Abstract
Background
Telomere shortening has been proposed as a marker of biological aging. Whether leukocyte telomere length is associated with mortality among patients with stable coronary artery disease (CAD) is unknown.
Methods and Results
We measured leukocyte telomere length in 780 patients with stable CAD in a prospective cohort study. Participants were categorized by quartiles of telomere length. Hazard Ratios (HRs) and 95% confidence intervals were calculated for all-cause mortality, heart failure (HF) hospitalization, and cardiovascular (CV) events. After 4.4 years of follow-up there were 166 deaths. Compared with participants in the highest telomere length quartile, those in the lowest quartile were at increased risk of death (age-adjusted HR 1.8; 95% CI 1.2–2.9). After multivariate adjustment for clinical (HR 2.1; CI 1.3–3.3), inflammatory (HR 2.0; CI 1.2–3.2), and echocardiographic (HR 1.9; CI 1.0–3.5) risk factors, patients in the lowest quartile of telomere length remained at significantly increased risk of death compared to those in the highest quartile. Patients in the lowest quartile of telomere length were also at significantly increased risk of HF hospitalization (HR 2.6; CI 1.1–6.0) but not CV events (HR 1.7; CI 0.9–3.5)
Conclusions
Reduced leukocyte telomere length is associated with all-cause mortality in patients with stable CAD. The prognostic value of short telomeres in predicting death is not completely captured by existing clinical, inflammatory, and echocardiographic markers of risk.
Keywords: Telomere, Aging, Leukocyte, Prognosis, Coronary
Telomeres are specialized tandem DNA repeat sequences (TTAGGG)n located at the ends of eukaryotic chromosomes which protect somatic cells from genomic instability during mitotic cell proliferation (1,2). During each cell division, DNA polymerase cannot fully replicate the 3′ end of linear DNA, resulting in progressive telomere shortening (3). After a critical degree of telomere shortening, loss of chromosomal integrity results in replicative senescence and apoptosis (4). Initial telomere length at birth is widely variable and determined by both genetic and environmental factors (5–10). In 1973 Olovnikov proposed that the process of telomere attrition may provide the basis for a “biological clock” which integrates the cumulative effect of environmental stressors independently of chronological age (11).
Several lines of evidence link telomere attrition with coronary artery disease (CAD). Inflammation and oxidative stress, two key factors in the pathogenesis of atherosclerosis, are both associated with accelerated telomere shortening (12, 13). Coronary endothelial cells in patients with CAD have shorter telomeres than those derived from age-matched non-CAD patients (14). The enzyme telomerase, which partially restores telomere length, is down-regulated in vascular smooth muscle cells in atherosclerotic plaques (15).
Prior studies have linked leukocyte telomere shortening with an increased risk of mortality and cardiovascular events in individuals with no prior history of atherosclerosis (16, 17). However, there are few longitudinal data examining the prognostic value of leukocyte telomere length in the context of other clinical, inflammatory, and echocardiographic variables. We sought to investigate the relationship between leukocyte telomere length and mortality in 780 patients with stable CAD in a prospective cohort study, and to determine whether leukocyte telomere length provides incremental prognostic value beyond existing clinical, inflammatory, and echocardiographic markers of risk.
METHODS
Participants
The Heart and Soul Study is a prospective cohort study investigating the influence of psychosocial factors on cardiovascular events in outpatients with stable CAD. The enrollment process for the Heart and Soul Study has been previously described (18). Eligible participants were recruited from outpatient clinics in the San Francisco Bay Area if they met at least one of the following inclusion criteria: 1) history of myocardial infarction, 2) angiographic evidence of at least 50% stenosis by area in at least 1 coronary artery, 3) evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging, 4) history of coronary revascularization. Individuals were excluded if they had a history of myocardial infarction in the past 6 months, deemed themselves unable to walk 1 block, or if they were planning to move out of the local area within 3 years.
The study protocol was approved by the following Institutional Review Boards: the University of California San Francisco Committee on Human Research, the Research and Development Committee at the San Francisco VA Medical Center, the Medical Human Subjects Committee at Stanford University, the Human Subjects Committee at the VA Palo Alto Health Care System, and the Data governance Board of the Community Health Network of San Francisco. All participants provided written informed consent. Between September 2000 and December 2002, a total of 1024 participants enrolled in the study. Of these, 960 provided DNA samples for analysis. A further 180 samples were excluded because significant evaporation occurred when heat-denaturing the first 3 plates of DNA, and the resulting telomere length data were poorly correlated (r=0.35) with triplicate (quality control) samples on other plates. Telomere length data for the remaining 780 samples correlated well with triplicate (quality control) samples (r=0.82). There were no differences in patient characteristics between subjects included and excluded from the telomere length analysis.
