Abstract
Objective
To compare the prognosis of stable coronary heart disease (CHD) patients with self-reported angina symptoms, inducible ischemia, or both.
Background
Current guidelines do not recommend routine cardiac stress testing in patients with stable CHD unless they report symptoms of angina.
Methods
We measured self-reported angina by questionnaire and inducible ischemia using treadmill stress echocardiography in 937 outpatients with stable CHD. We used Cox proportional hazards models to evaluate the independent association of angina and inducible ischemia with CHD events (myocardial infarction or CHD death) during an average 3.9 years of follow-up, adjusted for traditional cardiovascular risk factors.
Results
Of the study participants, 129 (14%) had angina alone, 188 (20%) had inducible ischemia alone, and 40 (4%) had both angina and ischemia. Recurrent CHD events occurred in 7% of participants without angina or inducible ischemia, 10% of those with angina alone, 21% of those with inducible ischemia alone, and 23% of those with both angina and inducible ischemia (p<0.0001). The presence of angina alone was not associated with recurrent CHD events (HR 1.4, 95% CI 0.7–2.9; p=.3). However, the presence of inducible ischemia without self-reported angina strongly predicted recurrent CHD events (HR 2.2, 95% CI, 1.4–3.5; p=.001).
Conclusions
We found that 24% of patients with stable CHD have inducible ischemia and over 80% of these do not report angina. The presence of inducible ischemia without self-reported angina is associated with a greater than 2-fold increased rate of recurrent CHD events.
Introduction
Current guidelines do not recommend routine cardiac stress testing in asymptomatic patients with known CHD1. However, the presence of asymptomatic ischemia has been shown to predict adverse outcomes following coronary artery bypass grafting2 or percutaneous coronary revascularization3, and in diabetics with CHD4. Some have suggested that ischemia-guided therapy may improve prognosis in patients with stable CHD 5, and that we should consider relief of myocardial ischemia rather than relief of angina symptoms as the goal of therapy.6, 7 Previous studies have found that inducible ischemia with or without angina is predictive of incident adverse cardiovascular events,8, 9 but it is unknown whether inducible ischemia in the absence of self-reported angina predicts recurrent events in patients with established CHD. 10
We previously demonstrated that 24% of outpatients with stable CHD have inducible ischemia by exercise stress echocardiography, and most of these patients do not report symptoms of angina.11 In the current study, we sought to compare the risk of recurrent CHD events associated with angina symptoms or inducible ischemia in outpatients with stable CHD. We assessed self-reported angina by questionnaire and measured inducible ischemia by exercise stress echocardiography in 937 outpatients with known CHD who were participating in the Heart and Soul study. We followed participants for an average of 3.9 years to determine the risk of recurrent CHD events (myocardial infarction or CHD death) associated with angina, inducible ischemia or both.
Methods
Participants
The Heart and Soul study is a prospective cohort study of psychosocial risk factors and cardiovascular outcomes in patients with established CHD. Details regarding our recruitment procedures have previously been published.11–14 Briefly, we enrolled outpatients with documented CHD from two Veterans Affairs Medical Centers (San Francisco and Palo Alto, CA), one university medical center (University of California, San Francisco), and nine community health clinics in Northern California. The presence of CHD was defined by having at least one of the following: a history of MI, angiographic evidence of at least 50% stenosis in one or more major coronary vessels, prior evidence of exercise-induced ischemia by EKG or nuclear perfusion imaging, or a history of percutaneous or surgical coronary artery revascularization. Patients were excluded if they were unable to walk one block, had an acute coronary syndrome within the prior 6 months, or were planning to move from the local area within three years.
