in risk-adjusted rate of HF. We used the Stata “LOWESS” command to generate a smoothed locally weighted scatterplot (LOWESS) curve to graphically depict the relationship between ESVI and HF outcome in the overall cohort.
To explore whether ESVI independently predicted HF hospitalizations in participants with preserved EF, we used an adjusted Cox proportional hazards model to determine if there was a difference in the risk of HF in the subgroup of patients with EF ≥ 50%. We also performed unadjusted subgroup analyses by age, gender, history of MI, history of HF, creatinine clearance, NT-proBNP, and EF, and constructed a hazard ratio (HR) plot to display our results. All analyses were performed using Stata (version 9, StataCorp LP, College Station, TX).
RESULTS
Of the 1024 total subjects enrolled in the Heart and Soul Study, we were able to measure ESV in the majority (989/1024, 97%). For the remaining 35 patients (3% of the cohort), ESVI could not be measured because of poor acoustic windows. Of the 989 subjects with ESVI data, 109 (11%) were hospitalized for HF during 3.6 ± 1.1 years (3485 person-years) of follow-up. The mean lengths of follow-up from the first to fourth quartiles of ESVI were 3.8 ± 0.9 years, 3.6 ± 0.9 years, 3.7 ± 1.1 years, and 3.2 ± 1.5 years, respectively ( P = 0001 by Kruskal-Wallis statistic). The highest quartile of ESVI had the shortest mean length of follow-up because more patients died in this subgroup. Compared with participants in the lower ESVI quartiles, those in the highest quartile were more likely to be male, to have a history of HF and MI, and to be receiving a renin-angiotensin system antagonist, beta-blocker, and diuretic therapy (Table 1). Those in the highest ESVI were also more likely to have a higher NT-proBNP level, a lower BMI, and lower systolic and diastolic blood pressures. Serum creatinine was similar between groups, but 24-hour urinary creatinine clearance was lower in participants in the highest ESVI quartile.
Table 1.
Baseline characteristics of participants by end-systolic volume index quartile
| ESVI quartile |
|||||
|---|---|---|---|---|---|
| Variable | I <13 mL/m2 (N = 247) | II 13–17 mL/m2 (N = 248) | III 17–25 mL/m2 (N = 246) | IV >25 mL/m2 (N = 248) | P value |
| Demographics: | |||||
| Age (y) | 67 ± 11 | 66 ± 10 | 66 ± 11 | 67 ± 11 | .71 |
| Male, n (%) | 171 (70) | 190 (77) | 225 (91) | 220 (89) | < .0001 |
| Medical history: | |||||
| Hypertension, n (%) | 171 (69) | 186 (75) | 171 (70) | 168 (68) | .36 |
| Diabetes mellitus, n (%) | 68 (28) | 65 (26) | 61 (25) | 64 (26) | .94 |
| Prior MI, n (%) | 114 (47) | 111 (45) | 128 (53) | 177 (72) | < .0001 |
| Smoker, n (%) | 47 (19) | 48 (19) | 46 (19) | 55 (22) | .82 |
| HF, n (%) | 26 (11) | 27 (11) | 38 (16) | 83 (34) | < .0001 |
| Alcohol use, n (%) | 66 (27) | 75 (30) | 73 (30) | 69 (28) | .82 |
| Physically active, n (%) | 168 (68) | 148 (60) | 152 (62) | 158 (64) | .24 |
| NYHA class, n (%) | .68* | ||||
