Abstract
Study Objectives:
To compare the efficacy of a mandibular advancement splint (MAS) and a novel tongue stabilizing device (TSD) in the treatment of obstructive sleep apnea (OSA).
Design:
A randomized crossover design was used.
Patients:
Twenty-seven patients (20 male, 7 female), recruited from a tertiary hospital sleep clinic.
Measurements and Results:
The apnea-hypopnea index (AHI) was reduced with MAS (11.68 ± 8.94, P = 0.000) and TSD (13.15 ± 10.77, P = 0.002) compared with baseline (26.96 ± 17.17). The arousal index decreased for MAS (21.09 ± 9.27, P = 0.004) and TSD (21.9 ± 10.56, P = 0.001) compared with baseline (33.23 ± 16.41). Sixty-eight percent of patients achieved a complete or partial response with MAS, compared with 45% with TSD. The Epworth Sleepiness Scale (ESS) score was decreased with MAS (P = <0.001) and TSD (P = 0.002). Subjective improvements in snoring and quality of sleep were reported, with a better response for MAS than TSD. Compliance was poorer for TSD, and the side effect profiles of the 2 modalities were different. All patients were satisfied with MAS compared to TSD, and 91% of patients preferred the MAS.
Conclusion:
Objective testing showed the MAS and TSD had similar efficacy in terms of AHI reduction. Patients reported improvements with both devices; however, better compliance and a clear preference for MAS was apparent when both devices were offered. Longer term studies are needed to clarify the role of TSD.
Citation:
Deane SA; Cistulli PA; Ng AT; Zeng B; Petocz P; Darendeliler MA. Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial. SLEEP 2009;32(5):648-653.
Keywords: mandibular advancement splint, tongue stabilizing device, obstructive sleep apnea
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMPLEX MULTIFACTORIAL CONDITION PRODUCED BY A COMBINATION OF ANATOMIC AND PHYSIOLOGICAL FACTORS.1 It is characterized by repetitive complete or partial closure of the upper airway during sleep resulting in sleep fragmentation and oxygen desaturation.2,3 Numerous risk factors including male gender and obesity,4 ethnicity,5 and craniofacial structure6 have been identified as increasing susceptibility to this disease. OSA has a significant associated morbidity and mortality and has been linked to cardiovascular7,8 and cerebrovascular disease,9,10 excessive daytime sleepiness,11 and increased risk for motor vehicle accidents.12–14 The prevalence of OSA varies depending on diagnostic criteria and population studied, and has been reported as affecting 4% of men and 2% of women in the middle-aged workforce.15 It was also found that among adults aged 30-69 years, 17% of adults had mild or worse sleep disordered breathing (AHI ≥ 5), and 5.7% of adults had moderate or worse sleep disordered breathing.16 As such, OSA is recognized as a significant public health issue.
While the gold standard of care combines conservative modalities such as weight loss and nasal continuous positive airway pressure (CPAP),17 interest in oral devices has been increasing possibly because of compliance difficulties with CPAP. Mandibular advancement splints (MAS) are the most common type of oral device; they protrude the mandible during sleep, thereby having a favorable impact on upper airway structure and function. Several designs have been extensively investigated and shown to be efficacious in a substantial number of patients, particularly those with mild to moderate OSA.18–21 The American Academy of Sleep Medicine practice parameters recommend the use of MAS as an alternative to CPAP for patients who prefer oral appliances or refuse or are unable to tolerate CPAP, particularly in mild to moderate OSA.22 A tongue stabilizing device (TSD) is a preformed appliance and uses suction to protrude the tongue and improve upper airway structure and function. The earlier designs were similar to a mouthguard, covering the upper and lower teeth to assist retention, with a flexible bulb into which the tongue was protruded.23 The current design has no dental coverage, reduced bulk, and has the bulb being retained in place only by suction. There are currently only limited data on the efficacy of the current device, which is commercially available.24 As they are not reliant on the teeth for retention, TSD have been proposed as an option for patients with a reduced number or absence of teeth (hypodontia, edentulism), or compromised dental health (periodontal disease). The aim of this study was to compare the efficacy of these 2 types of oral devices in typical OSA patients.
