Abstract
Traditional cardiac risk factors only partially explain the biological mechanisms by which persons of lower socioeconomic status (SES) have higher cardiovascular risk. Dietary factors, resulting in lower circulating levels of (n-3) fatty acids, may also contribute to the increased risk of cardiovascular disease (CVD) in patients with low SES. We tested whether low SES is associated with RBC levels of (n-3) fatty acids in patients with coronary heart disease. We performed a cross-sectional analysis of 987 adults with stable coronary artery disease (CAD) recruited from San Francisco area outpatient clinics. Four SES measures (household income, education, occupation, and housing status) were assessed by self-report. RBC fatty acid levels of 2 (n-3) fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), were measured in venous blood samples from fasting subjects. Participants with lower household income, education, occupation, and housing status had lower RBC levels of (n-3) fatty acids (P < 0.001 for all 4 measures). In multivariable models, household income, education, and occupation remained strongly associated with DHA and EPA levels after adjustment for demographic factors, BMI, physical activity, statin use, and kidney function (P < 0.001 for all 3 measures). Housing status was not associated with DHA or EPA after multivariable adjustment. Among patients with CAD, 3 indicators of low SES, household income, education, and occupation, were strongly associated with lower RBC levels of (n-3) fatty acids. Our results raise the possibility that (n-3) fatty acids may be an important mediating factor in the association between low SES and CVD.
Introduction
Effective prevention and treatment of cardiovascular disease (CVD)8 requires a complete understanding of the disease biology and an identification of associated risk factors. Patient populations with low socioeconomic status (SES) have higher rates of cardiovascular events and mortality, but the biological basis for this association has not been fully explained (1–3). Many studies have concluded that traditional risk factors for CVD (such as smoking, blood pressure, diabetes, or BMI) can provide only a partial explanation, suggesting that other mechanisms are involved in increasing the risk of heart disease in patients with low SES (1–3).
Low levels of particular (n-3) fatty acids, polyunsaturated fats that cannot be produced endogenously and thus must be obtained from the diet, are associated with increased coronary heart disease (CHD) risk and mortality (4–9). Most of this evidence points to 2 (n-3) fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), as being particularly important (10,11). Although some studies have challenged the assertion that (n-3) fatty acids are cardioprotective (12,13), more recent analyses (14) and organizations such as the AHA continue to recommend their intake for CVD prevention (10). Individuals with low SES report lower dietary intake of (n-3) fatty acids (15), but some have suggested that reporting bias may be responsible for this association (16). Two prior studies have examined the association of SES with serum levels of (n-3) fatty acids (17,18). Yeh et al. (17) found that lower occupational grade was associated with low serum levels of (n-3) fatty acids in Nigerian civil servants, excluding EPA levels. In the only known study to have evaluated this association in patients with CHD, Erkkila et al. (18) found no association between SES (measured by education level) and most serum (n-3) fatty acids.
We assessed 4 different SES variables (household income, education level, occupation, and housing status) and measured RBC levels of DHA and EPA in a cross-sectional study of 987 adults with CHD. We hypothesized that lower SES would be associated with lower levels of (n-3) fatty acids after adjusting for medication use, demographic characteristics, and other clinical indicators.
Materials and Methods
Participants
The Heart and Soul Study is a prospective cohort study examining psychosocial factors and health outcomes in patients with CHD. Detailed methods have been previously described (19). In summary, patients with documented coronary artery disease (CAD) were identified through several administrative databases: 2 Department of Veterans Affairs Medical Centers (San Francisco VA Medical Center and the Veterans Affairs Palo Alto Health Care System), 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco.
Between September 2000 and December 2002, a total of 1024 Bay Area residents with stable CHD enrolled. Participants completed a baseline study visit, which included a medical history, comprehensive health status questionnaire, physical examination, exercise treadmill test, and a fasting venous blood draw. Subjects for whom frozen blood samples were not available (n = 37) were excluded, resulting in a sample size of 987 for this analysis. The protocol was approved by the appropriate institutional review boards, including ethical approval from the Committee on Human Research at the University of California, San Francisco. All participants provided written, informed consent.
SES
Self-reported data on 4 SES variables, including household income, education level, housing status, and occupation, were collected in a questionnaire at the baseline interview. Methods for measuring both household income and education level have been previously described (20). For the regression analysis, we divided participants into 3 income groupings: <$20,000, $20,000 to $50,000, and >$50,000. We divided all participants into 3 education categories: ≤11 y of education, high school graduate (high school graduate, some college or vocational schooling), and ≥college degree (college graduate, or graduate or professional degree).
