Abstract
Objectives:
This study evaluated whether sleep over the first 6 months of life was more disturbed in infants born to mothers who were depressed compared with infants from nondepressed mothers.
Design:
Actigraphy was recorded for 7 consecutive days starting at 2 weeks postpartum and monthly thereafter until 6 months of age. Mothers completed daily sleep/wake diaries. Sleep data at 2 weeks and 6 months postpartum are presented here.
Setting:
The home environment.
Participants:
Eighteen healthy, full-term infants, 9 males and 9 females. Seven infants were born to women with no personal or family history of depression; 11 infants were born to women diagnosed with depression or with elevated levels of depression symptoms.
Interventions:
N/A.
Measurements and Results:
Total sleep time, sleep latency, sleep efficiency, and number and duration of sleep episodes were computed for nocturnal and daytime sleep in each 24-hour block. Data were coded for risk group (1 = low risk, 2 = high risk), and repeated-measures multivariate analysis of variance contrasted changes in sleep measures at Week 2 and Week 24, between risk groups. The high-risk infants took longer to fall asleep, had lower sleep efficiencies, and had more sleep bouts in the nocturnal sleep period than did low-risk infants. These effects persisted at 6 months postpartum.
Conclusions:
Maternal depression is associated with significant sleep disturbance in infancy at 2 weeks postpartum that continues through 24 weeks. It remains to be determined if sleep disturbance in infancy confers a greater risk of developing early-onset depression in childhood.
Citation:
Armitage R; Flynn H; Hoffmann R; Vazquez D; Lopez J; Marcus S. Early developmental changes in sleep in infants: the impact of maternal depression. SLEEP 2009;32(5):693-696.
Keywords: Infant sleep, circadian rhythms, depression, actigraphy, napping
MAJOR DEPRESSIVE DISORDERS (MDD) ARE ASSOCIATED WITH SIGNIFICANT SLEEP DISTURBANCES, INCLUDING PROLONGED SLEEP LATENCY, REDUCED total sleep time (TST), increased wakefulness after sleep onset (WASO), and in the timing and distribution of rapid eye movement (REM) and non-REM (NREM) sleep stages. Although the findings are more equivocal in children and adolescents with MDD, several large studies have shown both insomnia and hypersomnia in early-onset MDD.1 Weak circadian rest-activity cycles and poor synchronization of sleep electroencephalographic rhythms are also evident in early-onset MDD. In some studies, damped-amplitude rest-activity cycles were evident in MDD children as young as 6 years of age.2, 3 Anecdotal parent reports indicated that these children had a long history of sleep disturbance, suggesting to us that initial entrainment of sleep and circadian rhythms in infancy may play a role. Further, there is evidence that sleep disturbances may be an antecedent of depression with REM sleep and temporal coherence abnormalities in those with a family history of depression but no personal illness.4
These findings prompted us to evaluate sleep and circadian entrainment in infancy in those with and without a positive maternal history of MDD. Previous work has identified 2 to 4 months of age as a key period in the development of sleep/wake cycles and melatonin and temperature rhythms.5 Thus, we studied infants from 2 weeks postpartum through the first 6 months of life.
METHODS
Mothers were recruited during the last trimester of pregnancy, through perinatal mood disorders or obstetrics clinics at the University of Michigan. Two risk groups (defined below) were matched on ethnicity, age, socioeconomic status, and level of education. As a whole, the group was university educated, professional, and upper middle class. Structured Clinical Interviews for DSM-IV (SCID) were conducted in the last trimester of pregnancy. Edinburgh Postnatal Depression Scale (EPDS)6 and the Beck Depression Inventory (BDI-II)7 were administered at enrollment or within 2 weeks postpartum. Inclusion in the high-risk group required past or present MDD according to DSM-IV diagnostic criteria based on the SCID or an EPDS of 10 or greater.
