Abstract
Study Objectives:
To test the effects of exercise training on sleep and neurovascular control in patients with systolic heart failure with and without sleep disordered breathing.
Design:
Prospective interventional study.
Setting:
Cardiac rehabilitation and exercise physiology unit and sleep laboratory.
Patients:
Twenty-five patients with heart failure, aged 42 to 70 years, and New York Heart Association Functional Class I-III were divided into 1 of 3 groups: obstructive sleep apnea (n = 8), central sleep apnea (n = 9) and no sleep apnea (n = 7).
Interventions:
Four months of no-training (control) followed by 4 months of an exercise training program (three 60-minute, supervised, exercise sessions per week).
Measures and Results:
Sleep (polysomnography), microneurography, forearm blood flow (plethysmography), peak VO2, and quality of life were evaluated at baseline and at the end of the control and trained periods. No significant changes occurred in the control period. Exercise training reduced muscle sympathetic nerve activity (P < 0.001) and increased forearm blood flow (P < 0.01), peak VO2(P < 0.01), and quality of life (P < 0.01) in all groups, independent of the presence of sleep apnea. Exercise training improved the apnea-hypopnea index, minimum O2 saturation, and amount stage 3-4 sleep (P < 0.05) in patients with obstructive sleep apnea but had no significant effects in patients with central sleep apnea.
Conclusions.
The beneficial effects of exercise training on neurovascular function, functional capacity, and quality of life in patients with systolic dysfunction and heart failure occurs independently of sleep disordered breathing. Exercise training lessens the severity of obstructive sleep apnea but does not affect central sleep apnea in patients with heart failure and sleep disordered breathing.
Citation:
Ueno LM; Drager LF; Rodrigues ACT; Rondon MUPB; Braga AMFW; Mathias W; Krieger EM; Barretto ACP; Middlekauff HR; Lorenzi-Filho G; Negrão CE. Effects of exercise training in patients with chronic heart failure and sleep apnea. SLEEP 2009;32(5):637-647.
Keywords: Heart failure, exercise training, sleep apnea, forearm blood flow, muscle sympathetic nerve activity
IT IS NOW RECOGNIZED THAT SLEEP DISORDERED BREATHING IS EXTREMELY COMMON AMONG PATIENTS WITH HEART FAILURE (HF) IN THE SETTING of systolic dysfunction.1–3 Patients with HF have 2 distinct patterns of sleep disordered breathing, namely, obstructive sleep apnea (OSA) and central sleep apnea (CSA). CSA and OSA occur in approximately in 25% to 37% and 27% to 38% of the patients with HF, respectively.1,2 CSA is associated with a crescendo-decrescendo pattern of breathing, which is also known as Cheyne-Stokes respiration.4,5 OSA is primarily caused by upper airway instability and obstruction during sleep.6 In contrast, CSA has been hypothesized to be a consequence of the underlying heart disease, attributable to prolonged circulation time, pulmonary congestion, and increased chemosensitivity.5,7,8 Patients with HF and sleep disordered breathing, as compared with patients with similar heart function but no sleep disordered breathing, have greater levels of resting sympathetic activity.9 Moreover, treatment of OSA and CSA with continuous positive airway pressure (CPAP) seems to improve left ventricular ejection fraction10–12 and reduce mortality in patients with HF.13 These findings are consistent with the concept that sleep disordered breathing constitutes an additional burden to the cardiovascular system in patients with HF.
It is now well accepted that exercise training is safe and beneficial for patients with HF due to systolic dysfunction.14–17 This nonpharmacologic strategy significantly reduces muscle sympathetic nerve activity (MSNA) in patients with HF.17 Moreover exercise training has been shown to improve systemic endothelial function in patients with HF.18 The consequence of this dual effect of exercise training is the reduction in peripheral vascular resistance and an increase in blood flow, leading to amelioration in muscle oxidative stress and metabolism in patients with HF.19,20 Exercise training improves exercise tolerance and overall quality of life (QoL) in patients with HF.16 On the other hand, it has become clear from our previous study that the magnitude of neurovascular change caused by exercise training is variable among patients with HF.17 This heterogeneity may be explained by the unrecognized presence of sleep disordered breathing.9 However, the effect of exercise training in patients with HF according to the presence or absence of sleep disordered breathing has not been previously investigated.
It has been shown that exercise training increases left ventricular ejection fraction (LVEF),21,22 cardiac output23,24 and ventilatory pattern25 in patients with HF. In addition, exercise training ameliorates peripheral chemoreflex control in HF.26 All of these changes may favor improvement in sleep disturbances. Thus, we reasoned that exercise training would improve sleep disordered breathing in patients with HF.
In this study, we tested 2 hypotheses: (1) that the beneficial effects of exercise training on MSNA and forearm blood flow (FBF) would be modulated by the presence of sleep disordered breathing and (2) that exercise training would improve sleep apnea in patients with HF.
METHODS
Subjects
Patients with HF who were between the ages of 42 and 70 years and who had echocardiographic evidence of impaired left ventricular function from ischemic, idiopathic, and hypertensive etiology and who had stable HF duration of more than 3 months, were on optimal medical therapy, had New York Heart Association, Functional Class I-III, and had an ejection fraction of less than 45% were invited to participate in the study. Exclusion criteria included pulmonary disease, chronic renal disease, diabetes mellitus, atrial fibrillation, pacemaker, a history of stroke, body mass index (BMI) greater than 30 kg/m2, and recent (< 3 months) myocardial infarction, or unstable angina. Healthy, age-matched, control subjects also participated in the study. The study was approved by the institutional committee on human research and all patients gave written informed consent.
Experimental Design
Outpatients with HF and control subjects were invited to the study. Before study entry, all patients were followed for 2 months to ensure optimal medication dose and clinical stability of HF. After study entry (baseline), all patients with HF were followed for 4 months and instructed to avoid exercise (untrained period). The patients were then submitted to a 4-month period of exercise training. All evaluations were made at baseline and at the end of untrained and trained periods. During the entire study, the patients came to the hospital every 2 weeks to have their physical activity and medication status verified. Patients who needed changes in medication dose or were not compliant with medications were excluded. The normal control subjects were evaluated before (baseline) and after 4 months of exercise training.