Housing status responses included house, single room occupancy (SRO)/shelter, apartment/flat, and retirement community. For the regression analysis, “retirement community” was grouped with “apartment/flat.” Occupation categories included labor, service, protective service, manufacturing/transportation, craftsmen/foreman, clerical/sales, manager/official/proprietor, professional/technical, and other. For the regression analysis, we excluded participants (n = 150) who reported “other” occupation and divided the remaining participants into 4 categories: skilled/unskilled labor (labor, craftsman/foreman, and manufacturing/transportation), services/sales (clerical/sales, service, and protective services), manager/official/proprietor, and professional/technical.
RBC DHA and EPA levels
DHA and EPA were measured in the membranes of RBC from fasting venous blood samples. Upon enrollment, study participants refrained from smoking for 5 h, did not take aspirin for 1 wk, and completed an overnight 12-h fast (except for prescribed medications taken with water). RBC levels of fatty acids are thought to represent dietary intake and may act as a marker of tissue incorporation in general (21). We analyzed RBC levels of DHA and EPA as both continuous variables and ordinal variables divided into tertiles.
The fatty acid composition of RBC membranes was assessed by capillary GC using a GC2010 (Shimadzu) equipped with a SP2560, 100-m column (Supelco) after generation of fatty acid methyl esters (FAME) by treatment with boron trifluoride-methanol (22). FAME were identified by comparison with a weighed standard mixture consisting of 22 fatty acids characteristic of RBC membranes (GLC-727, Nuchek Prep). RBC levels of EPA and DHA were presented as a percentage composition of total FAME. Two RBC control pools were included with each batch to monitor analytical performance. Acceptable runs were those in which both controls fell within 2.5 SD. The inter-assay CV for EPA+DHA as a percentage of total RBC fatty acids was 5–6%, whereas the intra-assay CV was 2–3%.
Other variables
Age, sex, marital status, ethnicity, medical history, and history of tobacco and alcohol use were collected by self-report on baseline questionnaires. To denote their ethnicity, participants selected from 5 options: Hispanic, Asian, White, Black, or Other. We measured height and weight and calculated BMI. Participants rated their physical activity during the previous month using a 6-point Likert scale. Those responding “not at all active” or “a little active” were classified as physically inactive. Participants were also instructed to bring their medication bottles to the study appointment and the research team personnel recorded all current medications, including the use of statins and aspirin. Creatinine clearance was measured from 24-h urine collections using a Synchron LX 20 (Beckman Coulter). Fasting serum total cholesterol, HDL-cholesterol, and triglyceride concentrations were measured by enzymatic assays (23) using a Synchron LX 20 (Beckman Coulter). Serum LDL-cholesterol was calculated using Friedewald’s formula (24).
Statistical analysis
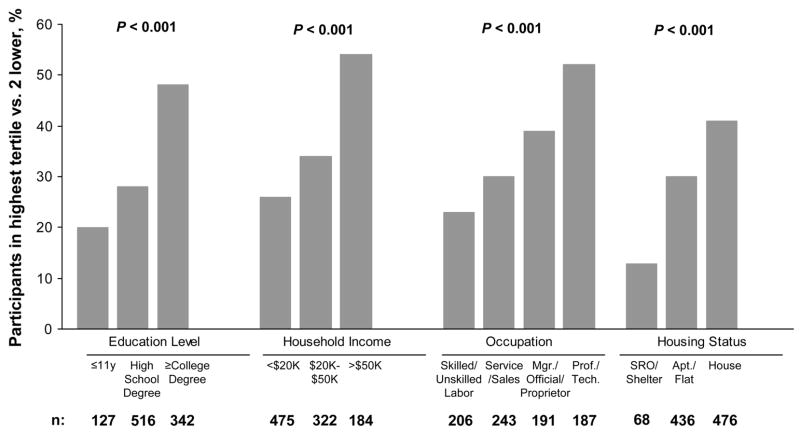
To describe the relevant differences in general characteristics of subjects by levels of income and education, we used ANOVA for continuous variables and chi-square tests for dichotomous variables. Values presented in the text are means ± SD. Unadjusted chi-square tests were used to calculate significance in Figure 1. All statistical tests were 2-sided and P < 0.05 was considered significant.
FIGURE 1.
Proportion of 987 study participants with CAD in the highest tertile (vs. 2 lower) for measured RBC levels of EPA and DHA combined, according to SES. From left to right, occupation categories include: skilled/unskilled labor, services/sales, manager/official/proprietor, and professional/technical. From left to right, housing categories include: SRO/shelter, apartment/flat, and house. P-values from chi square tests are shown.