A total of 1024 participants enrolled in the study between September 2000 and December 2002. Of the 1024 participants, 549 (54%) had a history of myocardial infarction (based on inpatient ICD-9 codes), 237 (23%) had a history of revascularization (based on inpatient ICD-9 codes) but no history of infarction, and 238 (23%) had a diagnosis of coronary disease documented by their physician (based on outpatient ICD-9 codes and review of medical records). All participants completed a daylong baseline study appointment that included a comprehensive medical history questionnaire and an exercise stress echocardiogram. Of the 1024 participants, 87 were unable to complete the exercise treadmill stress echocardiogram for orthopedic or other reasons, leaving 937 participants for this analysis. Of these 937 participants, 496 (53%) had a history of MI, 504 (54%) had a history of revascularization, and 228 (24%) had a history of CHD based on prior evidence of exercise-induced ischemia or an abnormal coronary angiogram. The protocol was approved by the appropriate institutional review boards, and all participants provided written, informed consent.
Inducible Ischemia
We assessed the presence of inducible cardiac ischemia using exercise treadmill testing with stress echocardiography.15 Participants were instructed to fast for at least four hours prior to exercise, except for taking their usual medications as prescribed. We performed a symptom-limited, graded exercise treadmill test according to a Standard Bruce Protocol. Participants were asked to walk on a treadmill beginning at a workload of 20–30 watts and increasing by 20–30 watts every 3 minutes until reaching dyspnea, symptom-limited fatigue, chest discomfort, or electrocardiographic changes suggestive of ischemia. To achieve maximum heart rate, participants who were unable to continue the Standard Bruce protocol (for orthopedic or other reasons) were switched to slower settings on the treadmill and encouraged to exercise for as long as possible.
We performed resting and stress echocardiograms using an Acuson Sequoia Ultrasound System (Mountain View, CA) with a 3.5 MHz transducer. Prior to exercise, standard two-dimensional parasternal long-axis and short-axis and apical two-chamber and four-chamber views were obtained and planimetered using a computerized digitization system to determine end-diastolic and end-systolic left-ventricular volume, and to calculate left ventricular ejection fraction. At peak exercise, parasternal long-axis and short-axis as well as apical two-chamber and four-chamber views were used to detect the development of left ventricular wall motion abnormalities. Inducible ischemia was defined as the presence of new wall motion abnormalities at peak exercise that were not present at rest. Results from the stress echocardiogram were interpreted by a single expert cardiologist (N.B.S) who was blinded to the presence of self-reported angina.
Angina Symptoms
We determined angina frequency using the question16: “Over the past four weeks, on average, how many times have you had chest pain, chest tightness, or angina?” Possible responses were none over the past 4 weeks, less than once a week, 1–2 times per week, 3 or more times per week, 1–3 times per day, or 4 or more times per day. Initially, we categorized participants as having “no angina” (none or less than once a week), “weekly angina” (1–2 times per week or more), or “daily angina” (1 or more times per day). However, too few participants reported daily angina (n=24) to power a separate category, so we instead dichotomized participants as having weekly angina (1–2 times per week or more) or no angina (none or less than once per week).
Outcome variable
The outcome variable was nonfatal MI or CHD death. We conducted annual telephone follow-up interviews with participants (or their proxy) to ask about death or hospitalizations. For any identified event, two independent and blinded adjudicators reviewed medical records, EKGs, death certificates, and coroner’s reports. If both adjudicators agreed on the outcome classification, their classification was binding. If there was disagreement in the classification, they conferred, reconsidered their classification, and, if necessary, requested consultation from a third adjudicator. All adjudicators were blinded to the presence of self-reported angina and ischemia by stress echocardiography.
Nonfatal MI was defined as the presence of cardiac biomarkers in a setting in which signs, symptoms, and/or EKG findings suggested acute cardiac ischemia, as outlined by standard diagnostic criteria.17 Death was considered CHD death if the participant died during the same hospitalization in which an acute MI was documented or if the participant experienced sudden CHD death defined as an unexpected, otherwise unexplained death within one hour of the onset of terminal symptoms. To evaluate whether the prognosis of inducible ischemia varied by whether the participant underwent revascularization, we defined revascularization as coronary artery bypass grafting or percutaneous transluminal coronary angioplasty with or without placement of an intracoronary stent during the follow-up period. Revascularization was not included as an outcome variable.
