Abstract
Neurokinin-1 receptor (NK-1R) antagonists suppress HIV-1 infection of macrophages in vitro. We have further investigated the anti-HIV-1 activity of aprepitant, a Food and Drug Administration-approved NK-1R antagonist, and its cytotoxic effect in the macrophage/microglia system. Aprepitant inhibited infection of macrophages with primary HIV-1 R5 strains (subtypes A, D, and H; UG275, BZ163, and BCF-KITA), while it had little effect on primary HIV-1 X4 strains (subtypes B and D, BZ167 and SE365). Aprepitant, when added to microglia cultures infected with CSF-derived HIV-1 strains (JAGO or JRFL), significantly inhibited viral replication. Aprepitant also enhanced the anti-HIV-1 activity of enfuvirtide (an HIV-1 fusion inhibitor) in HIV-1-infected macrophages. Over a concentration range of 10-9 to 10-5 M, aprepitant had little cytotoxic effect (less than 10%) on macrophages during the in vitro cultures. Autologous human serum (≤20%) had little effect on the anti-HIV-1 activity of aprepitant in macrophages. These observations provide additional evidence to support the potential use of NK-1R antagonists as therapeutic and immunomodulatory agents for the treatment of HIV-1 infection.
Keywords: aprepitant, macrophage, HIV-1, neurokinin 1 receptor
Introduction
Substance P (SP) is a neuropeptide that plays a crucial role in modulating the interactions between the nervous system and the immune system (Harrison and Geppetti 2001; Kennedy et al. 1997, 1998, 2003; McGillis et al. 1987). We have previously demonstrated that SP enhances HIV-1 infection of macrophages (Ho et al. 1996) and that SP activates HIV-1 replication in latently infected immune cells (Li et al. 2001). In addition, the interactions between SP and HIV-1 are bidirectional, as we showed that HIV-1 infection of human immune cells induces SP expression (Ho et al. 2002). Furthermore, the neurokinin-1 receptor (NK-1R) antagonist (CP96,345) inhibits HIV-1 replication in macrophages (Lai et al. 2001). Two potential mechanisms were suggested to be involved in the anti-HIV action of the NK-1R antagonist: one is that the NK-1R antagonists interrupt the SP autocrine loop that may have a role in maintaining high levels of CCR5 expression, permitting HIV R5 strain infection; the second mechanism is that the NK-1R antagonist directly inhibits SP-mediated upregulation of HIV replication at the transcriptional level, since the addition of SP to macrophage cultures did not affect CCR5 expression but did enhance HIV-1 LTR-driven gene expression (Lai et al. 2001).
These in vitro studies described above were based on the in vivo observations that HIV+ subjects had a greater incidence of abnormal patterns of immunoreactivity of SP in enteric nerves and enteroendocrine cells than HIV- subjects (Sharkey et al. 1992) and that HIV+ children had higher levels of SP plasma in comparison to HIV- children born to HIV+ mothers or healthy control children (Azzari et al. 1992). We also demonstrated that there are increased levels of plasma SP in HIV-infected men (Douglas et al. 2001) and women (Douglas et al. 2008) in comparison to uninfected control subjects. Although the cellular source of elevated levels of plasma SP in HIV-infected individuals is not yet defined, these in vivo findings in conjunction with the in vitro observations described above support the need for more studies on the possibility to use NK-1R antagonists as a therapeutic agent for the treatment of HIV-1 infection. We recently demonstrated that aprepitant, another highly selective non-peptide antagonist of NK-1R, inhibited HIV-1 infection of macrophages (Wang et al. 2007). In these studies, we further investigated the anti-HIV-1 activity of aprepitant in macrophage/microglia system. In addition, we determined the impact of human serum on aprepitant-mediated anti-HIV-1 activity and the cytotoxicity effect of aprepitant on human blood monocyte-derived macrophages.
