Abstract
Study Objectives:
The objectives of this study were to investigate possible neuropathology behind the Kleine-Levin Syndrome (KLS), a severe form of hypersomnia with onset during adolescence.
Design:
Functional magnetic resonance imaging (fMRI) applying a verbal working memory task was used in conjunction with a paper-and-pencil version of the task.
Participants:
Eight patients with KLS and 12 healthy volunteers participated in the study.
Results:
The results revealed a pattern of increased thalamic activity and reduced frontal activity (involving the anterior cingulate and adjacent prefrontal cortex) while performing a reading span task.
Discussion:
This finding may explain the clinical symptoms observed in KLS, in that the thalamus is known to be involved in the control of sleep. Given the increasing access to fMRI, this investigation may aid clinicians in the diagnosis of patients suffering from severe forms of hypersomnia.
Citation:
Engström M; Vigren P; Karlsson T; Landtblom AM. Working memory in 8 kleine-levin syndrome patients: an fmri study. SLEEP 2009;32(5):681–688.
Keywords: fMRI, Kleine-Levin Syndrome, working memory, hypersomnia, thalamus
THE KLEINE-LEVIN SYNDROME (KLS) IS A RARE BUT RELATIVELY WELL-DEFINED DISORDER CHARACTERIZED BY EXCESSIVE SLEEP PERIODS (PERIODIC hypersomnia) associated with behavioral disturbances such as binge eating (hyperphagia), irritability, and increased sexual interest (hypersexuality). Hence, KLS is an intriguing disorder that typically affects teenagers or young adults. At a first glance, KLS symptoms could be seen as features of a teenager's everyday life were it not for the fact that these cause severe suffering for the patient and his/her family. Despite the dramatic presentation and debilitating nature of KLS, it remains unclear how or even if the brain is affected. KLS has recently been the subject of intensified and organized research activity.1,2
Cognitive disturbances frequently occur during hypersomnia periods in KLS2, but are traditionally not associated with asymptomatic periods.3 Nevertheless, our team has shown working memory deficit during asymptomatic periods and also 6 years after remission.1,4 Our clinical experience of the disorder implicates that the symptomatic periods not only remit,2 but also change in character. Patients and their families typically describe periods of well-defined hypersomnia during the first years of active disease, whereas they describe the later symptomatic periods being episodes of behavioral changes until symptoms entirely cease to recur.
Our team has observed fronto-temporal hypoperfusion on single photon emission computed tomography (SPECT).1 We have also demonstrated enduring fronto-temporal hypoperfusion 7 years after remission.4 In other studies, hypoperfusion of both thalami, hypothalamus, basal ganglia, medial and dorsolateral frontal regions have been observed during symptomatic periods.5,6 Some patients show persistent hypoperfusion during asymptomatic periods, and it has been implicated that this might be connected to the duration of the syndrome. A few cases, based on SPECT or neuropathological examination, have implicated thalamic7 and possibly dorsolateral and inferior frontal lobe involvement.
We have noted that a distribution of neural networks similar to those networks implicated in KLS have been highlighted in recent investigations on working memory employing functional magnetic resonance imaging (fMRI).8,9 We were not aware of fMRI studies involving KLS, nor any other investigation of working memory in this specific form of primary hypersomnia. Hence, KLS patients and healthy controls were given a working memory task while being monitored by fMRI. The aim of the present study was to investigate if our previous finding of working memory deficit in KLS is reflected in the fMRI activation pattern.
METHODS
Subjects
We examined 8 patients, 5 men and 3 women, mean age was 27 years (standard error of mean (SEM) = 4.2 years) and the median age was 23.5 years. All patients fulfilled the criteria of KLS according to The International Classification of Sleep Disorders, revised by the American Academy of Sleep Medicine 2005. Diagnosed by an experienced neurologist, 7 patients had an active disease and one was in remission, the latter being the first patient with KLS to be diagnosed by us in the mid-1990s.4 Active disease was defined as at least one symptomatic period during the last one and a half years. All patients were in an asymptomatic period at the time of fMRI. One patient was treated with a selective serotonin reuptake inhibitors (SSRI, citalopram, 20 mg × 1), but considering the minimal effects on working memory shown for SSRIs10 and the small population of patients, we decided to include the patient in this study. In addition, no SSRI related effects on working memory brain activation pattern have been found in a previous study by Rose et al.11 The other patients had no medication. All patients had undergone extensive clinical investigations including repeated neurological examinations, MRI, CT, EEG, lumbar puncture, psychiatric evaluation, and comprehensive neuropsychological testing as important parts of the neurological diagnostic procedure. None of the patients had any other sleep disturbances or other detectable neurological disorders. The mean educational level was 11.8 studying years (SEM = 0.8). A description of the KLS patients included in this study is found in Table 1.
Table 1.