Eighteen infants participated in study. Seven infants were born to women with no personal or family history of depression, identified as the low-risk group. Eleven infants were born to high-risk mothers, 5 of whom were in an MDD episode at enrollment. Six infants were born to women with past MDD or EPDS greater than 10. Demographic data are summarized in Table 1. All infants were healthy at birth and full-term except for 2 infants born at 36.5 weeks gestation, 1 in each risk group. The majority of infants were breastfed throughout the study. Demographic characteristics of the sample are summarized in Table 1.
Table 1.
Demographic Characteristics of the Low- and High-Risk Sample
| Characteristic | Low Risk n = 7 |
High Risk n = 11 |
|---|---|---|
| Gestational age, wks | 39.5 ± 0.9 | 38.7 ± 1.1 |
| Birth weight, lb | 7.18 ± 0.3 | 7.38 ± 0.4 |
| Females | 4 (57.1) | 5 (45.4) |
| Breastfeeding | 6 (85.7) | 10 (90.9) |
| With siblings | 3 (42.8) | 7 (63.6) |
| In daycare | 5 (71.4) | 8 (72.7) |
| BDI-II at Wk 2a | 3.9 ± 1.2 | 17.8 ± 8.7 |
| Psychotropic useb | 1 (14.3) | 6 (54.5) |
| Cosleeping in parents' bedc | 1 (14.3) | 1 (9.1) |
| Sleeping in parents' roomc | 2 (28.6) | 2 (18.2) |
Data are presented as mean ± SD or number (%).
Refers to the score on the Mother's Beck Depression Inventory-II (BDI-II) at postpartum Week 2.
Refers to mothers' use of psychotrophic drugs during pregnancy. In the low-risk group, 1 mother used venlafaxine for treatment of panic disorder; in the high-risk group, 3 mothers used citalopram, 2 used escitalopram, and 1 used sertraline and clomipramine.
Missing data in 3 low-risk and 5 high-risk dyads
Sleep measures were derived from light- and motion-sensor actigraphy (AW64TM; Philips Respironics, Bend, OR) recorded for 7 consecutive days and nights in infants and mothers at 2 weeks postpartum and monthly thereafter for 6 months. Mothers wore the actigraphs on the nondominant wrist, and the infants wore the actigraphs on their ankles. Custom-made flannel straps were used for infants' actigraphs to minimize skin irritation. A second actigraph was attached to a small stuffed toy with a clip to ensure that light levels could be monitored even when the infants' legs were covered. Mothers were instructed to keep the toy with the infant at all times and to only remove the infant's actigraph during bathing. Mothers completed daily sleep/wake diaries, including information on when and where the actigraph was removed.
Actigraphs were set at a 1-minute sampling rate with a low motion-sensitivity threshold, corresponding to 0.01-g force following previously published convention.6 Light sensors were set to detect 0.1 to 150,000 lux. Actigraphs were calibrated for accuracy with a photometer and mechanical wheel prior to each recording session. Sleep variables were derived from the Mini Mitter Actiware 5TM software (Philips Respironics). Nocturnal sleep latency was defined as the first 10 minutes of no movement after the mother-defined sleep-diary entry of bedtime. Time in bed (TIB) was defined from bedtime to morning awakening, verified by diary entry. TIB minus sleep latency was defined as the total sleep period (TSP). TST was defined as the length of time, in minutes, from sleep onset to the morning awakening. Sleep efficiency was computed as TST relative to TIB, excluding time to sleep onset. TST, number of sleep episodes, sleep latency, and sleep efficiency were averaged across 24-hour blocks. The sleep variables were also divided into nocturnal sleep time (22:00-08:00) and daytime sleep (08:00-22:00), and averages were computed across the 7 days of recording for each infant.6
By examining the epoch by epoch sleep/wake scores for each infant, we determined that the factory default of a 5-minute minimum duration and a 30-minute maximum duration were not accurately assessing daytime sleep, during which periods could extend to 1 hour or more. We adjusted the duration criteria to the maximum of 180 minutes with a 20-minute interval required to begin a new sleep episode for all infants at all recording periods. We verified the settings with nap-diary data. We also analyzed data by the factory default settings, and although the number and duration of naps was higher with the factory default, mean differences between high- and low-risk groups were virtually identical with default and user-defined settings.