Materials and methods
Monocyte/microglia culture
Peripheral bloods obtained from five healthy adult donors were identified as HIV-1 antibody-negative by anonymous testing with the enzyme-linked immunosorbent assay (ELISA) method (Coulter Immunology, Hialeah, FL, USA). Informed consent was obtained, and the Institutional Research Board of our institution approved this study. Monocytes were purified according to our previously described technique (Hassan et al. 1986). Heparinized blood was separated by centrifugation over lymphocyte separation medium (Organon Teknika Corporation, Durham, NC, USA) at 400×g to 500×g for 45 min. The mononuclear cell layer was collected and incubated with Dulbecco’s modified Eagle medium (DMEM) (Life Technologies, Grand Island, NY, USA) in a 2% gelatin-coated flask for 45 min at 37°C, followed by removal of the non-adherent cells with DMEM. The non-adherent cells were peripheral blood lymphocytes (PBLs). PBLs were maintained in a culture of RPMI 1640 medium containing 10% fetal calf serum (FCS) and 1 μg/ml of phytohemagglutinin-P for 72 h. Cells were then transferred to a T-25 flask and treated with interleukin-2 (IL-2, 50 ng/ml). Adherent monocytes were detached with 10 mM ethylenediaminetetraacetic acid. Following the initial purification, greater than 97% of the cells were monocytes, as determined by nonspecific esterase staining and flow cytometry analysis using monoclonal antibody against CD14, a marker specific for monocytes and macrophages. Freshly isolated monocytes were plated in 48-well culture plates at a density of 5×105 cells/well in DMEM containing 10% FCS. Macrophages refer to 7-day-cultured monocytes in vitro. Macrophage viability was monitored by trypan blue exclusion and maintenance of cell adherence. Human microglia cells were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured under the same condition described above for monocytes/macrophages. The human microglia is isolated from primary human fetal brain cell culture and is characterized by immunofluorescent method with antibody to OX-42 (cd11b/c). The purity of isolated microglial cells was >95%.
Cell line culture
CHP212 cells (the neuroblastoma cell line) were purchased from American Type Tissue Culture (ATCC) (Manassas, VA, USA). CHP212 cells were cultured in Eagle’s MEM-Ham F12 (1:1) medium containing 10% heat-inactivated (HI) fetal bovine serum (HI-FBS) (Invitrogen), 0.1 mM non-essential amino acid, and 1.0 mM sodium pyruvate. U373-MG (human astroglioma cells), IM9 (human B lymphoblasts), and Jurkat E6-1 (human T lympholasts) were obtained from ATCC (Rockville, MD, USA). U373-MG cells were obtained from EMEM (Eagles MEM) containing 10% FCS, 2 mM l-glutamine, and 1 mM sodium pyruvate. IM9 or Jurkat E6-1 cells were cultured in RPMI 1640 (GIBCO) and supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 10% FBS. Huh 7 cells (human hepatoma cell line) were propagated in DMEM containing 10% FCS and 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg streptomycin.
Compounds and reagents
Aprepitant (Emend®), manufactured by Merck & Co., Inc. (Whitehouse Station, NJ, USA), was purchased through the pharmacy of the Children’s Hospital of Philadelphia. Aprepitant is a FDA-approved NK-1R antagonist that has been used clinically for the prevention of nausea and vomiting associated with cancer chemotherapy or following surgical procedures. For the in vitro experiments, aprepitant was dissolved in DMSO. The HIV-1 fusion inhibitor (enfuvirtide or T20) was obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health, Bethesda, MD, USA.
Cytotoxicity assay
Cytotoxicity was measured by the CellTiter 96® AQueous Assay (Promega, Madison, WI, USA). The assay is to measure the quantity of formazan products by the amount of 490 nm absorbance. The quantity of formazan is directly proportional to the number of living cells in culture. Briefly, different cell types (Table 2) were cultured in the absence or presence of various concentrations of aprepitant in triplicate in 96-well plates for 4 days. For HIV-infected macrophages, we cultured the cells in the absence or presence of HIV and/or anti-HIV drug aprepitant in triplicate in 96-well plates for 12 days. MTS assay was then carried out based on the instruction of the manufacturer. The percentage of viable cells was quantified at 590 nm in an ELISA reader (Elx800, Bio-Tek Instrument, USA). The concentration that caused the reduction of viable cells by 50% (CC 50) was determined using dose-response curve.
Table 2.