Sleep Study
All participants underwent overnight polysomnography, as has been previously described.27 Sleep stages, apneas, hypopneas, and arousals was defined and scored as has been previously described.27,28 Briefly, apnea was defined as complete cessation of airflow for at least 10 seconds with an oxygen desaturation of 3% or greater. Hypopnea was defined as a reduction in respiratory signal of at least 50% from baseline for at least 10 seconds and associated with oxygen desaturation of 3% or greater. The apnea-hypopnea index (AHI) was calculated by totaling the number of respiratory events (apneas and hypopneas) per hour of sleep. Sleep apnea was defined as an AHI of at least 10 events per hour of sleep.29,30 OSA was defined as a cessation of respiratory airflow for 10 seconds with thoracoabdominal effort, which was detected by piezoelectric respiratory-effort sensor. Patients who had more than 70% of events that were obstructive were defined as having OSA. CSA was defined as the absence of respiratory airflow at least 10 seconds without thoracoabdominal motion. Patients who had more than 70% of events without respiratory airflow were defined as having CSA. Patients with HF with an AHI of less than 10 events per hour of sleep were defined as having no sleep apnea (NoSA). A control group comprising age-matched subjects with an AHI less than 10 events per hour of sleep was also studied.
Muscle Sympathetic Nerve Activity
MSNA was directly recorded from the peroneal nerve using the technique of microneurography. Muscle sympathetic bursts were identified by visual inspection, blinded to the study protocol, and were expressed as burst frequency (bursts per minute).
Forearm Blood Flow
FBF was measured using venous occlusion plethysmography, as has been previously described16,17 FBF was determined on the basis of a minimum of 8 separate readings. Heart rate (electrocardiogram) and arterial blood pressure (FINAPRES, Ohmeda, Englewood, CO, model 2300) were continuously measured. Forearm vascular conductance (FVC) was estimated by dividing FBF by mean blood pressure. We used the FBF technique instead of calf blood flow to avoid leg movements that may interfere with MSNA measurements.
Cardiopulmonary Exercise Test
Maximal exercise capacity was determined by means of a maximal progressive exercise test on an electromagnetically braked cycle ergometer (Medifit 400L, Medical Fitness Equipment, Maarn, Netherlands), with work-rate increments of 10 to 15 watts and 15 to 30 watts every 3 minutes at 60 rpm until exhaustion for patients with HF and normal control subjects, respectively, as has been previously described.16,17 Peak VO2was defined as the maximum attained VO2 at the end of the exercise period in which the subject could no longer maintain the cycle ergometer velocity at 60 rpm. Anaerobic threshold was determined to occur at the break-point between the increase in the CO2 output and VO2 (V-slope) or the point at which the ventilatory equivalent for O2and end-tidal O2 partial pressure curves reached their respective minimum values and began to rise. Respiratory compensation was determined to occur at the point at which ventilatory equivalent for CO2was lowest before a systematic increase and when end-tidal CO2partial pressure reaches a maximum and begins to decrease.
Quality of Life
QoL was assessed by means of the Minnesota Living with Heart Failure Questionnaire.31 Recent studies have shown this questionnaire to be responsive to changes in QoL in patients with chronic HF after exercise training.16,32
Exercise Training Protocol
The exercise training protocol was identical to the one used in our prior study.17 The 4-month exercise training program consisted of three 60-minute, supervised, exercise sessions per week. Each session consisted of 5 minutes of stretching exercise, 25 minutes of cycling on the ergometer bicycle in the first month and up to 40 minutes min in the last 3 months, 10 minutes of local strengthening exercise, and 5 minutes of cool down with stretching exercises. The cycling exercise intensity was established by heart rate levels that corresponded to anaerobic threshold up to 10% below the respiratory compensation point obtained in the cardiopulmonary exercise test. Exercise compliance was assessed as percentage of exercise sessions attended.
Statistical Analysis
The data are presented as mean ± SEM. Statistical analysis was performed using one-way analysis of variance to compare possible group differences at baseline and the posttraining period. A χ2 test was used to test possible differences in sex, etiology, and medication use among the groups. One-way analysis of variance with repeated measures was used to compare within-group differences at baseline, the no-training control period, and the exercise training period. Paired student t-test was used to compare within-group differences at baseline and the posttraining period in the group of control subjects. In the case of significance, posthoc comparisons were performed by Tukey Honest Significant Difference test. Pearson correlation was used to investigate if changes in neurovascular function after exercise training correlated with changes in apnea severity. Probability values of 0.05 or less were considered to be statistically significant.
RESULTS
Characteristics of the Subjects
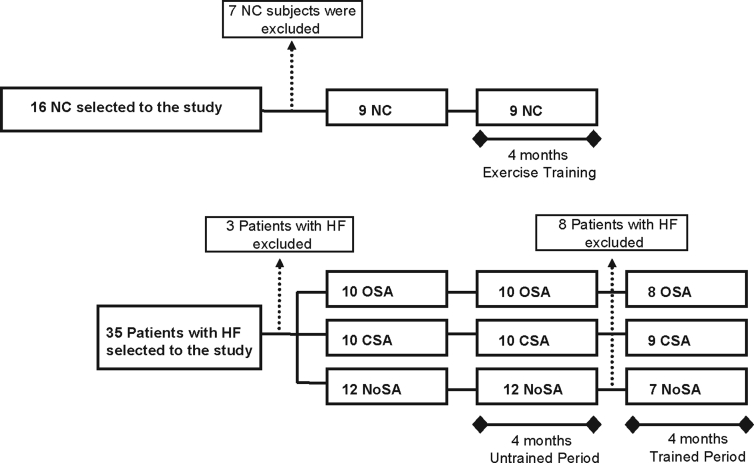
From a total of 35 patients with HF who were initially selected to participate in the study, 3 patients were excluded: 1 patient with mixed sleep apnea and 2 patients without MSNA recording at baseline measurements. Eight patients were excluded at the end of the untrained control period: 2 had unstable blood pressure levels, 2 had unstable HF, 2 patients dropped out, and 2 patients (1 with OSA and 1 without sleep apnea) died. From a total of 16 control subjects, 7 were excluded after the polysomnography study because of OSA (AHI ≥ 10/h of sleep). Thus, 24 patients with HF and 9 control subjects completed the exercise training period: 9 with CSA, 8 with OSA, 7 NoSA, and 9 control subjects (Figure 1). Compliance with the exercise program ranged from 86% to 98% of exercise session attended for both patients with HF and normal controls.