Clinical Data of KLS Patients Included in this Study
| Pat 1 | Pat 2 | Pat 3 | Pat 4 | Pat 5 | Pat 6 | Pat 7 | Pat 8 | |
|---|---|---|---|---|---|---|---|---|
| Hypersomnia | Yes | yes | yes | yes | yes | yes | yes | yes |
| Associated symptoms | Wm | Wm,H | Ha | H,Dp | H | H,Dp,Hs | H,Dp | Dp |
| Year of birth | 1988 | 1973 | 1991 | 1977 | 1990 | 1963 | 1989 | 1957 |
| Age of presentation | 16 | 16 | 13 | 16 | 14 | 22 | 15 | 16 |
| Triggers | A | A | none | A | none | A | I,Pa | none |
| Frequency of attacks (n/yr) | 2–4 | 6 | 12 | 4–12 | 3 | 3 | 20 | 4 |
| Mean duration of attacks | 1–2 w | 1 w | 1½ w | 1 w | 1–2 w | 1–2 w | 1 w | 4–6 w |
| Last attack prior to fMRI | 3 m | 13 y | 2 w | 1 y | 6 m | 1 m | 1 y | 3 m |
Abbrevations: Wm = subjective workning memory deficit, H = hyperphagia, Ha = hallucinations, Dp = depersonalisation (a sense of not being present), Hs = hypersexuality, A = alcohol ingestion, I = infection, Pa = physical activity
Twelve healthy controls (5 men and 7 women) were recruited from a cohort of students and non-students. Subjects from 18 to 30 years of age with no known sleep disorder, other neurological disease, or cognitive dysfunction (excluded in clinical interviews) were included in the control group. Other exclusion criteria were left-handedness and contraindication for MRI scanning. The mean age of the controls was 24 years, and the median age was 21.5 years. The mean educational level was 13.7 studying years (SEM = 0.6). The healthy controls underwent the same procedure as the KLS patients. The study was performed in compliance with the Declaration of Helsinki, and the participants gave informed consent to participate in the study.
MRI
Functional images were acquired with a blood oxygen level dependent (BOLD)-sensitive echo planar imaging (EPI) sequence using a Philips Achieva 1.5 T body scanner employing the following imaging parameters: TE = 40 ms, TR = 2.7 s, flip angle 90°, number of slices = 32 (with no slice gap), voxel size = 3 × 3 × 3 mm, dynamics = 302. Axial slices were acquired interleaved and aligned between the floor of the sella turcica and the posterior angle of the fourth ventricle.
Material
The fMRI paradigm consisted of a working memory task modeled after the Daneman and Carpenter reading span task.12 The material for the working memory tasks comprised 108 medium frequency words.13 Two sentences, one semantically correct and one incorrect, were created. The target word was always the last word of a sentence. An example of a correct sentence containing the word “snow” was “The boy played in the snow.” A corresponding incorrect sentence was “The boy started the snow.” The sentences comprised 5 words in Swedish. The material was previously used in a study involving young stroke victims.14
Procedure
The experiment comprised 3 parts. In the first, participants were given a paper-and-pencil version of the working memory task, the well-known digit-span task, as well as other neurocognitive tasks (not reported here). The working memory task was included because we wanted to make sure that any deficit shown by KLS patients in the fMRI task was also reflected in the performance of a classical version of the task, and because we wanted to familiarize participants with the general procedure prior to scanning. In this first version of the task, the listening span task, participants listened to sentences read out one by one by the investigator. Participants were instructed to respond right or wrong as soon as possible following the presentation of a sentence. Furthermore, participants were asked to remember the last word of each sentence. After a participant had given the final right-wrong response to 2, 3, 4 or 5 successive sentences, he/she was asked to recall the final words of the sentences in correct order. In keeping with the recommendations of a recent study comparing different measurements that can be computed from this procedure,15 we calculated the total number of words correctly recalled.
The second part of the experiment involved familiarization with the version of the working memory task used during the MR scan. In this task, participants were presented with sentences on a computer screen. Presentation of stimuli and timing of responses were controlled by means of Superlab Pro (Cedrus Corporation, San Pedro, CA, USA). Each sentence remained on the screen for 5 seconds. Participants were instructed to press a designated button on the keyboard with their right index finger if the sentence was correct, and another with their middle finger if the sentence was incorrect. Participants were presented with 1, 2, 3, or 4 sentences in this manner. It was emphasized that both speed of response and accuracy were important. Following the presentation of a varying number of sentences, a probe remained for one second. After the probe, 4 words were presented, one at a time and during 5 seconds. Half of the words had appeared as the last (target) word in a recently presented sentence, the other half were new words (lures). Participants were asked to indicate as quickly as possible if the word was new or old. Participants pressed their index finger to indicate targets or the middle finger for lures. This general procedure was repeated 4 times at each level of difficulty (i.e., 1 to 4 sentences).
