Abstract
Study Objectives:
Proton resonance spectroscopy (1H-MRS) allows noninvasive chemical tissue analysis in the living brain. As neuronal loss and gliosis have been described in narcolepsy, metabolites of primary interest are N-acetylaspartate (NAA), a marker of neuronal integrity and myo-Inositol (mI), a glial marker and second messenger involved in the regulation of intracellular calcium. One 1H-MRS study in narcolepsy found no metabolic changes in the pontomedullary junction. Another study showed a reduction in NAA/creatine-phosphocreatine (Cr) in the hypothalamus of narcolepsy patients with cataplexy. We aimed to test for metabolic changes in specific brain areas, “regions of interest,” thought to be involved in emotional processing, sleep regulation and pathophysiology of narcolepsy: hypothalamus, pontomesencephalic junction and both amygdalae.
Design:
We performed 1H-MRS using a 3T Philips Achieva whole body MR scanner. Single-voxel proton MR spectra were acquired and quantified with LCModel to determine metabolite concentration ratios.
Setting:
The participants in the study were recruited at the outpatient clinic for sleep medicine, Department of Neurology and magnetic resonance spectroscopy was performed at the MRI facility, University Hospital Zurich.
Participants:
1H-MRS was performed in fourteen narcolepsy patients with cataplexy, CSF hypocretin deficiency (10/10) and HLA-DQB1*0602 positivity (14/14) and 14 age, gender and body mass index matched controls. Patients were treatment naïve or off therapy for at least 14 days before scanning.
Measurements and Results:
No differences were observed in the regions of interest for (total NAA)/Cr ratios. Myo-Inositol (mI)/Cr was significantly lower in the right amygdala of the patients, compared to controls (P < 0.042). Significant negative correlations only in the patients group were found between (total NAA)/Cr in hypothalamus and mI/Cr in the right amygdala (r = −0.89, P < 0.001), between mI/Cr in hypothalamus and (total NAA)/Cr in the right amygdala (r = −63, P < 0.05) and between mI/Cr in the left amygdala and total NAA)/Cr in the pontomesencephalic junction (r = −0.69, P < 0.05).
Conclusion:
Our findings suggest amygdala involvement and possible hypothalamo-amygdala dysfunction in narcolepsy.
Citation:
Poryazova R; Werth E; Khatami R; Dydak U; Meier D; Boesiger P; Bassetti CL. Evidence for metabolic hypothalamo-amygdala dysfunction in narcolepsy. SLEEP 2009;32(5):607-613.
Keywords: Narcolepsy, cataplexy, magnetic resonance spectroscopy, hypocretin
NARCOLEPSY IS A DISABLING LIFE-LONG SLEEP-WAKE DISORDER, CHARACTERIZED BY EXCESSIVE DAYTIME SLEEPINESS AND SUDDEN LOSS OF MUSCLE tone (cataplexy),1 affecting between 25 and 50 per 100,000 people. Additional symptoms include sleep paralysis, hypnagogic hallucinations, and fragmented nighttime sleep. Most patients are positive for the HLA DQB1*0602. The diagnosis is clinical and is supported by the occurrence of 2 or more sleep-onset rapid eye movement (REM) sleep periods (SOREMPs) on the Multiple Sleep Latency Test (MSLT) and by the reduction or lack of hypocretin-1 in the cerebrospinal fluid (CSF).2,3
Hypocretin, a hypothalamic peptide, is involved in sleep-wake regulation, motor function, and food intake.4 Murine and canine narcolepsy-like features can be caused by mutations of the hypocretin precursor or hypocretin receptor genes. Human postmortem studies have shown a drastic reduction of hypocretin peptides5,6 and hypocretin-producing neurons and axons in hypothalamus,6–8 as well as signs of gliosis.6,7 In canine narcolepsy, neurodegeneration and gliosis have been reported not only in hypothalamus, but also in brain regions with vast hypocretin projections, including the amygdala.9 Brainstem structures involved in sleep regulation, motor control, and especially cataplexy, such as the locus coeruleus, dorsal raphe nuclei, and ventral tegmental area, also receive dense hypocretin projections but neither neuronal degeneration nor gliosis have been reported in these areas.