Cytotoxicity effect of aprepitant
| Cell names | Tissue origin | CC 50 (×10-5 M) |
|---|---|---|
| Primary cells | ||
| PBL | Peripheral blood | 4.3 |
| Macrophage | Peripheral blood | 4.9 |
| Cell lines | ||
| IM-9 | B-lymphoblastoid | >2 |
| Jurkat E6-1 | T lymphoblastic leukemia | >2.5 |
| Huh 7 | Hepatocellular carcinoma | 4 |
| CHP 212 | Neuroblastoma | 7 |
| U373-MG | Astrocytoma | 2.5 |
HIV-1 strains
The laboratory-adopted macrophage-tropic R5 strain (Bal) was obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health, Bethesda, MD, USA. The primary HIV-1 R5 isolates (UG275, BZ 163, and BCF-KITA) and the primary X4 isolates (BZ167 and SE365) were provided by Dr. Mark Manak and Dr. Dmitry Moshkoff (SeraCare BioServices, Gaithersburg, MD, USA, Integrated Preclinical Clinical Program Core-B). JAGO (CSF-derived isolate) and JRFL (brain tissue isolate) were obtained from the Center for AIDS Research at the University of Pennsylvania (CFAR of UPENN). Detailed information about these isolates is shown in Table 1.
Table 1.
HIV-1 primary isolates and laboratory-adopted strains
| Code | Isolate ID | HIV-1 subtype | Source of tissue | Country | NSI/SI | Co-receptor |
|---|---|---|---|---|---|---|
| 1 | Bal | B | Lung | USA | NSI | R5 |
| 2 | UG275 | A | PBMC | Uganda | NSI | R5 |
| 3 | BZ163 | F | PBMC | Brazil | NSI | R5 |
| 4 | BCF-KITA | H | PBMC | Zaire | NSI | R5 |
| 5 | BZ167 | B | PBMC | Brazil | SI | X4 |
| 6 | SE365 | D | PBMC | Senegal | SI | X4 |
| 7 | JAGO | NI* | CSF | USA | NSI | R5 |
| 8 | JRFL | B | Brain Tissue | USA | NSI | R5 |
NI: Not identified
HIV-1 reverse transcriptase
HIV reverse transcriptase (RT) activity was determined based on a modified version of the technique of Willey et al. (1988). In brief, 10 μl of collected culture supernatants were added to a cocktail containing poly (A), oligo (dT) (Pharmacia Inc., Piscataway, NJ, USA), MgCl2, and 32P dTTP (Amersham Corp., Arlington Heights, IL, USA) and then incubated for 20 h at 37°C. The cocktail (30 μl) was then spotted onto DE81 paper, dried, and washed five times with 2× saline-sodium citrate buffer and once with 95% ethanol. Radioactivity of the air-dried filter paper was counted in a liquid scintillation counter (Packard Instrument Inc., Palo Alto, CA, USA).
Aprepitant and/or anti-HIV-1 drug treatment and HIV-1 infection
Macrophages (5×105 cells/well in 48-well plates) or microglia (5×104 cells/well in 96-well plates) were incubated for 2 h with or without aprepitant (10-6 M) and/or the antiretroviral (enfuvirtide 10-8M) before infection with different strains of HIV-1. The cells were infected with equal amounts of cell-free based on p24 protein content (30 ng/106 cells) for 2 h at 37°C in the presence or absence of aprepitant and/or enfuvirtide. The cells were then washed three times with DMEM to remove unabsorbed virus and fresh medium containing aprepitant, and/or the antiretroviral was added to cell cultures. The final wash was tested for viral RT activity and shown to be free of residual inoculums of HIV-1. Untreated macrophages microglia cultures were used as controls. HIV-1 RT activity was analyzed at indicated time point post-infection.
RNA extraction, reverse transcription, and real-time polymerase chain reaction
Total cellular RNA was isolated from macrophages (106 cells) using Tri-reagent (Molecular Research Center, Cincinnati, OH, USA). In brief, the total RNA was extracted by a single-step, guanidium thiocyanate-phenol-chloroform extraction. After centrifugation at 13,000×g for 15 min at 4°C, the RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were then washed once in 75% ethanol and resuspended in 30 μl of RNase-free water. One microgram of total RNA was subjected to reverse transcription using the reverse transcription system (Promega, Madison, WI, USA) with specific primers (anti-sense) for HIV-1 coreceptor (CCR5) or SP genes for 1 h at 42°C. The reaction was terminated by incubating the mixture at 99°C for 5 min and then keeping it at 4°C. Real-time polymerase chain reaction (PCR) was performed with one tenth of complementary DNA derived from 1 μg RNA extracted from MDM using ABI Prism 7700 Sequence Detection System (Perkin Elmer), as described previously (Lai et al. 2002a, 2003). All controls and samples were run in duplicates in the same culture plate. The measurement of glyceraldehyde-3-phosphate dehydrogenase messenger RNA (mRNA) levels in the samples by real-time PCR performed on the same plate was used as a control to normalize the mRNA contents among the samples tested.