Telomere Length Assay
Genomic DNA was isolated according to standard procedures from peripheral blood leukocytes collected at the baseline study visit and stored at −70 C. Purified DNA samples were diluted in 96-well microtiter source plates to a fixed concentration of 1.75ng/ul. Relative mean telomere length was measured from DNA by a quantitative polymerase chain reaction (PCR) based assay that compares mean telomere repeat sequence copy number (T) to a reference single copy gene copy number (S) in each sample as previously described (19). Standard curves were derived from serially-diluted reference DNA for comparison with patient samples. The T/S ratio was determined as the average quantity of reference DNA found to match with each experimental sample for the copy number of the targeted template (the number of telomere repeats for T and the number of beta-globin gene copies for S. The T/S ratio has been confirmed previously to be highly consistent with independent measurement of telomere length by Southern blot terminal restriction fragment analysis (19). The reference single copy gene used in this study was human beta-globin. The telomere-specific primers were: forward, 5′CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT3′ and reverse, 5′GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT3′. The beta-globin-specific primers were: forward 5′GCTTCTGACACAACTGTGTTCACTAGC3′ and reverse, 5′CACCAACTTCATCCACGTTCACC′. The thermal cycling protocol for telomere PCR was: 95 degrees C for 5 minutes for initial denaturation followed by 25 cycles of 95 degrees C × 15 seconds, 54 degrees C × 60 seconds followed by signal acquisition. The thermal cycling protocol for beta-globin PCR was: 95 degrees C for 5 minutes for initial denaturation followed by 35 cycles of 95 degrees C × 15 seconds, 58 degrees C × 1 second, 72 degrees C × 15 seconds followed by signal acquisition.
All patient samples were assayed in triplicate. Samples were excluded from subsequent analysis when the coefficient of variation within person samples was greater than 20%. The coefficient of variation (average standard deviation divided by the mean) for the T/S ratio assay in this study was 9.5%. Determination of the T/S ratio was performed in a blinded fashion without knowledge of the clinical data.
Echocardiographic Measurements
All patients underwent complete resting 2-dimensional echocardiography and Doppler examination using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, CA) with a 3.5-MHz transducer. Standard parasternal short-axis and apical 2 – and 4-chamber views were obtained and planimetered to determine end-diastolic and end-systolic volumes. The left ventricular ejection fraction (LVEF) was calculated as (end diastolic volume – end systolic volume)/end diastolic volume. Diastolic dysfunction was defined as the presence of at least one of the following: impaired relaxation defined as a ratio of peak mitral early diastolic to atrial contraction velocity (E/A) of ≤ 0.75 with systolic dominant pulmonary vein flow; pseudonormal filling defined as 0.75 < E/A < 1.5 with diastolic dominant pulmonary vein flow; restrictive filling defined as an E/A of 1.5 or greater with diastolic dominant pulmonary vein flow. Left ventricular mass was calculated using a truncated ellipsoid equation as previously validated (20).
Other Measurements
Baseline demographics, age, sex, and self-reported ethnicity were recorded. Cardiovascular comorbidities including hypertension, diabetes, hyperlipidemia, and smoking status were determined by self-report of medical history. Medication use was determined by having participants bring bottles to the study appointment during which study personnel recorded all medications. Participants were weighed and measured without shoes. Body Mass Index (BMI) was calculated. All participants were instructed to bring their medication bottles to the study appointment where study personnel recorded all current medications. Fasting serum chemistry samples were used to measure HDL cholesterol, LDL cholesterol, and C-Reactive Protein (CRP). HDL- and LDL-cholesterol levels were measured in a clinical laboratory setting. CRP was measured using the Roche Integra high-sensitivity assay (Roche, Indianapolis, Indiana) in 229 participants and (due to a change in the laboratory) the Beckman Extended Range high-sensitivity CRP assay (Beckman, Galway, Ireland) in the remaining 551 participants. The Roche Integra assay uses an immunoturbidimetric technique, has been standardized against the World Health Organization reference, and compared with the Dade nephelometric method (correlation coefficient = 0.997). The lowest detectable CRP measurement with this assay was 0.025 mg/dl. The inter-assay coefficient of variation was 3.2%. The Beckman Extended Range assay also uses an immunoturbidimetric technique with a detection limit of 0.20 mg/l and a measuring range of 0.20 to 1440 mg/l. The inter-assay coefficient of variation was 6.7%. The Beckman Extended Range assay is highly correlated with the Roche Integra assay (correlation coefficient = 0.99).