Other variables
Age, sex, ethnicity, medical history, smoking status, alcohol use, and physical activity were determined by questionnaire. We measured weight and height and calculated body mass index (kg/m2). Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. Fasting serum samples were obtained for measurements of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), glycosylated hemoglobin, C-reactive protein, and NT-proBNP. Creatinine clearance was estimated using a 24-hour urine collection. Left ventricular ejection fraction was calculated using a resting echocardiogram as described above. Systolic and diastolic blood pressure was measured using a standard sphygmomanometer.
Analysis
Differences in characteristics between participants with and without exercise-induced ischemia were compared using two-tailed t-tests for continuous variables and Chi-squared tests for dichotomous variables. We then used multivariate Cox proportional hazards models to calculate the rate of nonfatal MI or CHD death in those with or without weekly angina, and in those with or without inducible ischemia. To determine the independent effects of angina and inducible ischemia on cardiovascular outcomes, we adjusted these models for the following covariates selected a priori because they were associated with inducible ischemia or known to predict recurrent CHD events: age, sex, race, history of MI, history of heart failure, glycosylated hemoglobin, creatinine clearance, LV ejection fraction, systolic blood pressure, diastolic blood pressure, and log C-reactive protein. Given the strong association of log NT-proBNP with recurrent cardiovascular events in this cohort 18, we further adjusted for NT-proBNP to see whether NT-proBNP levels might be in the pathway between ischemia and recurrent events. Finally, we assessed the risk of nonfatal MI or CHD death in participants with inducible ischemia, stratified by whether or not they underwent elective revascularization. For the above analyses, we report unadjusted and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). All analyses were performed using Statistical Analysis Software (Version 9.1, SAS Institute, Cary, N.C.)
Results
Of the 937 participants, 228 (24%) had exercise-induced ischemia by treadmill testing at the baseline examination. Compared with participants who did not have inducible ischemia, those with inducible ischemia were older, more likely to be male, and more likely to be white (Table 1). Those with inducible ischemia were more likely to have a history of MI or congestive heart failure, and more likely to be taking a renin-angiotensin inhibitor. Participants with inducible ischemia also had higher glycosylated hemoglobin, lower creatinine clearance, higher NT-proBNP, lower ejection fraction, and lower diastolic blood pressure. A total of 18% (20/228) of participants with inducible ischemia reported weekly or more angina, and 6% (13/228) stopped the treadmill due to chest pain. Thus, 82% of patients with inducible ischemia did not report daily or weekly angina, and 94% did not experience chest pain on the treadmill.
Table 1.
Baseline characteristics of 937 study participants with known coronary heart disease stratified by the presence of inducible ischemia.*
| Variable | Ischemia N=228 | No ischemia N=709 | P value |
|---|---|---|---|
| Age | 70±10 | 66±11 | < .0001 |
| Male sex | 200(88%) | 580(82%) | .04 |
| White race | 157(69%) | 412(58%) | .004 |
| History of: | |||
| Hypertension | 162(71%) | 493(70%) | .7 |
| Myocardial infarction | 150(66%) | 346(49%) | < .0001 |
| Congestive heart failure | 56(25%) | 100(14%) | .0003 |