METHODS
Subjects
Twenty-seven patients were recruited from a sleep clinic in a university teaching hospital. The study was approved by the institutional ethics committee, and all patients were provided with written informed consent. Inclusion criteria were age > 20 years, ≥ 2 symptoms of OSA (snoring, fragmented sleep, witnessed apneas, daytime sleepiness) and evidence of OSA on polysomnography with an apnea hypopnea index (AHI) > 10 per hour. Exclusion criteria were regular use of sedative medications, previous failure of an oral appliance for treatment of OSA, exaggerated gag reflex, edentulous patients, and < 10 teeth per jaw or evidence of periodontal disease.
Experimental Design
A randomized crossover design was used (Figure 1). The patients had an 8-week acclimatization period (4 weeks with each device), during which they were provided with the devices in random order and asked to complete questionnaires. Patients subsequently underwent 1-week intervention with each treatment in random order. One-week washout periods were applied prior to and between the treatment phases. Overnight polysomnography was performed with the designated device at the end of each 1-week treatment phase as per previous studies.18,19
Figure 1.
Schematic diagram summarizing the study design. PSG = polysomnography.
Two types of oral appliances were provided to the subjects. The MAS was a custom-made 2-piece device (Somnomed Ltd, Australia) with vertical extensions on the lower component and ramps on the upper component which induced a forward mandibular posture as previously described (Figure 2).18,20 For the current study, a non-titratable version of the device was used by not incorporating the usual adjustable screw mechanism. This was to permit patient participation in a separate magnetic resonance imaging research study with the device. A protrusive bite was taken with a 4-mm vertical inter-incisal opening at 75% of the maximal comfortable protrusive range.18 Patients were instructed on insertion and removal of the MAS and advised to wear it during the sleeping period as much as possible. The tongue stabilizing device used in this trial was a preformed, non-adjustable silicon appliance constructed by injection molding (Aveo-TSD, Innovative Health Technologies, New Zealand) (Figure 3). Patients were instructed to rinse the device with water, place the flanges of the TSD on the outside of the upper and lower lips, insert the tongue into the bulb as far as was comfortable, then squeeze and release the bulb to generate suction. Patients were advised to increase the suction by protruding the tongue further and/or squeezing the bulb more should the device loosen or be insufficiently retentive, or decrease the suction should there be excessive discomfort.
Figure 2.
Photograph of upper and lower plates of the mandibular advancement splint.
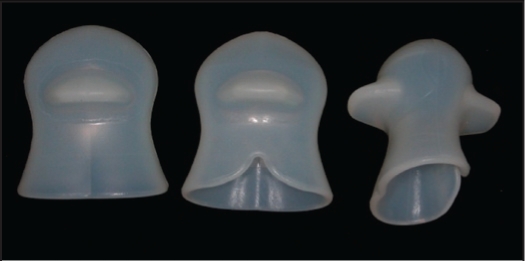
Figure 3.
Photograph of the tongue stabilizing device.
Treatment Outcome
The primary outcome for treatment efficacy was the apnea-hypopnea index (AHI) derived from standard nocturnal polysomnography (Compumedics Limited, Australia). Also measured were total sleep time, sleep time in REM, sleep time in NREM, arousal index (AI), sleep efficiency, minimum oxygen saturation (MinSO2), longest apnea, longest hypopnea, and mean duration of apneas and hypopneas. Apnea was defined as a cessation of airflow ≥ 10 s with oxygen desaturation > 3% and/or associated with arousal. Hypopnea was defined as a reduction in amplitude of airflow > 50% of the baseline tidal volume for > 10 s with an accompanying oxygen desaturation of ≥ 3% and/or associated arousal. Nasal airflow was measured using nasal prongs attached to a pressure transducer. Studies were scored by an experienced technician who was blinded to the treatment device.
OSA severity was classified according to the baseline AHI. Mild was defined as an AHI 5 to 15/h, moderate as AHI 15-30/h, and severe as AHI > 30/h. Treatment outcome was classified as follows: complete response (CR) was defined as a resolution of symptoms and a reduction in AHI to ≤ 5/h; partial response (PR) as an improvement in symptoms and ≥ 50% reduction in AHI, but where AHI remained > 5/h: and treatment failure (F) was defined as ongoing clinical symptoms and/or reduction in AHI < 50%. Compliance failure was defined as a patient who discontinued treatment.