Outcomes
We conducted annual telephone interviews with participants or their proxies regarding recent emergency room visits, hospitalizations, or death. Medical records, death certificates, and coroner’s reports were reviewed by two independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. If they disagreed, a third blinded adjudicator reviewed the event and determined the outcome classification.
All-cause mortality was determined by review of death certificates. Hospitalization for HF was defined as a minimum 1-night hospital stay for a clinical syndrome comprising at least 2 of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, and cardiomegaly or pulmonary edema on chest roentgenography. These clinical signs and symptoms must have represented a clear change from the baseline clinical status of the participant and must have been accompanied by either failing cardiac output as determined by peripheral hypoperfusion (in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary edema requiring intravenous diuretics, inotropes, or vasodilators. Nonfatal myocardial infarction (MI) was defined by the American Heart Association diagnostic criteria (21). Stroke was defined as a new neurological deficit not known to be secondary to brain trauma, tumor, infection, or other causes. CV death was defined as a death, occurring during the same hospitalization in which an acute MI was documented, or death occurring within 1 hour of the onset of terminal symptoms not explained by other etiologies. CV events were defined as the composite of nonfatal MI, stroke, and CV death. For all analyses, the outcome variable was time to first event. The mean length of follow-up was 4.4 years. Ascertainment of outcomes was achieved in 99% of participants.
Statistical Analysis
Since observed telomere lengths had a skewed distribution, the statistical analyses were performed on natural log-transformed data. We categorized leukocyte telomere length into quartile groups a priori. Differences in baseline characteristics were compared with the use of ANOVA for continuous variables and the chi-squared test for dichotomous variables, as appropriate. We used Cox proportional hazards models to examine the association between telomere length and cardiovascular outcomes, and verified the proportionality assumption of all models. For multivariable models, covariates were chosen a priori based on known clinical (age, gender, ethnicity, BMI, current smoking, systolic and diastolic blood pressure, LDL-cholesterol, HDL-cholesterol, diabetes, history of congestive heart failure), inflammatory (CRP), and echocardiographic (LVEF, Diastolic Dysfunction) markers of risk. Participants were censored at date of first event or last contact, whichever came first. To explore potential modifying effects of cardiac medications, we tested for statistical interactions between telomere length and the use of statins, aspirin, beta blockers, renin-angiotensin inhibitors (ACE-Is), and angiotensin receptor blockers (ARBs). We also tested for interactions between telomere length and ethnicity, and telomere length and gender. Statistical analysis was performed using SAS software version 9.1 (SAS Institute Inc, Cary, NC). The authors take responsibility for the integrity of the data. All authors had full access to the data, except R.M.C. who was blinded to the clinical data. All authors have read and agree to the manuscript as written.
RESULTS
The baseline characteristics of the study population categorized by telomere length quartiles are shown in Table 1. Participants with shorter telomere length were older than those with longer telomere length. Participants with shorter telomere length were also more likely to have lower BMI, lower BSA, prior stroke, lower LVEF, and echocardiographic evidence of diastolic dysfunction. There were no significant differences in gender, ethnicity, or history of hypertension, congestive heart failure (CHF), diabetes, or MI across quartiles of telomere length. Moreover there was no significant difference in CRP levels.
Table 1.
