Abstract
Purpose
Corneal collagen cross-linking through UVA-riboflavin photochemistry (UVAR) has been shown to be an effective treatment for keratoconus and related keratectasias. In recent studies using sclera, the authors observed that short-chain aliphatic β-nitro alcohols can cross-link collagenous tissue under physiologic conditions. Thus, this study was undertaken to evaluate these agents as potential pharmacologic alternatives to UVAR.
Methods
Porcine corneal strips (8 × 4 mm) and corneoscleral complexes were cross-linked using 1 to 100 mM 2-nitroethanol (2ne), 2-nitro-1-propanol (2nprop), and 3-nitro-2-pentanol (3n2pent) at pH 7.4, 34°C. Cross-linking by UVAR was carried out for comparison. Thermal shrinkage temperature analysis was used to evaluate cross-linking effects, and changes in corneal light transmission were determined with a fiber-optic spectrophotometer.
Results
At 10 and 100 mM for 96 hours, initial shrinkage temperature (Ti) was shifted by 3.3°C (P < 0.001) and 9.8°C (P < 0.001) for 2ne, 2.9°C (P = 0.008) and 4.9°C (P < 0.001) for 2nprop, and 3.8°C (P = 0.003) and 4.9°C (P < 0.001) for 3n2pent. Reacting at 1 mM through daily exchange of fluid over 7 days shifted Ti by 3.8°C (P < 0.001), 4.4°C (P = 0.002), and 3.2°C (P = 0.005), for 2ne, 2nprop, and 3n2pent, respectively. These shifts were greater than cross-linking using UVAR (Ti = 1.9°C; P = 0.012). In the blue light region (400−500 nm), transmission was decreased by 5.6% (P = 0.003), 2.1% (P = 0.260), and 0% (P = 0.428) for 2ne, 2nprop, and 3n2pent, respectively.
Conclusions
β-Nitro alcohols can induce corneal cross-linking in vitro better than the UVAR technique and can induce negligible effects on light transmission. These early results suggest that such compounds could be used as topical stiffening agents for keratoconus and related disorders.
Keratoconus has an estimated incidence from 1 in 435 to 2000 in the general population. In its classical form, keratoconus begins at puberty and progresses to the third to fourth decade of life.1 Thus, its overall impact is magnified by virtue of the younger population affected. Although the underlying etiology of keratoconus remains unclear, collagen interlamellar or intralamellar slippage is likely to play a role2 in the ultimate loss of biomechanical strength that occurs.3 Clinically, the disease is marked by progressive thinning of the corneal stroma with resultant bulging and distortion of the thinned, weakened areas. The bulging, distorted cornea creates an optically imperfect surface to the eye that produces increasingly irregular astigmatism and myopia. Current treatment options either mask the surface irregularity with a variety of contact lenses or attempt to improve the surface contour with intracorneal ring segments, lamellar keratoplasty, or excimer laser surgery.4
Corneal collagen cross-linking has recently been introduced as an effective, minimally invasive means to treat progressive keratoconus. The seminal works of Seiler and his coauthors, Spoerl et al.5 and Wollensak et al.,6 in the past decade have outlined the usefulness of photochemical cross-linking with UVA irradiation (λmax = 370 nm) with riboflavin as a photosensitizer (UVAR). This process is described by clinicians as CXL, an abbreviation for collagen cross-linking. Clinical trials are ongoing in Europe7-9 with encouraging progress reports. For example, after 5 years in the Dresden study, participants who have undergone this treatment protocol have shown no progression of keratoconus. With these exciting results, corneal cross-linking therapy has been extended to include patients with related disorders such as postsurgical ectasia, corneal edema, and corneal melting.10
Despite these successes, UVAR therapy poses attendant risks, particularly related to ultraviolet irradiation. As such, this therapy has only recently been granted approval by the Food and Drug Administration for the start of clinical trials in the United States. Because free oxygen radical formation occurs with riboflavin photolysis,11 this cross-linking method has a negative impact on cell viability. Indeed, keratocyte12 and corneal endothelial cell toxicity13 do occur with application of this therapy to the cornea. In lieu of such toxicity, it has been recommended that patients with particularly thin (<400 μm) central corneas not undergo this therapy because the depth of UVA penetration potentially exposes the endothelial cells to toxic photochemical damage. That said, several groups have recently promoted the use of hypo-osmotic riboflavin drops as a means of temporarily swelling the cornea before UVA irradiation. In this way, it may be possible to protect the underlying corneal endothelium during cross-linking of thin (<400a μm) corneas.