Figure 1.
Experimental design. OSA refers to patients with heart failure (HF) and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure without sleep apnea; NC, normal, healthy control subjects.
Baseline Measures
Baseline characteristics of patients with HF and control subjects are shown on Table 1. There were no significant differences in age, etiology, medication use, BMI, or abdominal circumference between patients with HF and control subjects. Sex was significantly different among groups. The number of women was greater and the number of men fewer in the NoSA group compared with the OSA and CSA groups. Neck circumference was significantly greater in the OSA group compared with control subjects and those in the NoSA group. As expected, patients with HF had lower LVEF, FBF, and FVC than did control subjects. Heart rate and blood pressure levels did not differ between patients with HF and control subjects. Peak VO2 was lower in patients with HF and CSA, OSA, and NoSA compared with control subjects. MSNA in bursts per minute or burst per 100 heart beats was higher in patients with HF when compared with control subjects. Furthermore, MSNA in bursts per in or burst per 100 heart beats in patients with HF with CSA and OSA were significantly higher than in patients with HF with NoSA (60 ± 3 and 51 ± 2 vs 37 ± 4 burst/min or 91 ± 3 and 81 ± 3 vs 59 ± 5 burst per 100 heart beats, respectively). QoL was not significantly different among groups of patients with HF.
Table 1.
Baseline Characteristics in Patients with Heart Failure and Normal Control Subjects
| OSA (n = 8) | CSA (n = 9) | NoSA (n = 7) | NC (n = 9) | |
|---|---|---|---|---|
| Age, y | 58 ± 2 | 60 ± 3 | 58 ± 4 | 50 ± 2 |
| Sex, M/F | 7/1 | 7/2 | 2/5c | 5/4 |
| BMI, kg/m2 | 27 ± 1 | 26 ± 1 | 26 ± 1 | 24 ± 1 |
| Abdominal circumference, cm | 92 ± 3 | 93 ± 3 | 89 ± 3 | 86 ± 3 |
| Neck circumference, cm | 40 ± 2,a | 37 ± 1 | 35 ± 1 | 35 ± 1 |
| HF etiology, No. | ||||
| Id/Isch/Hyp | 1/3/4 | 1/4/4 | 4/0/3 | |
| NYHA Functional Class | 2.5 | 2.6 | 2.0 | |
| Medications, No. (%) | ||||
| β-blocker | 8 (100) | 9 (100) | 7 (100) | |
| ACE inhibitors or ARBs | 8 (100) | 9 (100) | 7 (100) | |
| Digoxin | 6 (75) | 2 (22) | 3 (43) | |
| Diuretics | 6 (75) | 8 (89) | 5 (71) | |
| LVEF, % | 33 ± 2,b | 30 ± 3,b | 30 ± 4b | 64 ± 2 |
| Heart Rate, beats/min | 66 ± 3 | 63 ± 3 | 63 ± 2 | 63 ± 3 |
| SBP, mmHg | 117 ± 5 | 115 ± 8 | 118 ± 10 | 118 ± 6 |
| DBP, mmHg | 69 ± 4 | 60 ± 4 | 62 ± 4 | 64 ± 4 |
| Peak VO2, mL/kg/min | 18 ± 1b | 16 ± 1b | 17 ± 1b | 26 ± 3 |
| FBF, mL/min/100 mL | 1.7 ± 0.2b | 1.5 ± 0.1b | 1.6 ± 0.1b | 2.5 ± 0.3 |
| FVC, units | 2.0 ± 0.3b | 1.9 ± 0.1b | 2.0 ± 0.2b | 3.1 ± 0.4 |
| MSNA, bursts/min | 60 ± 3b | 51 ± 2b | 37 ± 4b,c | 20 ± 4 |
| MSNA, bursts/100 heart beats | 91 ± 3b | 81 ± 3b | 60 ± 5b,c | 32 ± 5 |
OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure and without sleep apnea; NC, normal, healthy control subjects; Id, idiopathic; Isch, ischemic; Hyp, hypertensive; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers. There was no significant difference in age, body mass index (BMI), abdominal circumference, heart failure etiology, New York Heart Association (NYHA) Functional Class, medications, heart rate, systolic blood pressure (SBP) and or diastolic blood pressure (DBP) among groups. Sex was significantly different among groups. Neck circumference was significantly greater in OSA compared with control subjects and NoSA. Left ventricle ejection fraction (LVEF), peak VO2, forearm blood flow (FBF), and forearm vascular conductance (FVC) were lower in patients with heart failure than in NC. Muscle sympathetic nerve activity (MSNA) was higher in patients with heart failure when compared with NC. Quality of life was not significantly different among HF patient groups.
vs NC and NoSA, P < 0.05;
vs NC, P < 0.001;
vs OSA and CSA, P < 0.05. One-way analysis of variance. Sex, etiology, and medication use were tested by χ2 test.
Regarding the sleep pattern (Table 2), stage 1-2 sleep was longer in patients with HF with OSA and CSA when compared with control subjects. Stage 3-4 was shorter in patients with HF with OSA than in control subjects. As expected, the AHI was higher and minimum O2 saturation lower in patients with HF with OSA and CSA, compared with patients with HF with NoSA and control subjects. In addition, minimum O2 saturation during sleep in patients with HF and OSA was lower than in patients with HF with NoSA and those with CSA. The arousal index was greater in patients with HF and OSA, compared with control subjects.
Table 2.