Proton resonance spectroscopy (1H-MRS) allows a noninvasive chemical tissue analysis of the living brain. Because neuronal loss and gliosis have been described in narcolepsy, metabolites of primary interest are N-acetylaspartate (NAA), a marker of neuronal integrity,10 and myoinositol (mI), a glial marker and second messenger involved in the regulation of intracellular calcium.11 The results of 2 1H-MRS studies have been previously reported. A first study found no metabolic difference between patients with narcolepsy and healthy control subjects in the pontomedullary junction.12 A second 1H-MRS study showed a reduction of NAA13 in the hypothalamus of patients with narcolepsy and cataplexy but not in patients with narcolepsy without cataplexy, compared with healthy control subjects. In the first study, only the pontomedullary junction was examined, and, in the second study, only the hypothalamus. In addition, in both studies, hypocretin CSF measurements were not available, and only a part of the population was tested for HLA association in the first study.
In the present study, we performed 1H-MRS to investigate metabolic changes in multiple regions of interest thought to be involved in the pathophysiology of narcolepsy and affected by the degeneration of the hypocretin system.
METHODS
Patients
Fourteen patients with narcolepsy (7 men, 7 women; 12 right-handed, 2 left-handed; aged 30.6 ± 2.3 years; body mass index [BMI], 29.6 ± 2.1; disease duration, 12.1 ± 2.2 years; mean ± SEM) and 14 healthy control subjects matched for age (31.4 ± 2.0), sex, handedness, and BMI (27.3 ± 2.1) participated in the study. All patients had a history of classic narcolepsy with cataplexy and findings typical for narcolepsy on polysomnography (SOREMPs in 13/14 patients, only patient number 14 did not present with SOREMP on polysomnography), MSLT ( > 2 SOREMPs in 12/12), or both polysomnography and MSLT. All patients were HLA DQB1*0602 positive,14 and 10 of 10 who were tested were hypocretin deficient. CSF hypocretin-1 levels were determined in a single radioimmunoassay, as has been previously described.15 Characteristics of our patients with narcolepsy-cataplexy are presented in Table 1. The patients had high scores on the Epworth Sleepiness Scale (mean ± SEM, 15.1 ± 1.1). A score of at least 14 is suggestive of narcolepsy. The Ullanlinna Narcolepsy Scale score was 22.1 ± 2.2. A score of at least 14 is suggestive of narcolepsy.16 The Swiss Narcolepsy Scale score was −43.6 ± 8.8; a score less than 0 is suggestive for narcolepsy.17 The Stanford Cataplexy Scale18 score was 70.1% ± 7.0%. Psychometric assessment was performed using standard tools, including the Beck Anxiety Inventory, Beck Depression Inventory, and Spielberger State-Trait Anxiety Inventory. In patients, significantly higher scores were observed in the Beck Anxiety Inventory (mean ± SEM, patients 14.2 ± 2.1, controls 5 ± 0.7, P < 0.001) and the Spielberger State-Trait Anxiety Inventory (patients 39.1 ± 1.6, controls 31.9 ± 1.9, P < 0.05). None of the patients had actual clinical signs of depression. The mean values ± SEM, including age, BMI, and various scores of the patients and control subjects are presented in Table 2.
Table 1.
Demographic, Clinical and Relevant MSLT Findings of 14 Patients with Narcolepsy and Cataplexy
| No. | Age, y and sex | Disease duration, y | Chronic drug treatment | Drugs attempted and stopped because of adverse effects/lack of efficacy | Current complaints | HLA-DQB1*0602 | Hypocretin pg/mL | SOREMPs on MSLT, no. | Reliable data: ROI/metabolites |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 29, f | 7 | no | Mazindol, clomipramine, methylphenidate for a few months | EDS, cataplexy,a sleep paralysis, hypnagogic hallucinations | positive | < 20 | 4/4 | all |
| 2 | 33, m | 27 | Ephedrine for 16 years | Pemolin, methylphenidate, imipramine, over a few months | EDS, partial cataplexya | positive | not measured | 3/5 | all except left amygdala for both metabolites |