The possibility of using a topical self-administered compound to produce a comparable degree of collagen cross-linking to UVAR therapy has several potential benefits. First, if no UVA irradiation is necessary, this could lessen the degree of cytotoxicity, allowing ophthalmologists to offer routine cross-linking treatment to patients regardless of corneal thickness (<400 μm). Second, patients would experience less discomfort because epithelial debridement would not be necessary (as it is with UVAR therapy). Third, because the compound could perhaps be self-administered, patients would benefit from the ease of application and the reduced costs. Fourth, dose modulation could supply an effect of controlled magnitude rather that the single effect currently produced with the UVAR procedure. Fifth, more complete cross-linking may be possible, particularly if water-soluble cross-linking compounds that can diffuse easily through the corneal stroma are used. The UVAR method cross-links only the anterior 200 μm of cornea, which correlates with the depth of penetration of UVA irradiation into the riboflavin-soaked cornea.14 Finally, a pharmacologic approach to corneal cross-linking could allow for treatment of the peripheral cornea in processes such as pellucid marginal degeneration. Cross-linking of the limbal region with the UVAR approach is prohibited because of the risk for damage to limbal stem cells.
In their early studies, Spoerl,5 Seiler,15 and Wollensak16 explored the possibility of using chemical cross-linking agents for corneal stabilization. They include some well-known cross-linking agents such as glutaraldehyde, formaldehyde, glyceraldehyde, ribose, and glucose.5,15,16 However, this line of investigation appears to have been largely abandoned because of concerns regarding compound toxicity and efficacy. Glyceraldehyde was singled out as a compound that could potentially provide efficacious cross-linking with low toxicity. Indeed, a recent study describes the use of glyceraldehyde for scleral cross-linking administered through retrobulbar injection in live rabbits.17
Our studies indicate that short-chain aliphatic β-nitro alcohols (Fig. 1) may be useful for in vivo tissue cross-linking. Shifts in thermal shrinkage temperature, a general index of nonenzymatic collagen cross-linking, were observed in studies using porcine and human sclera as a collagenous tissue substrate.18 The present study is an extension of those previous studies. Herein, we examine the changes in thermal shrinkage temperature effects of corneal tissue induced by these aliphatic β-nitro alcohols and characterize the effects on light transmission. The results indicate that under conditions of physiologic pH and temperature, corneal tissue cross-linking is feasible and has negligible effects on light transmission.
Figure 1.
Structures of the short-chain aliphatic β-nitro alcohols (A) 2-nitroethanol, (B) 2-nitro-1-propanol, and (C) 3-nitro-2-pentanol.
Materials and Methods
2-Nitroethanol (2ne), 2-nitro-1-propanol (2nprop), 3-nitro-2-pentanol (3n2pent) [mixture of (±) threo and (±) erythro], dextran T500, NaH2PO4, Na2HPO4, penicillin (5000 U/mL)/streptomycin (5000 μg/mL), and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma Chemical (St. Louis, MO). Millipore water was used in all experiments.
Reaction Conditions Using β-Nitro Alcohols
Fresh porcine eyes (6-month-old animals) with intact epithelia were obtained from Hatfield Meat Packing (Hatfield, PA) within 6 hours of kill, in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Strips (8 × 4 mm) from a superior to an inferior orientation in the central region were obtained from the corneas of approximately 100 eyes. Two strips could be obtained from each cornea. In all cases, the incubation solution consisted of 20% dextran (T500; to prevent tissue swelling) in 0.2 M NaH2PO4/Na2HPO4 (pH 7.4) with various concentrations (1, 10, and 100 mM) of β-nitro alcohols. Penicillin/streptomycin (10 μL/mL) was included to prevent bacterial growth, and EDTA (1 mM) was included to prevent endogenous proteolysis. Tissue samples were placed in 2-mL Eppendorf tubes containing 1 mL incubation solution. For light transmission studies, corneoscleral complexes containing a 0.5-cm ring of sclera were dissected from the intact globes and placed in the bottom of a 50-mL conical plastic centrifuge tube containing 5 mL incubation solution. Samples were then placed in a water bath and allowed to react for various reaction times at 34°C, which has been reported as the in vivo temperature of corneal stroma.19 After incubation, the samples were evaluated through thermal shrinkage temperature analysis to determine the degree of cross-linking. At these concentrations, pH is well maintained over the course of 96 hours and does not drift below 7.35. Corneas incubated at 34°C using the standard incubation solution without cross-linking agents for 96 hours were used as control samples and were compared with fresh tissue samples.