Baseline characteristics of Participants by Quartile of Telomere Length
| Quartile | I | II | III | IV | |
|---|---|---|---|---|---|
| Variable | N = 195 (0.50–0.94) | N = 195 (0.94–1.13) | N = 195 (1.14–1.35) | N = 195 (1.35–5.63) | P value |
| Age (yrs) | 70±10 | 68±11 | 67±11 | 65±11 | 0.0002 |
| Male sex | 152(78) | 166(85) | 157(81) | 159(82) | 0.33 |
| Body mass index (kg/m2) | 28.4±5.0 | 28.5±5.1 | 27.1±4.7 | 29.0±5.5 | 0.02 |
| Ethnicity | |||||
| - White | 120(62) | 118(61) | 119(61) | 118(61) | |
| - Black | 19(10) | 27(14) | 31(16) | 34(18) | 0.40 |
| - Asian | 30(15) | 22(11) | 24(12) | 17(9) | |
| - Other | 26(13) | 28(14) | 21(11) | 25(13) | |
| Medical history: | |||||
| Hypertension | 133(68) | 143(74) | 131(67) | 129(66) | 0.38 |
| MI | 100(52) | 116(60) | 100(51) | 99(51) | 0.24 |
| CHF | 33(17) | 37(19) | 27(14) | 39(20) | 0.37 |
| Stroke | 38(19) | 23(12) | 30(15) | 23(12) | 0.10 |
| Diabetes | 48(25) | 47(24) | 52(27) | 51(26) | 0.92 |
| COPD | 24(12) | 27(14) | 30(15) | 27(14) | 0.86 |
| Revascularization | 114(58) | 106(55) | 117(60) | 124(64) | 0.34 |
| Current Smoking | 29(15) | 35(18) | 41(21) | 38(20) | 0.44 |
| Former Smoking | 113(58) | 94(48) | 61(31) | 66(34) | 0.39 |
| Current Statin Use | 131(67) | 128(66) | 117(60) | 133(68) | 0.33 |
| Current Aspirin Use | 140(72) | 149(76) | 144(74) | 160(82) | 0.10 |
| Current Beta-Blocker Use | 102(52) | 115(59) | 116(59) | 120(62) | 0.28 |
| Current ACE-I/ARB Use | 84(43) | 99(51) | 101(52) | 112(57) | 0.04 |
| Systolic Blood Pressure(mmHg) | 132±20 | 133±23 | 133±21 | 132±21 | 0.86 |
| Diastolic Blood Pressure (mmHg) | 73±10 | 75±12 | 75±11 | 74±11 | 0.48 |
| LVEF | 61.9±8.9 | 60.0±11.0 | 62.0±9.6 | 62.5±9.3 | 0.07 |
| LV Mass Index (g/m2) | 95.9±25.4 | 97.5±22.7 | 99.3±28.2 | 97.6±25.6 | 0.63 |
| Diastolic Dysfunction | 130 (67) | 129 (66) | 124 (64) | 116 (59) | 0.02 |
| LDL Cholesterol (mg/dl) | 103.7±33.4 | 101.7±30.6 | 106.8±35.3 | 101.2±30.1 | 0.32 |
| HDL Cholesterol (mg/dl) | 46.3±13.5 | 46.0±14.8 | 46.9±14.7 | 43.9±13.1 | 0.19 |
| C-Reactive Protein (mg/l) | 4.5±10.6 | 4.2±7.1 | 4.5±8.5 | 4.7±7.6 | 0.94 |
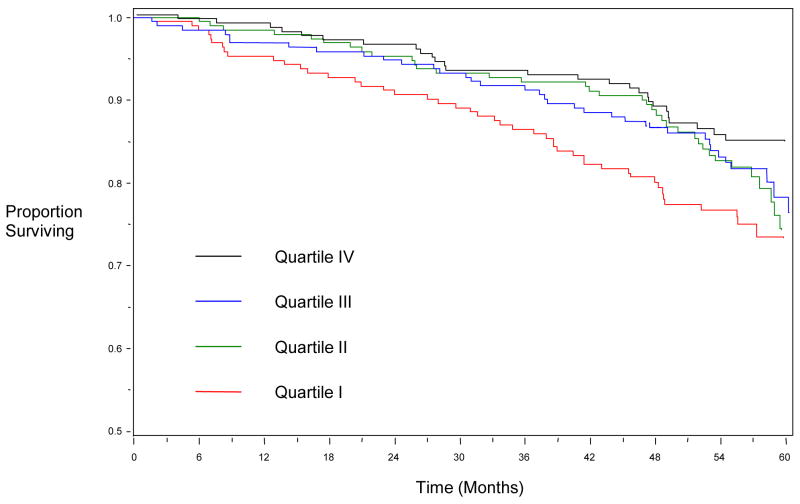
During a mean follow-up of 4.4 years there were 166 deaths, 99 hospitalizations for heart failure (HF), and 235 CV events. The number of participants with outcome events separated by telomere length quartile, and the corresponding age-adjusted HRs are shown in Table 2. After age-adjustment, patients in the shortest telomere quartile were at significantly increased risk of all-cause mortality (age-adjusted HR 1.8; 95% CI 1.2–2.9). Each standard deviation decrease in log telomere length was associated with a 20% greater risk of all-cause mortality (age-adjusted HR 1.2, 95% CI, 1.0–1.4; p=0.02).
