Abstract
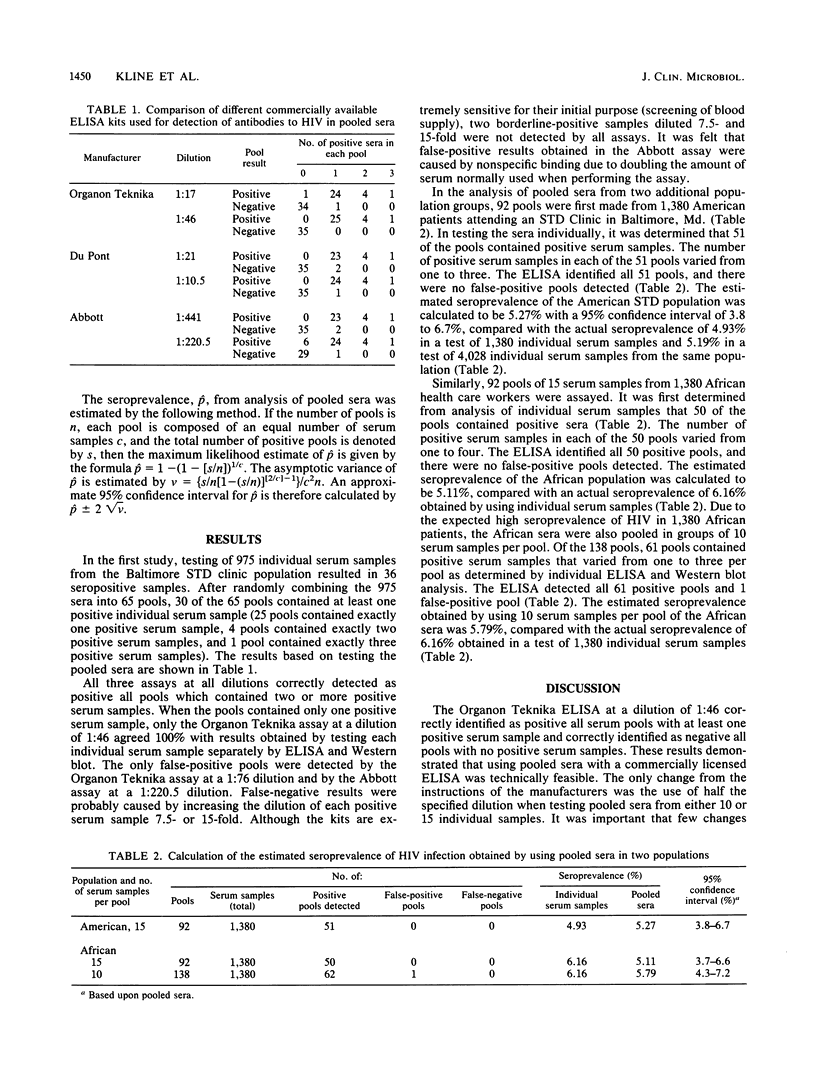
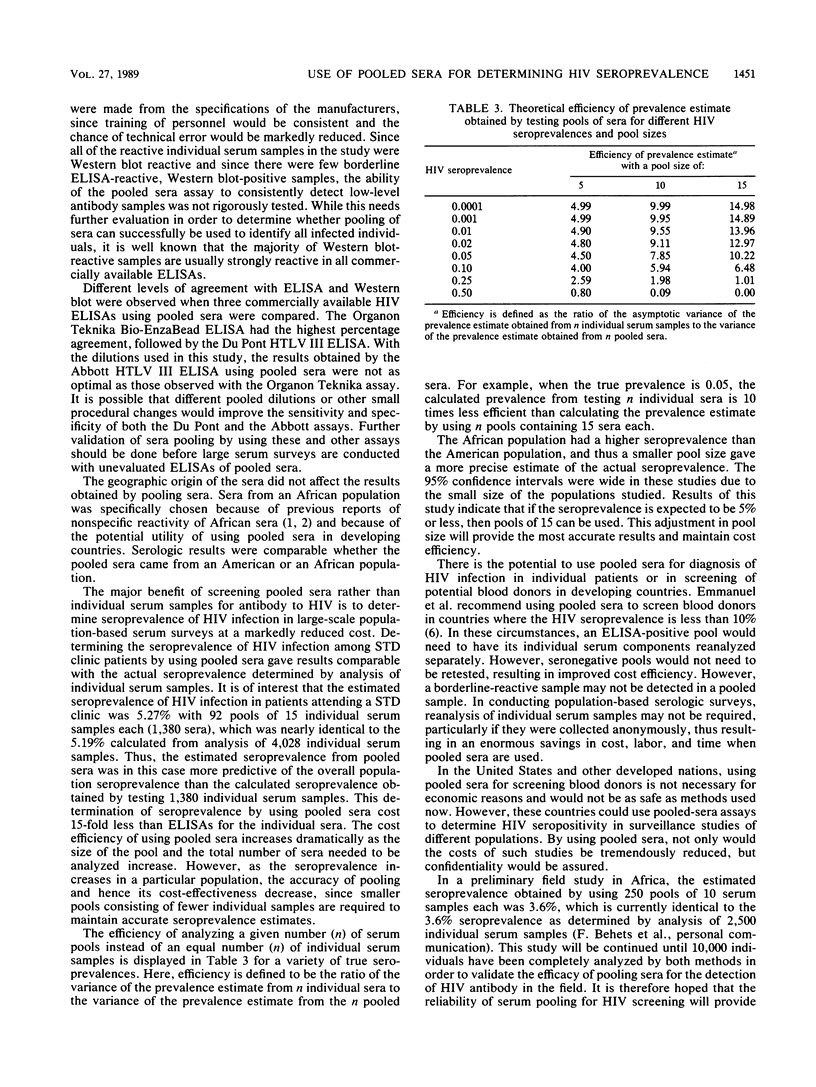
The pooling of individual serum samples to determine human immunodeficiency virus (HIV) seropositivity was examined to assess whether testing pooled sera was technically feasible, cost-effective, and accurate for estimating seroprevalence in large population surveys. The sensitivities and specificities of three commercially available HIV enzyme-linked immunosorbent assay (ELISA) kits were tested using 65 serum pools of 15 individual serum samples each (975 total serum samples) at two different dilutions. With pooled sera, the Organon Teknika Bio-EnzaBead ELISA at half the dilution recommended by the manufacturer showed the best agreement with ELISA and Western blot results of individual sera. In subsequently testing 92 pools, each containing 15 individual serum samples from a population of American patients attending a sexually transmitted diseases clinic, the estimated seroprevalence was 5.27 compared with 4.93% in a test of 1,380 individual serum samples and 5.19% in a test of 4,028 individual serum samples from the same population. In an evaluation of 1,380 African patients using 10 serum samples per pool, the estimated seroprevalence was 5.79 compared with 6.16% in a test of individual sera. These results indicate that ELISA testing with pooled sera is highly sensitive and specific and appears to be a cost-effective means for estimating HIV seroprevalence in large population-based surveys.