Baseline Sleep Pattern in Patients with Heart Failure and Normal Control Subjects
| OSA (n = 8) |
CSA (n = 9) |
NoSA (n = 7) |
NC (n = 9) |
|
|---|---|---|---|---|
| TST, min | 375 ± 11 | 376 ± 18 | 371 ± 30 | 365 ± 27 |
| Stage 1-2, % | 83 ± 3a | 78 ± 3a | 77 ± 2 | 68 ± 3 |
| Stage 3-4, % | 7 ± 1a | 11 ± 3 | 15 ± 2 | 17 ± 2 |
| REM, % | 10 ± 2 | 11 ± 2 | 8 ± 1 | 15 ± 2 |
| AHI, events/h | 34 ± 4a | 40 ± 7a | 5 ± 1b | 3 ± 1 |
| Arousals, events/h | 40 ± 8a | 28 ± 4 | 21 ± 3 | 18 ± 3 |
| Min O2sat | 80 ± 2a,c | 84 ± 1a | 86 ± 1 | 89 ± 1 |
OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure and without sleep apnea; NC, normal, healthy control subjects; TST, total sleep time. Stage 1-2 was longer in patients with heart failure with OSA and CSA when compared with NC. Stage 3-4 was shorter in patients with heart failure with OSA than in NC. The apnea-hypopnea index (AHI) was higher and minimum O2 saturation (min O2 sat) lower in patients with heart failure with OSA and CSA compared with patients with heart failure with NoSA and NC. Minimum O2 saturation during sleep in patients with heart failure and OSA was lower than in patients with heart failure with CSA and without sleep apnea. The arousal index was greater in patients with heart failure with OSA compared with NC.
vs NC, P < 0.05;
vs OSA and CSA, P < 0.05;
vs CSA and NoSA, P < 0.05. There was no significant difference in amount of total sleep time (TST) or rapid eye movement (REM) sleep among groups, P > 0.05. One-way analysis of variance.
Effect of Exercise Training on Neurovascular Parameters
Physical and physiologic parameters were unchanged after the untrained control period in all 3 HF groups (P > 0.05). Four months of exercise training caused no change in body weight, BMI, abdominal and neck circumferences, heart rate, or blood pressure in patients with HF or in control subjects (P > 0.05). Exercise training significantly improved Functional Class in all HF groups (P < 0.05, Table 3). Of note, LVEFs after exercise training showed a strong tendency toward increase in patients with HF with OSA (P = 0.056, Table 3). Exercise training caused an increase in peak VO2 in all patients with HF and control subjects studied. QoL significantly improved in all 3 HF groups after exercise training.
Table 3.
Physical and Physiologic Parameters in Patients with Heart Failure and OSA, CSA and No Sleep Apnea at Baseline and after the Untrained Period and Trained Period
| Baseline | Untrained | Trained | |
|---|---|---|---|
| NYHA Functional Class | |||
| OSA | 2.5 | 2.5 | 1.4a |
| CSA | 2.6 | 2.6 | 1.6a |
| NoSA | 2.0 | 1.9 | 1.0a |
| LVEF, % | |||
| OSA | 33 ± 2 | 33 ± 2 | 36 ± 2 |
| CSA | 30 ± 3 | 30 ± 3 | 31 ± 4 |
| NoSA | 30 ± 4 | 31 ± 4 | 30 ± 3 |
| Peak VO2,mL/kg/min | |||
| OSA | 18 ± 1 | 19 ± 1 | 24 ± 2a |
| CSA | 16 ± 1 | 16 ± 2 | 21 ± 2a |
| NoSA | 17 ± 1 | 17 ± 2 | 21 ± 2a |
| MLHF Score | |||
| OSA | 37 ± 7 | 35 ± 7 | 15 ± 5a |
| CSA | 44 ± 9 | 44 ± 9 | 17 ± 6a |
| NoSA | 24 ± 5 | 25 ± 6 | 8 ± 1a |
OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure and no sleep apnea; NYHA, New York Heart Association Functional Class; LVEF, left ventricle ejection fraction; MLHF Score, Minnesota Living with Heart Failure score. Physical and physiologic parameters were unchanged after untrained control period in all 3 heart failure groups. Exercise training caused no significant change in LVEF.
vs Baseline and Untrained period, P < 0.01. One-way analysis of variance with repeated measures.
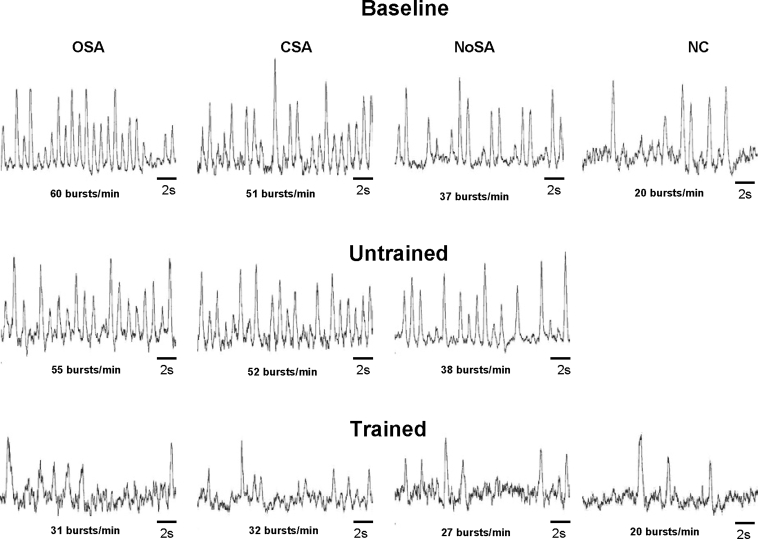
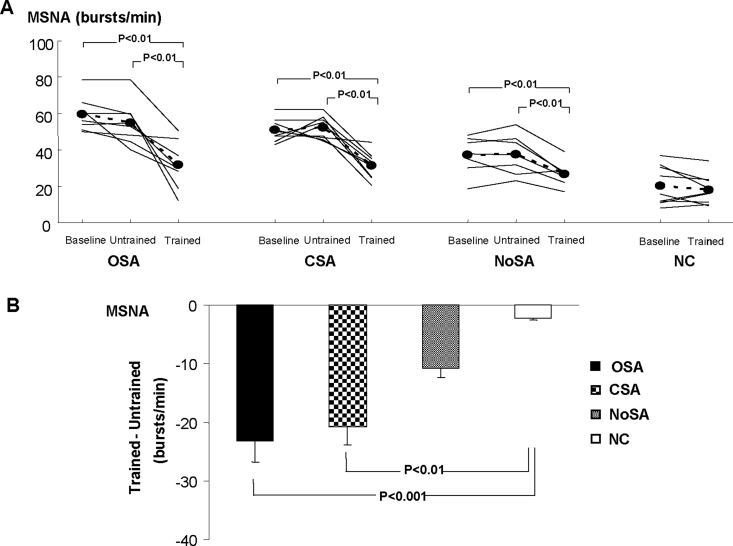
Examples of resting MSNA in patients with HF and control subjects at baseline, after the period without training, and after exercise training are shown in Figure 2. MSNA did not change in any of the 3 HF groups from baseline to after the period without training (Figure 3A). However, exercise training decreased MSNA in all 3 groups of patients with HF. The analysis of the mean difference in the change showed that the decrease in MSNA was greater in patients with HF with OSA and CSA than in control subjects but was not significantly greater compared to patients with HF but no sleep apnea (Figure 3B).
