Abstract
Study Objective:
Sleep disturbances have been associated with individual components of the metabolic syndrome (“syndrome X”), and, although the concept has been proposed, it is not known whether sleep disturbances actually cluster with features of the metabolic syndrome to produce a unifying trait, “syndrome Z”. Therefore, we evaluated a second-order factor model, whereby syndrome Z was described by 5 first-order factors – insulin resistance, obesity, hypertension, dyslipidemia, and sleep disturbance – with the sleep disturbance factor defined using the apnea-hypopnea index, arousal index, percentage of sleep time with oxygen saturation less than 90%, and percentage of slow wave sleep.
Design:
Observational, cross-sectional study.
Setting:
Clinical research center.
Participants:
Five hundred thirty-three adults from the Cleveland Family Sleep Study who underwent polysomnography and were not treated by continuous positive airway pressure.
Measurements and Results:
When modeling syndrome Z as a second-order factor unifying 5 first-order factors, we observed good overall model fit (χ2/df = 3.20; CFI = 0.96; RMSEA = 0.06; SRMR = 0.05) and found that obesity was the most important determining factor (standardized loading = 0.85 ± standard error = 0.02; P < 0.01) followed by sleep disturbance (0.82 ± 0.03; P < 0.01), insulin resistance (0.67 ± 0.03; P < 0.01), hypertension (0.64 ± 0.04; P < 0.01), and dyslipidemia (0.60 ± 0.05; P < 0.01). Simultaneous multiple group analyses revealed that this model was essentially generalizable across age, race, and sex subgroups.
Conclusions:
Our results demonstrate that sleep disturbance co-aggregates with other metabolic features to represent a single unifying trait, syndrome Z. Although our model awaits validation in other populations, it provides a tool for better understanding the synergistic risk of syndrome Z, compared with syndrome X, on type 2 diabetes and cardiovascular disease in future studies.
Citation:
Nock NL; Larkin EK; Patel SR; Redline S. Empirical evidence for “Syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. SLEEP 2009;32(5):615-622.
Keywords: Sleep, metabolic syndrome, factor analysis, Syndrome Z
THE METABOLIC SYNDROME IS A CO-OCCURRENCE OR CLUSTERING OF METABOLIC DISTURBANCES THAT RESULTS IN A HIGHER RISK OF TYPE 2 DIABETES and cardiovascular disease1 and may contribute to the pathogenesis of other complex diseases, including colon and other cancers.2 The metabolic syndrome is becoming increasingly more prevalent worldwide3–5 with approximately 25% to 40% of adult Americans (at least 47 million people) reported to have the syndrome.6,7 Although the metabolic syndrome has been shown to increase with age, recent studies have also indicated a rise in the prevalence among younger subjects, particularly women aged 20 to 39 years, a rise that appears to mirror the increasing rates of obesity among women compared to men in the United States.1 In terms of ethnicity, Mexican Americans appear to have the highest prevalence of the syndrome (31.9%) followed by Caucasians (23.8%) and African Americans (21.6%).6
The metabolic syndrome has been defined formally by several agencies, including the World Health Organization,8 National Cholesterol Education Program Third Adult Treatment Panel,9,10 American Heart Association/National Heart, Lung and Blood Institute,11,12 and the International Diabetes Federation.13 Although there is some disparity among these definitions, differences predominantly occur in the cutpoint values applied, the number of individual attributes required, the emphasis on central obesity, and consideration of medication use. Nevertheless, all of the definitions encompass some criteria of 4 key elements: insulin resistance/glucose dysregulation, obesity, hypertension, and dyslipidemia.
To help understand the underlying complex pathophysiology of the metabolic syndrome, researchers have utilized factor-analysis methods to determine the number of components (or factors) that best represent the syndrome statistically and to assess the strength of the relationships between each measurable attribute and the underlying latent factor, as well as relationships between factors, using factor loadings. Although some inconsistencies have been reported, most studies in adults using 8 to 10 metabolic measures (fasting insulin level, fasting glucose level, postchallenge insulin level, postchallenge glucose level, body mass index [BMI], waist circumference [waist], waist-to-hip ratio [WHR], high density lipoprotein-cholesterol level [HDL], triglyceride levels, systolic blood pressure [SBP], and diastolic blood pressure [DBP]) have shown that 4 factors— insulin resistance, obesity, blood pressure, and lipids—best describe the metabolic syndrome as a unifying, second-order construct.14–16
Recently, sleep disturbances, including obstructive sleep apnea (OSA), sleep deprivation, and sleep fragmentation, have been suggested to be involved in the development of the metabolic syndrome.17 OSA, a chronic illness characterized by repetitive episodes of partial or complete cessation of breathing during sleep, may affect up to 17% of middle-aged adults18 and has been associated with all 4 of the more established components of the metabolic syndrome,17 leading to the suggestion that a “syndrome Z” exists.19 Although the co-aggregation of OSA with the metabolic syndrome has been largely attributed to obesity, the exact mechanisms driving the association between OSA and the metabolic syndrome and its individual features remain to be elucidated. Tasali and Ip20 have suggested that OSA leads to chronic intermittent hypoxia and sleep fragmentation from arousals, which lead to a cascade of pathogenic mechanisms, such as oxidative stress and neurohumoral changes, that, in turn, lead to insulin resistance, hypertension, and dyslipidemia. Furthermore, both experimental and epidemiologic studies have implicated sleep deprivation and sleep fragmentation in the pathogenesis of impaired glucose tolerance21–23 and hypertension.24–26 In particular, suppression of deep or slow wave sleep (SWS), without any change in total sleep time, has recently been demonstrated to result in decreased insulin sensitivity and reduced glucose tolerance.27
Despite these suggested associations for the concept of syndrome Z, there is currently no evidence that sleep disturbance actually clusters with other traditionally recognized features of the metabolic syndrome to produce a unifying trait. Therefore, we used factor analysis to evaluate the fit of a second-order factor model for syndrome Z, whereby the second-order factor, syndrome Z, was defined by 5 first-order factors: insulin resistance, obesity, hypertension, dyslipidemia, and sleep disturbance. We defined the sleep-disturbance factor with 4 measures, the apnea-hypopnea index (AHI), the arousal index (ArI), the percentage of sleep time when oxygen saturation was less than 90%, and the percentage of sleep time in SWS. Because sleep disturbance and metabolic measures may vary by age, sex, and race,1,6,28,29 we also tested for generalizability of the model using simultaneous multiple group factor analysis methods.