Experiments conducted with 1 mM concentrations were conducted in a slightly different manner. For these studies, the incubation solution was exchanged daily over the course of 7 days. Our initial studies using 1 mM concentrations indicated that this concentration was ineffective at inducing cross-linking over 96 hours without fluid exchange. Based on cell toxicity studies with cultured bovine corneal endothelial cells, we determined that 1 mM was a concentration tolerated by those cells.20 Thus, we wanted to determine whether a shrinkage temperature shift could be induced with a concentration that could be tolerated by corneal endothelial cells.
UVAR Cross-Linking
In vitro tissue cross-linking through riboflavin photolysis was carried out according to the protocol of Wollensak et al.21 Corneal tissue strips were prepared from fresh porcine globes, as described, for β-nitro alcohol cross-linking. Corneas were deepithelialized using a blunt spatula. Tissue was soaked for 5 minutes in a solution containing 20% dextran (T500), 0.2 M NaH2PO4/Na2HPO4 (pH 7.4), and 0.1% riboflavin-5-phosphate (Sigma) and was placed under a double diode at a distance of 1 cm with output maxima at 370 nm for 30 minutes. Before and after the irradiation period, the diode output was measured with a UV light meter (HHUVA 1; Omega, Stamford, CT) with a wavelength peak of 365 nm ± 5 nm and was maintained between 5 and 6 mW/cm2.
Thermal Shrinkage Temperature Analysis
Thermal shrinkage temperature (Ts) or denaturation temperature analysis is a technique that has been used for decades in studying collagen cross-linking. Basically, as collagen fibers are progressively heated, a threshold temperature is reached at which disruption of the hydrogen bonding in the collagen molecule triple helix occurs, resulting in unwinding of the triple helices. The denaturation of tertiary protein structure results in rapid shrinkage of up to 80% of the tissue. This phenomenon can be expressed in various ways, including temperature at onset of tissue shrinkage,22 temperature at maximal change in tissue shrinkage,23 and temperature at shrinkage to one-third the initial length.24 Differential scanning calorimetry (DSC) is related to thermal shrinkage temperature analysis and has been widely used to evaluate biomaterial cross-linking.25 Thermal shrinkage temperature is essentially a simplified version of DSC and can be used effectively to screen the cross-linking efficacy of compounds.26 We determined the temperature at initial shrinkage (Ti), and the point of maximal rate of change in tissue shrinkage (T50). We use Ts as a general term to indicate thermal shrinkage temperature encompassing both Ti and T50. Graphs depicting the relationship between degree of shrinkage and temperature indicate differences in degree of cross-linking between groups. Thus, stabilization of collagen fiber structures through nonenzymatic cross-linking will increase Ti and T50.27 A 2.5°C upward shift in the onset of tissue shrinkage for the anterior corneal stroma has been shown previously for the UVAR method.28
We used a semiautomated system that increases the efficiency and accuracy of Ts data collection. This system could perform multiple Ts assays concurrently, allowing us to rapidly screen a number of potential cross-linking agents and conditions. The setup consisted of a custom-built polyethylene box into which tissue samples were placed in an insert. Inlet and outlet fittings allowed for constant circulation of heated water, modulated by a digitally controlled water bath equipped with a water pump. The entire box was placed on a digital photograph scanner (model G4010; Hewlett-Packard, Palo Alto, CA) connected to a Windows-based computer. Beginning at 50°C, the temperature was raised at a rate of 1°C/min, and serial scans were taken at every degree increase in temperature up to 80°C. Control pig corneas shrank at approximately 62.5°C and cross-linked tissue at higher temperatures. As the tissue shrank, the images were captured by the scanner. Image files were later analyzed for 2-dimensional area using National Institutes of Health Image J software, and percentage shrinkage calculations were made with a spreadsheet application (Excel XP; Microsoft, Redmond, WA) and with data analysis and technical graphics software (Origin version 6.0; Microcal, Northampton, MA) for graphing purposes.