| Stroke | 39(17%) | 88(12%) | .1 |
| Diabetes | 64(28%) | 169(24%) | .2 |
| Coronary revascularization | 147(64%) | 415(59%) | .1 |
| CABG | 112(49%) | 232(33%) | <.0001 |
| PCI | 82(36%) | 290(41%) | .17 |
| Current smoking | 40(18%) | 143(20%) | .4 |
| Regular alcohol use | 60(26%) | 214(30%) | .3 |
| Physically active | 147(64%) | 465(66%) | .7 |
| Body mass index | 28±5 | 28±5 | .2 |
| Medications | |||
| Beta blocker | 139(61%) | 406(57%) | .3 |
| Statin | 153(67%) | 458(65%) | .5 |
| Renin-angiotensin inhibitor | 139(61%) | 343(48%) | .0009 |
| Aspirin | 182(80%) | 554(78%) | .6 |
| Labs | |||
| Total cholesterol | 177±41 | 177±42 | .9 |
| HDL | 46±16 | 46±13 | .9 |
| LDL | 104±33 | 103±33 | .9 |
| Glycosylated hemoglobin | 6.2±1.3 | 5.9±1.1 | .0007 |
| Creatinine clearance | 74±26 | 85±28 | < .0001 |
| Log C-reactive protein | 0.77±1.3 | 0.65±1.3 | .2 |
| Log NT-proBNP | 5.85±1.2 | 4.96±1.2 | <.0001 |
| LV ejection fraction | 0.59±0.11 | 0.63±0.09 | <.0001 |
| Systolic blood pressure | 130±18 | 132±19 | 0.09 |
| Diastolic blood pressure | 70±10 | 75±10 | <.0001 |
| Weekly or more angina | 40(18%) | 131(18%) | 0.75 |
| Treadmill exercise capacity (METS) | 6.3±2.9 | 7.6±3.4 | <.0001 |
| Exercise stopped due to chest pain | 13(6%) | 21(3%) | .05 |
values are reported as % or mean ± standard deviation
Participants were followed for a mean of 3.9 (range 0.09–5.7) years. Eight participants (< 1%) were lost to follow up, leaving 929 for the analysis. Among participants with inducible ischemia, 21% (48/228) developed nonfatal MI or CHD death, compared with 8% (55/701) of those without inducible ischemia (unadjusted HR 2.9, 95% CI 1.9–4.2, p<.0001). This association remained strong after adjustment for age, sex, race, history of MI, history of heart failure, glycosylated hemoglobin, creatinine clearance, LV ejection fraction, systolic blood pressure, diastolic blood pressure, and log C-reactive protein (HR 2.2, 95% CI, 1.4–3.3, p=.0004).
Among participants who self-reported weekly or more angina, 13% (22/169) developed nonfatal MI or CHD death, compared with 11% (81/760) of those without weekly angina (HR 1.3, 95% CI 0.8–2.0; p=.3). Results were similar after multivariate adjustment for age, sex, race, history of MI, history of CHF, glycosylated hemoglobin, creatinine clearance, LV ejection fraction, systolic blood pressure, diastolic blood pressure, and log C-reactive protein (HR 1.4, 95% CI 0.9–2.4; p=.2).
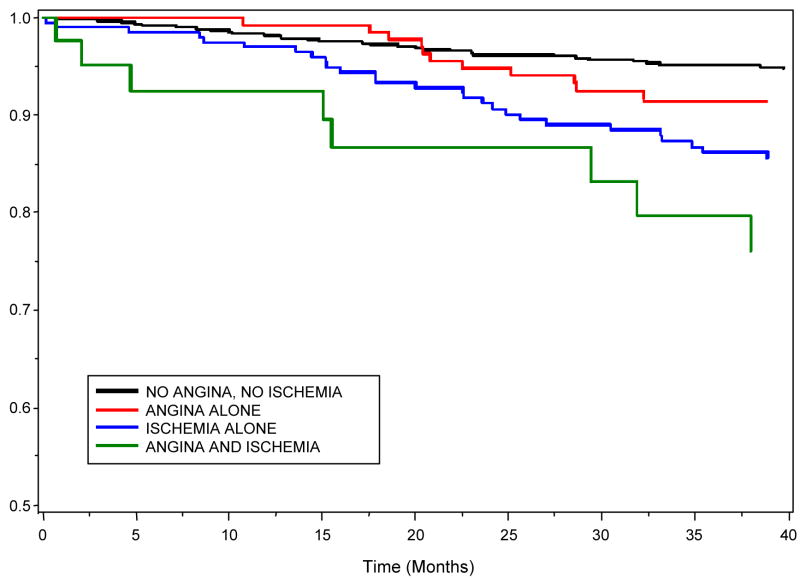
When stratifying the risk of CHD events by both self-reported angina and inducible ischemia, 572 (62%) participants had no angina or inducible ischemia, 129 (14%) had angina without inducible ischemia (angina alone), 188 (20%) had inducible ischemia without angina (ischemia alone), and 40 (4%) had both angina and inducible ischemia. CHD events occurred in 7% of participants without angina or inducible ischemia, 10% of those with angina alone, 21% of those with inducible ischemia alone, and 23% of those with both angina and inducible ischemia (p<.0001). The presence of angina alone was not associated with CHD events (Table 2). However, the presence of inducible ischemia alone was strongly associated with CHD events, and participants with both angina and inducible ischemia had the highest rate of CHD events (Figure 1). Further adjustment for logNT-proBNP did not eliminate the association between inducible ischemia alone and CHD events (HR 2.0, 95% CI, 1.2–3.2; p=.005) or the increased risk of events in patients with both angina and inducible ischemia (HR 2.4, 95% CI, 1.0–5.5; p=.04).