MATERIALS AND METHODS
Study Population
The Cleveland Family Sleep Study population has been previously described.30 Briefly, index probands with a laboratory-confirmed diagnosis of OSA and family members with and without OSA were recruited to participate in this study over a 15-year period. In the last examination (conducted in the years 2001-2006), a subset (n = 735) of individuals from families with siblings having extreme high or low values of AHI were selected for more detailed evaluation. Specifically, each participant completed overnight polysomnography, venipuncture before (∼22:00) and after sleep and after an overnight fast (∼07:00), an oral glucose test, and a standardized validated questionnaire, which assessed demographics, sleep habits and symptoms, medical history, medication use—including diabetic and antihypertensive medications—and other habits.31 The study was approved by the University Hospitals Institutional Review Board. The current analysis included 533 subjects (older than 16 years of age) from 133 families who participated in the most recent evaluation, were not treated for sleep apnea by continuous positive airway pressure, and had complete information on all metabolic and sleep measures (discussed below).
Metabolic Measures
Height was measured to the nearest 0.5 cm using a wall-mounted stadiometer, and weight was measured to the nearest 0.1 kg with a calibrated scale (Health-o-meter®, Jarden Consumer Solutions, Boca Raton, FL) in subjects without shoes. Waist circumference was measured to the nearest 0.5 cm in duplicate around the smallest circumference midway between the iliac crest and the lowest lateral portion of the rib cage using a nonstretchable tape and averaged. BMI was calculated as weight in kilograms (kg) divided by the square of height in meters (kg/m2).
Glucose tolerance testing involved oral administration of 75 grams of anhydrous glucose followed by venipuncture 2 hours later. Insulin levels were measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA), and glucose levels by enzymatic glucose oxidase method (YSI Life Sciences, Yellow Springs, OH). Lipid levels (triglycerides, HDL cholesterol) from blood serum obtained with the subjects fasting were measured by enzymatic methods using Centers for Disease Control and Prevention guidelines.32 Systolic and diastolic blood pressure were each determined using the average of 9 measurements following standardized guidelines using a calibrated sphygmomanometer33 and based on performance in triplicate at 3 different times: before bed (∼22:00); upon awakening (∼07:00); and, after sitting (∼11:00). Because of potential confounding by treatment, blood pressure and insulin and glucose measurements were also evaluated using values adjusted for the use of blood pressure and diabetes medications, respectively. Following the protocol of Cui et al,34 we added 10 mm Hg to SBP and 5 mm Hg to DBP in subjects who reported taking blood pressure medications within 3 days of their blood pressure being measured. No precedent exists on how to adjust for the use of diabetes medications. Therefore, subjects taking medications for diabetes were assigned a glucose value equal to the midpoint of the highest quintile of glucose level observed in subjects who were not taking medications for diabetes. Similarly, subjects taking medications for diabetes were assigned an insulin level equal to the midpoint of the highest quintile of insulin level observed in subjects who were not taking medications for diabetes. To evaluate the potential impact of adjusting for medication use, we performed analyses using the unadjusted and adjusted variables separately.
Sleep Measures
We objectively measured sleep parameters using overnight 14-channel polysomnography obtained in a clinical research unit, as previously described.31 An apnea was defined as a complete or almost complete cessation of airflow, as measured by a nasal-oral thermocouple, lasting 10 seconds or longer. Hypopneas were scored when the amplitude of the sum of the abdominal and thoracic inductance signals or the nasal pressure flow signal were clearly reduced for longer than 10 seconds and resulted in a 3% or greater desaturation. Overnight oxygen saturation (and degree of hypoxemia) were measured with a finger pulse oximeter (Nonin, Inc., Plymouth, MN) and quantified as the percentage of sleep time when oxygen saturation was less than 90%. Sleep stages were scored according to standardized guidelines,35 and stages 3 and 4 were combined to represent SWS. Arousals were identified according to standard criteria,36 and the ArI defined as the number of cortical arousals per hour of sleep.