Human serum and anti-HIV-1 activity of aprepitant in macrophage
Macrophages (1.5×105 cells/well in 96-well plates) were incubated for 2 h with or without aprepitant (10-6 M) in the presence of different percent of autologous human serum (HS) or 10% fetal calf serum. The cells were then infected with HIV-1 Bal strain (p24 30 ng/106 cells) for 2 h at 37°C. The cells were then washed three times with DMEM to remove the unabsorbed virus and fresh media containing aprepitant, and different concentrations of HS or 10% FCS were added to the cultures. HIV-1 RT activity was analyzed at day 12 post-infection. The cytotoxicity assay was carried out at day 4 post-treatment with aprepitant in the presence of autologous human serum or FCS.
Statistical analysis
The data were expressed as mean ± SD. For comparison of the means of both groups (aprepitant-treated vs. untreated macrophages), statistical significance was assessed by Student’s t test. Calculations were performed using Stata Statistical Software (Stata Corp., College Station, TX, USA). Statistical significance was defined as p<0.05.
Results
Aprepitant inhibits HIV-1 infection of macrophages with primary R5 isolates
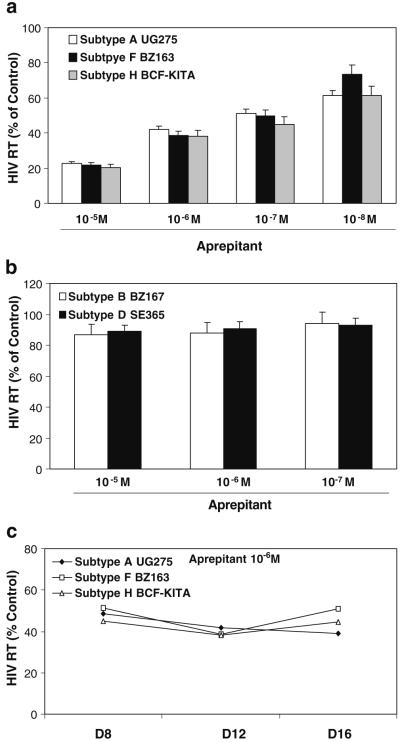
In order to determine whether aprepitant has a broad range of anti-HIV-1 activity, we determined whether aprepitant inhibits HIV-1 infection of macrophages with primary subtype isolates. Aprepitant inhibited replication of primary R5 isolates (subtype A, F, and H) of HIV-1 in macrophages (Fig. 1a,c). In contrast, aprepitant had little effect on HIV-1 infection of macrophages with primary X4 isolates (subtype B and D) (Fig. 1b).
Fig. 1.