Data from the 2- and 24-week recording periods are reported here primarily to conserve statistical degrees of freedom and to allow for the possibility that a developmental delay may have been evident in the high-risk group. A significant overall risk or time-period effect from multivariate analysis of variance (MANOVA), including all sleep measures was required before ANOVAs were computed on individual variables. Time period by risk group interactions were tested first, followed by time and risk group main effects when appropriate. Sex of the infant and maternal medication use during pregnancy were also used as statistical covariates in the analyses. Exact probabilities are reported below, but only those effects significant by Bonferroni adjusted experiment-wise probability of .05 are included, to protect against Type I errors. Cohen d effect sizes were also computed for each significant result. For reference, effects sizes greater than 0.8 are considered large.
RESULTS
No sex or medication-exposure main effects or interactions were evident by analysis of covariance for BDI-II or sleep measures, even before Bonferroni correction. As a result, there was no further analysis of these measures. The F ratios and effect sizes reported below are based on ANOVA outcomes, excluding covariates.
Symptom severity, as measured by the BDI-II, was significantly higher in the high-risk women compared with the low-risk group (17.8 ± 8.7 vs 3.9 ± 1.2 respectively; F1,16 = 17.6, P < 0.001) with an effect size of 2.25, confirming greater depression among the mothers in the high-risk group.
With regard to the infant sleep analysis, the first step was to evaluate differences within the high-risk group, comparing those infants whose mothers were in an index episode of depression at the time of enrollment (n = 5) with those who had mothers with a past history of MDD or who had elevated levels of depressed symptoms (n = 6) at the time of enrollment. No significant differences were obtained for any of the sleep measures (range of P = 0.26-0.89). Data were then combined into a single high-risk group of 11 infants for comparison with the 7 low-risk infants.
Average sleep time in 24 hours did not differ by risk group at 2 or 24 weeks (F1,15 = 2.3 P < 0.13). Low-risk infants had 547.7 ± 45.7 minutes of sleep at Week 2 and 523.7 ± 48.2 minutes at Week 24, compared with 520 ± 90.1 minutes at Week 2 and 502.8 + 32.5 minutes at Week 24 in the high-risk group. The distribution of sleep, however, differed significantly between groups. Nocturnal TST was 97 minutes longer in the low-risk infants at both recording periods, as evidenced by a risk-group main effect (F1,15 = 10.7, P < 0.007) with an effect size of 1.6. Further, the high-risk infants had significantly more nocturnal sleep episodes (indicating more awakenings during the night) than the low-risk infants at Week 2 (4.0 vs 1.9). Although both groups showed a reduction in the number of nocturnal sleep episodes, the high-risk infants continued to show more sleep episodes (2.8 vs 1.3). Both the risk (F1,15 = 49.9, P < 0.0001) and time (F1,15 = 9.85, P < 0.005) main effects were significant, with no interaction. The effect sizes were 3.5 and 1.2, respectively.
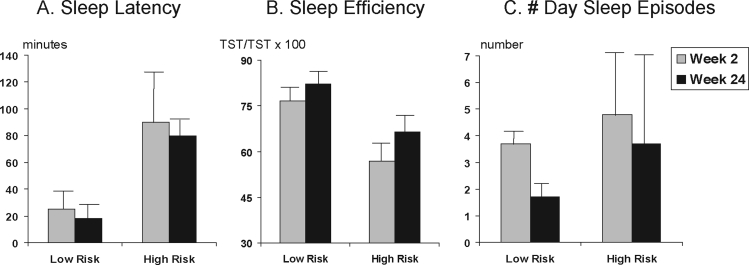
Nocturnal sleep latency was also significantly longer by more than 1 hour in the high-risk group at both 2 and 24 weeks, generating a risk main effect (F1,15 = 19.2, P < 0.0005) with an effect size of 2.36. This risk group difference is illustrated in Figure 1A.