Statistical Analysis
Because of the inherent complexity in the methods employed, we first summarize here the objective for each method and then describe the methods in more detail in the subsequent paragraphs. We used exploratory factor analysis (EFA) to confirm that 5 distinct factors would emerge when 4 new sleep disturbance measures were integrated with the 10 metabolic measures previously shown to represent the metabolic syndrome as a second-order factor. We used confirmatory factor analysis (CFA) to test the fit of our hypothesized second-order syndrome Z factor model and used CFA to compare this second-order syndrome Z model to a model that allowed all factors to correlate freely and imposed no constraints on how the factors were related to each other (i.e., a model that suggests that no unifying factor [syndrome Z] exists). We used simultaneous multiple-group CFA to test for generalizability of the model across age, race, and sex subgroups.
Before conducting factor analysis, we examined the distribution of all of the variables using SAS v9.1 (SAS Institute Inc., Cary, NC). Variables with extreme skew or kurtosis (fasting insulin level, postchallenge insulin level, triglyceride levels, AHI, percentage of time with an oxygen saturation below 90%, ArI, and percentage of SWS) were natural log transformed to approximate a normal distribution. The factor analysis method (i.e., EFA versus CFA) that should be used for examining the structure of the metabolic syndrome has been debated.37,38 Because we were integrating 4 new sleep measures with 10 previously established metabolic variables15,16 and because we had clustered (family) data (which cannot be readily handled in current EFA methods), we performed an initial evaluation with EFA (Promax rotation) to confirm our biologically driven hypothesis that 5 distinct factors would emerge using eigenvalue and factor pattern and pattern-loading criteria.39 Then we proceeded with CFA using a robust maximum likelihood estimator (MLR), which provides test statistics and standard errors robust to nonindependence of observations and nonnormality (Mplus v4.21; TYPE = COMPLEX; http://www.statmodel.com), to formally test our hypothesized 5-factor hierarchical model versus viable alternative nested model or models. CFA also enabled us to perform simultaneous multiple group analysis to test the generalizability (invariance) of the model across sex, ethnic, and age groups. The Lagrange multiplier test was used to detect significant differences in factor loadings between groups.40 All P values were from 2-sided tests, and statistical significance was set at ≤ 0.05.
To assess the overall goodness-of-fit of the model to the data, the χ2 test, comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean square residual (SRMR) were evaluated.41 The χ2 test, which evaluates whether the covariance matrix is equal to the model-implied covariance matrix predicted by the parameters, however, is very sensitive to sample size and model complexity. Thus, other fit indexes, including the ratio of the χ2 to the degrees of freedom (df) (χ2/df.), CFI, RMSEA, and SRMR have been proposed as alternative descriptive measures for evaluating overall model fit. Although no absolute standards exist, a χ2/df ratio of 2 has been suggested to represent a good model fit, whereas a ratio of 3.0 or as high as 5.0 may represent an acceptable model fit; however, the χ2/df index does not fully correct for the influence of sample size.41 Values for the CFI, which is relatively insensitive to sample size and model complexity, of at least 0.90 and at least 0.95 have been suggested to represent acceptable and good model fit, respectively.42,43 RMSEA is an index that is less sensitive to sample size, which favors more parsimonious models, and RMSEA values of 0.06 or less represent good model fit, whereas values exceeding 0.10 represent unacceptable fit.42,44 For the SRMR, which accounts for differences in scale in computing residuals, values of 0.08 or less and less than 0.10 represent good and acceptable fit, respectively.41,42 To compare nested models, we used the χ2 difference test with the Satorra-Bentler scaled χ2, which has scaling factors to correct for nonnormality (χ2c) (http://www.statmodel.com/chidiff.shtml).
RESULTS
The characteristics of the study population are presented in Table 1. The mean age was approximately 40 years, and the population was approximately 43% male, 43% Caucasian, and 57% African American. On average, the population was obese (mean BMI of 30.6 kg/m2), with a slightly larger mean BMI found in women than in men. Overall, OSA severity was mild to moderate based on the mean AHI (11.8 events/h). AHI and ArI were both higher in men, and percentage of time in SWS was lower in men and African Americans.
Table 1.