Secondary outcomes were assessed using a standardized questionnaire used in our previous published studies.18,19 These included subjective snoring frequency and intensity, quality of sleep, daytime sleepiness,25 side effects, patient satisfaction, and appliance preference. Each questionnaire was completed by the patient at the end of the acclimatization phase after wearing each device for 1 month. Compliance, assessed by the number of weeks the patient wore the device in the 1 month available to acclimatize to each device, was also investigated.
Statistical Analysis
Data were analyzed using SPSS (Version 14 and 16). The polysomnographic results were subjected to paired Student's t-Tests to demonstrate any difference between MAS and TSD. Cross tabulation and Pearson χ2 tests (using linear-by-linear P value) compared the percentage of patients within treatment outcome categories by appliance and OSA severity, as only 2 devices were involved. Analysis of variance (ANOVA) was used to test for period and order effects. All descriptive statistics are presented as mean ± standard deviation. The results of the questionnaires were assessed graphically. Due to the large number of tests carried out with unknown dependence, and allowing for multiple tests, we replaced the standard significance level of 0.05 with a P value of < 0.01. A priori power calculation indicated that a sample size of 21 subjects was required to give a 90% power to detect a 50% reduction in AHI (P = 0.05), based on the data from our group's previous study.18
RESULTS
Out of 27 patients initially recruited, 22 patients (16 male, 6 female) completed the protocol. Two patients failed to complete the study for medical and work-related reasons, and 3 patients attended the first consultation, then subsequently withdrew for personal and time concerns unrelated to the nature of the devices. The demographics and baseline data for the patients who completed the protocol (16 male, 6 female) are demonstrated in Table 1. Five patients had mild OSA, 11 had moderate OSA, and 6 had severe OSA. As 50% (3) of the female patients and 19% (3) of the male patients were classified as severe OSA, males and females were grouped together for analysis. The mean anteroposterior mandibular advancement with MAS was 77% of maximum protrusion (mean 4 mm, range 2–10 mm). There was no significant difference in body mass index (BMI) between severity groups.
Table 1.
Patient Characteristics at Baseline
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 49.4 ± 11.0 | 24.8–65.3 |
| Body mass index (kg/m2) | 29.3 ± 5.6 | 20.6–38.3 |
| Baseline AHI (/h) | 27.0 ± 17.2 | 10.3–75.7 |
| Baseline MinSaO2(%) | 84.3 ± 6.5 | 71–95 |
AHI = apnea-hypopnea index;MinSaO2= minimum oxygen saturation
Results of the polysomnography are detailed in Table 2. A decrease in AHI was recorded for 91% of the patients when using MAS and 77% of the patients when using TSD. Analysis of the effect of the appliances on AHI in supine and other body positions during sleep demonstrated that AHI between baseline and TSD, and baseline and MAS were significantly different (P < 0.001 in each case). There was a marginally significant reduction in REM sleep between baseline and TSD (P = 0.056), and between baseline and MAS (P = 0.051), but not between TSD and MAS (P = 0.72). Subgroup analyses comparing males and females demonstrated little difference between MAS and TSD (data not shown). Of note, in 2 patients the TSD was only tolerated for less than 2 hours during the polysomnographic study.
Table 2.
Comparison of Polysomnographic Variables Between Baseline, MAS, and TSD
| Variable | Baseline | MAS | P Value | TSD | P Value |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| TST, min | 400 ±51 | 347 ± 77 | ns | 320 ± 97 | ns |
| REM sleep, min | 53 ± 22 | 63 ± 28 | ns | 53 ± 31 | ns |
| NREM sleep, min | 286 ± 41 | 283 ± 64 | ns | 269 ± 76 | ns |
| Arousal index/h | 33 ± 16 | 21 ± 9 | 0.004 | 21 ± 11 | 0.001 |
| Sleep efficiency % | 80 ± 11 | 78 ± 17 | ns | 79 ± 11 | ns |
| AHI/h | 27 ± 17 | 12 ± 9 | 0.000 | 13 ± 11 | 0.002 |
| MinSaO2 % | 84 ± 7 | 87 ± 5 | ns | 88 ± 6 | ns |
TST = total sleep time;AHI = apnea hypopnea index;MinSaO2= minimum oxygen saturation.