| I | 84 (34) | 98 (40) | 101 (41) | 86 (35) | |
| II | 105 (43) | 95 (38) | 98 (39) | 100 (40) | |
| III | 43 (17) | 45 (18) | 39 (16) | 46 (19) | |
| IV | 15 (6) | 9 (4) | 9 (4) | 15 (6) | |
| Medication use: | |||||
| ACEI or ARB, n (%) | 111 (45) | 116 (47) | 118 (48) | 158 (64) | < .0001 |
| Beta-blocker, n (%) | 124 (50) | 142 (57) | 155 (63) | 149 (60) | .03 |
| Statin, n (%) | 152 (62) | 153 (62) | 159 (65) | 169 (68) | .37 |
| Diuretic,† n (%) | 54 (22) | 77 (31) | 59 (24) | 101 (40) | < .0001 |
| Laboratory data: | |||||
| Body mass index (kg/m2) | 28.6 ± 5.1 | 29.1 ± 5.1 | 28.5 ± 5.4 | 27.4 ± 5.1 | .004 |
| Total cholesterol (mg/dL) | 183.7 ± 41.5 | 175.8 ± 40.3 | 177.3 ± 38.9 | 174.1 ± 48.6 | .07 |
| LDL cholesterol (mg/dL) | 107.7 ± 33.3 | 103.8 ± 33.3 | 103.9 ± 31.4 | 101.7 ± 36.7 | .27 |
| HDL cholesterol (mg/dL) | 47.4 ± 13.9 | 45.2 ± 12.3 | 44.7 ± 14.0 | 45.1 ± 14.8 | .11 |
| Creatinine (mg/dL) | 1.1 ± 0.54 | 1.1 ± 0.72 | 1.2 ± 0.76 | 1.2 ± 0.68 | .13 |
| Creatinine clearance (mL/min) | 83.6 ± 27.5 | 82.1 ± 27.0 | 83.3 ± 29.2 | 76.6 ± 30.2 | .02 |
| NT-proBNP (pg/mL) | 181 ± 328 | 282 ± 361 | 509 ± 1414 | 1186 ± 2766 | < .0001 |
| Systolic blood pressure (mm Hg) | 133.3 ± 20.0 | 136.2 ± 20.5 | 131.8 ± 20.6 | 131.2 ± 22.4 | .04 |
| Diastolic blood pressure (mm Hg) | 75.1 ± 11.4 | 76.4 ± 10.3 | 74.2 ± 11.0 | 73.3 ± 12.0 | .01 |
| Pulse pressure (mm Hg) | 58.2 ± 16.2 | 59.8 ± 16.8 | 57.6 ± 15.5 | 57.9 ± 16.7 | .47 |
MI, Myocardial infarction; NYHA, New York Heart Association; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ESVI, end-systolic volume index; LDL, low-density lipoprotein; HF, heart failure; HDL, high-density lipoprotein; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Plus-minus values are mean ± standard deviation.
Across all groups of New York Heart Association class.
Diuretic therapy includes both loop and thiazide diuretics.
By echocardiography, participants in the highest ESVI quartile had a lower EF, higher EDVI, higher left atrial volume index, higher LV mass index, lower A-wave velocity, and a higher E/A ratio. Participants in the highest ESVI quartile were more likely to have diastolic dysfunction, mitral regurgitation of moderate or greater severity, and inducible ischemia (Table 2). Participants in the highest ESVI quartile had a mean ESVI of 37.5 ± 15.6 mL/m2. The median ESVI among participants in the highest quartile was 32 mL/m2, and the interquartile range was 26.7 to 41.8 mL/m2.
Table 2.
Echocardiographic characteristics of participants by end-systolic volume index quartile
| ESVI quartile |
|||||
|---|---|---|---|---|---|
| Variable | I <13 mL/m2 (N = 247) | II 13–17 mL/m2 (N = 248) | III 17–25 mL/m2 (N = 246) | IV >25 mL/m2 (N = 248) | P value |
| EF, n (%) | 69.0 ± 4.7 | 65.6 ± 4.5 | 61.7 ± 6.1 | 50.6 ± 9.8 | < .0001 |
| LV mass index (g/m2) | 84.6 ± 20.0 | 91.2 ± 18.6 | 99.1 ± 24.1 | 116.9 ± 30.3 | < .0001 |
| LV end-systolic dimension index (cm/m2) | 1.74 ± 0.42 | 1.76 ± 0.36 | 1.84 ± 0.39 | 1.97 ± 0.53 | < .0001 |