To further evaluate the association between SES and RBC DHA and EPA levels and control for potential confounding variables, we developed multivariable linear regression models using each of the 4 SES variables as predictors and RBC levels of DHA and EPA as continuous outcomes. We adjusted for the following covariates based on their potential association with the intake or metabolism of (n-3) fatty acids: age, sex, ethnicity, smoking, marital status, regular alcohol use, BMI, and physical activity (7,21). We also adjusted for statin use and creatinine clearance, because these factors have been shown to influence (n-3) fatty acid levels (25,26). Participants were excluded from the model if they did not provide socioeconomic information. We used ANOVA to calculate P-values for the multivariable models, comparing mean (n-3) fatty acid levels in different socioeconomic categories. Analyses were performed with SAS version 9.1 (SAS Institute).
Results
Patient characteristics
The study included 987 study participants with CAD, of whom 804 (~80%) were men. The age of the study participants was 67 ± 11 y. Compared with those in the higher levels of income or education, those with lower levels had lower (n-3) fatty acids (Table 1). Those with lower income or education were more likely to be Hispanic or black, to have a history of hypertension or diabetes mellitus, and to be physically inactive. Participants with lower income or education also had increased serum total cholesterol concentrations.
TABLE 1.
Characteristics of 987 participants with CAD according to household income and education levels1
| Household income level |
Education level |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | <$20,000 | $20,000–$50,000 | >$50,000 | P | ≤11 y | High school graduate | ≥College degree | P |
| n | 475 | 322 | 184 | 127 | 516 | 342 | ||
| EPA+DHA, g/100 g total fatty acids | 3.8 ± 1.9 | 4.1 ± 2.0 | 5.0 ± 2.4 | <0.001 | 4.0 ± 1.8 | 4.0 ± 1.7 | 5 ± 2.4 | <0.001 |
| Demographic | ||||||||
| Age, y | 65 ± 11 | 69 ± 11 | 67 ± 11 | <0.001 | 69 ± 11 | 66 ± 12 | 67 ± 10 | 0.005 |
| Men, % | 76 | 87 | 89 | <0.001 | 75 | 80 | 87 | 0.006 |
| Ethnicity, % | <0.001 | <0.0001 | ||||||
| Hispanic | 11 | 9 | 3 | 24 | 9 | 2 | ||
| White | 53 | 58 | 82 | 28 | 59 | 75 | ||
| Black | 20 | 17 | 4 | 32 | 18 | 7 | ||
| Asian | 12 | 12 | 10 | 10 | 10 | 15 | ||
| Other | 4 | 3 | 2 | 6 | 4 | 1 | ||
| Married, % | 24 | 52 | 75 | <0.001 | 36 | 36 | 56 | <0.001 |
| Medical history, % | ||||||||
| Hypertension | 74 | 71 | 63 | 0.02 | 83 | 71 | 66 | 0.002 |
| Myocardial infarction | 53 | 52 | 58 | 0.46 | 47 | 56 | 53 | 0.18 |
| Congestive heart failure | 19 | 16 | 16 | 0.46 | 17 | 20 | 15 | 0.21 |
| Stroke | 16 | 12 | 13 | 0.26 | 16 | 15 | 12 | 0.45 |
| Diabetes | 31 | 24 | 19 | 0.005 | 33 | 29 | 20 | 0.004 |
| Medication use, % | ||||||||
| Statins | 58 | 67 | 75 | <0.001 | 62 | 62 | 68 | 0.13 |
| Aspirin | 75 | 80 | 81 | 0.10 | 78 | 76 | 78 | 0.73 |
| Laboratory values | ||||||||
| Serum total cholesterol, mmol/L | 4.7 ± 1.1 | 4.6 ± 1.1 | 4.4 ± 0.9 | 0.007 | 4.6 ± 1.0 | 4.7 ± 1.2 | 4.5 ± 1.0 | 0.09 |
| Serum HDL-cholesterol, mmol/L | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.3 ± 0.4 | <0.001 | 1.1 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.14 |
| Serum triglycerides, mmol/L | 1.7 ± 1.5 | 1.7 ± 1.6 | 1.3 ± 1.0 | 0.03 | 1.4 ± 1.0 | 1.8 ± 1.7 | 1.4 ± 1.0 | 0.002 |
| Serum LDL-cholesterol, mmol/L | 2.8 ± 0.9 | 2.7 ± 0.9 | 2.5 ± 0.7 | 0.003 | 2.8 ± 0.9 | 2.7 ± 0.9 | 2.6 ± 0.8 | 0.30 |
| Creatinine clearance, mL/min | 80 ± 29 | 81 ± 28 | 86 ± 29 | 0.03 | 78 ± 29 | 80 ± 29 | 83 ± 28 | 0.24 |
| Other characteristics | ||||||||
| Current smoking, % | 28 | 14 | 9 | <0.001 | 22 | 26 | 10 | <0.001 |
| Regular alcohol use, % | 25 | 28 | 44 | <0.001 | 21 | 27 | 36 | 0.003 |
| Physically active, % | 60 | 66 | 66 | 0.21 | 53 | 61 | 70 | <0.001 |
| BMI, kg/m2 | 29 ± 6 | 29 ± 5 | 28 ± 5 | 0.09 | 29 ± 5 | 29 ± 6 | 28 ± 5 | 0.35 |
Values are means ± SD or %.