The treatment outcome with MAS demonstrated that 27.3% had a complete response, 40.9% had a partial response, and 31.8% failed. With TSD 22.7% had a complete response, 22.7% had a partial response, and 54.5% failed. Linear-by-linear χ2 tests demonstrated a trend towards a significant difference between TSD and MAS (P = 0.06). When assessing treatment outcome with both appliances compared with the patients OSA severity, no significant difference (exact linear-by-linear) was detected between the mild, moderate and severe OSA groups (MAS, P = 0.71; TSD, P = 0.23). Table 3 compares the number of patients in each category of treatment outcome for MAS and TSD. There were no significant period or order effects. Although the analysis showed that there was significant interpersonal variability, there was not enough difference between the 2 appliances to reach significance.
Table 3.
Cross-Tabulation Showing Treatment Response with MAS and TSD
|
MAS Response |
Total | |||
|---|---|---|---|---|
| Complete | Partial | Failure | ||
| TSD Response | ||||
| Complete | 3 | 1 | 1 | 12 |
| Partial | 1 | 4 | 0 | 5 |
| Failure | 2 | 4 | 6 | 5 |
| Total | 6 | 9 | 7 | 22 |
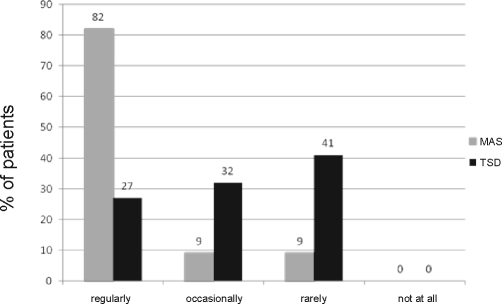
Snoring frequency improved from 81.8% of patients snoring 5–7 nights per week at baseline to 11.1% with MAS and 13.6% with TSD. MAS eliminated snoring in 40.9% of patients compared with 27.3% for TSD according to patient's perception. With MAS all patients reported improvement in snoring severity, with 27.3% being very much improved and 59.1% being much improved. With TSD, fewer patients indicated a favorable change in snoring severity, with 13.6% being very much improved, 13.6% being much improved, and 22.7% reporting no improvement. As an indicator of daytime sleepiness, the Epworth Sleepiness Scale (ESS) score decreased significantly with MAS (3.50 ± 2.41, P = 0.000) and TSD (5.86 ± 4.63, P = 0.002) compared with baseline (8.55 ± 5.12). Subjective compliance was better for MAS, with regular use (every night of the week for ≥ 6 h per night) reported by 81.8% of patients, compared with 27.3% for TSD (Figure 4). The incidence of patients involuntarily removing MAS during the night was 9% compared with 86.4% with TSD. At the 4-week follow-up appointment, MAS use was reported by 86.4% of the patients after 3 weeks, whereas 63.6% of patients discontinued use of TSD by 3 weeks. Side effects caused by MAS were jaw discomfort (59.1%) and dryness of mouth (50%), compared with TSD, with which excess salivation (86.4%), dryness of mouth (59.1%), and soft tissue irritation (50%) were problematic. All patients were satisfied with MAS, and 90.9% of patients preferred this device. Satisfaction with the TSD was indicated by 59.1% of the patients, with 3 patients being very dissatisfied.
Figure 4.
Comparison of compliance frequency reported by patients using MAS and TSD.
DISCUSSION
Oral appliances are increasingly being used in the management of OSA. The MAS is the more widely investigated oral appliance with an abundance of literature supporting its use in the management of OSA patients, particularly those with mild to moderate OSA.18–20,26 Across a number of studies, complete and partial response to MAS have been demonstrated in an average of 65% of OSA patients.27 There is good understanding of the indications for the prescription of MAS and supervising this treatment modality. In contrast, the role of TSD remains uncertain due to a paucity of evidence, with a limited number of studies with small sample sizes and using several appliance designs.24,28–30 In our short-term randomized controlled study, we demonstrated that MAS and TSD had similar effects on AHI, but that MAS was associated with greater symptomatic improvement, compliance, and patient preference.
The primary outcome in this study was AHI; we found that it decreased in 91% of patients when using MAS and 77% of patients when using TSD.