Characteristics of the Cleveland Family Sleep Study, Examination 2000-2006a
| Characteristic | All (N = 533) |
Men (n = 229) |
Women (n = 304) |
Caucasian (n = 229) |
African American (n = 304) |
|---|---|---|---|---|---|
| Age, ya | 39.94 (19.48) | 38.93 (20.41) | 40.70 (18.74) | 42.35 (19.88) | 38.12 (19.00)c |
| Menb | 229 (42.96) | - | - | 102 (44.54) | 127 (41.78) |
| African Americanb | 304 (57.04) | 127 (55.46) | 177 (58.22) | - | - |
| Families, no. | 133 | 115 | 103 | 60 | 73 |
| Medication use b | |||||
| Antihypertensive | 117 (21.95) | 49 (21.40) | 68 (22.37) | 33 (14.41) | 84 (27.63)c |
| Hypoglycemic | 37 (6.94) | 14 (6.11) | 23 (7.57) | 12 (5.24) | 25 (8.22) |
| Lipid-lowering | 61 (11.44) | 32 (13.97) | 29 (9.54) | 28 (12.22) | 33 (10.86) |
| Measures of insulin resistance, mg/dLd | |||||
| Fasting | |||||
| Glucose | 93.07 (14.60) | 96.70 (18.11) | 90.34 (10.44) c | 92.04 (11.78) | 93.90 (16.44) |
| Insuline | 12.34 (12.64) | 12.62 (14.26) | 12.12 (11.26) | 11.58 (15.98) | 12.93 (9.23)c |
| 2-h Postchallenge | |||||
| Glucose | 120.95 (46.73) | 127.60 (53.51) | 115.85 (40.13)c | 118.26 (42.23) | 123.03 (49.91) |
| Insuline | 65.40 (55.64) | 65.85 (58.35) | 65.06 (53.58) | 60.25 (55.31) | 69.41 (55.67)c |
| Measure of obesitya | |||||
| Waist, cm | 92.97 (18.30) | 95.55 (18.55) | 91.03 (17.89)c | 93.15 (17.75) | 92.83 (18.73) |
| BMI, kg/m2 | 30.64 (8.03) | 29.58 (7.47) | 31.44 (8.34)c | 29.90 (7.36) | 31.21 (8.47) |
| Lipid measures and dyslipidemia, mg/dL | |||||
| Triglyceridese | 110.20 (84.43) | 120.33 (97.70) | 102.57 (72.09)c | 131.07 (109.69) | 94.48 (53.72)c |
| HDL | 45.30 (12.62) | 41.18 (11.31) | 48.40 (12.67)c | 44.55 (12.10) | 45.86 (12.98) |
| Measures of blood pressure, mm Hgd | |||||
| Systolic | 121.01 (14.47) | 122.68 (13.24) | 119.73 (15.25)c | 118.43 (13.61) | 123.02 (14.83)c |
| Diastolic | 73.15 (9.39) | 74.77 (9.49) | 71.91 (9.144)c | 71.71 (8.07) | 74.27 (10.18)c |
| Measures of sleep and sleep disruptione | |||||
| AHI, no/h | 11.84 (18.55) | 16.80 (22.75) | 8.10 (13.51)c | 11.12 (17.21) | 12.38 (19.52) |
| % of sleep time o2 Sat < 90% | 2.95 (8.87) | 4.01 (9.44) | 2.16 (8.35) | 3.25 (8.89) | 2.73 (8.87) |
| Arousal Index, no/h | 15.57 (9.66) | 17.62 (11.21) | 14.20 (8.03)c | 14.87 (9.20) | 16.27 (9.97) |
| % time in SWS | 20.72 (13.17) | 18.83 (14.54) | 22.14 (11.86)c | 22.84 (13.05) | 19.12 (13.05)c |
Data are presented as mean (SD)a or
frequency (%)b.
Waist refers to waist circumference, BMI, body mass index; AHI, apnea-hypopnea index; Sat, saturation; SWS, slow-wave sleep.
cp Value ≤ 0.05 (t-test or χ2 test comparing groups: men vs women; Caucasian vs African American).
dVariable corrected for medication use (see Methods section of text).
eVariable natural log transformed due to extreme skew and/or kurtosis (untransformed values shown).
Before proceeding to CFA, we conducted EFA only to confirm the presence of 5 distinct factors when we integrated the 4 new sleep measures (AHI, ArI, percentage of time with an oxygen saturation < 90%, and percentage of time in SWS) with the 10 metabolic variables (fasting glucose level, fasting insulin level, postchallenge glucose level, postchallenge insulin level, BMI, WHR, triglyceride levels, HDL, SBP, and DBP) previously shown to represent the metabolic syndrome as a unifying, second-order factor model.15 However, because waist circumference appears to be a better measure in predicting the metabolic syndrome,16,45 we replaced WHR with waist circumference. We observed eigenvalues of 5.43, 1.67, 1.17, 1.08, and 1.03 and factor patterns that exhibited strong (> ± 0.40), significant (P < 0.001) loadings for 5 distinct factors that we labeled insulin resistance, sleep disturbance, obesity, lipids (dyslipidemia), and blood pressure (hypertension) (Table 2).
Table 2.
Factor Loadings from Exploratory Factor Analysis in Cleveland Family Sleep Studya
| Variable | Factor 1b Insulin Resistance |
Factor 2b Sleep Disturbance |
Factor 3b Obesity |
Factor 4b Lipids (Dyslipidemia) |
Factor 5b Blood pressure (Hypertension) |
|---|---|---|---|---|---|
| Fasting glucose | 0.71 | 0.13 | 0.15 | 0.06 | 0.01 |
| Fasting insulin | 0.44 | 0.13 | 0.04 | 0.15 | 0.05 |
| 2-h postchallenge glucose | 0.93 | 0.06 | 0.31 | 0.05 | 0.01 |
| 2-h postchallenge insulin | 0.50 | 0.10 | 0.16 | 0.15 | 0.01 |
| Waist circumference | 0.03 | 0.26 | 0.96 | 0.15 | 0.03 |
| BMI | 0.08 | 0.08 | 0.72 | 0.12 | 0.02 |
| Triglycerides | 0.02 | 0.08 | 0.08 | 0.79 | 0.01 |
| HDL | −0.05 | 0.02 | −0.08 | −0.72 | −0.03 |
| SBP | 0.19 | 0.07 | 0.01 | 0.04 | 0.68 |
| DBP | 0.08 | 0.01 | 0.03 | 0.05 | 0.91 |
| AHI | 0.01 | 0.73 | 0.18 | 0.07 | 0.02 |
| % of sleep time o2 sat < 90% | 0.10 | 0.60 | 0.03 | 0.09 | 0.07 |
| Arousal Index | 0.01 | 0.72 | 0.02 | 0.07 | 0.03 |
| % time in SWS | −0.01 | −0.61 | −0.08 | −0.01 | −0.05 |
Abbreviations: BMI refers to body mass index; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; AHI, apnea-hypopnea index; sat, saturation; SWS, slow wave sleep
aFactor loadings were obtained using an oblique (Promax) rotation.