SES and RBC DHA and EPA
The distribution of RBC DHA and EPA levels by tertile was determined for each socioeconomic variable (Fig. 1); 48% of 342 participants with a college degree were in the highest tertile compared with 20% of 127 participants with less than a high school degree, and 54% of 184 participants with a household income >$50,000 were in the highest tertile compared with 26% of 475 participants with household incomes <$20,000. Participants who lived in a house or had professional/technical occupations were also more likely to be in the highest tertile.
Prior to adjustment for potential confounding factors, both combined levels of DHA and EPA and individual levels of DHA and EPA were significantly associated with all 4 measures of SES (Table 2). Following multivariable adjustment (for age, sex, ethnicity, smoking, marital status, regular alcohol use, BMI, physical activity, statin use, and creatinine clearance), household income (P < 0.001), education level (P < 0.001), and occupation (P ≤ 0.004) remained associated with individual and combined RBC levels of DHA and EPA (Table 3). Housing status was not independently associated with RBC levels of DHA (P = 0.78), EPA (P = 0.95), or their combination (P = 0.89) after adjustment for potential confounders.
TABLE 2.
Unadjusted mean RBC levels of DHA and EPA in 987 study participants with CAD by category of SES1
| SES variable | n | DHA, g/100 g total fatty acids | P | EPA, g/100 g total fatty acids | P | DHA+EPA, g/100 g total fatty acids | P |
|---|---|---|---|---|---|---|---|
| Household income | <0.001 | <0.001 | <0.001 | ||||
| <$20,000 | 475 | 3.05 (2.93, 3.17) | 0.77 (0.70, 0.85) | 3.83 (3.65, 4.01) | |||
| $20,000–$50,000 | 322 | 3.26 (3.11, 3.40) | 0.87 (0.78, 0.96) | 4.13 (3.91, 4.35) | |||
| >$50,000 | 184 | 3.78 (3.60, 3.97) | 1.25 (1.13, 1.38) | 5.04 (4.75, 5.33) | |||
| Education | <0.001 | <0.001 | <0.001 | ||||
| ≤11 y | 127 | 2.96 (2.74, 3.19) | 0.69 (0.55, 0.84) | 3.66 (3.31, 4.00) | |||
| High school graduate | 516 | 3.05 (2.94, 3.16) | 0.76 (0.69, 0.83) | 3.81 (3.64, 3.99) | |||
| ≥College degree | 342 | 3.70 (3.56, 3.84) | 1.18 (1.09, 1.27) | 4.88 (4.67, 5.09) | |||
| Housing status | <0.001 | 0.003 | <0.001 | ||||
| SRO/shelter | 68 | 2.61 (2.29, 2.92) | 0.60 (0.40, 0.80) | 3.21 (2.72, 3.69) | |||
| Apartment/flat | 436 | 3.16 (3.03, 3.28) | 0.87 (0.79, 0.95) | 4.03 (3.84, 4.22) | |||
| House | 476 | 3.47 (3.35, 3.59) | 0.97 (0.89, 1.04) | 4.43 (4.25, 4.62) | |||
| Occupation | <0.001 | <0.001 | <0.001 | ||||
| Skilled/unskilled labor | 206 | 2.99 (2.81, 3.17) | 0.70 (0.59, 0.81) | 3.69 (3.42, 3.96) | |||
| Services/sales | 243 | 3.26 (3.10, 3.43) | 0.83 (0.73, 0.93) | 4.09 (3.85, 4.34) | |||
| Manager/official/proprietor | 191 | 3.41 (3.22, 3.59) | 0.93 (0.82, 1.04) | 4.34 (4.06, 4.61) | |||
| Professional/technical | 187 | 3.67 (3.48, 3.86) | 1.22 (1.10, 1.33) | 4.88 (4.60, 5.16) |
Values are means (95% CI).