| LV end-diastolic dimension index (cm/m2) | 2.61 ± 0.43 | 2.65 ± 0.41 | 2.73 ± 0.40 | 2.83 ± 0.57 | .0006 |
| LV EDVI (mL/m2) | 35.9 ± 5.9 | 43.9 ± 6.3 | 53.1 ± 7.2 | 73.8 ± 18.8 | < .0001 |
| LA volume index (mL/m2) | 26.5 ± 7.9 | 31.2 ± 8.8 | 34.6 ± 11.4 | 39.6 ± 14.7 | < .0001 |
| IVRT (msec) | 116.5 ± 25.5 | 117.5 ± 23.2 | 115.1 ± 25.5 | 118.7 ± 30.0 | .49 |
| E-wave velocity (m/s) | 0.77 ± 0.2 | 0.77 ± 0.2 | 0.77 ± 0.2 | 0.78 ± 0.3 | .94 |
| A-wave velocity (m/s) | 0.82 ± 0.3 | 0.80 ± 0.2 | 0.76 ± 0.2 | 0.76 ± 0.3 | .05 |
| E/A ratio | 1.0 ± 0.39 | 1.0 ± 0.35 | 1.1 ± 0.41 | 1.1 ± 0.66 | .005 |
| E-wave deceleration time (msec) | 247.4 ± 60.6 | 239.9 ± 54.8 | 242.8 ± 61.0 | 234.2 ± 73.6 | .13 |
| Right atrial pressure (mm Hg) | 5.2 ± 1.1 | 5.3 ± 1.5 | 5.3 ± 1.3 | 5.4 ± 1.7 | .27 |
| Aortic regurgitation, n (%) | 22 (9) | 30 (12) | 31 (13) | 33 (13) | .43 |
| Mitral regurgitation, n (%) | 20 (8) | 32 (13) | 44 (18) | 89 (36) | < .0001 |
| Diastolic function: | |||||
| Normal, n (%) | 146 (59) | 152 (61) | 138 (56) | 97 (39) | < .0001* |
| Impaired relaxation, n (%) | 57 (23) | 42 (17) | 46 (19) | 57 (23) | |
| Pseudo-normal, n (%) | 11 (4) | 14 (6) | 21 (8) | 26 (10) | |
| Restrictive, n (%) | 14 (6) | 18 (7) | 16 (6) | 38 (15) | |
| Indeterminate, n (%) | 19 (8) | 21 (9) | 26 (11) | 30 (12) | |
| Exercise-induced ischemia, n (%) | 32 (14) | 35 (16) | 53 (23) | 95 (43) | < .0001 |
LV, Left ventricular; ESVI, end-systolic volume index; EDVI, end-diastolic volume index; LA, left atrial; IVRT, isovolumic relaxation time; E, early mitral inflow; A, late (atrial) mitral inflow; EF, ejection fraction.
Plus-minus values are mean ± standard deviation.
Across all 5 groups of diastolic function.
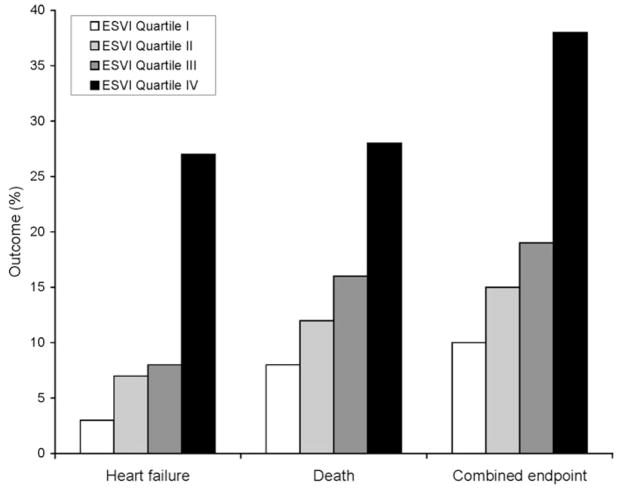
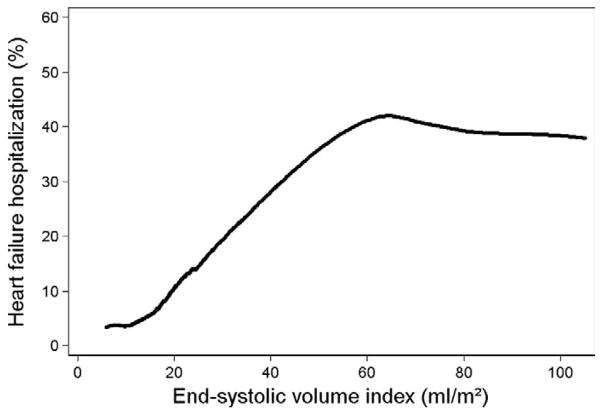
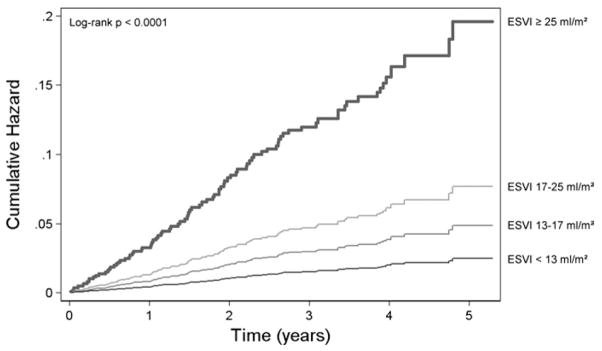
During 3.6 ± 1.1 years of follow-up, we observed increased HF hospitalization and mortality among participants in the highest ESVI quartile (Figure 1). In the unadjusted analysis, 67 (27%) participants in the highest ESVI quartile (ESVI > 25 mL/m2) were hospitalized with HF, compared with 8 (3%) of those participants in the lowest ESVI quartile (<13 mL/m2) (HR 9.7, 95% confidence interval [CI], 4.6–20.1; P < .0001). Figure 2 shows the unadjusted smoothed LOWESS plot relating ESVI to the proportion of participants hospitalized with HF. The association of ESVI with HF hospitalization was unchanged after adjustment for potential confounders and mediators (HR 5.0, 95% CI, 1.5–16.9; P = 01) (Table 3). Figure 3 compares the adjusted cumulative hazard of HF hospitalization for participants by ESVI quartile. In the above multivariable analyses, the referent group was the first quartile of ESVI. Using the first, second, and third quartiles as a combined referent group did not eliminate the association of ESVI > 25 mL/m2 with HF hospitalization (adjusted HR 3.1, 95% CI, 1.5–6.7; P = .003).