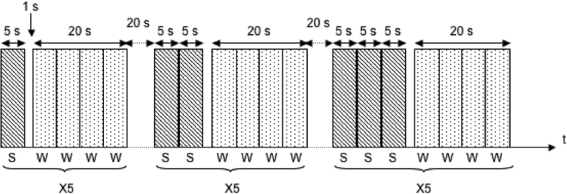
The third part of the experiment took part in the MR scanner. The procedure was identical to the procedure employed during parts 1 and 2 with a few notable exceptions. First, the trials were repeated 5 times instead of 4 at each difficulty level. An illustration of the fMRI paradigm is found in Figure 1. Next, stimuli were presented by means of high-resolution stereo-video goggles (Resonance Technology Inc, Northridge, CA, USA). Finally, participants indicated their responses by pressing 1 of 2 predefined buttons on a button response box (LUMItouch, Photon Control Inc., Burnaby, BC, Canada). The entire MRI session lasted approximately 30 min, and the part of the session involving administration of the fMRI task took 13 min.
Figure 1.
Illustration of the fMRI paradigm used for the working memory task. Difficulty levels were based on the recollection of the last word in each sentence after presentation of 1, 2, 3, or 4 sentences in a block. After sentence reading the participants were presented 4 words for 5 s each. The task was to indicate if the word was a lure or a target. Each difficulty level contained 5 Sentence/Word blocks. S = Sentences, W = Words.
fMRI Analysis
The fMRI images were preprocessed and analyzed using SPM5 software (Wellcome Department of Imaging Neuroscience, University College, London, UK). All functional images were realigned to correct for movement during scanning, but realignment parameters were not included as a confounder in the statistical analysis. In addition, images were normalized and re-sliced to a standard EPI template in SPM5. Finally, the normalized EPI images were smoothed with 8 mm Gaussian kernels to correct for differences in intersubject localization. Functional images were analyzed applying the hemodynamic response function. A high pass filter with a cut-off period of 128 s was used. Second level analysis, which was based on contrast images, was performed using a random effect model with one-sample and 2-sample t-tests for in-group and between-group analysis, respectively. The threshold was set at P < 0.001, uncorrected for family wise error. Activation in areas with 5 or more activated voxels was taken into account to exclude small volume activation.
Different task difficulties were taken into consideration (i.e., word recognition after 1, 2, 3, or 4 sentences) when analyzing blood oxygen level dependent (BOLD) fMRI data. Significant differences between the 4 difficulty levels in memory retrieval were explored using a step contrast function. Level weighting was chosen in order to make the weighting sum equal to zero and maintain equal interval between weights. The contrast vector for positive correlation with increased task difficulty was accordingly set to: 0 −3 −1 1 3 and the corresponding vector for negative correlation was 0 3 1 −1 −3.
Region of interest analysis and anatomical recognition of activated areas were based on the WFU PickAtlas Tool,16 using the coordinates from normalized images.
RESULTS
Working Memory Performance
Before scanning participants were administered a paper-and-pencil version of the working memory task and the digit-span task. Results from these 2 tasks are shown in Table 2. As we expected, KLS participants performed as well as controls on the digit span task (P > 0.1). However, controls recalled more words in the more taxing working memory task, listening span: t = 2.9, P < 0.01.
Table 2.
Demographic Characteristics of Patients with Kleine-Levin Syndrome (KLS) and Healthy Controls. Digit and Listening Span Data Refer to Results Obtained Prior to fMRI. Standard Error of Mean (SEM) Values in Brackets.
| KLS | Control | |
|---|---|---|
| Age | 27.0 (4.2) | 23.9 (1.2) |
| Education | 11.8 (0.8) | 13.7 (0.6) |
| Digit Span (Total Correct) | 9.3 (0.5) | 10.1 (0.5) |
| Listening Span (Total Correct) | 15.9* (1.3) | 20.1 (0.8) |
Note: Demographic characteristics were assessed by means of t-test or Mann-Whitney U (the Age variable).
P < 0.01.
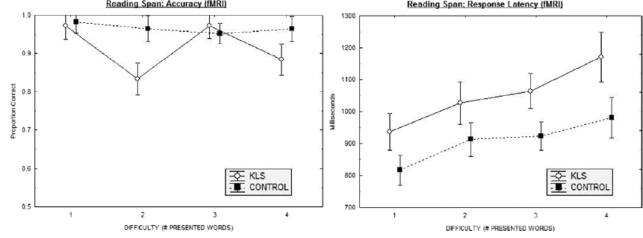
When performing the working memory task during fMRI, KLS patients showed both lower accuracy and longer response latency than healthy subjects (Figure 2, Tables 3 and 4). The results with respect to hits (previously presented words that subsequently were correctly recognized; Table 3) were analyzed by means of a 2 × 4 × 2 (Group by Difficulty Level by Semantic Correctness) split-plot ANOVA. This analysis yielded significant effects of Group (F1,21 = 8.3, P < 0.01) and Difficulty Level (F3,63 = 4.3, P < 0.01). In addition, the Group by Difficulty Level was significant; F3,63 = 4.0, P < 0.05. Post-hoc comparisons, using the Dunn-Sidak procedure, showed differences between groups as regards presentation of 2 and 4 sentences (P < 0.05). We also analyzed the results pertaining to correctly rejected new words. Given the fact that performances in this respect were highly accurate, there was no significant effect.
Figure 2.
Results from the reading span working memory task show performance accuracy (left panel) and response latency (right panel) for KLS and controls as a function of difficulty (number of words to retain).