TABLE 3.
Mean RBC levels of DHA and EPA in 987 study participants with CAD by category of SES, adjusted for age, sex, ethnicity, marital status, BMI, physical activity, smoking, alcohol use, statin use, and creatinine clearance1
| SES variable | n | DHA, g/100 g total fatty acids | P | EPA, g/100 g total fatty acids | P | DHA+EPA, g/100 g total fatty acids | P |
|---|---|---|---|---|---|---|---|
| Household income | <0.001 | <0.001 | <0.001 | ||||
| <$20,000 | 475 | 3.18 (3.01, 3.34) | 0.85 (0.74, 0.96) | 4.06 (3.81, 4.31) | |||
| $20,000–$50,000 | 322 | 3.05 (2.87, 3.24) | 0.80 (0.67, 0.92) | 3.88 (3.60, 4.17) | |||
| >$50,000 | 184 | 3.53 (3.30, 3.76) | 1.13 (0.98, 1.28) | 4.70 (4.34, 5.05) | |||
| Education | <0.001 | <0.001 | <0.001 | ||||
| ≤11 y | 127 | 2.84 (2.59, 3.09) | 0.70 (0.53, 0.86) | 3.56 (3.17, 3.95) | |||
| High school graduate | 516 | 3.13 (2.97, 3.28) | 0.82 (0.72, 0.92) | 3.98 (3.74, 4.21) | |||
| ≥College degree | 342 | 3.55 (3.37, 3.74) | 1.11 (0.98, 1.23) | 4.68 (4.40, 4.97) | |||
| Housing status | 0.78 | 0.95 | 0.89 | ||||
| SRO/shelter | 68 | 3.12 (2.78, 3.46) | 0.86 (0.64, 1.08) | 4.01 (3.49, 4.53) | |||
| Apartment/flat | 436 | 3.20 (3.02, 3.37) | 0.89 (0.78, 1.00) | 4.12 (3.86, 4.38) | |||
| House | 476 | 3.24 (3.07, 3.40) | 0.88 (0.77, 0.99) | 4.15 (3.89, 4.40) | |||
| Occupation | 0.004 | <0.001 | <0.001 | ||||
| Skilled/unskilled labor | 206 | 3.07 (2.84, 3.29) | 0.79 (0.65, 0.93) | 3.88 (3.54, 4.23) | |||
| Services/sales | 243 | 3.22 (3.02, 3.42) | 0.84 (0.72, 0.96) | 4.09 (3.79, 4.38) | |||
| Manager/official/proprietor | 191 | 3.33 (3.10, 3.56) | 0.92 (0.78, 1.06) | 4.28 (3.93, 4.62) | |||
| Professional/technical | 187 | 3.54 (3.31, 3.77) | 1.16 (1.02, 1.30) | 4.73 (4.39, 5.07) |
Values are means (95% CI).
Discussion
We found that 3 measures of low SES, household income, education level, and occupation, were associated with low RBC levels of 2 (n-3) fatty acids in 987 outpatients with CHD. After multivariable adjustment, participants with less than a high school education had mean DHA+EPA levels that were 24% lower than those with a college degree, participants with a household income <$20,000 had DHA+EPA levels that were 14% lower than those with a household income >$50,000, and laborers had DHA+EPA levels that were 18% lower than participants with professional or technical occupations. These results raise the possibility that low (n-3) fatty acid levels, an easily modifiable risk factor, may contribute to the increased risk of CVD in patients with low SES. Our study extends the existing literature by examining 4 different measures of SES, measuring RBC levels of fatty acids rather than using dietary questionnaires, and providing comprehensive assessment of potential confounding variables.
Prior studies examining the association between SES and (n-3) fatty acids have relied on self-reported dietary intake questionnaires to estimate (n-3) levels (15,16). Such questionnaires may not capture hidden dietary fats and are susceptible to reporting errors (16,27,28). Computer simulations or published correlation coefficients were often used to convert dietary data to fatty acid levels. One prior study reported an association between occupation and measured serum levels of fatty acids in Nigerian civil servants (17). Another study, the only one known to address this question in patients with established CHD who are at highest risk for cardiovascular events, reported no association between education and serum levels of EPA or DHA (18). However, >85% of surveyed CHD patients in this study had <12 y of education (in contrast to <15% in our study) and >60% had <9 y of education, potentially limiting the ability to capture the true benefits of higher education.