bGiven the factor patterns shown, we labeled the 5 factors: Insulin Resistance, Sleep Disturbance, Obesity, Lipids (dyslipidemia), and Blood Pressure (hypertension).
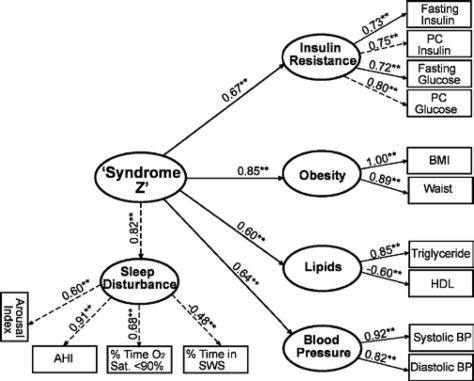
We next conducted CFA to formally test the fit of our hypothesized, second-order (hierarchical) 5-factor model of syndrome Z. This model, depicted in Figure 1, had good overall model fit (χ2/df = 3.20; CFI = 0.96; RMSEA = 0.06; SRMR = 0.05) and highly significant (P < 0.001) loadings for all measures on their respective factors and for all first-order factors on the second-order factor when including paths for residual correlations between postchallenge glucose level and postchallenge insulin level and between fasting insulin and BMI, as previously reported,15,16 and between ArI and percentage of time in SWS and between fasting insulin level and AHI. The magnitude and significance of the factor loadings can be used to evaluate the strength of the relationship between the observed measure and the underlying factor and between the factors. In the second-order factor model (Figure 1), the obesity factor had the strongest loading on the syndrome Z factor (standardized loading (λs) = 0.85 ± standard error of λs = 0.02) followed by sleep disturbance (0.82 ± 0.03; P < 0.001), insulin resistance (0.67 ± 0.03; P < 0.001), hypertension/blood pressure (0.64 ± 0.04; P < 0.001), and dyslipidemia/lipids (0.60 ± 0.05; P < 0.001). The loadings between the observed variables and their respective factors were all approximately greater than ± 0.5 and highly significant (P < 0.001), which lends further support to the validity of the factors.43 In addition, the loadings indicate that AHI is the most important determining variable of the sleep disturbance factor (0.91 ± 0.02; P < 0.001), followed by the percentage of sleep time with an oxygen saturation < 90% (0.68 ± 0.03; P < 0.001), ArI (0.60 ± 0.04; P < 0.001), and percentage of sleep time in SWS (−0.48 ± 0.03; P < 0.001), which is generally consistent with the order of importance found in the EFA results (Table 2). The standardized residual covariance was 0.33 (P < 0.01) between postchallenge glucose and insulin levels, 0.24 (P < 0.01) between fasting insulin level and BMI, −0.37 (P < 0.01) between ArI and SWS, and −0.23 (P < 0.01) between fasting insulin level and AHI. The second-order syndrome Z factor explained 72%, 67%, 44%, 42%, and 37% of the variance (R2) in the obesity, sleep disturbance, insulin resistance, lipids, and blood pressure factors, respectively. In a 5-factor model integrating measures (SBP, DBP, fasting insulin level, fasting glucose level, postchallenge insulin level, and postchallenge glucose level) that were unadjusted for the use of hypertension or diabetes medications, we obtained similar results in overall model fit (χ2/df = 2.76, CFI = 0.96, RMSEA = 0.06, and SRMR = 0.05) and in the factor loadings (obesity: 0.85 ± 0.02, P < 0.01; sleep disturbance: 0.81 ± 0.03, P < 0.01; insulin resistance: 0.63 ± 0.03, P < 0.01; hypertension: 0.61 ± 0.04, P < 0.01; dyslipidemia: 0.60 ± 0.05, P < 0.01).
Figure 1.
Hypothesized second-order (hierarchical), 5-factor model of Syndrome Z. Confirmatory factor analysis (CFA) results indicate good to adequate model fit: \?\χc2 = 217.58; degrees of freedom (df) = 68; χ2/df = 3.20; Comparative Fit Index (CFI) = 0.96; Root Mean Square Error of Approximation (RMSEA) = 0.06; Standardized Root Mean Square Residual (SRMR) = 0.05. Standardized factor loadings are shown above with directional arrows. Residual terms are not shown for clarity. **P < 0.01; PC refers to postchallenge; BP, blood pressure; HDL, high-density lipoprotein; AHI, apnea-hypopnea index; SWS, slow wave sleep; Sat, saturation.