Table 3.
Proportion Correct Responses in the fMRI Working Memory Tasks for Patients with Kleine-Levin Syndrome and Healthy Controls
| Difficulty Level | KLS |
Controls |
||||
|---|---|---|---|---|---|---|
| Previously Studied |
New | Previously Studied |
New | |||
| Correct Sentences | Incorrect Sentences | Correct Sentences | Incorrect Sentences | |||
| One Item | 0.94 (0.05) | 1.0 (0.01) | 0.99 (0.01) | 0.96 (0.04) | 1.0 (0.01) | 0.98 (0.01) |
| Two Items | 0.83 (0.03) | 0.83 (0.04) | 0.97 (0.01) | 0.96 (0.02) | 0.96 (0.03) | 1.00 (0.01) |
| Three Items | 0.94 (0.03) | 1.0 (0.04) | 1.00 (0.01) | 0.98 (0.02) | 0.93 (0.03) | 1.00 (0.01) |
| Four Items | 0.91 (0.04) | 0.86 (0.04) | 0.99 (0.01) | 0.96 (0.03) | 0.96 (0.03) | 0.96 (0.01) |
Performance is expressed as a function of level of difficulty (i.e., the number of words to be stored before responses were probed) and semantic correctness of sentences containing to-be-remembered words.
Table 4.
Latency Data as to Correctly Recognized Items in the fMRI Working Memory Tasks for Patients with Kleine-Levin Syndrome (KLS) and Healthy Controls
| Difficulty Level | KLS |
Controls |
||||
|---|---|---|---|---|---|---|
| Previously Studied |
New | Previously Studied | New | |||
| Correct Sentences | Incorrect Sentences | Correct Sentences | Incorrect Sentences | |||
| One Item | 896 (48) | 977 (51) | 977 (51) | 785 (38) | 848 (41) | 848 (41) |
| Two Items | 1130 (60) | 922 (46) | 922 (46) | 999 (48) | 828 (37) | 828 (37) |
| Three Items | 1126 (53) | 1002 (38) | 1002 (38) | 986 (43) | 862 (31) | 862 (31) |
| Four Items | 1139 (54) | 1203 (79) | 1202 (79) | 965 (43) | 996 (63) | 996 (63) |
Performance is expressed as a function of level of difficulty (i.e., the number of words to be stored before responses were probed) and semantic correctness of sentences containing to-be-remembered words.
We also carried out similar analyses with respect to latency data. Beginning with previously studied items that were correctly identified, a 2 × 4 × 2 (Group by Difficulty Level by Semantic Correctness) ANOVA revealed statistically significant main effects of Group (F1,21 = 7.0, P < 0.05), Difficulty (F3,63 = 17.69, P < 0.01), and Semantic Correctness (F1,21 = 39.76, P < 0.01. The Group by Semantic Correctness interaction also was significant, F1,21 = 4.74, P < 0.05. The effect was due to the fact that KLS participants showed longer latency following the presentation of semantically incorrect sentences (P < 0.05), an effect which was less pronounced in controls and absent in terms of statistical significance. Finally, the Difficulty Level by Semantic Correctness interaction was significant, F3,63 = 2.78, P < 0.05. This 2-way interaction came about since participants made speedier responses to correct sentences when presentation involved 1 or 2 sentences; when participants were required to store 3 or 4 sentences, latencies became longer and neared latencies for incorrect sentences.
In contrast to accuracy data, a 2 × 4 (Group by Difficulty) ANOVA involving correctly rejected novel words evidenced a significant main effect of Group: F1,21 = 7.34, P < 0.05. Furthermore, the Difficulty main effect was significant: F3,63 = 12.90, P < 0.01. No other effects were significant.