Figure 1.
Adverse cardiovascular outcomes by ESVI quartile. *Combined end point = HF hospitalization or death.
Figure 2.
LOWESS plot of the proportion of patients hospitalized with HF during follow-up according to baseline LV ESVI. LOWESS, Locally weighted, smoothed scatterplot.
Table 3.
Association of end-systolic volume index with heart failure hospitalization
| ESVI quartile | Proportion hospitalized for HF | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value* |
|---|---|---|---|---|---|
| I | 3% | 1 | — | 1 | — |
| II | 7% | 2.1 (0.9–4.8) | .096 | 1.1 (0.3–3.5) | .88 |
| III | 8% | 2.4 (1.1–5.6) | .035 | 1.8 (0.5–4.9) | .47 |
| IV | 27% | 9.7 (4.6–20.1) | < .0001 | 5.0 (1.5–16.9) | .01 |
ESVI, End-systolic volume index; HF, heart failure; CI, confidence interval.
Adjusted for sex, body mass index, blood pressure, creatinine clearance, log NT-proBNP, history of HF or MI, medications, LV mass index, left atrial volume index, mitral regurgitation, diastolic function, LV EDVI, LV EF, and inducible ischemia.
Figure 3.
Cumulative risk of HF hospitalization by quartile of ESVI. Curves were adjusted for sex, body mass index, history of HF, history of MI, medication use, measured creatinine clearance, log NT-proBNP, and echocardiographic variables (EF, LV EDVI, left atrial volume index, LV mass index, valvular regurgitation, and diastolic function). ESVI, End-systolic volume index.
The highest quartile of ESVI predicted mortality on unadjusted analyses (HR 3.4, 95% CI, 2.1–5.6; P < .0001) but not on multivariate analysis (P = .70). The highest quartile of ESVI did, however, predict the combined end point of HF hospitalization or death on both unadjusted (HR 9.9, 95% CI, 4.8–20.6; P < .0001) and multivariable-adjusted analyses (HR 4.4, 95% CI, 1.3–15.4; P = .019). To examine the utility of ESVI as a predictor of incident HF hospitalization, we conducted an analysis of the 815 patients without a history of HF hospitalization. On unadjusted analysis, the highest quartile of ESVI predicted HF hospitalization (HR 9.7, 95% CI, 3.8–24.9; P < .0001). The association of ESVI with incident HF hospitalization persisted after adjustment for known cardiovascular risk factors, medication use, blood pressure, BNP, and renal function (adjusted HR 4.6, 95% CI, 1.3–16.1; P = .02). The highest quartile of ESVI did not predict incident HF hospitalization when other echocardiographic variables were included in multivariable Cox regression analysis.
To explore the association between ESVI and HF hospitalization among participants with preserved EF, we excluded all 109 participants with an EF less than 50%. Because participants in this subgroup had smaller LV volumes, we calculated new quartiles of ESVI that were specific to this subgroup (fourth quartile mean 27 ± 6 mL/m2, range 21–59 mL/m2). In those with EF ≥ 50%, even with a lower cutoff of ESVI for this subgroup, ESVI continued to predict increased HF hospitalization. Of those with an EF ≥ 50%, 37 participants (17%) in the highest ESVI quartile were hospitalized with HF, compared with 7 (3%) among those participants in the lowest ESVI quartile (unadjusted HR 5.4, 95% CI, 2.4–12.2; P < .0001). This association persisted after multivariable adjustment (adjusted HR 4.8, 95% CI, 1.0–23.2; P = .048). Of note, for all of the above multivariate analyses (for all outcomes and for all subgroup analyses), removing EDVI and EF to avoid multicollinearity had no effect on our results.