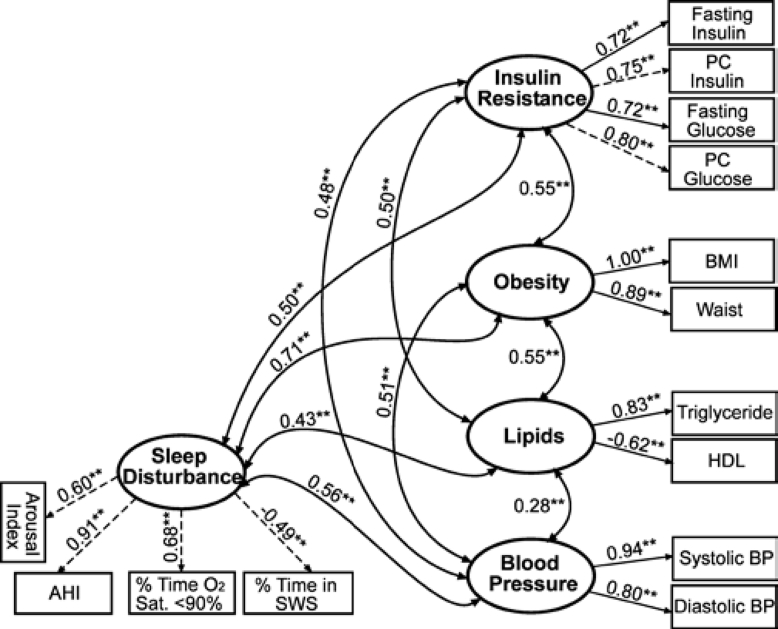
We next compared the hierarchical 5-factor model (Figure 1) to a 5-factor model that allowed all factors to correlate freely (Figure 2). The overall fit of this model (χ2/df = 3.23, CFI = 0.96, RMSEA = 0.06, and SRMR = 0.05) was similar to the hierarchical 5-factor model. However, performing a χ2 difference test revealed that the model with all factors correlating freely (Figure 2) was not significantly better than the more restricted second-order factor model (Figure 1) \?\(χc2 diff = 6.54, P = 0.26), which suggests that the more parsimonious, hierarchical 5-factor model (Figure 1) would be favored.
Figure 2.
Alternative 5-factor model without Syndrome Z as a second-order, unifying construct. Confirmatory factor analysis (CFA) results indicate good to adequate model fit: \?\χc2 = 261.21; degrees of freedom (df) = 63; χ2/df = 3.24; Comparative Fit Index (CFI) = 0.96; Root Mean Square Error of Approximation (RMSEA) = 0.07; Standardized Root Mean Square Residual (SRMR) = 0.05. Standardized factor loadings are shown above with directional arrows. Residual terms are not shown for clarity. **P < 0.01; PC refers to postchallenge; BP, blood pressure; HDL, high-density lipoprotein; AHI, apnea-hypopnea index; SWS, slow wave sleep; Sat, saturation.
We performed simultaneous multiple group CFA to test the generalizabilty of the hierarchical 5-factor model and to explore possible differences by sex, race, and age subgroups. When examining male and female subgroups simultaneously, the hierarchical 5-factor syndrome Z structure (Figure 1) was similar except for 3 factor loadings. The factor loading for BMI on obesity was significantly stronger in women (unstandardized loading = 0.41) than in men (0.35) (χ2 diff = 39.06; P < 0.01), and the loading for triglycerides on dyslipidemia/lipids was greater in women (0.46) than in men (0.43) (χ2 diff = 29.93, P < 0.01). We also observed a significant difference in the loading of fasting glucose on the insulin resistance factor among men (0.55) compared to women (0.45) (χ2 diff = 13.26, P < 0.01). After allowing for these 3 differences by sex, we obtained an adequate to good fitting model (χ2/df = 2.57; CFI = 0.94; RMSEA = 0.08; SRMR = 0.07). When examining Caucasian versus African American subgroups, we found significant differences in 3 loadings. The triglyceride loading on the lipids factor was higher in Caucasians (0.37) than in African Americans (0.32) (χ2 diff = 10.73; P < 0.01), but the BMI loading on the obesity factor was higher in African Americans (0.40) than in Caucasians (0.36) (χ2 diff = 16.89; P < 0.01). The loading of percentage of time in SWS on sleep disturbance was stronger among African Americans (−0.16), compared with Caucasians (−0.13) (χ2 diff = 10.32; P < 0.01). After allowing for these differences by race, we obtained a good fitting model (χ2/df = 2.27; CFI = 0.95; RMSEA = 0.07; SRMR = 0.07). Using a cutpoint of 55 years to define older and younger age subgroups,46 we observed several differences by age, including a higher loading of SBP on the hypertension/blood pressure factor in the older (0.73) versus younger (0.55) age group (χ2/df = 43.57; P < 0.01). We also found that BMI loaded higher on obesity in younger (0.35), compared with older (0.31), subgroups (χ2/df = 26.19; P < 0.01), and the loading of triglyceride level on dyslipidemia/lipid levels was higher in younger (0.36) than in older (0.19) subgroups (χ2/df = 13.85; P < 0.01). The AHI loading on sleep-disruption construct was also higher in the older (0.65), compared with the younger (0.58) group (χ2 diff = 11.95; P < 0.01). After allowing for these differences by age, we obtained a good fitting model (χc2/df = 2.31; CFI = 0.95; RMSEA = 0.07; SRMR = 0.06).