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggar R. J., Gigase P. L., Melbye M., Kestens L., Sarin P. S., Bodner A. J., Demedts P., Stevens W. J., Paluku L., Delacollette C. ELISA HTLV retrovirus antibody reactivity associated with malaria and immune complexes in healthy Africans. Lancet. 1985 Sep 7;2(8454):520–523. doi: 10.1016/s0140-6736(85)90461-1. [DOI] [PubMed] [Google Scholar]
- Biggar R. J. The AIDS problem in Africa. Lancet. 1986 Jan 11;1(8472):79–83. doi: 10.1016/s0140-6736(86)90728-2. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Brundage J. F., Redfield R. R., Damato J. J., Schable C. A., Putman P., Visintine R., Kim H. I. Measurement of the false positive rate in a screening program for human immunodeficiency virus infections. N Engl J Med. 1988 Oct 13;319(15):961–964. doi: 10.1056/NEJM198810133191501. [DOI] [PubMed] [Google Scholar]
- Emmanuel J. C., Bassett M. T., Smith H. J., Jacobs J. A. Pooling of sera for human immunodeficiency virus (HIV) testing: an economical method for use in developing countries. J Clin Pathol. 1988 May;41(5):582–585. doi: 10.1136/jcp.41.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert J. J. Testing for human immunodeficiency virus. Ann Intern Med. 1986 Oct;105(4):609–610. doi: 10.7326/0003-4819-105-4-609. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Mann J. M., Curran J. W., Piot P. AIDS in Africa: an epidemiologic paradigm. Science. 1986 Nov 21;234(4779):955–963. doi: 10.1126/science.3022379. [DOI] [PubMed] [Google Scholar]