| 3 | 43, f | 15 | no | Methylphenidate, modafinil, reboxetine over a few months | EDS, partial cataplexy | positive | < 20 | 3/5 | all except hypothalamus for mI/Cr |
| 4 | 38, f | 18 | no | no | EDS, cataplexy,a sleep paralysis, hypnagogic hallucinations | positive | not measured | N.A. | all except right amygdala for both metabolites |
| 5 | 35, f | 18 | no | At diagnosis, mazindol for 3 years, then clomipramine for 3 years | EDS, cataplexy, sleep paralysis, hypnagogic hallucinations | positive | not measured | 3/5 | all except right amygdala for mI/Cr and pontomesencephalic junction for both metabolites |
| 6 | 19, m | 1 | no | no | EDS, cataplexy,a sleep paralysis, hypnagogic hallucinations | positive | < 20 | 4/4 | all except left and right amygdala for mI/Cr |
| 7 | 22, f | 4 | no | Modafinil for 4 months | EDS, cataplexy,a sleep paralysis, hypnagogic hallucinations | positive | 107 | 4/4 | all |
| 8 | 33, f | 19 | Modafinil 100-200 mg/d for 2 y | Mazindol, imipramine, dexamphethamine over a few months | EDS, cataplexy,a sleep paralysis, hypnagogic hallucinations | positive | < 20 | N.A. | all |
| 9 | 25, m | 9 | Modafinil 200 mg/d for 5 y | Mazindol for 2 years before modafinil | EDS, partial cataplexy a | positive | < 20 | 4/4 | all |
| 10 | 20, f | 3 | Modafinil 200 mg/d and fluoxetine 20 mg/d for 2 y | no | EDS, cataplexy,a sleep paralysis, hypnagogic hallucinations | positive | < 20 | 4/4 | all |
| 11 | 27, m | 20 | Modafinil 100-200 mg/d for 4 y | Methylphenidate, clomipramine for a few months | EDS, partial cataplexy a | positive | not measured | 5/5 | all except right amygdala for both metabolites |
| 12 | 39, m | 18 | Clomipramine 100 mg/d for 10 y | Modafinil for 6 years (stopped in 2002), dexamphethamine, reboxetine over a few months | EDS, cataplexy, sleep paralysis, hypnagogic hallucinations | positive | 118 | 4/4 | all |
| 13 | 45, m | 3 | Modafinil 200 mg/d and venlafaxine 75 mg/d for 3 y | no | EDS, cataplexy,a sleep paralysis, hypnagogic hallucinations | positive | < 20 | 4/4 | all |
| 14 | 25, f | 8 | Modafinil 100 mg/d for 1 y | no | EDS, partial cataplexy, sleep paralysis | positive | < 20 | 3/5 | all |
MSLT refers to the multiple sleep latency test;
ROI, region of interest;
SOREMPs, sleep-onset rapid eye movement sleep periods
EDS, excessive daytime sleepiness;
mI/Cr; myoinositol/creatine.
aUnequivocal cataplexy observed by our team during visits.
Table 2.
Demographic and Clinical Data of the 14 Patients with Narcolepsy and Cataplexy and the 14 Control Subjects
| Variable | Patients | Controls | P Value |
|---|---|---|---|
| Age, y | 30.6 ± 2.3 | 31.4 ± 2.0 | 0.8 |
| BMI, kg/m2 | 29.6 ± 2.1 | 27.3 ± 2.01 | 0.4 |
| ESS | 15.1 ± 1.1 | 6.6 ± 0.7 | < 0.001 |
| UNS | 22.1 ± 2.2 | 5.8 ± 0.8 | < 0.001 |
| SNS | −43.6 ± 8.8 | 23.6 ± 2.7 | < 0.001 |
| SCQ | 70.1 ± 7.0 | 2.9 ± 2.3 | < 0.001 |
| BAI | 14.2 ± 2.1 | 5.0 ± 0.7 | 0.001 |
| BDI | 13.1 ± 2.6 | 6.4 ± 1.3 | 0.036 |
| SS-TAI | 39.1 ± 1.6 | 31.9 ± 1.9 | 0.009 |
Data are presented as mean ± SEM.
BMI refers to body mass index;
ESS, Epworth Sleepiness Scale;
UNS, Ullanlinna Narcolepsy Scale;
SNS, Swiss Narcolepsy Scale;
SCQ, Stanford Cataplexy Questionnaire;
BAI, Beck Anxiety Inventory;
BDI, Beck Depression Inventory;
SS-TAI- Spielberger State-Trait Anxiety Inventory.
Six patients were treatment naïve or drug free for years; the other 8 patients stopped taking narcolepsy-specific medication 14 days prior to 1. Concomitant medications prior to the study included modafinil (n = 6, in 4 patients as a monotherapy), fluoxetine (n = 1, in combination with modafinil), venlafaxine (n = 1, in combination with modafinil), clomipramine (n = 1), and ephedrine (n = 1).
The study was approved by the local ethics committee, and written informed consent was obtained from all subjects.