Table 2.
Number (%) of participants with adverse outcomes by quartile of telomere length
| Quartiles of Telomere Length | I N=195 | II N=195 | III N=195 | IV N=195 | P-value for trend | Age-adjusted HR (95% CI) Quartile I vs IV | P-value | Age-Adjusted HR (95% CI) per SD decrease in log telomere length | P-value |
|---|---|---|---|---|---|---|---|---|---|
| All-cause Mortality | 54 (28) | 41 (21) | 40 (21) | 31 (16) | 0.04 | 1.8 (1.2–2.9) | 0.008 | 1.2 (1.0–1.4) | 0.02 |
| HF Hospitalization | 31 (16) | 26 (13) | 24 (12) | 18 (9) | 0.25 | 1.6 (0.9–2.9) | 0.10 | 1.2 (0.9–1.4) | 0.16 |
| CV Events (MI, Stroke, or CV Death) | 35 (18) | 24 (12) | 37 (19) | 22 (11) | 0.08 | 1.5(0.9–2.6) | 0.11 | 1.1(0.9–1.3) | 0.46 |
After multivariate adjustment for clinical risk factors, participants in the shortest telomere quartile remained at significantly increased risk of all-cause mortality (adjusted HR 2.1; 95% CI 1.3–3.3) than participants in the longest telomere quartile (Table 3, Model 1). Following the addition of a marker of systemic inflammation (CRP) or echocardiographic indices of systolic and diastolic function into the Cox regression model, participants in the shortest quartile remained at significantly increased risk of death (HR 2.0; CI 1.2–3.2 and HR 1.9; CI 1.0–3.5 respectively). In the same multivariable model, patients in the lowest quartile of telomere length were also at significantly increased risk of HF hospitalization (HR 2.6; CI 1.1–6.0) but not CV events (HR 1.7; CI 0.9–3.5).
Table 3.
Adjusted HRs for adverse outcomes after adjustment for Clinical (model 1), Clinical + CRP (model 2), and Clinical + Echocardiographic variables (model 3).
| OUTCOME | Model 1* | p value | Model 2† | p value | Model 3‡ | p value |
|---|---|---|---|---|---|---|
| All Cause Mortality | ||||||
| - Quartile I vs IV | 2.1(1.3–3.3) | 0.003 | 2.0(1.2–3.2) | 0.004 | 1.9(1.0–3.5) | 0.04 |
| - Per SD ↓ in log telomere length | 1.3(1.1–1.5) | 0.005 | 1.2(1.1–1.4) | 0.009 | 1.2(1.0–1.5) | 0.12 |
| HF Hospitalization | ||||||
| - Quartile I vs IV | 2.1(1.1–3.8) | 0.02 | 2.0(1.1–3.7) | 0.03 | 2.6(1.1–6.0) | 0.03 |
| - Per SD ↓ in log telomere length | 1.3(1.0–1.5) | 0.04 | 1.2(1.0–1.5) | 0.06 | 1.4(1.0–1.9) | 0.03 |
| CV Events (MI, Stroke, or CV Death) | ||||||
| - Quartile I vs IV | 1.7(1.0–3.0) | 0.07 | 1.7(0.9–2.9) | 0.08 | 1.7(0.9–3.5) | 0.12 |
| - Per SD ↓ in log telomere length | 1.1(0.9–1.4) | 0.20 | 1.1(0.9–1.4) | 0.22 | 1.1(0.9–1.4) | 0.33 |
Adjusted for Age, Gender, Ethnicity, LDL Cholesterol, HDL Cholesterol, Systolic Blood Pressure, Diastolic Blood Pressure, BMI, Stroke, Smoking, Diabetes, CHF
Adjusted for all variables in Model 1 + Log CRP
Adjusted for all variables in Model 1 + LVEF, Diastolic Dysfunction
We found no evidence that the association of telomere length with adverse outcomes differed in users and non-users of statins, aspirin, beta-blockers or renin-angiotensin inhibitors (all p values for interaction >0.05 for adverse outcomes in age-adjusted models). There was no difference in mean telomere length between users and non-users of statins, aspirin, beta-blockers, or renin-angiotensin inhibitors (all p values > 0.05). We also found no significant interaction between telomere length and ethnicity (p = 0.52) or male gender (p=0.37) for adverse outcomes in age-adjusted models.