Common Features Regarding Working Memory Activation in KLS and Controls
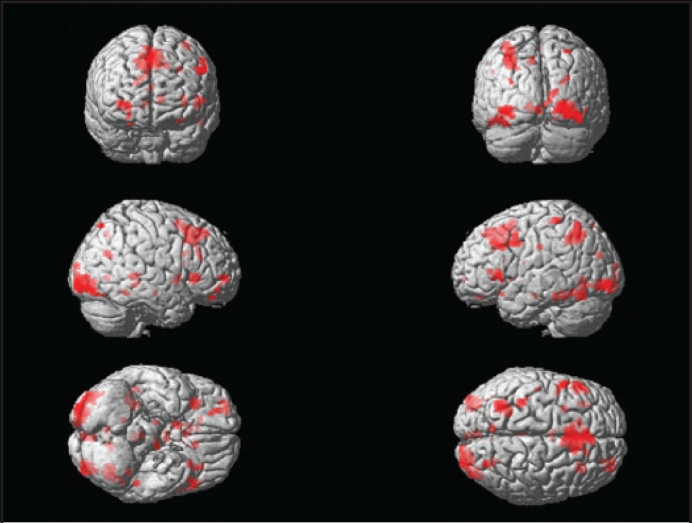
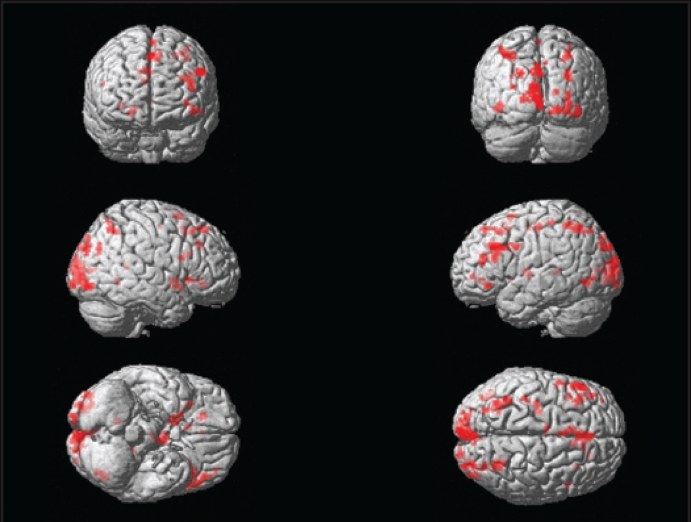
In the present study, we analyzed the BOLD response to increasing working memory load. KLS patients and controls demonstrated similar activation patterns in some brain areas. Both groups evidenced substantial activation in the superior parietal and (possibly related to visual presentation) the occipital lobes (Figures 3 and 4). KLS patients (activation peak at −36 −60 50, Z = 4.3) and the control group (−30 −56 46, Z = 4.7) activated similar areas in the left superior parietal lobe. KLS patients, however, tended to activate the right parietal lobe more than control subjects (32 −58 50, Z = 4.1), according to the between-group analysis. Common activation in both groups was also observed in several frontal regions and in the basal ganglia (Tables 5 and 6). Both KLS and controls had significant activation in the right striatum (ZKLS = 4.2, Zcontrol = 4.3) and in the right globus pallidus (ZKLS = 3.6, Zcontrol = 3.7). This pattern fits well with previous neuroimaging studies of working memory.8,17
Figure 3.
Activation in healthy controls during working memory performance assessed by random effects analysis (one-sample t-test).
Figure 4.
Cerebral activation in patients with Kleine-Levin syndrome during working memory performance using fMRI assessed by random effects analysis (one-sample t-test).
Table 5.
Activated Areas (MNI Coordinates) in 12 Healthy Controls During Working Memory Performance Assessed by Random Effects Analysis (one-sample t-test)
| Activation sitea | x | y | z | Z score | No. voxels |
|---|---|---|---|---|---|
| R. Inferior occipital gyrus | 42 | −82 | −14 | 5.0 | 410 |
| L. Superior parietal lobe | −30 | −56 | 46 | 4.7 | 285 |
| M. Superior frontal gyrus b | 0 | 28 | 46 | 4.6 | 730 |
| L. Post central gyrus | −34 | −38 | 62 | 4.5 | 45 |
| Vermis | −2 | −38 | −2 | 4.4 | 41 |
| R. Insula | 30 | 24 | 0 | 4.4 | 95 |
| R. Hippocampus | 32 | −40 | 2 | 4.3 | 50 |
| R. Lentiform nucleus | 10 | 8 | −4 | 4.3 | 46 |
| L. Fusiform gyrus | −52 | −36 | −22 | 4.2 | 15 |
| L. Inferior frontal gyrus, triangularis | −48 | 22 | 2 | 4.1 | 58 |
M = Medial, R = Right, L = Left
This area includes the pre-frontal cortex and cingulate gyrus.
Table 6.