Figure 1.
Means and standard deviations (error bars) in (A) nocturnal sleep latency, (B) nocturnal sleep efficiency, and (C) number of sleep episodes during the day at 2 and 24 weeks postpartum in 7 infants from nondepressed, low-risk mothers (low risk) and 11 infants from mothers with depression or who had significant symptoms of depression (high risk). TST refers to total sleep time.
Sleep efficiency, an additional index of awakenings from sleep, was also significantly lower in the high-risk group at both Week 2 and 24, as evidenced by a significant group effect (F1,15 = 16.8, P < 0.0009) with an effect size of 2.3. The risk-group difference can be seen in Figure 1B. The improvement in sleep efficiency from Week 2 to 24 was not significant.
Daytime sleep measures were also compared across groups. Total day sleep time did not differ between groups. As seen with nocturnal sleep, high-risk infants had significantly more daytime sleep episodes of a shorter average duration. The number of daytime sleep episodes showed a significant risk-group effect (F1,15 = 34.6, P < 0.0001) with an effect size of 3.04. The time main effect was also significant (F1,15 = 58.3, P < 0.0001), with a very large effect size (3.9). No significant time by risk interaction was obtained. Both groups of infants showed a decrease in the number of daytime naps from Week 2 to 24, as sleep became more consolidated to the nocturnal period. However, the high-risk group continued to show significantly more daytime sleep episodes than the low-risk infants. These effects can be seen in Figure 1C.
DISCUSSION
Sleep was more disturbed, based on several indexes, in the infants of mothers with depression risk as compared with low-risk infants. Nocturnal sleep latency was longer, sleep episodes shorter, and sleep efficiency lower in the high-risk group at 2 weeks postpartum, an effect that persisted at 24 weeks. The high-risk infants also had more daytime sleep than the low-risk infants at both 2 and 24 weeks, suggesting a delay in the consolidation of sleep to the nocturnal period in the high-risk infants. Further, there were more day and night sleep episodes in the high-risk infants, indicating multiple awakenings throughout sleep periods and, perhaps, an inability to sustain sleep. The effect sizes were very large for the sleep measures, indicating statistical robustness despite the small sample size. ANCOVA indicated that neither the sex of the infant nor medication exposure influenced the differences between risk groups.
That sleep is more disturbed in infants from depressed mothers is consistent with previous work on behavioral assessment of sleep, indicating that quiet sleep is decreased and fussing during sleep episodes is higher in infants from depressed mothers compared with those with no maternal history of depression.8 Previous work has also shown that decreased sleep duration is also associated with difficult temperament over the first year of life, even in infants without maternal depression,9 further supporting the relationship between sleep and mood. Although it is not clear from the current study how maternal depression influences sleep in infants, it is clear that it is not just current symptomatology that is associated with poor sleep in infants. The high-risk group of infants in this study included both those whose mothers were in an index episode at the time of enrollment and those whose mothers had only a past history of depression. It remains to be determined if the infants designated as high risk do go on to develop depression at rates higher than the general population. Nevertheless, differences in mother-infant bonding, parenting styles, genetics, environmental noise, light exposure, or other factors could be contributing to the observed sleep differences. However, the sleep disturbances in the high-risk group were already evident at 2 weeks postpartum, making it unlikely that differences in maternal behavior explained the results. Alternatively, several studies have identified increased cortisol levels during pregnancy and after delivery in depressed mothers, suggesting that the maternal hypothalamic pituitary adrenal axis in utero may impact infant sleep. This would serve to prime the stress response in infants and subsequently interfere with the initiation and maintenance of sleep.9
Although there are many environmental and social factors that can influence infant sleep and behavior, this study is a first step toward characterizing the influence of maternal depression. Future work with a larger sample size is necessary to evaluate what other factors influence sleep in infancy and whether those high-risk infants with the most disturbed sleep go on to develop early-onset depression. If so, it will be necessary to determine whether sleep in infancy is modifiable and to define the optimal conditions for entrainment of sleep to the nocturnal period. There is already evidence to indicate that education and behavior sleep interventions improve sleep in both mothers and infants.10
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Armitage has received discounted actigraphs from Mini Mitter division of Respironics. Dr. Flynn has consulted for the state of Michigan to conduct psychotherapy training. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We sincerely appreciate the data-collection efforts and research support of Holli Bertram, Jared Fordyce, Susan Hamilton, Rena Menke, Joan Stephens, and Katrina Wilburn. Most of all, we thank the dedication of the mothers who volunteered for this study.