The present study is the first comparative study for TSD and demonstrates that TSD can yield an improvement in AHI, although in a lesser percentage of patients than MAS. Treatment outcome in this study was based on rigorous definitions, as previously reported by our group, which is a strength of our study. The percentage of patients with complete and partial response for TSD was lower than MAS, although this did not quite reach statistical significance. In addition the arousal index decreased significantly with MAS and TSD which is consistent with previous studies on MAS18,19,31 and TSD.24
The MAS used in this study has been rigorously evaluated in previous studies. In contrast to previous studies of this device, we used a titration strategy aimed at achieving mandibular advancement at approximately 75% of maximal jaw protrusion, rather than the maximal comfortable limit of advancement. This was because the MAS design was modified to remove the titration screws for the purpose of undertaking MRI scans in a separate study. An actual advancement of 77% ± 8% (range 2–10 mm) was achieved in this study. An important limitation of this study was that patients did not have further mandibular advancement as they acclimatized to MAS, which may have limited improvement in OSA in some patients. In support of this possibility is the lower complete response rate than previously reported by studies using this MAS design. Despite this limiting factor, the MAS provided significant clinical benefit.
The TSD appliance was non-adjustable, with the patient controlling the amount of tongue protrusion and suction generated by the device. It was noted that patients protruded the tongue into the appliance by differing amounts and squeezed the bulb with differing force. There was no method to standardize the application of TSD, with each individual having to establish his or her own comfort level. The forward tongue posture and stretching of the related soft tissues (especially the lingual frenum) may have caused discomfort, which in turn may have limited the amount of tongue protrusion and their potential response to TSD. The use of TSD in this manner reflects the real-life use of the device, which is intended by the manufacturer to be available over the counter for use in an unsupervised manner. We believe that clinical supervision is required to ensure patient safety and optimal outcome.
When the sequence of appliance provided first in the acclimatization and trial phases was considered, no significant difference was detected in the treatment outcome, although there was variation between individuals. It was observed that patients who were provided with the TSD as their first device were more willing to persist with the appliance than those who received MAS first, and subsequently appeared less enthusiastic about TSD. Patients appeared to find the MAS more comfortable and easier to manipulate than the TSD. In addition, 13 of 22 patients achieved the same category of response to treatment with both MAS and TSD. This suggests that some patients may consistently respond to either form of oral appliance. and raises the issue of predictive parameters that could be used to identify such “responders.”
Subjective evaluation of snoring frequency and severity showed both MAS and TSD were regarded by the patients (and their partners) as providing an improvement, however TSD was reported to produce less improvement than MAS. O'Sullivan and colleagues32 found a reduction of 18% in snoring frequency and 15.8% in snoring intensity using MAS. Kingshott and colleagues24 found the TSD significantly reduced snoring frequency in the 61–70 decibel range, but did not alter snoring in other decibel ranges.
Quality of sleep was improved with oral appliance therapy as has been demonstrated in other studies.18,27,33 Mehta et al.18 demonstrated a combined improvement in snoring, sleep quality and daytime sleepiness in 83% of the patients with MAS. In an evidence-based review of 87 articles by Ferguson et al,27 it was concluded that effects on sleepiness and quality of life were apparent yet improvements in other neuro-cognitive outcomes were less consistent when using oral appliances. In this study all patients reported improvement in quality of sleep with MAS, compared with 45% with TSD.
Objective compliance with oral appliances in the management of OSA is difficult to ascertain. While there has been a report of an objective compliance measuring device, this is not routinely available.34 Hence we relied on self-report for this study. Compliance, as indicated by the number of nights per week worn (minimum 6 hours per night), was assessed over a 4 week period and divided into 4 groups: regularly (worn every night of the week), occasionally (worn 3–4 nights of the week), rarely (worn 1–2 nights of the week), and not at all (device never worn). Compliance was better for MAS and was comparable to other MAS studies. Mehta and colleagues18 reported a compliance rate of 87.5% with MAS at one month. There is no literature to assess compliance with TSD but it can be assumed that like MAS, compliance rates are likely to decrease relative to the length of follow-up. To further appreciate the compliance issues patients were asked if they unknowingly removed the appliances during the night. Patients involuntarily removed MAS during the night in 9% of cases compared with 86.4% of cases for TSD, with the device often ending up on the bedside table. This was further evidenced by the fact that 2 patients were only able to tolerate the TSD for less than 2 hours during the efficacy polysomnograph. This appears to be a major limitation of TSD. In addition, MAS use was reported by 86.4% of the patients after 3 weeks, however 63.6% of patients discontinued use of TSD by 3 weeks. Patient satisfaction is likely to be a key influencer of compliance. In our short-term study all patients were satisfied with MAS and 91% of patients preferred this device. Satisfaction with TSD was indicated by only 60% of the patients, with 3 patients being very dissatisfied.