Statistical Analysis
Each Ts curve (a minimum of three curves for each condition) was individually fitted using Boltzmann sigmoidal fit equation: y = A2 + (A1 − A2)/{1 + exp[(x − x0)/dx]}, where A1 = initial value (left horizontal asymptote), A2 = final value (right horizontal asymptote), x0 = center (point of inflection), and dx = width (the change in x corresponding to the most significant change in y values; Origin version 6.0 [Microcal]). From the best-fit curves (R2 > 0.99) were derived values for temperature at initial shrinkage (Ti), as derived from the temperature corresponding to 1% shrinkage, and temperature at maximal change in shrinkage (T50), as derived from the point midway between the starting size and maximally shrunken size. T50 represents the point of inflection of the sigmoidal curve and was designated T50 because it was 50% of the maximal shrinkage change. Ti values were then used to determine statistical significance by averaging three independent values for each condition and comparing them with controls using nonpaired Student's t-test (assuming normative data). Statistical significance was taken as P ≤ 0.05.
Light Transmission Studies
The effects on corneal light transmission induced by cross-linking with β-nitro alcohols were evaluated using a method adopted from Dillon et al.29 After a 96-hour incubation period at 10 mM, the corneoscleral complexes were mounted between 2 pieces of quartz, previously determined to have no ultraviolet absorption. This cross-linking condition was chosen because it represents a target level of cross-linking as determined by thermal shrinkage temperature. In other words, the tissue cross-linking effects induced by these conditions are commensurate with those reported for UVAR cross-linking and, as such, represent a level of cross-linking that would be predicted to have therapeutic value. The sample was then mounted in a vertical, adjustable-stage apparatus specifically designed to accommodate a spectrometer (PC 1000 Fiber Optic Spectrometer; Ocean Optics, Dunedin, FL). A xenon lamp was used as a light source and was connected to the upper half of the stage. The lower half was connected to a high-sensitivity charge-coupled device (CCD) mounted on a card installed in a personal computer. The CCD array detector captures a full-wavelength spectrum (200−1000 nm).
The absorption spectrum for each sample was recorded and exported (Origin version 6.1; Microcal) for processing. Correction of the absorption spectra for light scattering (Rayleigh and Tyndall) was performed as described by Dillon et al.29 The scattering component was subtracted from the observed absorption spectrum by first fitting the scattering (nonabsorption) portion of the spectrum (e.g., 700−800 nm) to the formula A = aλb, where A is the absorbance, λ is the wavelength, b is the order of the relationship between absorbance and wavelength, and a is a constant. The background signal attributed to scattering for all other wavelengths is estimated using the coefficients determined from the fit. These values are then subtracted from the spectrum to generate the true absorption spectrum. After scatter correction, the transmission spectrum for each absorption spectrum was calculated according to the Beer-Lambert law (T = 10−A × 100%), where T is the transmission and A is the absorbance at each wavelength. Finally, the effects on blue light transmission were determined by integrating the transmission spectra from 400 to 500 nm. A percentage decrease was then calculated with respect to the area calculations using control samples. Each condition was performed in triplicate.
Results
Visual Stiffening Effects
Previous work from Spoerl et al.30 has shown that corneal cross-linking using UVAR induces a dramatic stiffening of tissue that is readily apparent simply by holding the tissue against gravity (i.e., cantilevering). The visual effects using the aliphatic β-nitro alcohols were reminiscent of these effects. As shown in Figure 2, all three compounds were able to stiffen corneal tissue to the extent that the effects were readily apparent by holding the tissue against gravity. At 10 mM concentration, the effects with all three compounds (Figs. 2A-2C) were striking. These tissues maintained a concave shape against gravity in marked contrast to control tissues, which lacked the ability to resist gravity force (Fig. 2D).
Figure 2.

Stiffening effect induced by short-chain aliphatic β-nitro alcohols. Porcine cornea samples were reacted as described for Figure 2. (A) 2-nitroethanol, (B) 2-nitro-1-propanol, and (C) 3-nitro-2-pentanol cross-linked samples all showed significant gross stiffening effects at 10 mM concentration. (D) The control sample had no stiffening and simply “flopped over” when handled with a forceps (i.e., cantilevering).