DISCUSSION
Although others have suggested that OSA may be a component of the metabolic syndrome,17,19 to our knowledge, this is the first report providing empirical evidence for syndrome Z as a hierarchical 5-factor model unifying 5 traits: sleep disturbance, obesity, insulin resistance, hypertension, and dyslipidemia. We observed a good overall fit in this second-order factor model of syndrome Z and found that obesity was the most important determining factor, followed by sleep disturbance, insulin resistance, hypertension, and dyslipidemia.
Because our 5-factor model is not nested within 4-factor models,43 the χ2 difference test could not be utilized to directly compare our model with previously described models of the metabolic syndrome.15,16 However, our model performed similarly in terms of the overall model fit indexes. In terms of the factor loadings, in our study population, obesity had the highest loading on the second-order syndrome Z factor (0.85), followed by sleep disruption (0.82), insulin resistance (0.67), blood pressure (0.64) and lipids (0.60), but prior 4-factor models15,16 have reported that insulin resistance is the most important factor (0.83, 0.87), followed by obesity (0.80, 0.80), lipids (0.59, 0.72), and blood pressure (0.33, 0.59). These differences may be attributed, in part, to the relative overrepresentation of overweight individuals in our study population and the strong association between obesity and OSA.47
Our multiple-group CFA results were also generally consistent with prior 4-factor models. Differences by sex have been reported for triglyceride levels and obesity loadings,16 which we also observed, with both being stronger in women than in men. However, we also found that fasting glucose was significantly higher in men than in women. The differences we observed by sex are not unexpected because elevated triglyceride levels may be a greater risk factor for cardiovascular disease in women than in men, impaired glucose tolerance has been more commonly observed among men, and increasing rates of BMI may be contributing to the rise of the metabolic syndrome in women.1 Although a prior study noted differences in insulin resistance and waist circumference loadings between African Americans and Caucasians,16 we observed lower loadings for triglyceride levels and higher loadings for BMI and percentage of time in SWS in African Americans, compared with in Caucasians. Triglyceride levels are generally lower in African Americans, and this may be driven, in part, by certain apolipoprotein genotypes.48 African Americans also appear to have reduced amounts of SWS,28 and the importance of SWS in glucose homeostasis is becoming increasingly more evident.27 Differences by age using a cutpoint of 60 years have been previously reported for the triglyceride level loading,15 and we observed similar results using a cutpoint of 55 years, which is consistent with the decline in triglyceride levels with advancing age.49,50 The higher AHI loading in the older group was anticipated, since sleep apnea may peak at around 55 years of age, particularly in men,46 and the stronger loading we observed for SBP may be attributed, in part, to a greater prevalence of isolated systolic hypertension in older compared to younger subjects and because SBP is a more potent predictor of cardiovascular events in older compared to younger adults.51
Strengths of our study include the use of objective measures for all variables, including the sleep variables, which were obtained via overnight polysomnography and scored using standardized measures by a single certified scorer to maximize reliability. However, validation of our model in other studies, particularly in populations with less severe OSA and those not selected on the basis of sleep disorders, is needed to better understand the role of sleep disruption in the metabolic syndrome. Furthermore, much larger sample sizes are needed to evaluate other clinically important subgroups (e.g., men older than 55 years of age). We used paths for correlated residuals, which suggest the existence of “small factors” not explained by the common factor; however, these were justified,52 since they were limited to those previously utilized in 4-factor models (between fasting insulin levels and BMI16 and between postchallenge glucose and insulin levels15) and those involving sleep-metabolic relations in which strong theoretical and empirical evidence exists (between arousal and SWS53 and between AHI and fasting insulin level54,55).
In summary, we provide here the first empirical evidence for the concept of syndrome Z, as described by a second-order factor unifying 5 first-order factors: insulin resistance, obesity, hypertension, dyslipidemia, and sleep disturbance. Our second-order syndrome Z factor model had good overall fit, which was better than the fit of a competing model with no factor structure imposed, providing evidence that these 5 components co-aggregate as a single unifying trait. Although we cannot directly infer causality from our results, the magnitude of the factor loadings suggests that sleep disturbance is the second-most important factor, behind obesity, in determining syndrome Z and that AHI is the strongest measure of sleep disturbance in this population. Given the rising rates of obesity and sleep disorders, our results advocate for further research in other populations specifically aimed at validating the co-aggregation of sleep and metabolic traits, syndrome Z. This model could then be used to better predict the potential synergistic risk of syndrome Z, compared with syndrome X, on incident type 2 diabetes and cardiovascular disease.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported in part by NHLBI HL 46380, NCI Career Award (K07-CA129162), NCI R25 Training Grant (R25T-CA094186), Case Center for Transdisciplinary Research on Energetics and Cancer (U54 CA-116867) and KL2-RR024990.