Both MAS and TSD resulted in patients reporting side effects, with each appliance producing a different type and severity of problems. For MAS the main concerns were jaw discomfort (59%) and dryness of mouth (50%). These side effects were largely mild in nature, resolved within about 30 minutes of removing the device, did not persist beyond 1 to 2 weeks and did not prevent the patients from using the MAS. The main side effects caused by TSD were reported as excess salivation (86.4%), dryness of mouth (59.1%), and soft tissue irritation (50%). The problems generated with TSD varied from mild to severe in nature, and appeared to persist for up to or longer than 3 weeks. Several patients described a temporary tingling sensation to the tongue lasting approximately 30 minutes to 1 hour. Minor ulceration of the lingual frenum also occurred, and was addressed by enlargement of the “notch” to accommodate the frenum. If the patient's lingual frenum inhibited their ability to protrude their tongue into the TSD, their response may have been suboptimal and in turn affected compliance. Patients also indicated swallowing was more difficult with TSD due to the vertical mouth opening. The reduction of AHI in supine sleep and slight reduction in REM sleep may have been influenced by the side effects, in particular excess salivation, however there was no statistically significant difference between MAS and TSD. The side effects were severe enough to prevent nearly half of the sample continuing with the TSD, compared with MAS where the side effects did not prevent any of the subjects from using the device.
Our study had a number of potential limitations. The sample population was recruited from a sleep disorders clinic known for its research interest in dental treatments for OSA, and this could have resulted in a referral bias. As the characteristics of the sample population were consistent with other sleep apnea populations reported in the literature, it would appear that bias was minimal. Our sample size was small, raising the possibility of a type 2 error, although our priori sample size calculation suggested the sample size was adequate. Despite this, the results from the study usefully inform clinical practice in this area. Reduced titration of the MAS may have resulted in a lower response rate, thereby reducing the apparent difference in treatment effects. Similarly, the inability to standardize the TSD suction levels and degree of tongue protrusion, which are unable to be measured, may have affected treatment outcome. The randomized crossover design, while an important strength of this study in eliminating between-patient variability, had a relatively short duration. Questionnaires were useful in providing feedback on the patients experience with the devices, but they relied on the accuracy of patient reporting, which may have resulted in responses that minimized or exaggerated the experience of patients. More information may have been obtained if the patients had been able to use each appliance for a longer period prior to the testing phase of the study. The important question of clinical effectiveness was not resolved by our short-term study.
In conclusion, this study showed that 4 weeks of MAS and 4 weeks of TSD can improve the parameters of OSA, including daytime and nocturnal symptoms. Although the findings suggest similar treatment effects of the two appliances in terms of reducing AHI, the higher complete response rate, overall acceptance, and compliance with MAS suggest it is a superior treatment for OSA in the clinical setting. However for subjects who are able to tolerate TSD, or are inappropriate for MAS (e.g., insufficient teeth), this may be a viable treatment option, and further work is required to evaluate the role of TSD in the management of OSA.
DISCLOSURE STATEMENT
This was not an industry sponsored study. The Mandibular Advancement Splints used in the study were provided at no cost by SomnoMed Ltd. Australia and the Tongue Stabilizing Devices used in the study were provided at no cost by Innovative Health Technologies, New Zealand. Dr. Cistulli contributed to the development of the mandibular advancement splint used in this study. He has consulted for and has been on the advisory board for SomnoMed and has financial interest in the company. He has received research support from ResMed and is a board member of the ResMed Foundation a non-profit, charitable organization. Dr. Ng has participated in industry supported studies. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Innovative Health Technologies, New Zealand, who provided the TSD but had no other role in the study.
Staff at Sydney Dental Hospital Department of Orthodontics Staff at St George Hospital Centre for Sleep Disorders and Respiratory Failure.
Research was undertaken at Sydney Dental Hospital and St George Hospital, Sydney, Australia.