Thermal Shrinkage Temperature Effects
As shown in Table 1 and Figure 3, 2ne was found to be the most potent cross-linker of the group, followed by 2nprop and 3n2pent. At 10 and 100 mM for 96 hours, the temperature of initial shrinkage (Ti) of porcine cornea was shifted by 3.3°C (P < 0.001) and 9.8°C (P < 0.001) for 2ne, 2.9°C (P = 0.008) and 4.9°C (P < 0.001) for 2nprop, and 3.8°C (P = 0.003) and 4.9°C (P < 0.001) for 3n2pent. Control cornea showed Ti of 62.6°C, which was similar to that (62.5°C) reported by Spoerl et al.28 in 2004. No significant differences were noted when comparing control samples kept at 34°C for 96 hours with fresh controls. Furthermore, the cross-linking effects induced by β-nitro alcohols with corneal tissue were similar to those induced in scleral tissue. In a series of parallel experiments using reaction conditions similar to those reported herein, the temperature shifts in porcine sclera were as follows: at 10 and 100 mM for 96 hours, Ti was shifted by 4.3°C (P < 0.001) and 7.8°C (P < 0.001) for 2ne, 1.5°C (P = 0.009) and 6.6°C (P = 0.001) for 2nprop, and 2.4°C (P = 0.058) and 5.4°C (P < 0.001) for 3n2pent. Complete results of those experiments have been reported separately.18
Table 1.
Thermal Shrinkage Temperature Shifts Induced by β-Nitro Alcohols and UVA Riboflavin in Porcine Corneas
| Compound/Condition | Reagent Concentration | Ti*(°C) ± SE | Ti Δ†(°C) | T50‡(°C) ± SE | T50 Δ§(°C) | P (for Ti) |
|---|---|---|---|---|---|---|
| 2-Nitroethanol | 100 mM (4 d) | 72.4 ± 0.33 | 9.8 | 76.8 ± 0.34 | 8.7 | <0.001 |
| 10 mM (4 d) | 65.9 ± 0.19 | 3.3 | 72.4 ± 0.86 | 4.3 | <0.001 | |
| 1 mM (7 d serial) | 66.4 ± 0.39 | 3.8 | 71.1 ± 0.68 | 3.0 | <0.001 | |
| 2-Nitro-1-propanol | 100 mM (4 d) | 67.4 ± 0.32 | 4.9 | 74.4 ± 0.70 | 6.3 | <0.001 |
| 10 mM (4 d) | 65.5 ± 0.60 | 2.9 | 72.2 ± 0.68 | 4.1 | 0.009 | |
| 1 mM (7 d serial) | 67.0 ± 0.57 | 4.4 | 71.2 ± 0.51 | 3.1 | 0.002 | |
| 3-Nitro-2-pentanol | 100 mM (4 d) | 67.5 ± 0.28 | 4.9 | 73.3 ± 0.36 | 5.2 | <0.001 |
| 10 mM (4 d) | 66.4 ± 0.32 | 3.8 | 70.4 ± 0.54 | 2.3 | 0.003 | |
| 1 mM (7 d serial) | 65.8 ± 0.56 | 3.2 | 70.2 ± 0.76 | 2.2 | 0.005 | |
| UVA riboflavin | 0.1% 30 min | 64.5 ± 0.43 | 1.9 | 68.8 ± 0.28 | 0.7 | 0.012 |
| UVA riboflavin anterior cornea28 | – | 65 | 2.5 | – | – | – |
| Control porcine cornea | – | 62.6 ± 0.05 | 68.1 ± 0.13 | – | – | |
| Control porcine cornea28 | – | 62.5 | – | – | – | – |
Ti, temperature at 1% absolute shrinkage.
Ti Δ, change in Ti as compared to control.
T50, temperature at 50% of maximal shrinkage (or maximal rate of shrinkage change).
T50 Δ, change in T50 as compared to control.
Figure 3.
Concentration-dependent shifts in thermal shrinkage temperature curves using three different aliphatic β-nitro alcohols. (A) Concentration-dependent shift in Ti using 2-nitroethanol. Porcine corneal strips (8 × 4 mm) were incubated in solutions of 20% dextran containing various concentrations of 2ne at pH 7.4. After 96 hours of incubation at 34°C for 10 and 100 mM, the thermal shrinkage temperature was determined. Reaction at 1 mM was carried out over 7 days with daily exchange of the incubation solution. Ts shifts observed for 10 mM and 1 mM were comparable to each other and greater than the effect observed for UVAR cross-linking; 100 mM cross-linking induced a considerably greater shift in Ts. Specific values for Ti and T50 can be found in Table 1. (B) Concentration-dependent shift in Ts using 2-nitro-1-propanol. Similar conditions were used as in (A) except that 2nprop was used as a cross-linking agent. As with 2ne, a concentration-dependent shift in Ts was noted for 2nprop, with 10 mM and 100 mM producing upward shifts in Ti and T50, respectively. Again the effect at 1 mM 2nprop with serial fluid exchange was comparable to 10 mM 2nprop and superior to UVAR cross-linking. (C) Concentration-dependent shift in Ts using 3-nitro-2-pentanol (3n2pent). Similar conditions were used as in (A) except that 3n2pent was used as a cross-linking agent. As with 2ne and 2nprop, there was a concentration-dependent shift in Ts noted for 3n2pent at 10 mM and 100 mM producing upward shifts in Ti and T50, respectively. Reaction at 1 mM with serial fluid exchange induced effects slightly lower than those of the 10-mM samples and was comparable to UVAR cross-linking. Overall, these results indicated that cross-linking with β-nitro alcohols can occur in a cumulative fashion because the shifts in Ts through serial fluid exchange at 1 mM are similar to those observed through reaction at 10 mM for 96 hours without daily fluid exchange. In addition, the shifts in Ts are commensurate and, in most cases, superior to cross-linking with the UVAR technique. (D) Example of sigmoidal curve fitting used to derive Ti and T50 values. To determine statistical significance for each condition, each sample analyzed was individually fitted according to a Boltzmann sigmoidal curve fit. The derived equation was then used to calculate Ti and T50.