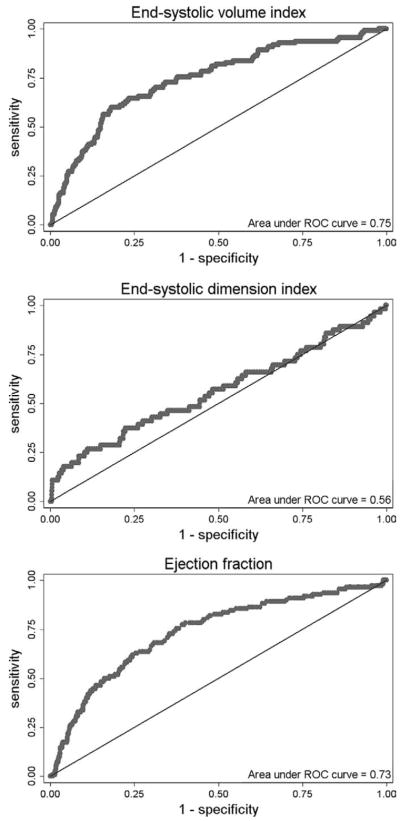
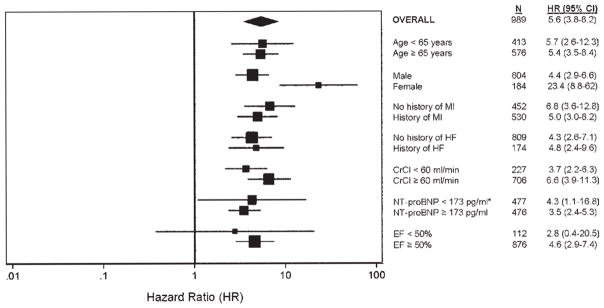
To examine the relative utility of ESVI as a diagnostic test to predict HF hospitalization, we analyzed ROC curves for LV end-systolic dimension index, ESVI, EDVI, and EF as predictors of this outcome. Figure 4 shows that the area under the ROC curve for ESVI was far superior to LV end-systolic dimension index (0.78 [95% CI, 0.71–0.85] vs 0.56 [95% CI, 0.47–0.65], respectively, P < .0001). The area under the ROC curve for ESVI was also superior to EDVI (0.71, 95% CI, 0.65–0.76; P < .0001) and EF (0.73, 95% CI, 0.68–0.78; P < .0001). Finally, we constructed a HR plot (Figure 5) to display the utility of an ESVI ≥ 25 mL/m2 as a predictor of HF hospitalization in various subgroups (HRs compare ESVI ≥ 25 mL/m2 vs ESVI < 25 mL/m2). This unadjusted analysis shows that ESVI predicts risk for HF hospitalization in a wide variety of patient subgroups.
Figure 4.
ROC curves for LV ESDI, ESVI, and EF as predictors of hospitalization for HF. LV, Left ventricular.
Figure 5.
HR plot for the risk of hospitalization for HF in ESVI ≥ 25 mL/m2 (vs ESVI < 25 mL/m2) in specified subgroups. *Cutoffs for NT-proBNP were determined by median BNP (173 pg/mL). †Only 4 patients with EF < 50% had an ESVI < 25 mL/m2, which limited the diagnostic utility of ESVI 25 mL/m2 in this subgroup. ESVI, End-systolic volume index; MI, myocardial infarction; HF, heart failure; CrCl, creatinine clearance; NT-proBNP, N-terminal pro-B-type natriuretic peptide; EF, ejection fraction.
DISCUSSION
In this prospective cohort study of 989 outpatients with established CHD, we found that participants with higher ESVI had increased rates of hospitalization for HF and death. Compared with those in the lowest quartile of ESVI, participants in the highest ESVI quartile (ESVI > 25 mL/m2) had a 4.6-fold higher rate of HF hospitalization during 3.6 ± 1.1 years of follow-up. This association persisted after adjustment for multiple potential confounding variables, including known cardiovascular risk factors, history of HF and MI, NT-proBNP, and echocardiographic abnormalities (valvular abnormalities; diastolic function; structural parameters such as EDV, EF, and LV mass; and inducible ischemia). These findings suggest that ESVI is a robust, independent predictor of HF hospitalization among patients with established CHD.
When compared with another echocardiographic measure of LV end-systolic enlargement, LV end-systolic dimension index, ESVI was a better predictor of HF hospitalization as assessed by area under the ROC curve. This important finding likely reflects the superior discriminative power of biplane volumetric measurements in detecting subtle changes in LV size; the fact that end-systolic dimension does not characterize the full extent of ventricular enlargement in some patients; and the possibility that 1-dimensional measurements are more prone to errors because fewer data points are analyzed compared with the biplane ESVI. In addition, ESVI was statistically superior to other echocardiographic measures of ventricular enlargement and systolic function (EDVI and EF) on ROC analysis. Our analyses suggest that ESVI is more effective than other markers of LV volume and function in predicting HF outcomes. This finding has practical importance because some patients with CHD have poor acoustic windows with endocardial borders only visible at end systole, thus precluding accurate EDV and EF calculation.