Figure 2.
Sympathetic neurograms in patients with heart failure at baseline, untrained, and trained periods. OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure without sleep apnea; NC, normal, healthy control subjects.
Figure 3.
Muscle sympathetic nerve activity (MSNA) at baseline, untrained, and trained periods (A). Comparison among groups in the mean difference in the change (Trained – Untrained ) in MSNA (B). OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure without sleep apnea; NC, normal, healthy control subjects.
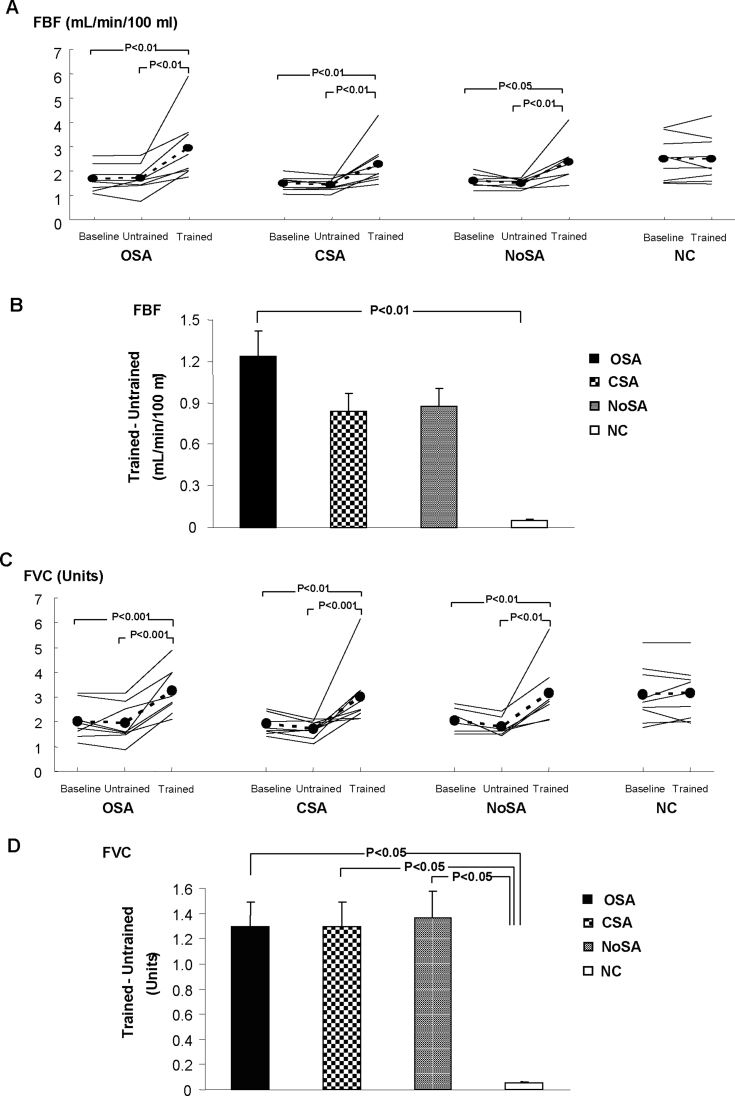
FBF and FVC were unchanged after the untrained period. Exercise training increased FBF and FVC in all 3 HF groups studied (Figure 4A, C, respectively), and the differences between patients with HF and control subjects were no longer observed (P > 0.05). Further analysis showed that the mean difference in the change in FBF after exercise training was greater in patients with HF and OSA, compared with control subjects (Figure 4B), and the mean difference in the change in FVC (Figure 4D) was greater in all 3 HF groups when compared with the group of control subjects.
Figure 4.
Forearm blood flow (FBF) and forearm vascular conductance (FVC) at baseline, untrained, and trained periods (A and C). Comparison among groups in the mean difference in the change (Trained – Untrained) in FBF (B), and FVC (D). OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure without sleep apnea; NC, normal healthy control subjects.
Effect of Exercise Training on Indexes of Sleep Apnea
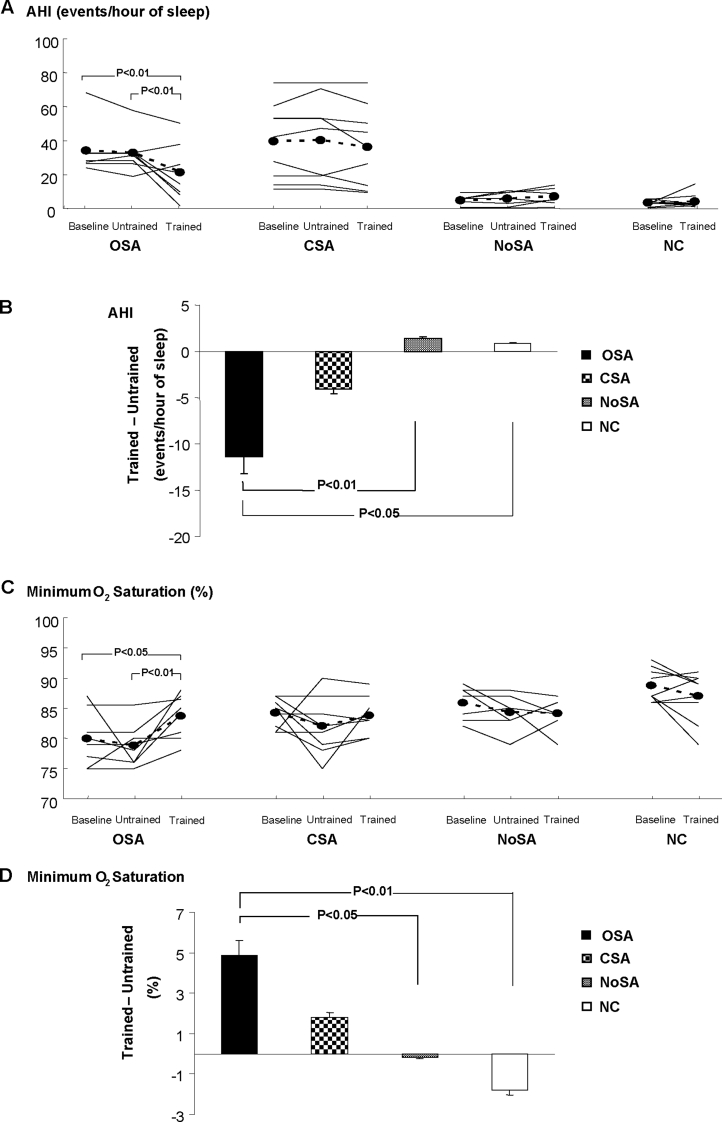
Sleep parameters were unchanged after the untrained period in all 3 HF groups (Table 4). In patients with HF and OSA, exercise training increased the amount of stage 3-4 sleep and decreased the number of arousals. Exercise training decreased the AHI and improved the levels of minimum O2 saturation during sleep only in patients with HF and OSA (Figure 5A and C, respectively). Furthermore, the mean difference in the change in AHI and minimum O2 saturation during sleep in patients with HF and OSA was greater than in control subjects and in subjects with HF and no sleep apnea (Figure 5B and D, respectively).