1 was performed on a 3T Philips Achieva whole-body magnetic resonance scanner. Ellis et al12 reported that time of acquisition does not influence the results, so data were randomly acquired throughout the day (08:00 to 18:00) in both patients and control subjects groups. After the acquisition, subjects were asked whether or not they fell asleep. All but 1 patient with narcolepsy reported falling asleep, as did 6 of 14 control subjects. Single-voxel proton magnetic resonance spectra were acquired from hypothalamus (a volume of interest [VOI] of 1 cm3 was selected to include bilateral gray matter), both amygdalae (VOI of 1 cm3), and the pontomesencephalic junction (VOI of 1.9 cm3). Water-suppressed proton MR spectra were acquired using a PRESS single-voxel localization sequence19,20 (repetition time [TR] 1500 ms; echo time 34 ms; number of acquisitions, 512). Second-order shimming (3T Philips Achieva) was used. Acquisition time for each VOI was 19 minutes. Levels of NAA, N-acetylaspartate-glutamate (NAAG), NAA+NAAG (total NAA), mI, creatine-phosphocreatine (Cr), and choline were analyzed by fitting a linear combination of a basis set of metabolite model spectra to the data (LCModel).21 The basis set was simulated using the GAMMA (a general approach to magnetic resonance mathematical analysis) library.22 The spectra were calculated with a pulse sequence identical to that as used for the in vivo measurement, using prior knowledge, such as chemical shifts and coupling constants, with the values reported by Govindaraju et al23 used. The in vivo spectra were then analyzed as a linear combination of the simulated spectra. The metabolite concentrations were expressed as ratios relative to Cr peak. Figure 1 shows representative spectra of the analysis with LCModel and the location of each VOI. The patient group was compared with the control group on the basis of these results. Artifacts were rejected in 2 steps: first, after a visual inspection by a spectroscopy specialist, whole spectra were rejected because of artifacts. Second, based on the goodness-of-fit statistics, individual metabolites were rejected from some spectra. The goodness-of-fit statistics are shown in Table 3.
Figure 1.
Regions of interest: A: hypothalamus, B: pontomesencephalic junction, C: left amygdala, D: right amygdala and representative spectra. mI refers to myoinositol; Cho, choline; Cr, creatine; NAA, N-acetylaspartate.
Table 3.
Goodness-of-Fit Statistics
| Volume of interest | Patients |
Control subjects |
||
|---|---|---|---|---|
| No. | Results | No. | Results | |
| mI/Cr, hypothalamus | 13 | 15.7 ± 1.2 | 11 | 15.5 ± 0.6 |
| (total NAA)/Cr, hypothalamus | 14 | 16.3 ± 1.2 | 12 | 15.2 ± 0.9 |
| mI/Cr, pontomesencephalic junction | 13 | 11.8 ± 1.1 | 14 | 13.4 ± 1.5 |
| (total NAA)/Cr, pontomesencephalic junction | 14 | 7.9 ± 0.6 | 14 | 7.4 ± 0.4 |
| mI/Cr, right amygdala | 10 | 20.1 ± 2.0 | 13 | 14.7 ± 1.2 |
| (total NAA)/Cr, right amygdala | 12 | 13.7 ± 1.2 | 13 | 11.8 ± 0.5 |
| mI/Cr, left amygdala | 12 | 16.2 ± 1.4 | 12 | 16.8 ± 1.7 |
| (total NAA)/Cr, left amygdala | 13 | 12.8 ± 1.3 | 14 | 12.0 ± 1.0 |
Data are presented as mean ± SEM.
NAA refers to N-acetylaspartate;
Cr, creatine;
mI, myoinositol
Statistical Analysis
Mann-Whitney and Wilcoxon signed ranks tests were used for the between-group comparison. Correlations were obtained using Pearson correlation coefficient. Significance level was determined as P < 0.05.
RESULTS
Hypothalamus
No differences were found in hypothalamus between patients with narcolepsy and control subjects for the ratios of (total NAA)/Cr (patients 1.25 ± 0.06, controls 1.3 ± 0.14, P = 0.86) and mI/Cr (patients 1.92 ± 0.14, controls 1.67 ± 0.12, P = 0.23) (Figure 2A). Reliable data were available for mI/Cr in 13 patients and 11 controls and for (total NAA)/Cr in 14 patients and 12 controls.
Figure 2.