In the 815 participants without a history of HF, ESVI predicted incident HF hospitalization on unadjusted analyses, and this association persisted after multivariate adjustment for known cardiovascular risk factors, medication use, blood pressure, BNP, and renal function. Compared with those in the lowest quartile of ESVI, participants in the highest ESVI quartile (ESVI > 25 mL/m2) had a 4.6-fold higher rate of incident HF hospitalization after multivariate adjustment. Addition of other echocardiographic variables into a Cox multivariable regression analysis resulted in loss of the statistically significant association between ESVI and incident HF hospitalization. We suspect that the reduced sample size and decreased number of HF events reduced the statistical power to detect an association between ESVI and incident HF hospitalization.
In a subgroup analysis of 879 participants with normal EF (>50%), we found that ESVI remained predictive of increased HF hospitalization. Those participants in the highest ESVI quartile (ESVI > 21 mL/m2) had a 4.3-fold increased risk of HF hospitalization when compared with those in the lowest ESVI quartile after multivariate adjustment. These findings suggest that ESVI is associated with HF hospitalization even in patients with preserved EF.
EF is commonly used clinically as a means of assessing global LV function and risk stratifying patients with CHD and HF. Some investigators have questioned the validity of this practice on the basis that EF does little to identify the underlying cause of HF.28–30 EF is also affected by factors extrinsic to the LV (eg, preload, afterload, and heart rate), thus decreasing its accuracy as a measure of cardiac function.31 Some have argued that, instead of reflecting myocardial contractility, EF is largely driven by the degree of LV dilatation.32,33 Experimental data suggest that ESV is less sensitive to cardiac loading and varies greatly in response to changes in contractility.34–37 If so, it would perhaps be more accurate to categorize patients on the basis of ESV.
Maladaptive LV remodeling resulting in chamber dilatation is central to the pathogenesis of HF in patients with CHD.4 Myocardial infarction and ischemia can lead to myocyte loss, myocardial fibrosis, and LV dilatation. Along with neurohormonal activation, these changes lead to maladaptive LV remodeling and progressive deterioration of the remaining myocardium.38 Prior studies, which have shown that increased ESV post-MI is associated with increased mortality,6,39,40 indicate that larger ESV is an important predictor of adverse events in patients with CHD. In addition, reduction in ESV has been associated with improved mortality, reduced HF symptoms, and improved quality of life in cardiac resynchronization trials.41
Our results show that even slight increases in ESVI (ESVI > 25 mL/m2) predict adverse cardiovascular events in this population. The finding that subtle LV enlargement predicts HF hospitalization represents a distinct departure from prior studies, which required a much greater degree of LV enlargement (ESVI > 50–70 mL/m2) to detect an association between ESV and cardiovascular outcomes. The mean ESVI among participants in the highest ESVI cohort was 37.5 ± 15.6 mL/m2, and only 5% of patients in the highest ESVI cohort had an ESVI > 70 mL/m2. Our study suggests that a minor increase in ESVI represents an important pathophysiologic change that may predispose to HF and increased cardiovascular mortality.
Studies using 3-dimensional echocardiography and cardiac magnetic resonance (CMR) parallel our findings by demonstrating subtle LV volumetric and functional abnormalities in patients characterized as having normal systolic function on the basis of EF.33 Although 3-dimensional echocardiography and CMR are less accessible clinically, 2D echocardiography is widely available, and our findings support routine 2D biplane quantification of LVESV as a predictive tool for HF in patients with CHD. Complementary to our results, a recent study found that ESVI was a major echocardiographic predictor of adverse events in patients with asymptomatic aortic regurgitation (even in those with EF > 50%).42 Although our study included comprehensive measurement of clinical and echocardiographic risk factors for cardiovascular outcomes, as well as detailed adjudication of outcome events, limitations must be considered in interpreting our results. First, the determination of ESVI was based on manual tracing of echocardiographic images. All tracings were performed by a trained research sonographer and verified by a cardiologist, a practice that has been shown to minimize variability.43,44 It should also be noted that measurement of ESVI by 2D echocardiography has been shown to underestimate true ventricular ESVI when compared with 3-dimensional and CMR-based volumetric assessments.22 This tendency toward underestimation of ESVI would result in a systematic misclassification of ESVI that would not change the association between ESVI and HF. Although outcomes were adjudicated for HF hospitalization, we do not have data on LV assist device placement, cardiac transplantation, or cause of HF hospitalization. Finally, our definition of diastolic dysfunction, although comprehensive, did not include tissue Doppler imaging. However, our study did include measurement of NT-proBNP, which has been correlated with both LV diastolic dysfunction and increased LV filling pressures.