Activated Areas (MNI Coordinates) in 8 Patients with Kleine-Levin Syndrome During Working Memory Performance Assessed by Random Effects Analysis (one-sample t-test)
| Activation sitea | x | y | z | Z score | No. voxels |
|---|---|---|---|---|---|
| M. Cuneus | 2 | −90 | 30 | 5.0 | 78 |
| L. Middle frontal gyrus | −52 | 24 | 32 | 4.9 | 70 |
| L. Middle occipital gyrus | −8 | −100 | 16 | 4.6 | 285 |
| L. Inferior frontal gyrus, triangularis | −42 | 26 | 18 | 4.4 | 65 |
| L. Superior parietal lobe | −36 | −60 | 50 | 4.3 | 104 |
| R. Cuneus | 30 | −86 | 28 | 4.3 | 19 |
| R. Putamen | 14 | 8 | −4 | 4.2 | 57 |
| L. Thalamusb | −6 | −6 | 6 | 4.2 | 63 |
| M. Superior frontal gyrus | −2 | 10 | 64 | 4.2 | 22 |
| R. Middle occipital gyrus | 32 | −92 | 0 | 4.0 | 110 |
M = Medial, R = Right, L = Left
Activation in ventral anterior, ventral lateral, and medial dorsal nuclei
Altered Working Memory Patterns in KLS
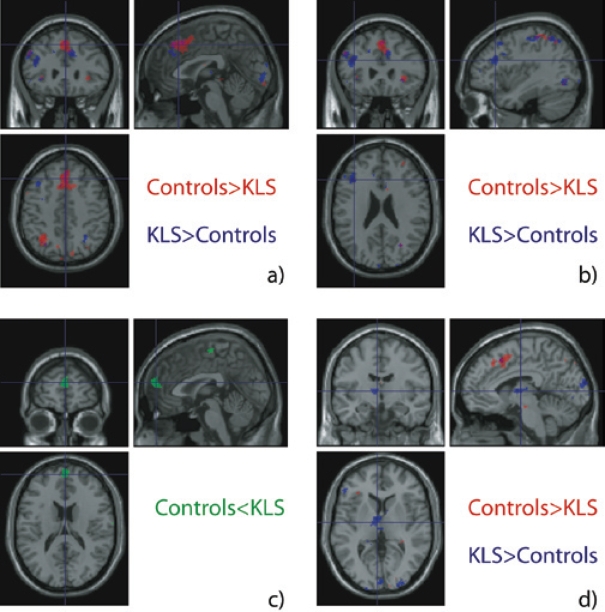
The aim of the present study was to investigate if previous findings of working memory deficit in KLS are reflected in the fMRI activation pattern. Therefore, differences between KLS patients and the group of healthy controls regarding working memory activation were explored. Images that point out the main differences between the two groups are shown in Figure 5. When the two groups were compared, healthy participants activated more voxels in the anterior cingulate gyrus (2 22 46, Z = 4.5) and adjacent prefrontal cortex (0 24 48, Z = 4.8) than KLS patients (Figure 5a). However, KLS patients showed significantly greater activation volume in the left triangular part of inferior frontal gyrus (−40 28 22, Z = 4.7, Figure 5b), than the control group. KLS patients also showed increased task difficulty correlated activation in the left thalamus (−6 −4 6, Z = 4.4, Figure 5d). The increased left thalamus activation in KLS patients was found in the ventral anterior (−6 −4 6, Z = 4.4), ventral lateral (−8 −10 4, Z = 3.6), and medial dorsal nuclei (−4 −16 8, Z = 3.7). No activation in the thalamus was found in controls at the group level (both in- and between-group analysis). The pattern of data regarding BOLD was reflected at the behavioral level in that KLS patients showed working memory deficits (Tables 2–4).
Figure 5.
Results showing regions that are more activated by one group compared to the other. The healthy subjects (Controls) activated the cingulate gyrus and adjacent dorsomedial prefrontal cortex (a) more than patients with Kleine-Levin syndrome (KLS). Patients activated the inferior frontal gyrus, pars triangularis (b) and thalamus (d) more than controls. A significant negative correlation with task difficulty was observed in the control group (b).
Ancillary Findings: Negative Correlation in Controls
Negative correlation implies areas with decreased BOLD response at increased cognitive load. A predominantly negative correlation was found in the anterior medial superior frontal cortex (0 62 20, Z = 5.0) in the healthy control group. This result was also maintained when comparing with KLS patients in the between-group analysis (0 62 20, Z = 5.5). We did not register any negative correlation in this area in KLS patients.
DISCUSSION
Our results confirm the previous finding of reduced working memory capacity in KLS. We have shown that this deficit correlates to lower activation of the anterior cingulate cortex and adjacent dorsomedial prefrontal cortex and stronger activation in the medial and anterior thalamus and possibly the inferior frontal gyrus. These alterations involve areas of the brain previously implicated in neuropathology studies on a few KLS patients. Damage to the medial thalamus also results in KLS-like symptoms following thalamic stroke, crack poisoning or Kearns-Sayre syndrome.18–20 Investigations of disorders of wakefulness, including KLS, could help us delineate the neural system behind these symptoms, and could even advance the understanding of more frequent ailments, such as bipolar disorder, serotonin transporter polymorphism, or attention deficit hyperactivity disorder—conditions that have similarities with KLS and where the thalamus and fronto-thalamic circuitry are of importance.21
Much work is, however, needed to clarify the exact implication of fMRI changes seen on activation in KLS. In comparison to effects of sleep deprivation,22–24 KLS patients evidence no parietal deficit. However, similar to sleep deprivation, KLS patients demonstrate evidence of frontal deficits. Although less consistently than in KLS, thalamic activation is also amplified in sleep deprivation, especially when participants remain alert.22 Thus, increased thalamic reactivity could also represent a compensatory response: through recruitment of thalamic networks, the brain is trying to compensate for increasing processing demands invoked by disturbed sleep or attenuated wakefulness.
In contrast to the effects of sleep deprivation, there is a paucity of data in the literature on working memory and attention, not only in KLS, but in most forms of dyssomnia (including primary hypersomnia).25 Along with the results in this report, we are aware of only 2 investigations, involving obstructive sleep apnea (OSA)26 and narcolepsy27 that directly are dealing with working memory. In comparison to OSA, KLS related BOLD differences seem to highlight inferior frontal and anterior cingulate deficits, rather than the dorsolateral and parietal deficits that were observed in OSA.26
In narcolepsy, working memory deficit is related to reduced BOLD signals in frontal, anterior cingulate, and parietal areas. Hence, in comparison to narcolepsy, the KLS alteration linked to working memory leaves parietal areas unaffected but suggests that anterior cingulate and prefrontal deficits may be similar in other forms of hypersomnia.28–30 It is of profound interest that a thalamic injury can give rise to prefrontal and anterior cingulate damage.31–33 It is thus possible that the cingulate and prefrontal changes, which in turn cause working memory problems, are consequences of thalamic pathology.