Effect of aprepitant on infection of macrophages with different primary HIV-1 subtypes: R5 isolates (a) and X4 isolates (b). Seven-day-cultured macrophages were incubated with or without aprepitant at indicated concentrations for 2 h and then infected with the indicated HIV-1 subtypes. HIV-1 RT activity was determined at day 12 post-infection. c Time-course effect of aprepitant on replication in macrophages with different primary HIV-1 R5 isolates. Seven-day-cultured macrophages were incubated with or without aprepitant at indicated concentrations for 2 h and then infected with the indicated HIV-1 subtypes. HIV-1 RT activity was determined at days 8, 12, and 16 post-infection. The data shown are the mean ± SD of triplicate cultures representative of three experiments using cells from three different donors
Aprepitant inhibits HIV-1 infection of microglia
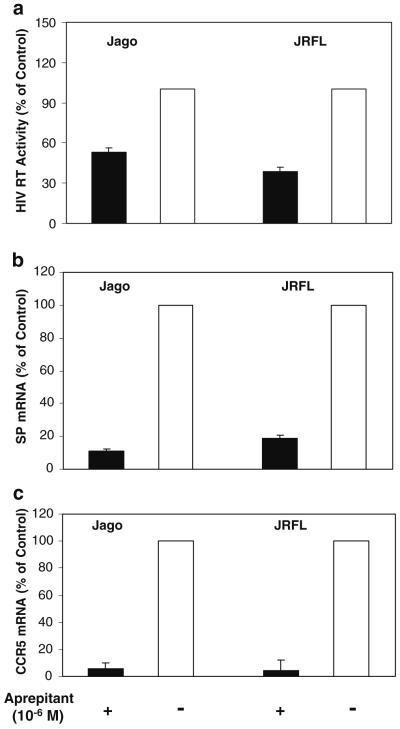
Since the primary target cells for HIV-1 infection in the central nervous system (CNS) are microglia cells, we examined whether aprepitant has the ability to suppress HIV-1 infection of microglia. When infected by HIV-1, aprepitant-treated microglia cells had significantly lower levels of HIV RT activity than the control cultures (Fig. 2a). This inhibitory effect of aprepitant was observed in the cell cultures infected with two CNS-derived HIV-1 R5 isolates (JAGO and JRFL). In order to investigate the mechanism of anti-HIV-1 activity of aprepitant in microglia, we also examined the expression of endogenous SP and CCR5 in HIV-1-infected microglia treated with or without aprepitant. Aprepitant-treated microglia expressed lower levels of mRNA of SP and CCR5 than untreated cells (Fig. 2b,c).
Fig. 2.
Effect of aprepitant on HIV-1 infection of human microglia. Seven-day-cultured microglia were treated with or without aprepitant (10-6 M) for 2 h and then infected with CNS-derived HIV-1 strains (JAGO or JRFL) in the presence or absence of aprepitant. Cultures were re-fed with fresh media containing aprepitant every 3-4 days. Culture supernatants were collected for HIV RT activity at day 12 post-infection (a). Total cellular RNA was extracted and subjected to the real-time RT-PCR for SP (b) and CCR5 (c) mRNA expression. The data shown are the mean ± SD of triplicate cultures, representative of two experiments
Aprepitant enhances the anti-HIV-1 activity of enfuvirtide
We previously demonstrated that aprepitant significantly enhanced the anti-HIV-1 activity of the antiretrovirals (AZT, efavirenz, and indinavir) (Wang et al. 2007). We further determined whether aprepitant has the ability to enhance the anti-HIV-1 activity of enfuvirtide (T20, a HIV-1 fusion inhibitor) in macrophages. While aprepitant or enfuvirtide treatment individually significantly inhibited HIV-1 (Bal) infection of macrophages, the cells treated with both aprepitant and enfuvirtide had the lowest levels of HIV-1 RT among the cell cultures tested (Fig. 3). These drugs at the concentrations that had anti-HIV-1 activity had no cytotoxic effects on macrophages (Fig. 3).
Fig. 3.
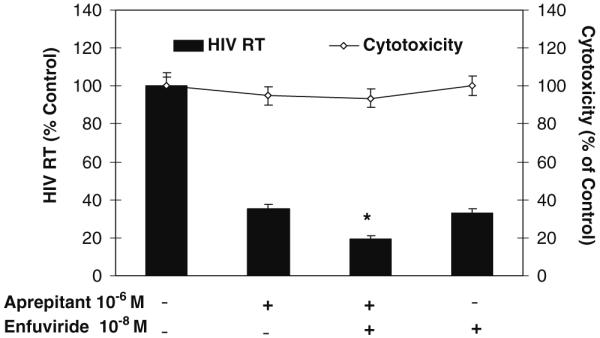
Effect of aprepitant and/or enfuvirtide on HIV-1 (Bal) infection of macrophages in vitro. Macrophages were treated with aprepitant (10-6 M) and/or antiretroviral (enfuvirtide, 10-8 M) at indicated concentrations for 2 h and then infected with HIV-1 Bal strain. The drug cytotoxicity effects were measured by the MTS assay on day 12 post-infection. HIV-1 RT activity was also determined at day 12 post-infection. The data are presented as the mean ± SD of triplicate cultures, representative of three experiments using cells from three different donors (aprepitant vs. aprepitant + enfuvirtide or enfuvirtide vs. aprepitant + enfuvirtide: *p<0.05)
Cytotoxicity effect of aprepitant
We further determined whether the anti-HIV-1 activity of aprepitant is due to its cytotoxic effect on the macrophages. Parallel to the determination of antiviral activity of aprepitant, the cytotoxicity of aprepitant was analyzed. There was no cytotoxicity effect observed after incubation of macrophages with aprepitant over a concentration range of 10-9 to 10-5 M for up to 12 days (Fig. 4). In addition to macrophages, effects of aprepitant on a variety of human cells, including B-lymphoblastoid cell line (IM-9), lymphoblastic leukemia T cell line (Jurkat, Clone E6-1), hepatic cell line (Huh 7), neuroblastoma line (CHP 212), and astrocytoma line (U373-MG) were checked. The 50% cytotoxicity concentrations (CC 50) for aprepitant on these cell types are shown in Table 2.