COMMENT
In this large prospective study of patients with stable CAD, we found that leukocyte telomere length is associated with mortality independently of chronological age, clinical factors, CRP, and echocardiographic variables. Additionally, we found no significant interactions between the use of cardioprotective medications and leukocyte telomere length for adverse outcomes.
Cawthon et al measured leukocyte telomere length in 143 healthy blood donors age 60–97 years, and found poorer survival in those with shorter telomeres, largely attributable to increased deaths from infection and cardiovascular disease (16). However, two subsequent studies found that leukocyte telomere length was not associated with morbidity and mortality in very elderly individuals when chronological age was taken into account (22, 23). Our results support an independent association between telomere shortening and all-cause mortality, and extend these findings for the first time to a prospectively-studied cohort of patients with stable CAD. These observations support the hypothesis that, in CAD, leukocyte telomere length reflects biological aging and may integrate multiple genetic and environmental factors as a final common pathway of cellular stress. Several factors important in the pathogenesis and progression of atherosclerosis are known to accelerate telomere attrition. These include inflammation, oxidative stress, and endocrine aberrations (24, 25). Further basic and epidemiologic studies are needed to elucidate the underlying mechanisms of telomere attrition in this patient population.
Our results differ from prior studies in individuals at risk of atherosclerosis in that we observed no relationship between telomere length and CRP (26). One possible explanation is that in the preclinical phase of disease, systemic inflammation promotes both atherogenesis and leukocyte telomere attrition. However, once CAD is established other genetic and environmental factors have greater influence in determining the rate of telomere shortening. Whether leukocyte telomere shortening contributes directly to the progression of CAD at the cellular and molecular level cannot be determined from our results.
We also found a significant inverse association between telomere length and hospitalization for heart failure. This finding provides prospective validation of a recent study by van der Harst et al which demonstrated shorter telomeres in patients with CHF compared with age-balanced controls (27). In that study, telomere length was incrementally shorter in the presence of advanced disease and an ischemic etiology. Further studies are warranted to investigate the interplay between systemic inflammation, atherogenesis, telomere shortening, and heart failure.
Brouillette et al used a nested case-control approach in the West of Scotland Primary Prevention Study to show that the risk of developing CAD was highest in individuals with short telomeres, and that this risk was substantially attenuated by treatment with pravastatin (28). By contrast, in the present study, we found no interaction between statin use and telomere length, and no difference in mean telomere length between users and non-users of statins. Prior studies have also shown an association between telomere attrition and obesity (9, 29). In contrast, we found lower body mass index in patients with shorter telomeres. The explanation for this discrepancy is unclear but suggests an inverse U-shaped relationship between BMI and telomere length. Among our study population, lower body mass index (and shorter telomere length) may be a marker of greater cardiovascular disease severity.
Among the strengths of the present study is the \measurement of multiple potential confounding variables including inflammatory markers and echocardiographic parameters of systolic and diastolic function. The study design allowed us to prospectively investigate the prognostic value of leukocyte telomere length in a large cohort of comprehensively-phenotyped patients with CAD. However, several limitations should be considered in the interpretation of our results. First, telomere length in leukocytes derived from peripheral blood sampling does not necessarily reflect alterations in telomere length and function in the atherosclerotic plaque or myocardium (30, 31). There are limited data to suggest general correlation between telomere length in leukocytes and other tissues. Although no studies have directly compared leukocyte telomere length with endothelial cell telomere length, there is evidence that telomere shortening contributes to endothelial cell senescence, and may be accelerated by oxidative stress (32,33). Second, our results are derived from a single measurement of telomere length at the outset of a prospective cohort study. As such, we are unable to determine the rate of change of telomere length which would likely provide further insights into the significance of telomere attrition in this population (34). Furthermore, a single measurement of leukocyte telomere length cannot distinguish between chronic low-level stressors and a highly-stressful single prior event. Third, we did not evaluate the impact of telomere-associated proteins such as telomerase on the prognostic value of leukocyte telomere length. Fourth, we are unable to account for inherited differences in leukocyte telomere length between individuals. However, recent evidence suggests that early telomerase activity may “homogenize” telomere lengths such that longer telomeres shorten more quickly than short telomeres. (35,36). Finally, the question of whether telomere shortening is merely an epiphenomenon, or whether it plays an active role in the progression of coronary atherosclerosis, cannot be answered on the basis of our results (37).