The level of cross-linking as estimated by shifts in thermal shrinkage temperature for all three β-nitro alcohols spans a range below and above values previously reported for cross-linking of the anterior corneal stroma with UVAR (2.5°C shift for onset of tissue shrinkage; see Table 1).28 Our comparison studies using the UVAR cross-linking method have shown that photochemical cross-linking produces a mild degree of thermal shrinkage temperature shift by comparison with the β-nitro alcohols. Cross-linking using UVAR induced a Ti shift of 1.9°C (P = 0.01). These shifts were not altered by increasing the photolyzing time from 30 to 60 minutes.
Reacting at 1 mM through daily exchanges of fluid resulted in Ti shifts of 3.8°C (P < 0.001), 4.4°C (P = 0.002), and 3.2°C (P = 0.005) for 2ne, 2nprop, and 3n2pent, respectively. Again, these results are in agreement with experimental data obtained using sclera.18 In those experiments, a time-dependent effect was shown using 2ne. Reacting over the course of 6, 10, and 14 days resulted in increasing shifts in Ti of 0.7°C (P = 0.203), 2.5°C (P = 0.048), and 5.3°C (P = 0.001), respectively. These values, which were obtained through daily exchange of the incubation solution, were comparable to those obtained using higher concentrations of β-nitro alcohols reacted over 96 hours but without daily fluid exchanges. The 1-mM experiments were designed to simulate an in vivo situation in which application of cross-linking agents over a relatively long time course (i.e., weeks) may be used. In this case, cross-linking effects are believed to occur in a cumulative manner. Furthermore, the concentration of 1 mM was chosen to conduct these experiments based on preliminary cell toxicity studies. Recent experiments have been conducted in our laboratory with primary bovine corneal endothelial cells. With this cell culture model, we determined that a 1-mM concentration of 2ne is tolerated by cells after 48 hours of continuous exposure. However, 3 mM resulted in cell death in an all-or-nothing fashion.20
Light Transmission Studies
Initial studies using 100-mM concentrations of 2ne reacted over the course of 96 hours showed a significant yellowing effect. This observation raised a concern that cross-linking using this compound may induce negative effects on blue light perception. At 100 mM concentration, a significant yellowing effect was observed using 2ne such that it was not possible to obtain absorption spectra at this level of cross-linking. However, the 10-mM concentration induced more modest yellowing and was chosen for study. Figure 4 shows the yellowing effect induced by 2ne (Fig. 4A) in pig cornea. This yellowing was not observed when using 2nprop (Fig. 4B) or 3n2pent (Fig. 4C). The rationale for choosing the 10-mM concentration samples for analysis was that this concentration induced cross-linking effects (as determined by thermal shrinkage temperature) commensurate with cross-linking using riboflavin photolysis and, as such, are clinically relevant. Although the highest concentration (100 mM) used in this study shifted the Ts parameter far to the right, this degree of cross-linking would be neither necessary nor desirable in a clinical setting.
Figure 4.