CONCLUSIONS
Maladaptive ventricular remodeling and depressed cardiac contractility, as determined by increased ESVI, are predictive of HF hospitalization in a general cohort of outpatients with CHD. ESVI was more effective than more established markers of left-ventricular systolic function (EF) and remodeling (EDVI) in its ability to predict HF. In addition, ESVI retained its ability to predict HF in patients with normal EF. Our work underscores the importance of subtle maladaptive LV remodeling in the pathogenesis of HF and mortality in patients with CHD and supports the routine measurement of ESVI in clinical practice.
Acknowledgments
The Heart and Soul Study was supported by grants from the Department of Veterans Affairs (Epidemiology Program), Robert Wood Johnson Foundation, American Federation for Aging Research, and National Institutes of Health (R01 HL079235). Dr Shah was supported by a Heart Failure Society of America Research Fellowship Award.
Footnotes
Conflicts of Interest: None.
References
- 1.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–9. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Ruzumna P, Borzak S, Havstad S, Ali A, Goldstein S. Decline in the rate of hospital mortality from acute myocardial infarction: impact of changing management strategies. Am Heart J. 1996;131:250–6. doi: 10.1016/s0002-8703(96)90349-x. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202–13. doi: 10.1161/CIRCULATIONAHA.106.623199. [DOI] [PubMed] [Google Scholar]
- 5.Migrino RQ, Young JB, Ellis SG, White HD, Lundergan CF, Miller DP, et al. End-systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. The Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO)-I Angiographic Investigators. Circulation. 1997;96:116–21. doi: 10.1161/01.cir.96.1.116. [DOI] [PubMed] [Google Scholar]
- 6.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 7.Kjoller-Hansen L, Steffensen R, Grande P. Beneficial effects of ramipril on left ventricular end-diastolic and end-systolic volume indexes after uncomplicated invasive revascularization are associated with a reduction in cardiac events in patients with moderately impaired left ventricular function and no clinical heart failure. J Am Coll Cardiol. 2001;37:1214–20. doi: 10.1016/s0735-1097(01)01118-4. [DOI] [PubMed] [Google Scholar]
- 8.Hamer AW, Takayama M, Abraham KA, Roche AH, Kerr AR, Williams BF, et al. End-systolic volume and long-term survival after coronary artery bypass graft surgery in patients with impaired left ventricular function. Circulation. 1994;90:2899–904. doi: 10.1161/01.cir.90.6.2899. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi A, Ino T, Adachi H, Mizuhara A, Murata S, Kamio H. Left ventricular end-systolic volume index in patients with ischemic cardiomyopathy predicts postoperative ventricular function. Ann Thorac Surg. 1995;60:1059–62. doi: 10.1016/0003-4975(95)00488-7. [DOI] [PubMed] [Google Scholar]
- 10.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–5. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick JN, Vannan MA, Narula J, Lang RM. Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol. 2007;50:381–96. doi: 10.1016/j.jacc.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 15.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erbel R, Schweizer P, Lambertz H, Henn G, Meyer J, Krebs W, et al. Echoventriculography—a simultaneous analysis of two-dimensional echocardiography and cineventriculography. Circulation. 1983;67:205–15. doi: 10.1161/01.cir.67.1.205. [DOI] [PubMed] [Google Scholar]
- 17.Folland ED, Parisi AF, Moynihan PF, Jones DR, Feldman CL, Tow DE. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–6. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg PS, Berge RD, Johnson KD, Bible M, Ellestad MH, Hayes M, et al. Use of end-systolic volume changes with exercise to detect left ventricular dysfunction in patients with coronary artery disease. Clin Cardiol. 1982;5:409–14. doi: 10.1002/clc.4960050704. [DOI] [PubMed] [Google Scholar]
- 19.Parisi AF, Moynihan PF, Feldman CL, Folland ED. Approaches to determination of left ventricular volume and ejection fraction by real-time two-dimensional echocardiography. Clin Cardiol. 1979;2:257–63. doi: 10.1002/clc.4960020404. [DOI] [PubMed] [Google Scholar]
- 20.Schiller NB, Acquatella H, Ports TA, Drew D, Goerke J, Ringertz H, et al. Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation. 1979;60:547–55. doi: 10.1161/01.cir.60.3.547. [DOI] [PubMed] [Google Scholar]