Financial Support was provided by the Australian Society of Orthodontics Foundation for Research and Education
References
- 1.Cistulli P, Sullivan C, editors. In: Sleep and breathing. New York: Marcel Dekker Inc; 1994. Pathophysiology of sleep apnea. [Google Scholar]
- 2.Anonymous. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 3.Qureshi A, Ballard RD. Obstructive sleep apnea. J Allergy Immunol. 2003;112:643–51. doi: 10.1016/j.jaci.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Redline S. Epidemiology of sleep-disordered breathing. Semin Respir Crit Care Med. 1998;19:113–22. [Google Scholar]
- 5.Ancoli-Israeli S, Kripke D, Klauber M, Mason W, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battagel J, L'Estrange P. The cephalometric morphology of patients with obstructive sleep apnoea. Eur J Orthod. 1996;18:557–69. doi: 10.1093/ejo/18.6.557. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–84. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Peppard P, Palta M, et al. Population based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 9.Spriggs DA, French JM, Murdy JM, Bates D, James OF. Historical risk factors for stroke: a case control study. Age Aging. 1990;19:280–7. doi: 10.1093/ageing/19.5.280. [DOI] [PubMed] [Google Scholar]
- 10.Palomaki H. Snoring and the risk of ischaemic brain infarction. Stroke. 1991;22:1021–25. doi: 10.1161/01.str.22.8.1021. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, Nino-Murcia G. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988;94:32–7. doi: 10.1378/chest.94.1.32. [DOI] [PubMed] [Google Scholar]
- 12.Aldrich M. Automobile accidents in patients with sleep disorders. Sleep. 1989;6:487–94. doi: 10.1093/sleep/12.6.487. [DOI] [PubMed] [Google Scholar]
- 13.Liam CK, How LG, Tan CT. Road traffic accidents in patients with obstructive sleep apnoea. Med J Malaysia. 1996;51:143–5. [PubMed] [Google Scholar]
- 14.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badra S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 16.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea the continuous positive airway pressure applied through the nares. Lancet. 1981;18:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 18.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized controlled study of a mandibular advancement splint for obstructive sleep apnoea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 19.Pitsis AJ, Darendeliler MA, Gotsopolous H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:860–64. doi: 10.1164/rccm.200204-342OC. [DOI] [PubMed] [Google Scholar]
- 20.Gotsopolous H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 21.Ng AT, Gotsopolous H, Qian J, Cistulli PA. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;168:238–41. doi: 10.1164/rccm.200211-1275OC. [DOI] [PubMed] [Google Scholar]
- 22.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 23.Cartwright RD. Predicting response to the tongue retaining device for sleep apnea syndrome. Arch Otolaryngol. 1985;111:385–9. doi: 10.1001/archotol.1985.00800080071008. [DOI] [PubMed] [Google Scholar]
- 24.Kingshott RN, Jones DR, Taylor DR, Robertson CJ. The efficacy of a novel tongue-stabilising device on polysomnographic variables in sleep-disordered breathing: a pilot study. Sleep Breath. 2002;6:59–66. doi: 10.1007/s11325-002-0069-1. [DOI] [PubMed] [Google Scholar]
- 25.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 26.Bloch KE, Iseli A, Zhang JN, et al. A randomized, controlled crossover trial of two oral appliances for sleep apnea treatment. Am J Respir Crit Care Med. 2000;162:246–51. doi: 10.1164/ajrccm.162.1.9908112. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 28.Samelson CF. CDS Review . 1988. The role of tongue retaining device in treatment of snoring and obstructive sleep apnea; pp. 44–7. [PubMed] [Google Scholar]
- 29.Cartwright RD, Samelson CF. The effects of a nonsurgical treatment for obstructive sleep apnea: the tongue-retaining device. JAMA. 1982;248:705–9. [PubMed] [Google Scholar]
- 30.Higurashi N, Kikuchi M, Miyazaki S, Itasaka Y. Effectiveness of a tongue-retaining device. Psychiatry Clin Neurosci. 2002;56:331–2. doi: 10.1046/j.1440-1819.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109:1269–75. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan RA, Hillman DR, Mateljan R, Pantin C, Finucane KE. Mandibular advancement splint: an appliance to treat snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:194–8. doi: 10.1164/ajrccm.151.1.7812552. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt-Nowara W, Lowe AA, Wiegand L, Cartwright R, Perez-Guerra F, Menn S. Oral appliances for the treatment of snoring and obstructive sleep apnea: a review. Sleep. 1995;18:501–10. doi: 10.1093/sleep/18.6.501. [DOI] [PubMed] [Google Scholar]
- 34.Lowe AA, Sjoholm TT, Ryan CF, et al. Treatment, airway and compliance effects of a titratable oral appliance. Sleep. 2000;23:S172–8. [PubMed] [Google Scholar]