Table 4.
Sleep Pattern in Patients with Heart Failure and Normal Control Subjects at Baseline and after the Untrained and Trained Periods
| Baseline | Untrained | Trained | |
|---|---|---|---|
| TST, min | |||
| OSA | 375 ± 11 | 359 ± 14 | 375 ± 19 |
| CSA | 376 ± 18 | 368 ± 14 | 376 ± 17 |
| NoSA | 371 ± 30 | 361 ± 29 | 391 ± 10 |
| NC | 365 ± 27 | 407 ± 7 | |
| Stage 1-2, % TST | |||
| OSA | 83 ± 3 | 82 ± 2 | 75 ± 3 |
| CSA | 78 ± 3 | 78 ± 6 | 79 ± 4 |
| NoSA | 77 ± 2 | 78 ± 3 | 74 ± 4 |
| NC | 68 ± 3 | 70 ± 3 | |
| Stage 3-4, % TST | |||
| OSA | 7 ± 1 | 7 ± 1 | 13 ± 1a |
| CSA | 11 ± 3 | 8 ± 3 | 8 ± 3 |
| NoSA | 15 ± 4 | 13 ± 8 | 17 ± 10 |
| NC | 17 ± 2 | 13 ± 1 | |
| REM, % TST | |||
| OSA | 10 ± 2 | 11 ± 2 | 13 ± 2 |
| CSA | 11 ± 1 | 12 ± 2 | 13 ± 2 |
| NoSA | 8 ± 2 | 10 ± 2 | 12 ± 3 |
| NC | 15 ± 2 | 17 ± 2 | |
| Arousals, no./h of sleep | |||
| OSA | 40 ± 8 | 38 ± 8 | 21 ± 5a |
| CSA | 28 ± 4 | 32 ± 7 | 23 ± 6 |
| NoSA | 21 ± 3 | 21 ± 4 | 17 ± 5 |
| NC | 18 ± 3 | 14 ± 3 |
OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure and no sleep apnea; NC, normal, healthy control subjects. Sleep parameters were unchanged after the untrained period in all 3 groups with heart failure. Exercise training caused no significant change in total sleep time (TST), Stage 1-2, or and rapid eye movement (REM) sleep in both patients with heart failure and control subjects.
vs Baseline and Untrained period, P < 0.05. One way analysis of variance with repeated measures for within-group differences. Paired Student t test for within-group differences in control subjects. One-way analysis of variance for post-training between-group differences.
Figure 5.
Apnea-hypopnea index (AHI) and minimum O2 saturation during sleep in heart failure patients at baseline, untrained, and trained periods (A and C). Comparison among groups in the mean difference in the change (Trained – Untrained) in AHI (B), and minimum O2 saturation (D). OSA refers to patients with heart failure and obstructive sleep apnea; CSA, patients with heart failure and central sleep apnea; NoSA, patients with heart failure without sleep apnea; NC, normal healthy control subjects.
Correlation Between Changes in MSNA and Sleep Parameters
There were no significant correlations between delta changes (trained period − untrained period) in MSNA and AHI (r = 0.41; P > 0.05), and FBF and AHI (r = −0.33; P > 0.05) in patients with HF. Similarly, there were no significant correlations between delta changes (trained period − untrained period) in MSNA and time of SaO2 < 90% (r = 0.39; P > 0.05), and FBF and time of SaO2 < 90% (r = 0.1; P > 0.05).
DISCUSSION
The main findings of the present study are that (1) exercise training significantly improves MSNA, FBF, functional capacity, and QoL in patients with HF with and without sleep disordered breathing; (2) exercise training improves OSA severity, minimum O2 saturation, and sleep architecture in patients with HF independently of changes in body weight; (3) exercise training has no significant impact on CSA severity and sleep pattern in patients with HF; and (4) the improvement in MSNA and FBF in patients with HF caused by exercise training does not correlate with the improvement in sleep parameters.
In this study, we report for the first time that 4 months of regular exercise improves OSA severity and sleep architecture in patients with HF. Exercise training shifted patients with HF from severe to moderate OSA, independent of changes in BMI. Exercise training provoked a 36% reduction in AHI, 5% improvement in minimal O2 saturation (from 79% to 84%), and a significant increase in slow wave sleep stages. Our results are to some extent in line with the observation that exercise training reduces OSA severity in obese patients with OSA without cardiac disease.33,34 In contrast, exercise training had no significant effects on CSA severity and sleep parameters. Moreover, our results are in contrast with those of a recent investigation that showed a beneficial effect of exercise training on CSA severity but not on OSA.29 Possible explanations for different results may relate to classification of sleep disordered breathing, since, in contrast with our study, the previous investigation did not monitor sleep by standard polysomnography.29
The exact mechanism by which exercise training is beneficial to OSA remains speculative. Exercise training may reduce fluid accumulation in the neck, which in turn attenuates the upper airway narrowing and collapse.35 Interestingly enough, exercise training showed a trend to increase LVEF only in the subgroup of patients with HF and OSA (P = 0.056). Therefore, it is possible that exercise training improves cardiac function and reduces fluid edema in the upper airway in patients with HF with OSA.