Table 2.
Relative rate of myocardial infarction or CHD death, stratified by the presence of self-reported weekly or more angina and inducible ischemia
| Proportion with MI or CHD death | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI)* | P value | |
|---|---|---|---|---|---|
| No angina or ischemia | 7% (42/572) | 1.0 | -- | 1.0 | -- |
| Angina alone | 10% (13/129) | 1.4 (0.7–2.6) | .3 | 1.4 (0.7–2.9) | .3 |
| Ischemia alone | 21% (39/188) | 2.9 (1.9–4.5) | <.0001 | 2.2 (1.4–3.5) | .005 |
| Angina and ischemia | 23% (9/40) | 3.7 (1.8–7.6) | .0004 | 3.2 (1.4–7.2) | .006 |
Adjusted for age, sex, race, history of MI, history of CHF, glycosylated hemoglobin, creatinine clearance, LV ejection fraction, systolic blood pressure and diastolic blood pressure, and log CRP
Figure 1.
Survival free of myocardial infarction or CHD death by presence of weekly angina or inducible ischemia at baseline, adjusted for age, sex, race, history of MI, history of CHF, glycosyolated hemoglobin, creatinine clearance, LV ejection fraction, systolic blood pressure and diastolic blood pressure (p=.0003).
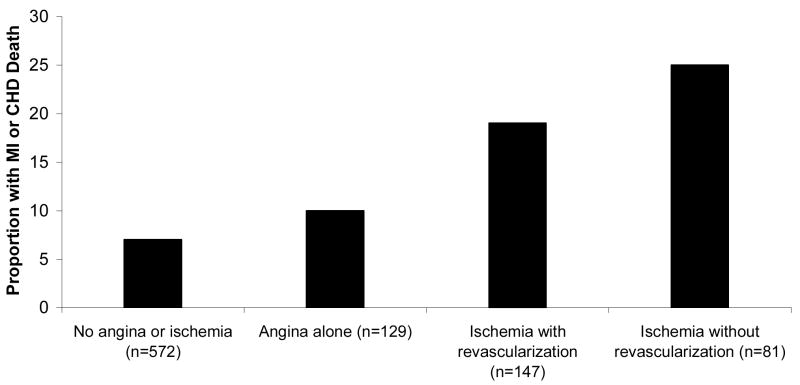
Among the 228 participants with inducible ischemia, 147 (64%) underwent revascularization during the follow-up period. Patients with inducible ischemia who were not revascularized appeared to have the highest risk of CHD events (Table 3, Figure 2).
Table 3.