Although the results of our study clearly suggest the involvement of working memory related networks in KLS, it should perhaps be kept in mind that working memory presumably refers to a complicated combination of skills related to attention and temporary representation of information, including the dynamic, willful, and often effortful regulation of these activities by the individual. There are several ways of describing and studying working memory.34–37 Our experiment highlights the expenditure of effort in controlling temporary storage and manipulation.38,39 Although the question of effort always has been central to theories about working memory, attention, and consciousness, there are few reports that have used fMRI or other modes of brain imaging to address this topic. Recently, however, Jansma et al.40 described an association between effortful processing and activity in several regions in the frontal cortex and the anterior cingulate. Our data seem to indicate a similar pattern. On the other hand, Jansma et al. highlighted the right ventral prefrontal cortex in relation to cognitive effort. We did not observe such a specific response in that more extensive regions of the frontal cortex were implicated. The task in our study may have been more taxing than that in Jansma's study. Also, Jansma's study used a more elaborate paradigm to separate effort from more automatized processing. Hence, despite differences between tasks, both studies may provide important basal data as to the brain's online self-organization.
Another observation in this study was the significant negative BOLD signal in the anterior frontal cortex in the control group. Although inhibitory processes are of fundamental importance in the understanding of neural mechanisms, little research on fMRI deactivation patterns has been performed. A possible reason for this inattention is the difficulty in interpreting negative correlations in fMRI.41 Recent studies, however, have demonstrated that a negative BOLD signal is associated with a decrease in neuronal activity.42,43 A negative BOLD response can thus be interpreted as active inhibition to minimize task-irrelevant neural processes. Another interpretation of this negative correlation is that the redistribution of blood flow upon activation causes the negative response in less active regions; in other words, a negative BOLD signal could be the result of a passive deflection process in regions adjacent to activated areas. Thus the negative correlation to increased working memory load that was found in the anterior frontal cortex of the control group in the present study may be interpreted in two ways. One possible explanation is that the negative response is a result of redistribution of blood flow from the anterior frontal cortex to adjacent areas in the pre-frontal cortex—areas that have been demonstrated to be important for working memory function.17 Another explanation is that the negative correlation is a result of active inhibition of frontal regions reducing non-relevant neural processes to maximize the efficiency of processes necessary for a well-functioning working memory.
In conclusion, patients suffering from KLS show a working memory deficit related to changes on fMRI that are in keeping with what is known about sleep and working memory from previous studies. Results from a small population of patients suffering from a rare disorder like KLS, can nevertheless make substantial contributions to our understanding of sleep and working memory.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by Linköping University Hospital funds and the strategic research area for medical image science and visualisation.
This work was performed at Center for Medical Image Science and Visualization (CMIV), Linköping University, Sweden.
REFERENCES
- 1.Landtblom AM, Dige N, Schwerdt K, Säfström P, Granerus G. Short-term memory dysfunction in Kleine-Levin syndrome. Acta Neurol Scand. 2003;108:363–7. doi: 10.1034/j.1600-0404.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 2.Arnulf I, Zeitzer JM, File J, Farber N, Mignot E. Kleine-Levin syndrome: a systematic review of 186 cases in the literature. Brain. 2005;128:2763–76. doi: 10.1093/brain/awh620. [DOI] [PubMed] [Google Scholar]
- 3.Lishman WA. Organic psychiatry: the psychological consequences of cerebral disorder. 3rd ed. Oxford: Blackwell Science; 1998. pp. 732–33. [Google Scholar]
- 4.Landtblom AM, Dige N, Schwerdt K, Säfström P, Granerus, G A case of Kleine–Levin syndrome examined with SPECT and neuropsychological testing. Acta Neurol Scand. 2002;105:318–21. doi: 10.1034/j.1600-0404.2002.1c162.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang YS, Guilleminault C, Kao PF, Liu FY. SPECT findings in Kleine–Levin syndrome. Sleep. 2005;28:955–60. doi: 10.1093/sleep/28.8.955. [DOI] [PubMed] [Google Scholar]
- 6.Hong SB, Joo EY, Tae WS, Lee J, Han SJ, Lee HW. Episodic diencephalic hypoperfusion in Kleine-Levin syndrome. Sleep. 2006;29:1091–3. doi: 10.1093/sleep/29.8.1091. [DOI] [PubMed] [Google Scholar]
- 7.Billiard M. The Kleine–Levin syndrome: A paramedian thalamic dysfunction? Sleep. 2005;28:915–6. doi: 10.1093/sleep/28.8.915. [DOI] [PubMed] [Google Scholar]