We have previously demonstrated that exercise training causes a significant reduction in MSNA in patients with HF.17 However, in that study, sleep was not monitored. In the present study, we extend this finding by showing that the beneficial effects of exercise training on sympathetic nerve activity in patients with HF occur regardless of disordered breathing. Moreover, we provide evidence that this beneficial effect is independent of the positive effects on sleep disordered breathing. Despite the significant reduction in OSA severity, the neurovascular improvement did not correlate with changes in sleep apnea severity. In addition, despite the nonsignificant improvement in AHI in the patients with CSA, the fall in MSNA in patients with CSA was similar to that observed in patients with OSA.
The reduction in MSNA by exercise training has clinical importance, since sympathetic nerve activity has been associated with poor prognosis in patients with HF. In a classic study, Cohn et al.36 showed that augmented plasma norepinephrine levels were related to poor prognosis in patients with HF. And, more recently, we found that MSNA predicts mortality rate in patients with HF.37 In addition, sympathetic excitation contributes to skeletal muscle myopathy, which explains, in great part, the exercise intolerance in chronic HF.19
The mechanism by which exercise training reduces sympathetic nerve activity in HF is outside the scope of the present study. However, we may suggest that the improvement in carotid chemoreflex control is implicated in this exercise training benefit. Li et al.26 demonstrated that the enhancement in peripheral chemoreflex control was normalized after exercise training in rabbits with HF. Since chemoreflex control integrates in the central nervous system (CNS), it is reasonable to conclude that exercise leads to changes in the CNS. In fact, previous studies have shown that exercise training reduces angiotensin II levels and increases the production of endothelial nitric oxide synthase in endothelial cells and nitric oxide synthase isoform in the CNS.38 Both angiotensin II and nitric oxide are mutually inhibitory within the CNS associated with sympathetic modulation.38 Independent of the exact mechanism, our findings support exercise training as an important strategy to treat sympathetic overactivity in patients with chronic HF. The new finding of the present study is that this beneficial effect is independent of sleep apnea and actually seems even more pronounced in patients with coexistent sleep apnea in whom MSNA is exacerbated (Figure 3B). In fact, the powerful effects of exercise training on the cardiovascular system override the burden represented by sleep apnea in patients with HF.
Another interesting piece of information in our study is that exercise training causes a remarkable increase in muscle blood flow and vascular conductance in all patients with HF. The potential mechanisms underlying this peripheral vascular adaptation are an enhancement in endothelium function,18 an attenuation in peripheral inflammatory process,39,40 and a reduction in sympathetic outflow.17,25 The results of previous studies41,42 have suggested that proinflammatory cytokines play an important role in modulating the function and structure of the heart. Cytokines increase inducible nitric oxide synthase isoform, which provokes excessive intracellular nitric oxide production and formation of oxygen free radicals. These cellular alterations may inhibit key aerobic enzymes and, in consequence, oxygen consumption.43 In contrast, exercise training significantly reduces plasma tumor necrosis factor-α levels in patients with symptomatic HF.44 In addition, there is evidence that exercise training decreases skeletal muscle tumor necrosis factor-α, interleukin-6, IL-1-β and inducible nitric oxide synthase expression and increases in Cu/Zn superoxide dismutase and glutathione peroxidase expression in patients with stable HF.20,45 The reduction in sympathetic nerve activity also plays a role in the alleviation of peripheral vasoconstriction. We have consistently shown that exercise training reduces muscle sympathetic nerve activity and muscle blood flow in patients with HF.16,17,25 Moreover, these findings have been demonstrated in patients with HF who are receiving treatment with β-adrenergic receptor-blocking agents.25 This information has clinical implications, since treatment with β-adrenergic receptor-blocking agents for 6 months has not been shown to cause changes in FBF.46 The peripheral vascular changes observed in our study in response to exercise training may help to explain the improvement in exercise capacity in patients with HF with and without sleep apnea. Moreover, these findings may also explain the improvement in QoL presently observed after exercise training. In a previous study, Bellardinelli et al.32 showed that the improvement in QoL paralleled the improvement in peak VO2 responses in patients with HF. More recently, other investigators have reported that exercise intensity is an important factor for improvement in aerobic capacity and QoL in patients with HF.22
Our study has some limitations. Finapres measurements of blood pressure do not always agree with intraarterial or cuff pressures. To control this possible limitation, Finapres measures of blood pressure were always compared with automatic blood pressure measures at the beginning of each study. There was a significant correlation between systolic blood pressure measured by Finapres and systolic blood pressure measured by an automatic method (P = 0.037), and no significant difference between these 2 measures was found (P > 0.05). The patients were not randomly assigned to a control group or exercise-training group. However, we included 4 months of untrained period, and each patient served as his or her own control. Exercise training did not cause any significant reduction in heart rate, which might suggest that our training strategy was insufficient. This does not seem to be the case. The absence in resting bradycardia can be attributed to the treatment with β-adrenergic receptor-blocking agents, since all studied patients were receiving treatment with β-adrenergic receptor-blocking agents. In addition, exercise training provoked a significant increase in peak VO2, which is a good marker of exercise training adaptation. Finally, we excluded participants with BMI greater than 30 kg/m2 to avoid the potentially confounding influence of obesity, realizing that this strategy may have limited the extrapolation of the present findings to obese patients with HF and OSA. Interestingly, a recent prospective study of the prevalence of sleep disordered breathing in patients with HF with systolic dysfunction reported that the mean BMI in this group was 28.9 kg/m.2,3 Perhaps the prevalence of obesity in patients with HF with sleep disordered breathing is less than that in individuals with normal ventricular function and with sleep disordered breathing.
In conclusion, exercise training improves neurovascular control, functional capacity, and overall QoL in patients with HF with systolic dysfunction with and without sleep apnea. Exercise training improves sleep apnea severity and sleep architecture in patients with HF with OSA but does not do so in patients with CSA. These findings provide compelling evidence for prescribing exercise training in the treatment of patients with HF with sleep apnea, particularly in those with OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by Fundaçcãao de Amparo àa Pesquisa do Estado de Sãao Paulo (FAPESP # 2005/59740-7), Conselho Nacional de Pesquisa (CNPq #474621/2004-9) and, in part, Fundaçcãao Zerbini. Dr. Linda M Ueno was supported by FAPESP as a postdoctoral fellow (# 03/10881-2). Carlos E Negrao and Maria U Rondon were supported by CNPq # 304304/2004-2 and #305159/2005-4, respectively. The authors are indebted to the polysomnography technologists for performing the overnight polysomnography and for excellent patient care.