Relative rate of MI or CHD death, stratified by the presence of self-reported angina or inducible ischemia (with or without revascularization) during an average 3.9 years of follow-up.
| Proportion with MI or CHD death | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI)* | P value | |
|---|---|---|---|---|---|
| No angina or ischemia | 7% (42/572) | 1 | -- | 1 | -- |
| Angina alone | 10% (13/129) | 1.4 (0.7–2.6) | .3 | 1.4 (0.7–2.0) | .3 |
| Ischemia with revascularization | 19% (28/147) | 2.8 (1.7–4.5) | <.0001 | 2.1 (1.3–3.5) | .005 |
| Ischemia without revascularization | 25% (20/81) | 3.6 (2.1–6.1) | <.0001 | 2.6 (1.5–4.5) | .0009 |
Adjusted for age, sex, race, history of MI, history of CHF, glycosylated hemoglobin, creatinine clearance, LV ejection fraction, systolic blood pressure and diastolic blood pressure, and log CRP
Figure 2.
Proportion of participants who developed nonfatal MI or CHD death, stratified by self-reported angina or inducible ischemia with or without subsequent revascularization (p <.0001 for trend)
Discussion
We found that 24% percent of patients with stable CHD have inducible ischemia, and over 80% of patients with inducible ischemia did not report significant symptoms of angina. In addition, the presence of inducible ischemia without angina was associated with a greater than 2-fold increased risk of recurrent CHD events (MI or CHD death), while the presence of angina symptoms did not adequately predict recurrent events. This association between inducible ischemia and recurrent events was independent of traditional cardiac risk factors. Our findings suggest that further study into the potential benefit of routine stress testing in outpatients with stable CHD regardless of angina symptoms may be warranted.
Although there was a trend toward worse outcomes in patients with weekly angina compared with patients without weekly angina, this trend did not reach statistical significance. This is in contrast to prior studies of angina frequency which have shown both an increase in admission for acute coronary syndrome19 and an increase in mortality19, 20 in patients with greater angina burden. It is possible that our study was underpowered to detect a difference in outcomes associated with angina. It is also possible that some patients may minimize their symptoms or not exert themselves to the point of developing angina. Nonetheless, our results suggest that the presence of inducible ischemia is a much stronger predictor of adverse events than self-reported angina, and over 80% of patients with inducible ischemia may not report the presence of weekly or more angina.
Several prior studies have demonstrated that patients who have inducible ischemia (with or without associated symptoms of angina) have an increased risk of adverse cardiovascular events. However, prior studies have not concurrently evaluated the predictive value of self-reported angina symptoms (outside of the stress test setting) in patients with CHD, nor have they compared the risk of recurrent events associated with self-reported angina symptoms versus inducible ischemia. Mark et al demonstrated in a series of 1698 patients with CHD undergoing exercise electrocardiography that those with asymptomatic ischemia during stress testing had an intermediate prognosis between those patients with no ischemia and those with symptomatic ischemia.8 In a study of 521 patients with CHD undergoing exercise radionuclide imaging, Pancholy et al showed that those with symptomatic or asymptomatic ischemia during exercise had a worse prognosis than those with no ischemia, and that the extent of perfusion abnormality was most predictive of risk. Our study evaluated the prognosis of patient-reported symptoms (rather than angina experienced during a stress test) because current guidelines recommend referral for stress testing based on patient-reported symptoms
The precise mechanism by which inducible ischemia, resulting from obstructive coronary atherosclerosis, predicts MI or death, generally resulting from rupture of a mildly stenotic plaque, is unclear. Most likely, extent of obstructive disease predicts MI and death because patients with more extensive obstructive plaques are more likely to have mildly stenotic or nonstenotic plaques that are potential sites for acute coronary events.{Nakagomi, 1996 #125} Another possibility is that the presence of an obstructive lesion may increase the likelihood that a more proximal plaque rupture would lead to infarction. A third possibility is that the presence of obstructive plaques may limit collateral blood flow to adjacent areas affected by the ruptured plaque.