- 21.Weiss JL, Eaton LW, Kallman CH, Maughan WL. Accuracy of volume determination by two-dimensional echocardiography: defining requirements under controlled conditions in the ejecting canine left ventricle. Circulation. 1983;67:889–95. doi: 10.1161/01.cir.67.4.889. [DOI] [PubMed] [Google Scholar]
- 22.Wyatt HL, Meerbaum S, Heng MK, Gueret P, Corday E. Cross-sectional echocardiography. III. Analysis of mathematic models for quantifying volume of symmetric and asymmetric left ventricles. Am Heart J. 1980;100:821–8. doi: 10.1016/0002-8703(80)90062-9. [DOI] [PubMed] [Google Scholar]
- 23.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 24.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 25.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 26.Gehi AK, Ali S, Na B, Schiller NB, Whooley MA. Inducible ischemia and the risk of recurrent cardiovascular events in outpatients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2008;168:1423–8. doi: 10.1001/archinte.168.13.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 28.Brutsaert DL, De Keulenaer GW. Diastolic heart failure: a myth. Curr Opin Cardiol. 2006;21:240–8. doi: 10.1097/01.hco.0000221587.02114.da. [DOI] [PubMed] [Google Scholar]
- 29.De Keulenaer GW, Brutsaert DL. Systolic and diastolic heart failure: different phenotypes of the same disease? Eur J Heart Fail. 2007;9:136–43. doi: 10.1016/j.ejheart.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Petrie MC, Caruana L, Berry C, McMurray JJ. “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction? Heart. 2002;87:29–31. doi: 10.1136/heart.87.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–12. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 32.Konstam MA. “Systolic and diastolic dysfunction” in heart failure? Time for a new paradigm J Card Fail. 2003;9:1–3. doi: 10.1054/jcaf.2003.9. [DOI] [PubMed] [Google Scholar]
- 33.Maurer MS, El Khoury Rumbarger L, King DL. Ventricular volume and length in hypertensive diastolic heart failure. J Am Soc Echocardiogr. 2005;18:1051–7. doi: 10.1016/j.echo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Bombardini T. Myocardial contractility in the echo lab: molecular, cellular and pathophysiological basis. Cardiovasc Ultrasound. 2005;3:27. doi: 10.1186/1476-7120-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagawa K, Suga H, Shoukas AA, Bakalar KM. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am J Cardiol. 1977;40:748–53. doi: 10.1016/0002-9149(77)90192-8. [DOI] [PubMed] [Google Scholar]
- 36.Suga H. End-systolic pressure-volume relations. Circulation. 1979;59:419–20. doi: 10.1161/01.cir.59.2.419. [DOI] [PubMed] [Google Scholar]
- 37.Suga H, Kitabatake A, Sagawa K. End-systolic pressure determines stroke volume from fixed end-diastolic volume in the isolated canine left ventricle under a constant contractile state. Circ Res. 1979;44:238–49. doi: 10.1161/01.res.44.2.238. [DOI] [PubMed] [Google Scholar]
- 38.St John Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moye LA, Dagenais GR, et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68–75. doi: 10.1161/01.cir.89.1.68. [DOI] [PubMed] [Google Scholar]
- 39.Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39:30–6. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 40.Norris RM, White HD, Cross DB, Wild CJ, Whitlock RM. Prognosis after recovery from myocardial infarction: the relative importance of cardiac dilatation and coronary stenoses. Eur Heart J. 1992;13:1611–8. doi: 10.1093/oxfordjournals.eurheartj.a060113. [DOI] [PubMed] [Google Scholar]
- 41.Cleland JG, Daubert JC, Erdmann E, Freesmantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 42.Detaint D, Messika-Zeitoun D, Maalouf J, Tribouilloy C, Mahoney DW, Tajik AJ, et al. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. J Am Coll Cardiol. 2008;1:1–11. doi: 10.1016/j.jcmg.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Himelman RB, Cassidy MM, Landzberg JS, Schiller NB. Reproducibility of quantitative two-dimensional echocardiography. Am Heart J. 1988;115:425–31. doi: 10.1016/0002-8703(88)90491-7. [DOI] [PubMed] [Google Scholar]
- 44.Kuecherer HF, Kee LL, Modin G, Cheitlin MD, Schiller NB. Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications. J Am Soc Echocardiogr. 1991;4:203–14. doi: 10.1016/s0894-7317(14)80020-5. [DOI] [PubMed] [Google Scholar]