REFERENCES
- 1.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 2.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley TD, Floras JS. Sleep apnea and heart failure. Part II: central sleep apnea. Circulation. 2003;107:1822–6. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzi-Filho G, Genta PR, Figueiredo AC, Inoue D. Cheyne-Stokes respiration in patients with congestive heart failure: causes and consequences. Clinics. 2005;60:333–44. doi: 10.1590/s1807-59322005000400012. [DOI] [PubMed] [Google Scholar]
- 6.Bradley TD, Floras JS. Sleep apnea and heart failure. Part I. Obstructive sleep apnea. Circulation. 2003;107:1671–8. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzi-Filho G, Bradley TD. New York, NY: Marcel Dekker, Inc; 2002. Cardiac function in sleep apnea. In: Pack AI, ed. Sleep Apnea. Pathogenesis, Diagnosis, and Treatment; pp. 377–9. [Google Scholar]
- 8.Lorenzi-Filho G, Rankin F, Bies I, Bradley TD. Effects of inhaled carbon dioxide and oxygen in Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 1999;159:1490–8. doi: 10.1164/ajrccm.159.5.9810040. [DOI] [PubMed] [Google Scholar]
- 9.Spaak J, Egri ZJ, Kubo T, et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46:1327–32. doi: 10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 11.Usui K, Bradley TD, Spaak J, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45:2008–11. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 12.Bradley TD, Logan AG, Kimoff RJ, et al. CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 13.Arzt M, Floras JS, Logan AG, et al. CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 14.Whellan DJ, O'Connor CM, Lee KL, et al. HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Boudreau M, Genovese J. Cardiac rehabilitation: a comprehensive program for the management of heart failure. Prog Cardiovasc Nurs. 2007;22:88–92. doi: 10.1111/j.0889-7204.2007.05242.x. [DOI] [PubMed] [Google Scholar]
- 16.deMello Franco FG, Santos AC, Rondon MU, et al. Effects of home-based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail. 2006;8:851–5. doi: 10.1016/j.ejheart.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Roveda F, Middlekauff HR, Rondon MU, et al. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol. 2003;42:854–60. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 18.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 19.Negrão CE, Middlekauff HR. Adaptations in autonomic function during exercise training in heart failure. Heart Fail Rev. 2008;13:51–60. doi: 10.1007/s10741-007-9057-7. [DOI] [PubMed] [Google Scholar]
- 20.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erb S, Yu J. Anti-inflammatory effects of exercise traning in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–8. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 21.Erbs S, Linke A, Gielen S, Fiehn E, et al. Exercise training in patients with severe chronic heart failure: impact on left ventricular performance and cardiac size. A retrospective analysis of the Leipzig Heart Failure Training Trial. Eur J Cardiovasc Prev Rehabil. 2003;10:336–44. doi: 10.1097/01.hjr.0000099031.38268.27. [DOI] [PubMed] [Google Scholar]
- 22.Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 23.Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–31. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 24.Hambrecht R, Niebauer J, Fiehn E, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. 1995;25:1239–49. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 25.Fraga R, Franco FG, Roveda F, et al. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail. 2007;9:630–6. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Li YL, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol. 2008;105:782–90. doi: 10.1152/japplphysiol.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drager LF, Bortolotto LA, Lorenzi MC, et al. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 28.Rechtstaffen A, Kales A. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of Standardized Terminology Techniques and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 29.Yamamoto U, Mohri M, Shimada K, et al. Six-month aerobic exercise training ameliorates central sleep apnea in patients with chronic heart failure. J Cardiol Fail. 2007;13:825–9. doi: 10.1016/j.cardfail.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–25. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 32.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional, quality of life, and clinical outcome. Circulation. 1999;99:1173–82. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 33.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–5. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 34.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 35.Shiota S, Ryan CM, Chiu KL, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–72. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS. Plasma norepinephrine as a guide to prognosis in patients with chronic heart failure. N Engl J Med. 1984;311:819–23. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 37.Barretto AC, Santos AC, Munhoz R, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.03.056. Jun 25. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Zucker IH, Wang W, Pliquett RU, Liy JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide and exercise training. Ann NY Acad Sci. 2001;940:431–43. [PubMed] [Google Scholar]
- 39.Adamopoulos S, Parissis J, Kroupis C, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–7. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 40.Karavidas AI, Raisakis KG, Parissis JT, et al. Functional electrical stimulation improves endothelial function and reduces peripheral immune responses in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:592–7. doi: 10.1097/01.hjr.0000219111.02544.ff. [DOI] [PubMed] [Google Scholar]
- 41.Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH, Polak JM. Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet. 1996;347:1151–5. doi: 10.1016/s0140-6736(96)90610-8. [DOI] [PubMed] [Google Scholar]
- 42.Kukielka GL, Smith CW, LaRosa GJ, et al. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest. 1995;95:89–103. doi: 10.1172/JCI117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hambrecht R, Adams V, Gielen S, Linke A, Mobius-Winkler S, Yu J. Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J Am Coll Cardiol. 1999;33:174–9. doi: 10.1016/s0735-1097(98)00531-2. [DOI] [PubMed] [Google Scholar]
- 44.Larsen AI, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of Tumor Necrosis Factor Alpha in patients with heart failure. Am J Cardiol. 2001;88:805–8. doi: 10.1016/s0002-9149(01)01859-8. [DOI] [PubMed] [Google Scholar]
- 45.Ennezat PV, Malendowicz LS, Testa M, et al. Physical training in patients with chronic heart failure enhances the expression of genes antioxidative enzymes. J Am Coll Cardiol. 2001;38:194–8. doi: 10.1016/s0735-1097(01)01321-3. [DOI] [PubMed] [Google Scholar]
- 46.De Matos LD, Gardenghi G, Rondon MU, et al. Impact of 6 months of therapy with carvedilol on muscle sympathetic nerve activity in heart failure patients. J Card Fail. 2004;10:496–502. doi: 10.1016/j.cardfail.2004.03.006. [DOI] [PubMed] [Google Scholar]