Other investigators have considered the utility of a routine stress for identifying patients with a worse prognosis after revascularization, but none have examined the prognostic utility of routine stress testing in a broad selection of outpatients with stable CHD. Weiner et al performed stress testing in 174 participants from the Coronary Artery Surgery Study before and 6 months after CABG surgery. They found that survival 12 years after surgery was decreased in patients with both symptomatic or asymptomatic ischemia compared with those with no ischemia.21 In a study of 873 asymptomatic patients after CABG surgery, Lauer et al found that those with inducible ischemia were more likely to die or suffer a nonfatal MI compared with those without ischemia.2 Pfisterer et al used radionuclide stress testing to assess 490 asymptomatic patients for the presence of ischemia who had undergone successful coronary angioplasty.3 Inducible ischemia was present in 28% of these asymptomatic patients and was predictive of recurrent ischemic events. However, in a study of 936 patients between 1 and 6 months after a coronary event, exercise radionuclide stress testing was found to add little prognostic information at 1 year of follow-up.22
If a routine stress test identifies a patient who may be at increased risk for adverse events, would this change the approach to management? Several studies have addressed this question. In a randomized, placebo-controlled study of 360 outpatients with asymptomatic ischemia, Pepine et al showed that treatment with atenolol reduced the burden of asymptomatic ischemia and improved event free survival.23 The Asymptomatic Cardiac Ischemia Pilot study randomized 558 patients with ischemia during stress testing to angina-guided drug therapy, angina plus ischemia-guided drug therapy, or revascularization.5 At 2 years follow-up, those treated with a revascularization-based strategy had the best prognosis, those in the ischemia-guided strategy had an intermediate prognosis, and those in the angina-guided strategy had the worst prognosis.
In the present study, we stratified the risk of events by whether or not patients underwent elective revascularization. We found that patients with inducible ischemia who underwent elective revascularization had a better prognosis than those who were not revascularized, suggesting that an aggressive treatment strategy in patients with inducible ischemia may be beneficial. However, ours was an observational study and not a randomized trial, and thus our results are subject to potential bias. For instance, sicker patients may be less likely to undergo revascularization, and such selection bias could have the appearance of improving the prognosis of patients undergoing revascularization. Moreover, the recently reported Clinical Outcomes Utilizing Revscularization and Aggressive Drug Evaluation (COURAGE) trial suggests that revascularization may not reduce the long-term rates of adverse cardiovascular events compared with optimal medical therapy.24 Thus, a randomized trial would be required to evaluate whether a strategy of routine stress testing improves patient outcomes.
There are several potential limitations to this study. First, inducible ischemia was defined by exercise testing and not confirmed anatomically by coronary angiography. However, because findings on angiography do not necessarily correlate with the risk for future acute coronary events25, functional studies may be more predictive of subsequent events than anatomical studies of coronary disease. Second, we used the presence or absence of inducible ischemia as our predictor variable and did not further characterize the extent of ischemia evident by stress echocardiography. Although it is likely that those patients with more extensive ischemia have a poorer prognosis9, we felt that the mere presence of ischemia would be a simple and reliable indicator that would not require further expert interpretation for clinical use. Third, although our findings suggest that aggressive treatment of inducible ischemia even in the absence of self-reported angina may improve prognosis, as previously mentioned, this analysis is subject to bias and is not necessarily supported by the literature. Fourth, our findings suggest that a routine stress test in the asymptomatic patient may have some clinical utility. However, our results cannot determine whether or how often a stress test should be performed. Finally, the participants in this study were mostly urban men with known CHD and thus our results may not generalize to women or to other patient populations.
In conclusion, in a large prospective study of outpatients with stable CHD, we have shown that inducible ischemia, in the absence of self-reported angina, is both prevalent and predicts a poor prognosis. Our findings suggest that further study into the potential benefit of routine stress testing in outpatients with stable CHD regardless of symptoms may be warranted.
Acknowledgments
This study was supported by the Department of Veterans Affairs, Washington, DC, the National Heart Lung and Blood Institute (R01 HL079235), the American Federation for Aging Research (Paul Beeson Scholars Program), New York, NY, the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, NJ, the Ischemia Research and Education Foundation, and the Nancy Kirwan Heart Research Fund, San Francisco, CA.