Fig. 4.
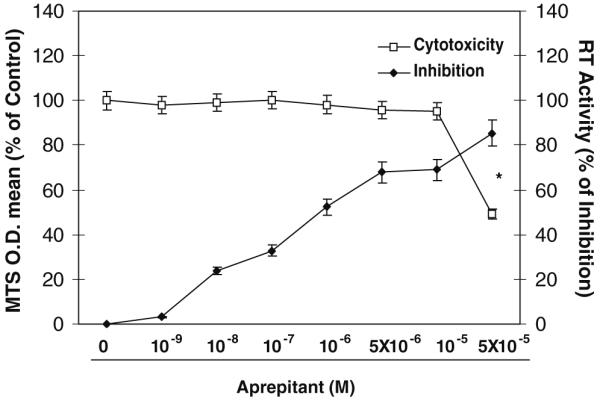
Aprepitant in dose-dependent fashion inhibits HIV-1 (Bal) infection of macrophages. Seven-day-cultured macrophages were treated with aprepitant at indicated concentrations (10-9 M to 5×10-5 M) for 2 h and then infected with HIV-1 Bal strain. Cell viability was detected with MTS assay on day 12 post-infection. HIV-1 RT activity was determined at day 12 post-infection. The data shown are presented as the mean ± SD of triplicate cultures, representative of three experiments using macrophage from three donors (aprepitant vs. control: *p<0.05)
Impact of human serum on anti-HIV-1 activity of aprepitant in macrophages
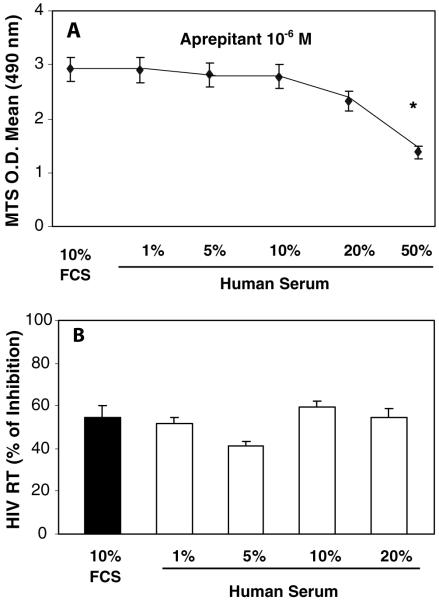
In order to determine whether the presence of human serum in macrophages cultures affects the anti-HIV-1 activity of aprepitant that may bind to serum proteins, we cultured macrophages with different concentrations of autologous serum. Although autologous serum had cytotoxicity effect on macrophages at concentrations greater than 20% (v/v), the anti-HIV-1 activity of aprepitant in macrophages cultured in media containing less than 20% autologous serum was not affected (Fig. 5b).
Fig. 5.