Because (n-3) fatty acids cannot be synthesized in the body de novo, they must be obtained through dietary sources. The best sources for DHA and EPA are oily, coldwater fish (such as salmon, sardines, mackerel, and albacore tuna) and fish oils (10), as well as nonhydrogenated vegetable oils. However, the quantity of (n-3) fatty acids present varies greatly, depending on fish size and species, season, geography, and preparation methods (29,30). Vegetable oils, especially canola or soybean, contain 7–10% α-linolenic acid, a short-chain (n-3) fatty acid that can be converted to EPA and DHA in the body but with very limited efficiency (31). The extent to which the differences in RBC EPA and DHA levels in the present study were the result of differences in the intake of fish or fish oil supplements is not known, because these data were not collected. There is, however, a clear dose-response relationship between fish intake (21) or fish oil supplementation (32) and EPA and DHA levels. Because lower levels of (n-3) fatty acids are associated with increased CHD risk (6) and increased intake reduces this risk (14), it seems reasonable to hypothesize that increased fish oil intake could reduce risk for CHD in lower SES populations.
Previous epidemiological studies have found significant differences in the diets and nutrient intakes of different socioeconomic classes (18,33–37). Specifically, reduced fish consumption has been associated with lower education levels and less-skilled, lower-paying occupations (15,38). Several explanations have been suggested to explain such dietary disparities, including the increased cost of healthy foods (39) and decreased health knowledge in lower SES individuals (40). However, such explanations are not yet conclusive. It has also been suggested that poorer social support and limited availability of affordable healthy foods in socioeconomically disadvantaged neighborhoods negatively influence diet (41).
Our study results raise the possibility that (n-3) fatty acids may be an important mediating factor in the association between low SES and CVD. Although a Cochrane meta-analysis has challenged the view that (n-3) fatty acids reduce adverse cardiac outcomes (12,13), the review has been criticized due to the controversial inclusion of the DART-2 trial (42), the use of composite endpoints, the wide range of intakes included, and the questionable exclusion of many potential cohorts (43,44). Despite such challenges, major cardiac societies and national health agencies continue to endorse the intake of (n-3) fatty acid for CVD prevention and treatment. The AHA currently recommends all adults eat fish at least twice a week and that all patients with CAD take 1 g/d of DHA and EPA through dietary and/or supplemental sources (45). It is not clear what levels of RBC DHA+EPA would represent an increased risk for CVD, but prior studies may provide some guidance. In a nested case-control analysis among men followed for up to 17 y in the Physicians’ Health Study, baseline levels of EPA+DHA were significantly lower in 94 men who had sudden cardiac death than in 184 controls matched for age and smoking status (median EPA+DHA 3.84% vs. 4.22%) (46). The median level of EPA+DHA in our participants with known CHD was 3.6%, which is even lower than the cases from the Physician’s Health Study and consistent with the high risk of our study population.
Current dietary recommendations to increase (n-3) fatty acid intake, a modifiable risk factor, are especially relevant to our cohort of patients with diagnosed CHD. In comparison to the general public, CHD patients would likely have more encounters with the healthcare system and should be more knowledgeable about their health condition and the cardioprotective role of (n-3) fatty acids. Our results demonstrate that significant socioeconomic disparities in (n-3) fatty acid levels still exist in this high-risk population, suggesting even stronger disparities may be affecting the general population. Current cardiovascular prevention strategies need to more adequately address the socioeconomic barriers to (n-3) consumption, especially in patients with CHD. More effective methods should be devised to uniformly disseminate knowledge regarding the benefits of (n-3) fatty acids. The RBC (n-3) fatty acid level has been proposed as a new risk factor for CHD (47) and a growing body of literature has been exploring the value and cost-effectiveness of using supplements, fortified foods, or bioengineered plant foods to increase (n-3) consumption (48–50). These dietary alternatives may help address economic, geographic, social, and cultural barriers to consuming foods rich in (n-3) fatty acids.