A: metabolite concentration ratios in hypothalamus, not significant; B: metabolite concentration ratios in the pontomesencephalic junction, not significant; C: metabolite concentration ratios in the right amygdala, significant (P < 0.042) for myoinositol (mI)/creatine (Cr) and not significant for (total N-acetylaspartate [NAA])/Cr; D: metabolite concentration ratios in the left amygdala, not significant.
Pontomesencephalic Junction
No differences were found in the pontomesencephalic junction between patients with narcolepsy and control subjects for the ratios of (total NAA)/Cr (patients 1.76 ± 0.12, controls 1.6 ± 0.07, P = 0.31) and mI/Cr (patients 1.47 ± 0.08, controls 1.31 ± 0.09, P = 0.16) (Figure 2). Reliable data were available for mI/Cr in 13 patients and 14 controls and (total NAA)/Cr in 14 patients and 14 controls.
Amygdala
A significantly lower mI/Cr ratio was found in the right amygdala of patients (1.15 ± 0.11), compared with control subjects (1.42 ± 0.07, P = 0.042). No significant differences were observed concerning (total NAA)/Cr (patients 1.15 ± 0.05, controls 1.23 ± 0.09, P = 0.852) (Figure 2C). Reliable data was available for mI/Cr in 10 patients and 13 controls and for (total NAA)/Cr for 12 patients and 13 controls. In the left amygdala, no significant metabolic differences were observed between patients and control subjects for either (total NAA)/Cr (patients 1.45 ± 0.12, controls 1.22 ± 0.06, P = 0.256) or mI/Cr (patients 1.69 ± 0.2, controls 1.24 ± 0.08, P = 0.16) (Figure 2D). Reliable data were available for mI/Cr in 12 patients and 12 controls and for (total NAA)/Cr for 13 patients and 14 controls. All data are expressed as mean ± SEM.
Metabolite Correlations Between Different Brain Areas
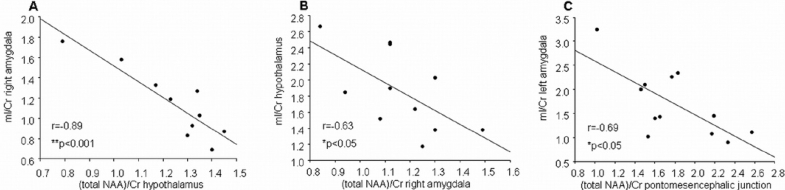
A highly significant negative correlation was found between (total NAA)/Cr in the hypothalamus and the mI/Cr in the right amygdala of the patients (r = −0.89, P < 0.001) (Figure 3A). There was also a significant negative correlation between the mI/Cr in the hypothalamus and (total NAA)/Cr in the right amygdala of the patients (r = −63, P < 0.05) (Figure 3B).
Figure 3.
A: correlation between (total N-acetylaspartate [NAA])/ creatine (Cr) in hypothalamus and the myoinositol (mI)/Cr in the right amygdala of the patients (r = −0.89, P < 0.001, B: correlation between the mI/Cr in hypothalamus and (total NAA)/Cr in the right amygdala of the patients (r = −63, P = 0.039), C: correlation between mI/Cr in the left amygdala and the (total NAA)/Cr in the pontomesencephalic junction in the patients (r = −0.69, P = 0.012)
The mI/Cr in the left amygdala correlated significantly and negatively with the (total NAA)/Cr in the pontomesencephalic junction in the patient group (r = −0.69, P < 0.05) (Figure 3C).
In the control group, however, no correlations between the metabolite ratios were observed.
Metabolite Ratios and Characteristics of Narcolepsy
No association was found between the metabolite concentration ratios and any demographic, psychometric, neurophysiologic parameter, or specific narcolepsy scores and symptoms, such as cataplexy or excessive daytime sleepiness.
DISCUSSION
In this study, the presence of neurochemical changes was assessed using 1 in 14 patients with narcolepsy-cataplexy and 14 healthy control subjects. Compared with the results of 2 previous studies, the present work has several advantages. First, multiple regions of interest were examined, which is a prerequisite to search for possible interactions between different brain areas. Second, we selected a homogenous group of patients with cataplexy, hypocretin deficiency, HLA DQB1*0602 positivity and control subjects matched for age, sex, handedness and BMI. Third, only subjects aged 18 to 45 years were included.
There are 2 main results of the study. First, metabolic changes in the right amygdala were found. This is in agreement with other data, suggesting an involvement of the right amygdala in the pathophysiology of narcolepsy.