Effect of human serum on anti-HIV-1 activity of aprepitant in macrophages. Seven-day-cultured macrophages were incubated with or without aprepitant (10-6 M) at different concentrations of autologous human serum or 10% FCS for 2 h. The cells were infected with HIV-1 (Bal) for 2 h at 37°C in the presence or absence of aprepitant. The human serum cytotoxicity effects were measured by the MTS assays on day 4, following the protocol of manufactory (a). HIV-1 RT activity was analyzed at day 12 post-infection (b). The data shown were presented as the mean ± SD of triplicate cultures, representative of three experiments using cells from three different donors (aprepitant vs. control: *p<0.05)
Discussion
Microglia and brain macrophages are the primary cell types that support productive HIV-1 infection in the CNS (Gendelman et al. 1989; Kramer-Hammerle et al. 2005; Price et al. 1988; Takahashi et al. 1996; Watkins et al. 1990). In addition, the neuronal damage observed among patients with NeuroAIDS is mediated by microglia/macrophages-produced cytokines/chemokines (Gelbard et al. 1994; Genis et al. 1992; Giulian et al. 1990; Kielian 2004; Pulliam et al. 1997). Therefore, we further examined the anti-HIV-1 effectiveness of the FDA-approved NK-1R antagonist, aprepitant (Emend®), on HIV-1 infection of macrophages and microglia. In parallel, we determined the cytotoxic effect of aprepitant on macrophages. Our findings that aprepitant inhibited HIV-1 infection of macrophages with primary isolates (Fig. 1) support and extend our earlier observations that the NK-1R antagonists inhibited HIV-1 infection of macrophages with the laboratory-adopted Bal strain (Bal) (Lai et al. 2001, 2002b) as well as with antiretroviral resistant strains (Wang et al. 2007). Similar to these earlier studies (Lai et al. 2001; Wang et al. 2007), we also observed the variability (10-80%) of macrophages from different donors with regard to the anti-HIV effect of aprepitant (data not shown). The variation of anti-HIV activity by aprepitant is currently under investigation. In the cell cultures where high-level inhibition of HIV RT by aprepitant was observed, a 40% suppression of viral replication remained in spite of a three-log reduction in aprepitant concentration. This finding suggests that the antiviral effect at low concentration may not be directly related to aprepitant. Thus, we investigated whether aprepitant, through its immunomodulating effects, inhibits HIV infection of macrophages. This speculation was supported by the data showing that aprepitant inhibited infection of macrophages with HIV-1 primary R5 isolates (UG275, BZ163, and BCF-KITA), while it had little effect on primary X4 isolates of HIV-1 (BZ167 and SE365) (Fig. 1). These observations also suggest that CCR5 receptor is involved in aprepitant-mediated anti-HIV-1 activity in macrophages. More importantly, the anti-HIV-1 ability of aprepitant was observed in microglial cells infected with the CNS-derived HIV-1 isolates (JAGO and JRFL), as aprepitant-treated microglia had significantly lower levels of HIV-1 RT activity than untreated cells (Fig. 2a). We also examined the mechanisms involved in the aprepitant action on HIV-1, showing that aprepitant suppressed the expression of CCR5 and endogenous SP in microglial cells (Fig. 2b,c). These findings not only support our earlier studies using macrophages (Lai et al. 2001, 2002b) but also are highly significant since microglial cells are the primary target for HIV-1 infection in the CNS and have a key role in HIV-mediated neuronal injury.
In order to further determine whether aprepitant has the ability to enhance the anti-HIV activity of antiretrovirals, we investigated the impact of the combination of aprepitant with a HIV-1 fusion inhibitor (enfuvirtide) on HIV-1 infection of macrophages. We demonstrated that aprepitant enhanced enfuvirtide-mediated anti-HIV-1 activity in macrophages (Fig. 3). The activity of aprepitant is not caused by its cytotoxic effect, as aprepitant over a concentration range of 10-9 to 10-5 M had no cytotoxicity effects on macrophages (Fig. 4).
Furthermore, autologous human serum at concentrations ≤20% has little effect on the anti-HIV-1 activity of aprepitant in cultured macrophages (Fig. 5b).
In summary, this study provides additional and compelling in vitro evidence to support the concept that aprepitant may have therapeutic and immunomodulatory effects on HIV-1 infection. In addition to its benefit to individuals who are infected with HIV-1 strains resistant to the current and standard HIV therapy, aprepitant, which is currently in a phase IB clinical trial (http://www.clinicaltrails.gov/ct2/show/NCT00428519), may have a role in the treatment of HIV-1 infection in the CNS.
Acknowledgment
We are currently doing a phase I clinical trial (IND# 75558) of aprepitant in HIV-1-positive individuals. This work/investigation was supported by the National Institutes of Health grants DA-012815 and DA-022177 (to W.Z.H), and PO1 MH 076388 and MH 049981 (to S.D.D.). We thank Dr. Mark Manak and Dr. Dmitry Moshkoff (SeraCare BioServices, IPCP Core B) for providing primary HIV-1 strains.