This research was supported by the Jack L. and Barbara A. Berman Depression Center Fund and the Cohen Family Fund.
REFERENCES
- 1.Robert J, Hoffmann R, Emslie G, et al. Gender and age differences in sleep macroarchitecture in childhood and adolescent depression. Sleep. 2005;29:351–8. doi: 10.1093/sleep/29.3.351. [DOI] [PubMed] [Google Scholar]
- 2.Armitage R, Hoffmann R, Emslie G, Rintelmann J, Moore J, Lewis K. Rest-activity cycles in childhood and adolescent depression. J Am Acad Child Adolesc Psychiatry. 2004;43:761–9. doi: 10.1097/01.chi.0000122731.72597.4e. [DOI] [PubMed] [Google Scholar]
- 3.Armitage R, Hoffmann R, Emslie G, Rintelmann J, Robert J. Sleep microarchitecture in childhood and adolescent depression: temporal coherence. Clin Electroencephalogr Neurosci. 2006;37:1–9. doi: 10.1177/155005940603700103. [DOI] [PubMed] [Google Scholar]
- 4.Morehouse RL, Kusumakar V, Kutcher SP, LeBlanc J, Armitage R. Temporal coherence in ultradian sleep EEG rhythms in a never-depressed, high-risk cohort of female adolescents. Biol Psychiatry. 2002;51:446–56. doi: 10.1016/s0006-3223(01)01297-5. [DOI] [PubMed] [Google Scholar]
- 5.McGraw K, Hoffmann R, Harker C, Herman JH. The development of circadian rhythms in a human infant. Sleep. 1999;22:3. doi: 10.1093/sleep/22.3.303. [DOI] [PubMed] [Google Scholar]
- 6.So K, Buckley P, Adamson TM, Horne RSC. Actigraphy correctly predicts sleep behavior in infants who are younger than six months, when compared with polysomnography. Pediatr Res. 2005;58:761–6. doi: 10.1203/01.PDR.0000180568.97221.56. [DOI] [PubMed] [Google Scholar]
- 7.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of the Beck Depression Inventories IA and II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 8.Field T, Diego M, Hernandez-Reif M, Figueiredo B, Schanberg S, Kuhn C. Sleep disturbances in depressed pregnant women and their newborns. Infant Behav Dev. 2007;30:127–33. doi: 10.1016/j.infbeh.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Spruyt K, Aitken RJ, So K, Charlton M, Adamson TM, Horne RSC. Relationship between sleep/wake patterns, temperment and overal development in term infants over the first year of life. Early Hum Dev. 2008;84:289–96. doi: 10.1016/j.earlhumdev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Stremler R, Hodnett E, Lee K, et al. A behavioral-educational intervention to promote maternal and infant sleep: a pilot randomized, controlled trial. Sleep. 2006;29:1609–15. doi: 10.1093/sleep/29.12.1609. [DOI] [PubMed] [Google Scholar]