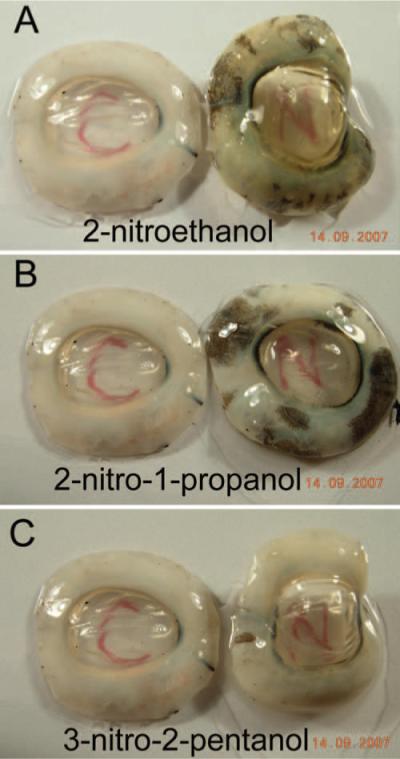
Transparency of porcine corneas cross-linked with aliphatic β-nitro alcohols. Fresh porcine corneoscleral complexes were cross-linked for 96 hours using 10 mM concentrations at pH 7.4, 34°C. Three β-nitro alcohols were tested, (A) 2ne, (B) 2nprop, and (C) 3n2pent. Each panel shows the control sample on the left and the treated sample on the right. The incubation solution contained 20% dextran (T500) to prevent tissue swelling. At this concentration, a slight yellowing effect was most pronounced in the samples treated with 2ne, with minimal effects seen for 2nprop and 3n2pent.
Incubations were set up to maintain equal proportions of tissue to incubation solution. Thus, an 8 × 4-mm tissue strip incubated in 1 mL was roughly one-fifth the size of the corneoscleral ring complexes incubated in 5 mL. In addition, the specific shift in shrinkage temperature induced in the corneoscleral complexes was studied independently by first reacting the corneoscleral complex with a β-nitro alcohol, followed by tissue trimming to the prescribed 8 × 4-mm size, followed by shrinkage temperature analysis. The shifts in Ts using either tissue strips incubated in 1 mL or corneoscleral complexes incubated in 5 mL were in agreement for all three of the β-nitro alcohol compounds tested (data not shown).
Under these conditions, a modest decrease in light transmission was noted for each of the three compounds (Fig. 5). Decreased transmission was greatest for 2ne, followed by 2nprop and 3n2pent. Integration of the 400- to 500-nm blue light region revealed total absorption readings (±SE) in arbitrary units of 7761 (±111) for 2NE, 8046 (±140) for 2nprop, 8288 (±67) for 3n2pent, and 8222 (±44) for controls. This corresponds to changes of −5.6% (P = 0.003), −2.1% (P = 0.260), and +0.8% (P = 0.428) for 2ne, 2nprop, and 3n2pent, respectively (Fig. 6).
Figure 5.
Absorption/transmission spectra of porcine cornea cross-linked with aliphatic β-nitro alcohols. Samples were cross-linked, as described in the Figure 2 legend, with the use of 10 mM β-nitro alcohols for 96 hours. Changes in light absorption/transmission were studied with a fiber-optic spectrometer. (A) Raw absorption data (black) are corrected for light scatter. Small dotted line: scatter correction (y = axb). Dashed line: corrected absorption curve. Arrow: calculated transmission curve, also indicated by the accompanying formula. (B) Examples of corrected absorption and transmission curves for the three β-nitro alcohols studied. The greatest effects were seen using 2ne, followed by 2nprop and 3n2pent.
Figure 6.

Comparison of transmission effects in the blue light region (400−500 nm) induced by three β-nitro alcohols. Transmission spectra such as those shown in Figure 5 were taken from porcine corneas cross-linked with 10 mM 2ne, 2nprop, and 3n2pent at 34°C for 96 hours, as previously described. Integration of the spectra from 400 to 500 nm was performed (n = 6) and compared with controls. The greatest effects were seen for 2ne (−5.6%; P = 0.003), followed by 2nprop (−2.1%; P = 0.260) and 3n2pent (+0.8%; P = 0.428), which did not alter the transmission.