References
- Azzari C, Rossi ME, Resti M, Caldini AL, Lega L, Galli L, et al. Changed levels of substance P and somatostatin in HIV-positive children. Pediatr Med Chir. 1992;14:577–581. [PubMed] [Google Scholar]
- Douglas SD, Ho WZ, Gettes DR, Cnaan A, Zhao HQ, Leserman J, et al. Elevated substance P levels in HIV-infected men. AIDS. 2001;15:2043–2045. doi: 10.1097/00002030-200110190-00019. [DOI] [PubMed] [Google Scholar]
- Douglas SD, Cnaan A, Lynch KG, Benton T, Gettes DR, Evans DL. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res Hum Retrovir. 2008;24:375–378. doi: 10.1089/aid.2007.0207. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Nottet HS, Swindells S, Jett M, Dzenko KA, Genis P, et al. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Baca LM, Weiser B, Burger H, Kalter DC, et al. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989;3:475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Harrison S, Geppetti P. Substance P. Int J Biochem Cell. 2001;33:555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Cnaan A, Li YH, Zhao H, Lee HR, Song L, et al. Substance P modulates human immunodeficiency virus replication in human peripheral blood monocyte-derived macrophages. AIDS Res Hum Retrovir. 1996;12:195–198. doi: 10.1089/aid.1996.12.195. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Li Y, Douglas SD. HIV enhances substance P expression in human immune cells. FASEB J. 2002;16:616–618. doi: 10.1096/fj.01-0655fje. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Rodgers J, Jennings FW, Murray M, Leeman SE, Burke JM. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc Natl Acad Sci U S A. 1997;94:4167–4170. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J Neuroimmunol. 1998;92:98–108. doi: 10.1016/s0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Rodgers J, Bradley B, Hunt SP, Gettinby G, Leeman SE, et al. Clinical and neuroinflammatory responses to meningoencephalitis in substance P receptor knockout mice. Brain. 2003;126:1683–1690. doi: 10.1093/brain/awg160. [DOI] [PubMed] [Google Scholar]
- Kielian T. Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Front Biosci. 2004;9:732–750. doi: 10.2741/1266. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Lai J, Ho W, Zhan G, Yi Y, Collman R, Douglas S. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci U S A. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Shaheen F, Pleasure DD, Ho WZ. Quantification of substance P mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol. 2002a;9:138–143. doi: 10.1128/CDLI.9.1.138-143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Ho WZ, Yang JH, Wang X, Song L, Douglas SD. A non-peptide substance P antagonist down-regulates SP mRNA expression in human mononuclear phagocytes. J Neuroimmunol. 2002b;128:101–108. doi: 10.1016/s0165-5728(02)00164-9. [DOI] [PubMed] [Google Scholar]
- Lai JP, Yang JH, Douglas SD, Wang X, Riedel E, Ho WZ. Quantification of CCR5 mRNA in human lymphocytes and macrophages by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol. 2003;10:1123–1128. doi: 10.1128/CDLI.10.6.1123-1128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Douglas SD, Song L, Sun S, Ho WZ. Substance P enhances HIV-1 replication in latently infected human immune cells. J Neuroimmunol. 2001;121:67–75. doi: 10.1016/s0165-5728(01)00439-8. [DOI] [PubMed] [Google Scholar]
- McGillis JP, Organist ML, Payan DG. Substance P and immunoregulation. Fed Proc. 1987;46:196–199. [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Sutherland LR, Davison JS, Zwiers H, Gill MJ, Church DL, The GI/HIV Study Group of the University of Calgary Peptides in the gastrointestinal tract in human immunodeficiency virus infection. Gastroenterology. 1992;103:18–28. doi: 10.1016/0016-5085(92)91090-q. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Lai JP, Tuluc F, Tebas P, Ho WZ. Neurokinin-1 receptor antagonist (aprepitant) inhibits drug-resistant HIV-1 infection of macrophages in vitro. J Neuroimmune Pharmacol. 2007;2:42–48. doi: 10.1007/s11481-006-9059-6. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Dorn HH, Kelly WB, Armstrong RC, Potts BJ, Michaels F, et al. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- Willey RL, Smith DH, Lasky LA, Theodore TS, Earl PL, Moss B, et al. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]