Several limitations must be considered in interpreting our results. First, the lack of data on dietary intake or use of dietary supplements limited our ability to confirm that dietary intake was responsible for the low RBC DHA and EPA levels found in patients with low SES. However, given that (n-3) fatty acids must be obtained through dietary sources and cannot be synthesized de novo in humans, it follows that RBC (n-3) fatty acid levels should be an accurate reflection of dietary intake. Second, the cross-sectional nature of our study precludes any definitive conclusions of causality in the association between SES and RBC (n-3) fatty acids. In this study, reverse causality is unlikely given that it is less plausible that adult (n-3) fatty acid consumption could significantly affect SES. Third, the study participants were mostly urban men and are thus not completely reflective of the general population. Finally, we cannot eliminate the possibility of residual confounding by factors correlated with both SES and RBC (n-3) levels. Although many factors can affect SES, it is unlikely these same factors alter RBC (n-3) levels, which are primarily a function of diet.
In summary, we found that 3 indicators of low SES, household income, education level, and occupation, were strongly associated with low RBC levels of 2 (n-3) fatty acids, EPA and DHA, among patients with CHD. These observations provide evidence that (n-3) fatty acids may be an important mediating factor in the association between lower SES and CVD. Future prevention efforts to raise awareness about and increase the dietary intake of (n-3) fatty acids in patients with CHD may help reduce existing socioeconomic disparities.
Footnotes
Supported by the Department of Veterans Affairs, Washington, DC, the National Heart Lung and Blood Institute (R01 HL079235), Bethesda, MD, the American Federation for Aging Research (Paul Beeson Scholars Program), New York, NY, the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, NJ, the Ischemia Research and Education Foundation, South San Francisco, CA, and the Nancy Kirwan Heart Research Fund, San Francisco, CA.
Author disclosures: B. E. Cohen, S. K. Garg, S. Ali, W. S. Harris, and M. A. Whooley, no conflicts of interest.
Abbreviations used: CAD, coronary artery disease; CHD, coronary heart disease; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FAME, fatty acid methyl esters; SES, socioeconomic status; SRO, single room occupancy.
Literature Cited
- 1.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–98. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 3.Marmot MG, Shipley MJ, Rose G. Inequalities in death: specific explanations of a general pattern? Lancet. 1984;1:1003–6. doi: 10.1016/s0140-6736(84)92337-7. [DOI] [PubMed] [Google Scholar]
- 4.Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–8. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–61. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 6.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–25. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 8.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 10.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev. 2004;4:CD003177. doi: 10.1002/14651858.CD003177.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–60. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 15.Johansson LR, Solvoll K, Bjorneboe GE, Drevon CA. Intake of very-long-chain n-3 fatty acids related to social status and lifestyle. Eur J Clin Nutr. 1998;52:716–21. doi: 10.1038/sj.ejcn.1600632. [DOI] [PubMed] [Google Scholar]
- 16.Stallone DD, Brunner EJ, Bingham SA, Marmot MG. Dietary assessment in Whitehall II: the influence of reporting bias on apparent socioeconomic variation in nutrient intakes. Eur J Clin Nutr. 1997;51:815–25. doi: 10.1038/sj.ejcn.1600491. [DOI] [PubMed] [Google Scholar]
- 17.Yeh LL, Kuller LH, Bunker CH, Ukoli FA, Huston SL, Terrell DF. The role of socioeconomic status and serum fatty acids in the relationship between intake of animal foods and cardiovascular risk factors. Ann Epidemiol. 1996;6:290–8. doi: 10.1016/s1047-2797(96)00023-3. [DOI] [PubMed] [Google Scholar]
- 18.Erkkila AT, Sarkkinen ES, Lehto S, Pyorala K, Uusitupa MI. Diet in relation to socioeconomic status in patients with coronary heart disease. Eur J Clin Nutr. 1999;53:662–8. doi: 10.1038/sj.ejcn.1600829. [DOI] [PubMed] [Google Scholar]
- 19.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubbock LA, Goh A, Ali S, Ritchie J, Whooley MA. Relation of low socioeconomic status to C-reactive protein in patients with coronary heart disease (from the Heart and Soul Study) Am J Cardiol. 2005;96:1506–11. doi: 10.1016/j.amjcard.2005.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–7. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 22.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res. 1964;5:600–8. [PubMed] [Google Scholar]