References
- 1.Mitrakou A. Women's health and the metabolic syndrome. Ann N Y Acad Sci. 2006;1092:33–48. doi: 10.1196/annals.1365.003. [DOI] [PubMed] [Google Scholar]
- 2.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006;169:1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RJ, Appleton S, Wilson DH, et al. Population comparison of two clinical approaches to the metabolic syndrome: implications of the new International Diabetes Federation consensus definition. Diabetes Care. 2005;28:2777–9. doi: 10.2337/diacare.28.11.2777. [DOI] [PubMed] [Google Scholar]
- 4.Elabbassi WN, Haddad HA. The epidemic of the metabolic syndrome. Saudi Med J. 2005;26:373–5. [PubMed] [Google Scholar]
- 5.Milani RV, Lavie CJ. Prevalence and profile of metabolic syndrome in patients following acute coronary events and effects of therapeutic lifestyle change with cardiac rehabilitation. Am J Cardiol. 2003;92:50–4. doi: 10.1016/s0002-9149(03)00464-8. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–9. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 11.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.International Diabetes Federation. 2006. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; pp. 1–14. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. [Google Scholar]
- 14.Lafortuna CL, Adorni F, Agosti F, Sartorio A. Factor analysis of metabolic syndrome components in obese women. Nutr Metab Cardiovasc Dis. 2008;18:233–41. doi: 10.1016/j.numecd.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Shen B.J., Todaro JF, Niaura R, McCaffery JM, Zhang J, Ward KD. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol. 2003;157:701–11. doi: 10.1093/aje/kwg045. [DOI] [PubMed] [Google Scholar]
- 16.Shen BJ, Goldberg RB, Llabre MM, Schneiderman N. Is the factor structure of the metabolic syndrome comparable between men and women and across three ethnic groups: the Miami Community Health Study. Ann Epidemiol. 2006;16:131–7. doi: 10.1016/j.annepidem.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 17.Wolk R, Somers VK. Sleep and the metabolic syndrome. Exp Physiol. 2007;92:67–78. doi: 10.1113/expphysiol.2006.033787. [DOI] [PubMed] [Google Scholar]
- 18.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox I, McNamara SG, Collins FL, Grunstein RR, Sullivan CE. “syndrome Z”: the interaction of sleep apnoea, vascular risk factors and heart disease. Thorax. 1998;53:S25–S28. [PMC free article] [PubMed] [Google Scholar]
- 20.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 21.Chaput JP, Després JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 24.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–24. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 25.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 27.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Am J Hum Biol. 2002;14:321–6. doi: 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- 29.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 30.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:682–7. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 31.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 32.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–70. [PubMed] [Google Scholar]
- 33.Kirkendall WM, Feinleib M, Freis ED, Mark AL. Recommendations for human blood pressure determination by sphygmomanometers. Subcommittee of the AHA Postgraduate Education Committee. Circulation. 1980;62:1146A–55A. [PubMed] [Google Scholar]
- 34.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–10. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- 35.Rechtschaffen A, Kales A. Washington, DC: NIH; 1968. A manual of standardized techniques and scoring system for sleep stages of human subjects. [DOI] [PubMed] [Google Scholar]
- 36.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 37.Hu FB. Re: “Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome”. Am J Epidemiol. 2001;153:717–8. doi: 10.1093/aje/153.7.717. [DOI] [PubMed] [Google Scholar]
- 38.Lawlor DA, Ebrahim S, May M, Davey SG. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol. 2004;159:1013–8. doi: 10.1093/aje/kwh150. [DOI] [PubMed] [Google Scholar]
- 39.Hair JF, Anderson RE, Tatham RM, Black WC. Multivariate data analysis: With readings. Fourth Ed. New Jersey: Prentice Hall; 1995. [Google Scholar]
- 40.Muthén LK, Muthéen BO. Los Angeles: Muthén – Muthén; 2004. Confirmatory factor analysis and structural equation modeling: Mplus User's Guide. [Google Scholar]
- 41.Kline RB. Principles and Practice of Structural Equation Modeling. Second Ed. New York: Guilford Press; 2005. Measurement models and confirmatory factor analysis; pp. 133–45. [Google Scholar]
- 42.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 43.Hair JF, Black B, Babin B, Anderson AB, Tatham RL. 6th. Upper Saddle River, NJ: Prentice Hall; 2005. Multivariate Data Analysis. [Google Scholar]
- 44.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; 1993. pp. 136–62. [Google Scholar]
- 45.Shen W, Punyanitya M, Chen J, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity. 2006;14:727–36. doi: 10.1038/oby.2006.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–24. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 48.Shin MJ, Kanaya AM, Krauss RM. Polymorphisms in the peroxisome proliferator activated receptor alpha gene are associated with levels of apolipoprotein CIII and triglyceride in African-Americans but not Caucasians. Atherosclerosis. 2008;198:313–9. doi: 10.1016/j.atherosclerosis.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattila KS, Marniemi J, Maki J, Juva K. Lipids, lipoproteins and apolipoproteins in the elderly. Scand J Clin Lab Invest. 1986;46:131–6. doi: 10.3109/00365518609083648. [DOI] [PubMed] [Google Scholar]
- 50.Shoukry MI, Fareed S. Plasma lipid and lipoprotein concentrations in an Egyptian male sample. Lipids. 1982;17:692–5. doi: 10.1007/BF02534653. [DOI] [PubMed] [Google Scholar]
- 51.Franklin SS, Jacobs MJ, Wong ND, L'Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–74. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 52.EQS Structural Equations Program Manual. Encino, CA: Multivariate Software; 1995. [Google Scholar]
- 53.Bonnet MH, Arand DL. EEG arousal norms by age. J Clin Sleep Med. 2007;3:271–4. [PMC free article] [PubMed] [Google Scholar]
- 54.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 55.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]