During REM sleep, cortical and subcortical areas,24 including both amygdalae,25,26 are activated. Right amygdala hyperperfusion during cataplexy, triggered by emotions, has been reported in 2 patients with narcolepsy in a SPECT study.27 Another case study in a single patient could not confirm these findings28; however, in this study, the patient's cataplexy attack was not triggered by any particular emotion. In narcoleptic dogs, neurodegeneration with gliosis has been reported in the amygdalae.9 Cataplexy-related neurons in the amygdalae of narcoleptic dogs have been identified, firing before and during cataplexy.29 Finally, the absence of aversive startle reflex potentiation has recently been described in patients with narcolepsy,30 a finding that has been previously documented in patients with right amygdala lesions.
A few observations may explain why metabolic changes were seen only in the right amygdala. There is evidence that the right hemisphere shows the strongest relationship with affect. Predominant right amygdala activation has been described in response to laughing and crying stimuli,31 and strong emotions are well-known triggers for cataplexy.32 Human studies using monoaural acoustic startle response has also demonstrated predominantly right hemisphere activation.33 The laterality of the reflex and the importance of the right amygdala in emotional processing have been demonstrated in rats34 and humans.35
Although a metabolic change in the right amygdala of narcoleptic patients supports the hypothesis of amygdala involvement in the pathophysiology of narcolepsy, the decrease of mI/Cr is more difficult to explain. Because neurodegeneration and gliosis are reported in canine narcolepsy, an increase, rather than a decrease, of mI/Cr would be expected. Considering the physiologic role of myoinositol as a second messenger involved in the regulation of intracellular calcium,11 disturbed cell signaling in the right amygdala of patients with narcolepsy may be suggested.
Second, a reciprocal negative correlation between mI/Cr and (total NAA)/Cr in hypothalamus and in the right amygdala and a negative correlation between mI/Cr in the left amygdala and (total NAA)/Cr in the pontomesencephalic tegmental region were found. The reciprocal negative correlation between mI/Cr and (total NAA)/Cr in hypothalamus and in the right amygdala in patients with narcolepsy probably originates from their close interaction. Amygdala and hypothalamus are anatomically connected via stria terminalis and the amygdalofugal tract. Hypothalamic hypocretin peptides in acute rat brain slices excite a specific class of neurons in the central medial nucleus of the amygdala. It has been suggested that the hypocretin system can act through the amygdala to augment arousal and evoke the autonomic and behavioral responses associated with fear, stress, or emotion.36 A recent functional magnetic resonance imaging study demonstrated a dysfunction of hypothalamic-amygdala interactions during positive emotions in patients with narcolepsy. A reduced hypothalamic activation and an exaggerated amygdala response to humour was observed, suggesting the possibility that the hypothalamus might have modulatory influences on amygdala activity during positive emotions, possibly via direct projections from hypothalamic hypocretin neurons.37
As a marker of neuronal integrity in the hypothalamus decreases, a glial marker and second messenger involved in the regulation of intracellular calcium in the amygdala increases. It can be hypothesized that deficient hypocretin signalling from hypothalamus impairs intracellular regulation in the amygdala. Tissue segmentation analysis might help to better understand the metabolite alterations and interactions in different regions of interest.
In narcoleptic dogs, cataplexy-related neuron populations in amygdala are thought to mediate or modulate cataplexy through interactions with pontomesencephalic regions controlling atonia.34 The metabolic association between mI/Cr in the left amygdala and (total NAA)/Cr in the pontomesencephalic tegmental area found in this study suggests a similar relationship in humans.
The findings of Lodi et al.,13 who reported reduced NAA/Cr in hypothalamus of patients with narcolepsy and cataplexy, were not confirmed by our data. This may be explained if the hypothalamic hypocretin neuron degeneration only leads to subtle changes, not detectable by 1. On the other hand, the data might also be influenced by patient selection and technical approach. In the study of Lodi et al, 1536 repetitions were used. With a TR of 1500 ms, this means that the duration of the measurement itself was 38.4 minutes without shimming. Different shimming protocols may have different durations. Our acquisition with 512 repetitions, and a TR of 1500 lasted 12.8 minutes, plus 6 minutes of shimming, yielding an overall duration for a measurement of a VOI of about 19 minutes. With 4 regions of interest, a duration of the measurement itself of 38.4 minutes was not feasible for us. Additionally, prolonged duration of the scanning increases the rate of motion artifacts. Before the actual study, we performed a number of pilot examinations, in which the choice of 512 repetitions yielded the best results with regard to motion artifacts, duration, signal-to-noise ratio, and goodness-of-fit statistics.