- 8.Wager, TD, Smith, EE Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 9.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions NeuroImage. 2007;37:343–60. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 10.Wadsworth EJ, Moss SC, Simpson SA, Smith AP. SSRIs and cognitive performance in a working sample. Hum Psychopharmacol. 2005;20:561–72. doi: 10.1002/hup.725. [DOI] [PubMed] [Google Scholar]
- 11.Rose EJ, Simonotto E, Spencer EP, Ebmeier KP. The effects of escitalopram on working memory and brain activity in healthy adults during performance of the n-back task. Phychopharmacology. 2006;185:339–47. doi: 10.1007/s00213-006-0334-2. [DOI] [PubMed] [Google Scholar]
- 12.Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verbal Learn Verbal Behavior. 1980;19:450–66. [Google Scholar]
- 13.Allén S. Swedish word frequency norms. Stockholm: Almqvist – Wiksell; 1997. [Google Scholar]
- 14.Malm J, Kristensen B, Karlsson T, Carlberg B, Fagerlund M, Olsson T. Cognitive impairment and prognosis in young adults with infratentorial infarcts. Neurology. 1998;51:443–40. doi: 10.1212/wnl.51.2.433. [DOI] [PubMed] [Google Scholar]
- 15.Friedman NP, Miyake A. Comparison of four scoring methods for the reading span test. Behav Res Methods. 2005;37:581–90. doi: 10.3758/bf03192728. [DOI] [PubMed] [Google Scholar]
- 16.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 17.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 18.Bassetti C, Mathis J, Gugger M, Lovblad KO, Hess CW. Hypersomnia following paramedian thalamic stroke: a report of 12 patients. Ann Neurol. 1996;39:471–80. doi: 10.1002/ana.410390409. [DOI] [PubMed] [Google Scholar]
- 19.Guilleminault C, Quera-Salva MA, Goldberg MP. Pseudo-hypersomnia and pre-sleep behavior with bilateral paramedian thalamic lesions. Brain. 1993;116:1549–63. doi: 10.1093/brain/116.6.1549. [DOI] [PubMed] [Google Scholar]
- 20.Lovblad KO, Bassetti C, Mathis J, Schroth G. MRI of paramedian thalamic stroke with sleep disturbance. Neuroradiology. 1997;39:693–8. doi: 10.1007/s002340050488. [DOI] [PubMed] [Google Scholar]
- 21.Young KA, Holcomb LA, Bonkale WL, Hicks PB, Yazdani U, German DC. 5HTTLPR polymorphism and enlargement of the pulvinar: Unlocking the backdoor to the limbic system Biol Psychiatry. 2007;61:813–8. doi: 10.1016/j.biopsych.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 22.Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 24.Tomasi D, Wang RL, Telang F, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. doi: 10.1093/cercor/bhn073. doi: 10.1093/cercor/bhn073 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desseilles M, Dang-Vu TD, Schabus M, Sterpenich V, Maquet P, Schwartz S. Neuroimaging insights into the pathophysiology of sleep disorders. Sleep. 2008;31:777–94. doi: 10.1093/sleep/31.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–34. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 27.Thomas RJ. Fatigue in the executive cortical network demonstrated in narcoleptics using functional magnetic resonance imaging - a preliminary study. Sleep Med. 2005;6(399):406. doi: 10.1016/j.sleep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 29.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 30.Naumann A, Bellebaum C, Daum I. Cognitive deficits in narcolepsy. J Sleep Res. 2006;15:329–38. doi: 10.1111/j.1365-2869.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 31.Mutarelli EG, Omuro AM, Adoni T. Hypersexuality following bilateral thalamic infarction: case report. Arq Neuropsiquiatr. 2006;64:146–8. doi: 10.1590/s0004-282x2006000100032. [DOI] [PubMed] [Google Scholar]
- 32.Sandson TA, Daffner KR, Carvalho PA, Mesulam MM. Frontal lobe dysfunction following infarction of the left-sided medial thalamus. Arch Neurol. 1991;48:1300–3. doi: 10.1001/archneur.1991.00530240106031. [DOI] [PubMed] [Google Scholar]
- 33.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 34.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–39. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 35.Cowan N. Working memory capacity. New York: Psychology Press; 2005. [Google Scholar]
- 36.Ericsson KA, Kintsch W. Long-term working memory. Psychol Rev. 1995;102:211–45. doi: 10.1037/0033-295x.102.2.211. [DOI] [PubMed] [Google Scholar]
- 37.Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. New York: Cambridge University Press; 1999. [Google Scholar]
- 38.Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol General. 1979;108:356–88. [Google Scholar]
- 39.Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- 40.Jansma JM, Ramsey NF, de Zwart JA, van Gelderen P, Duyn JH. fMRI study of effort and information processing in a working memory task. Hum Brain Map. 2007;28:431–40. doi: 10.1002/hbm.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankenstein U, Wennerberg A, et al. Activation and deactivation in blood oxygenation level dependent functional magnetic resonance imaging. Concepts in Magnetic Resonance, A. 2003;16:63–70. [Google Scholar]
- 42.Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Map. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]