Footnotes
Presented as an oral abstract at the American College of Cardiology Scientific Sessions, March 2007, New Orleans, LA
References
- 1.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–92. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 2.Lauer MS, Lytle B, Pashkow F, Snader CE, Marwick TH. Prediction of death and myocardial infarction by screening with exercise-thallium testing after coronary-artery-bypass grafting. Lancet. 1998;351:615–22. doi: 10.1016/S0140-6736(97)07062-1. [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer M, Rickenbacher P, Kiowski W, Muller-Brand J, Burkart F. Silent ischemia after percutaneous transluminal coronary angioplasty: incidence and prognostic significance. J Am Coll Cardiol. 1993;22:1446–54. doi: 10.1016/0735-1097(93)90556-g. [DOI] [PubMed] [Google Scholar]
- 4.Zellweger MJ, Hachamovitch R, Kang X, et al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543–50. doi: 10.1016/j.ehj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Davies RF, Goldberg AD, Forman S, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation. 1997;95:2037–43. doi: 10.1161/01.cir.95.8.2037. [DOI] [PubMed] [Google Scholar]
- 6.Conti CR. Silent myocardial ischemia: prognostic significance and therapeutic implications. Clin Cardiol. 1988;11:807–11. doi: 10.1002/clc.4960111202. [DOI] [PubMed] [Google Scholar]
- 7.Mulcahy DA. The return of silent ischaemia? Not really Heart. 2005;91:1249–50. doi: 10.1136/hrt.2004.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mark DB, Hlatky MA, Califf RM, et al. Painless exercise ST deviation on the treadmill: long-term prognosis. J Am Coll Cardiol. 1989;14:885–92. doi: 10.1016/0735-1097(89)90459-2. [DOI] [PubMed] [Google Scholar]
- 9.Pancholy SB, Schalet B, Kuhlmeier V, Cave V, Heo J, Iskandrian AS. Prognostic significance of silent ischemia. J Nucl Cardiol. 1994;1:434–40. doi: 10.1007/BF02961597. [DOI] [PubMed] [Google Scholar]
- 10.Cohn PF, Fox KM, Daly C. Silent myocardial ischemia. Circulation. 2003;108:1263–77. doi: 10.1161/01.CIR.0000088001.59265.EE. [DOI] [PubMed] [Google Scholar]
- 11.Gehi AK, Rumsfeld JS, Liu H, Schiller NB, Whooley MA. Relation of self-reported angina pectoris to inducible myocardial ischemia in patients with known coronary artery disease: the Heart and Soul Study. Am J Cardiol. 2003;92:705–7. doi: 10.1016/s0002-9149(03)00831-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. Jama. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165:2508–13. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2005;62:661–6. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TH, Boucher CA. Clinical practice. Noninvasive tests in patients with stable coronary artery disease. N Engl J Med. 2001;344:1840–5. doi: 10.1056/NEJM200106143442406. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 17.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–9. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 18.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. Jama. 2007;297:169–76. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–9. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–22. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 21.Weiner DA, Ryan TJ, Parsons L, et al. Prevalence and prognostic significance of silent and symptomatic ischemia after coronary bypass surgery: a report from the Coronary Artery Surgery Study (CASS) randomized population. J Am Coll Cardiol. 1991;18:343–8. doi: 10.1016/0735-1097(91)90584-v. [DOI] [PubMed] [Google Scholar]
- 22.Krone RJ, Gregory JJ, Freedland KE, et al. Limited usefulness of exercise testing and thallium scintigraphy in evaluation of ambulatory patients several months after recovery from an acute coronary event: implications for management of stable coronary heart disease. Multicenter Myocardial Ischemia Research Group. J Am Coll Cardiol. 1994;24:1274–81. doi: 10.1016/0735-1097(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 23.Pepine CJ, Cohn PF, Deedwania PC, et al. Effects of treatment on outcome in mildly symptomatic patients with ischemia during daily life. The Atenolol Silent Ischemia Study (ASIST) Circulation. 1994;90:762–8. doi: 10.1161/01.cir.90.2.762. [DOI] [PubMed] [Google Scholar]
- 24.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 25.Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]