Voxel-based morphometry studies have also shown inconsistent results, with respect to structural brain changes in the hypothalamus of patients with narcolepsy. Two voxel-based morphometry studies have shown structural changes in hypothalamus in the form of reduced grey matter.38,39 In 1 of these studies,38 however, no details about the clinical and laboratory characteristics of the patients were provided. Three other studies,40–42 1 of which included a highly homogenous group of patients (all hypocretin deficient, all HLA DQB1*0602 positive40) found no hypothalamic structural abnormalities in narcoleptic subjects.
Possible limitations of our study are the small size of the examined regions of interest and, therefore, the small MRS volume size, which requires longer scanning time. The longer scanning time itself leads to agitation of the subjects and increases possible movement artifacts. On the other hand, a small volume size has the advantage of better magnetic field homogeneity, leading to smaller peak line widths and easier peak identification and quantification. Absolute quantification of the metabolites would yield more precise values. This issue should be addressed by future studies, developing technical sequences that would allow such measurements. Because narcolepsy is a fairly rare disease, large patient samples are difficult to obtain. Although our patients stopped all narcolepsy-specific medication 14 days prior to scanning, the previous long-term use of medication may have long-lasting effect.
In conclusion, our findings suggest an amygdala involvement and a possible hypothalamo-amygdala dysfunction in narcolepsy. Future work will include tissue classification within the spectroscopic VOI to judge whether the metabolic alterations are due to grey matter, white matter, or CSF changes.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Dydak has received research support from Siemens Medical Solutions. Dr. Boesiger has received research support form Philips Health Care. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The participants in the study were recruited at the outpatient clinic for sleep medicine, Department of Neurology, University Hospital Zurich. Magnetic resonance spectroscopy was acquired at the MRI facility, University Hospital Zurich, and data analysis was performed at the Institute for Biomedical Engineering, University and ETH Zurich, and at the Department of Neurology, University Hospital Zurich.
The study was supported by a Swiss National Science Foundation grant and by an EFNS (European Federation of Neurological Societies) grant in 2006 (to RP).
REFERENCES
- 1.Bassetti C, Aldrich MS. Narcolepsy. Neurol Clin. 1996;14:545–71. doi: 10.1016/s0733-8619(05)70273-5. [DOI] [PubMed] [Google Scholar]
- 2.Baumann CR, Bassetti CL. Hypocretins (orexins) and sleep-wake disorders. Lancet Neurol. 2005;4:673–82. doi: 10.1016/S1474-4422(05)70196-4. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders. [Google Scholar]
- 4.Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–52. doi: 10.1016/S0079-6123(06)53014-6. [DOI] [PubMed] [Google Scholar]
- 5.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 6.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol. 2003;13:340–51. doi: 10.1111/j.1750-3639.2003.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology. 2005;65:1189–92. doi: 10.1212/01.wnl.0000175219.01544.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel JM, Nienhuis R, Gulyani S, et al. Neuronal degeneration in canine narcolepsy. J Neurosci. 1999;19:248–57. doi: 10.1523/JNEUROSCI.19-01-00248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubaek E, Ross D. New York, NY: Dekker Publishing; 1999. Magnetic resonance spectroscopy diagnosis of neurological diseases. [Google Scholar]
- 11.Berridge MJ, Irvine RF. Inositol phosphates and cell signaling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 12.Ellis CM, Simmons A, Lemmens G, Williams SC, Parkes JD. Proton spectroscopy in the narcoleptic syndrome. Is there evidence of a brainstem lesion? Neurology. 1998;50:S23–6. doi: 10.1212/wnl.50.2_suppl_1.s23. [DOI] [PubMed] [Google Scholar]
- 13.Lodi R, Tonon C, Vignatelli L, et al. In vivo evidence of neuronal loss in the hypothalamus of narcoleptic patients. Neurology. 2004;63:1513–5. doi: 10.1212/01.wnl.0000142259.94107.4c. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Hungs M, Mignot E. Narcolepsy and the HLA region. J Neuroimmunol. 2001;117:9–20. doi: 10.1016/s0165-5728(01)00333-2. [DOI] [PubMed] [Google Scholar]