- 23.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Das UN. Essential fatty acids as possible mediators of the actions of statins. Prostaglandins Leukot Essent Fatty Acids. 2001;65:37–40. doi: 10.1054/plef.2001.0285. [DOI] [PubMed] [Google Scholar]
- 26.Lauretani F, Semba RD, Bandinelli S, Miller ER, III, Ruggiero C, Cherubini A, Gualnik JM, Ferrucci L. Plasma polyunsaturated fatty acids and the decline of renal function. Clin Chem. 2008;54:475–81. doi: 10.1373/clinchem.2007.095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter LM, Whiting SJ. Underreporting of energy intake, socioeconomic status, and expression of nutrient intake. Nutr Rev. 1998;56:179–82. doi: 10.1111/j.1753-4887.1998.tb06134.x. [DOI] [PubMed] [Google Scholar]
- 28.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):S925–32. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 29.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:S179–88. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 30.Racine RA, Deckelbaum RJ. Sources of the very-long-chain unsaturated omega-3 fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Curr Opin Clin Nutr Metab Care. 2007;10:123–8. doi: 10.1097/MCO.0b013e3280129652. [DOI] [PubMed] [Google Scholar]
- 31.Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002;5:127–32. doi: 10.1097/00075197-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–22. [PubMed] [Google Scholar]
- 33.Bolton-Smith C, Smith WC, Woodward M, Tunstall-Pedoe H. Nutrient intakes of different social-class groups: results from the Scottish Heart Health Study (SHHS) Br J Nutr. 1991;65:321–35. doi: 10.1079/bjn19910093. [DOI] [PubMed] [Google Scholar]
- 34.Deshmukh-Taskar P, Nicklas TA, Yang SJ, Berenson GS. Does food group consumption vary by differences in socioeconomic, demographic, and lifestyle factors in young adults? The Bogalusa Heart Study. J Am Diet Assoc. 2007;107:223–34. doi: 10.1016/j.jada.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hupkens CL, Knibbe RA, Drop MJ. Social class differences in women’s fat and fibre consumption: a cross-national study. Appetite. 1997;28:131–49. doi: 10.1006/appe.1996.0070. [DOI] [PubMed] [Google Scholar]
- 36.Popkin BM, Siega-Riz AM, Haines PS. A comparison of dietary trends among racial and socioeconomic groups in the United States. N Engl J Med. 1996;335:716–20. doi: 10.1056/NEJM199609053351006. [DOI] [PubMed] [Google Scholar]
- 37.Shimakawa T, Sorlie P, Carpenter MA, Dennis B, Tell GS, Watson R, Williams OD. Dietary intake patterns and sociodemographic factors in the atherosclerosis risk in communities study. ARIC Study Investigators. Prev Med. 1994;23:769–80. doi: 10.1006/pmed.1994.1133. [DOI] [PubMed] [Google Scholar]
- 38.Galobardes B, Morabia A, Bernstein MS. Diet and socioeconomic position: does the use of different indicators matter? Int J Epidemiol. 2001;30:334–40. doi: 10.1093/ije/30.2.334. [DOI] [PubMed] [Google Scholar]
- 39.French SA. Pricing effects on food choices. J Nutr. 2003;133:S841–3. doi: 10.1093/jn/133.3.841S. [DOI] [PubMed] [Google Scholar]
- 40.Ball K, Crawford D, Mishra G. Socio-economic inequalities in women’s fruit and vegetable intakes: a multilevel study of individual, social and environmental mediators. Public Health Nutr. 2006;9:623–30. doi: 10.1079/phn2005897. [DOI] [PubMed] [Google Scholar]
- 41.Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med. 2002;22:23–9. doi: 10.1016/s0749-3797(01)00403-2. [DOI] [PubMed] [Google Scholar]
- 42.Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC. Lack of benefit ofdietaryadvice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- 43.He K, Song Y. Risks and benefits of omega 3 fats: a few thoughts on systematic review. BMJ. 2006;332:915–6. doi: 10.1136/bmj.332.7546.915-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98:4A:3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–2. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 46.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 47.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 48.Franzosi MG, Brunetti M, Marchioli R, Marfisi RM, Tognoni G, Valagussa F. Cost-effectiveness analysis of n-3 polyunsaturated fatty acids (PUFA) after myocardial infarction: results from Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto (GISSI)-Prevenzione Trial. Pharmacoeconomics. 2001;19:411–20. doi: 10.2165/00019053-200119040-00008. [DOI] [PubMed] [Google Scholar]
- 49.Metcalf RG, James MJ, Mantzioris E, Cleland LG. A practical approach to increasing intakes of n-3 polyunsaturated fatty acids: use of novel foods enriched with n-3 fats. Eur J Clin Nutr. 2003;57:1605–12. doi: 10.1038/sj.ejcn.1601731. [DOI] [PubMed] [Google Scholar]
- 50.Schmier JK, Rachman NJ, Halpern MT. The cost-effectiveness of omega-3 supplements for prevention of secondary coronary events. Manag Care. 2006;15:43–50. [PubMed] [Google Scholar]