In summary, we found that leukocyte telomere length in peripheral blood leukocytes is associated with mortality among ambulatory individuals with stable CAD. The prognostic value of leukocyte telomere length in this population is not fully captured by existing clinical, inflammatory, and echocardiographic markers of increased risk. Future studies should be aimed at investigating the mechanisms and significance of the association between telomere length and adverse outcomes in patients with stable CAD.
Figure 1.
Kaplan-Meier Curve showing survival by Quartiles of Leukocyte Telomere Length.
Acknowledgments
The authors acknowledge Ms. Teresa Stepanek for her assistance in measuring telomere lengths.
FUNDING SOURCES
The Heart and Soul study was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung and Blood Institute (R01 HL079235), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and the Nancy Kirwan Heart Research Fund.
Footnotes
DISCLOSURES
No conflicts of interest declared.
References
- 1.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–667. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.Blasco MA. Telomeres and human disease: ageing, cancer, and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 3.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- 5.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 6.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 7.Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci. 2004;101(50):17323–17324. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 10.Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 11.Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol. 1996;31(4):443–448. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 12.Von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez R, Carracedo J, Soriano S, Jimenez R, Martin-Malo A, Rodriguez M, Blasco M, Aljama P. Stress-induced premature senescence in mononuclear cells from patients on long-term hemodialysis. Am J Kidney Dis. 2005;45(2):353–359. doi: 10.1053/j.ajkd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M. Telomere shortening in human coronary artery diseases. Arterioscer Thromb Vasc Biol. 2004;24:546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 15.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 18.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life : the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiller NB, Skioldebrand CG, Schiller EJ, Mavroudis CC, Silverman NH, Rahimtoola SH, Lipton MJ. Canine left ventricular mass estimation by two-dimensional echocardiography. Circulation. 1983;68(1):210–216. doi: 10.1161/01.cir.68.1.210. [DOI] [PubMed] [Google Scholar]
- 21.Luepker RV, Apple FS, Christenson RS, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 22.Bischoff C, Petersen HC, Graakjaer J, Andersen-Ran berg K, Vamped JW, Bohr VA, Cholera S, Christensen K. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 24.Schonland SO, Lopez C, Widmann T, Zimmer J, Beryl E, Goony JJ, Weygand CM. Premature telomere loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci. 2003;100(23):13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen activates telomerase. Cancer Res. 1999;59:5917–5921. [PubMed] [Google Scholar]
- 26.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 27.van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, van Veldhuisen DJ. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49(13):1459–64. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Brouillette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 29.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GM, Aviv A. Rise in Insulin resistance is Associated with Escalated Telomere Attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 30.Martens UM, Zijlmans JM, Poon SS, Dragowska W, Yui J, Chavez EA, Ward RK, Lansdorp PM. Short telomeres on human chromosome 17p. Nat Genet. 1998;18(1):76–80. doi: 10.1038/ng0198-018. [DOI] [PubMed] [Google Scholar]
- 31.Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, Oshimura M, Nakamura K. Telomere lengths are characteristic in each human individual. Exp Gerontol. 2002;37(4):523–31. doi: 10.1016/s0531-5565(01)00218-2. [DOI] [PubMed] [Google Scholar]
- 32.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–1514. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 33.Voghel G, Thorin-Trescases N, Farhat N, Nguyen A, Villeneuve L, Mamarbachi AM, Fortier A, Perrault LP, Carrier M, Thorin E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev. 128(11–12):662–671. doi: 10.1016/j.mad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;25:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 35.Gilson E, Londono-Vallejo A. Telomere Length Profile in Humans. All Ends are Not Equal. Cell Cycle. 2007;6(20):2486–2494. doi: 10.4161/cc.6.20.4798. [DOI] [PubMed] [Google Scholar]
- 36.Londono-Vallejo JA, DerSarkissian H, Cazes L, Thomas G. Differences in telomere length between homologous chromososmes in humans. Nucleic Acids Res. 2001;29:3164–3171. doi: 10.1093/nar/29.15.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornsby PJ. Short telomeres : cause or consequence of aging? Aging Cell. 2006;5:577–578. doi: 10.1111/j.1474-9726.2006.00249.x. [DOI] [PubMed] [Google Scholar]