- 15.Baumann CR, Dauvilliers Y, Mignot E, Bassetti CL. Normal CSF hypocretin- 1 (orexin A) levels in dementia with Lewy bodies associated with excessive daytime sleepiness. Eur Neurol. 2004;52:73–6. doi: 10.1159/000079749. [DOI] [PubMed] [Google Scholar]
- 16.Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikkilä K. The Ullanlinna Narcolepsy Scale: validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res. 1994;3:52–59. doi: 10.1111/j.1365-2869.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 17.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 18.Anic-Labat S, Guilleminault C, Kraemer H C, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disordered patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- 19.Bottomley PA. Selective volume method for performing localized NMR spectroscopy. U.S. patent 4 480 228. 1984
- 20.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci. 1987;508:333–48. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 21.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 22.Smith SA, Levante TO, Meier B. H, Ernst RR. Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. J Magn Reson. 1994;106:75–105. [Google Scholar]
- 23.Govindaraju V, Young K, Maudsley A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H215O PET study. Brain. 1997;120:1173–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 25.Maquet P, Peters J, Aerts J, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–6. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 26.Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: and FDG PET study. Brain Res. 1997;770:192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- 27.Hong SB, Tae WS, Joo EY. Cerebral perfusion changes during cataplexy in narcolepsy patients. Neurology. 2006;66:1747–9. doi: 10.1212/01.wnl.0000218205.72668.ab. [DOI] [PubMed] [Google Scholar]
- 28.Chabas D, Habert MO, Maksud P, et al. Functional imaging of cataplexy during status cataplecticus. Sleep. 2007;30:153–6. doi: 10.1093/sleep/30.2.153. [DOI] [PubMed] [Google Scholar]
- 29.Gulyani S, Wu MF, Nienhuis R, John J, Siegel JM. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112:355–65. doi: 10.1016/s0306-4522(02)00089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatami R, Birkmann S, Bassetti CL. Amygdala dysfunction in narcolepsy-cataplexy. J Sleep Res. 2007;16:226–9. doi: 10.1111/j.1365-2869.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 31.Sander K, Brechmann A, Scheich H. Audition of laughing and crying leads to right amygdala activation in a low-noise fMRI setting. Brain Res Brain Res Protocol. 2003;11:81–91. doi: 10.1016/s1385-299x(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 32.Siegel JM, Boehmer LN. Narcolepsy and the hypocretin system--where motion meets emotion. Nat Clin Pract Neurol. 2006;2:548–56. doi: 10.1038/ncpneuro0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley MM, Cuthbert BN, Lang PJ. Startle and emotion: lateral acoustic probes and the bilateral blink. Psychophysiology. 1991;28:285–95. doi: 10.1111/j.1469-8986.1991.tb02196.x. [DOI] [PubMed] [Google Scholar]
- 34.Coleman-Mesches K, McGaugh JL. Differential involvement of the right and left amygdalae in expression of memory for aversively motivated training. Brain Res. 1995;670:75–81. doi: 10.1016/0006-8993(94)01272-j. [DOI] [PubMed] [Google Scholar]
- 35.Angrilli A, Mauri A, Palomba D, et al. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119:1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- 36.Bisetti A, Cvetkovic V, Serafin M, et al. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz S, Ponz A, Poryazova R, et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131:514–22. doi: 10.1093/brain/awm292. [DOI] [PubMed] [Google Scholar]
- 38.Draganski B, Geisler P, Hajak G, et al. Hypothalamic gray matter changes in narcoleptic patients. Nat Med. 2002;8:1186–8. doi: 10.1038/nm1102-1186. [DOI] [PubMed] [Google Scholar]
- 39.Buskova J, Vaneckova M, Sonka K, Seidl Z, Nevsimalova S. Reduced hypothalamic gray matter in narcolepsy with cataplexy. Neuro Endocrinol Lett. 2006;27:769–72. [PubMed] [Google Scholar]
- 40.Overeem S, Steens SC, Good CD, et al. Voxel-based morphometry in hypocretin-deficient narcolepsy. Sleep. 2003;26:44–6. [PubMed] [Google Scholar]
- 41.Brenneis C, Brandauer E, Frauscher B, et al. Voxel-based morphometry in narcolepsy. Sleep Med. 2005;6:531–6. doi: 10.1016/j.sleep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Kaufmann C, Schuld A, Pollmacher T, Auer DP. Reduced cortical gray matter in narcolepsy: preliminary findings with voxel-based morphometry. Neurology. 2002;58:1852–5. doi: 10.1212/wnl.58.12.1852. [DOI] [PubMed] [Google Scholar]