Discussion
Results of the present study indicate that corneal collagen cross-linking through reactions with short-chain aliphatic β-nitro alcohols can occur under conditions of physiologic pH and temperature. Furthermore, a comparison with published values for shrinkage temperature shifts induced in the porcine anterior cornea by UVAR indicated that a comparable degree of cross-linking can be induced through the use of β-nitro alcohols (see Table 1). Spoerl et al.28 reported on the thermal shrinkage temperature changes induced by UVAR in porcine cornea. In that report, the shift in onset of thermal shrinkage (ΔTi) for the anterior stroma was reported to be 5°C, which is considerably higher that what we found. There are two reasons for this discrepancy. First, the method used by Spoerl et al.28 was less precise than ours. Length measurements were taken every 2.5°C. This temperature interval was significantly greater than ours (every 1°C), limiting the precision of the measurements. Second, the onset of shrinkage for the control was taken as the last baseline value (62.5°C). This is in contrast to the UVAR samples, in which the onset of shrinkage was taken as the temperature at which the first change in length was noted (67.5°C). Thus, if the same criteria for onset of shrinkage are taken for controls and UVAR-treated samples in Spoerl et al.,28 then the shift in Ti would be 2.5°C (confirmed with E. Spoerl, personal communication, April 14, 2008). This value of ΔTi = 2.5°C is then in better agreement with the values we determined (ΔTi = 1.9°C).
Previous work using fibroblast-populated collagen gels in a biaxial mechanical tester showed that mechanical stiffness could be induced by cross-linking of the collagen matrix using sodium nitrite. These studies were directed at delineating a potential mechanism for age-related connective tissue protein damage because nonenzymatic collagen cross-linking is a hallmark of the aging process.31 Recent interest in therapeutic collagen cross-linking with riboflavin photochemistry prompted us to explore the possibility of using nitrite and related agents for therapeutic cross-linking. However, because the neutral nitrite reaction is exceedingly slow, we wanted to identify nitrite-related agents that were more efficient cross-linkers at neutral pH. To do so, we selected the assay of thermal shrinkage temperature (which is a relatively simple, straightforward assay), rather than mechanical testing, to screen compounds related to nitrite for cross-linking efficacy. A number of compounds were initially screened using this assay on sclera and included representative compounds from several chemical classes. They include the nitrite esters, diazeniumdiolates, nitro alkanes, aliphatic β-amino alcohols, aromatic β-nitro alcohols, and nitrous acid.18 From the screening, the class of aliphatic β-nitro alcohols was identified. The present work is an extension of this initial screening using the thermal shrinkage temperature assay. Studies are in progress examining the biomechanical strength changes induced by these compounds in corneal tissue and will be the subject of a subsequent report.
One important question that remains unanswered is whether these compounds can be used to access the corneal stroma. Although their size (<10 Å) should not be a limiting factor, they could be excluded based on hydrophilicity. In other words, because these compounds are water soluble, they may not be able to pass through the lipid bilayer of the corneal epithelial cells by passive mechanisms. Several active transport systems are present in the corneal epithelium. They facilitate the transport of various amino acids and other small hydro-philic compounds such as taurine.32 Thus, these active transporters could provide a means for access of these compounds to the corneal stroma. In addition, small molecules can pass through hydrophilic pathways such as intercellular junctions.33 In the stroma, the aqueous environment would predict an even compound distribution through passive mechanisms. To obtain an initial impression regarding the ability of β-nitro alcohols to pass through the corneal epithelium, we performed preliminary permeability experiments with isolated rabbit cornea using a method adopted from Schoenwald et al.34 In these studies apparent permeability (Ptot) was calculated to be Ptot = 1.61 × 10−5 (±4.26 × 10−6) cm/s for 2ne and Ptot = 6.38 × 10−6 cm/s for free nitrite (unpublished observations, February 2008). With these values, it would take approximately 10 minutes to achievea1mM stromal tissue concentration using 100 mM (∼1%) eye drops. These preliminary studies support the notion that small hydrophilic compounds such as 2ne (MWt, 91) can access the corneal stroma through a transepithelial route. Other hydrophilic compounds of comparable size, such as glycerol (MWt, 92), have been studied with regard to corneal permeability. The reported permeability coefficient for glycerol (4.5 × 10−6 cm/s)33 is comparable to the values we obtained in our preliminary studies using 2ne and free nitrite.
The usefulness of these cross-linking agents in vivo will be determined not only by their ability to induce cross-linking in a living eye but by any potential cytotoxic effects. As described in Results, recent cell toxicity studies using bovine corneal endothelial cells suggest that these compounds are relatively well tolerated by cells.20 In addition, published results from other groups suggest a reasonable safety profile for these agents. Related nitro agents have favorable mutagenicity35 and animal toxicity profiles.36 As such, they have been proposed for use in animal feeds to control food-borne pathogens in ruminants and chickens, in which they exhibit bacteriostatic activity.37
The main potential drawback to using a topical pharmacologic means of corneal cross-linking is the inability to localize the effect. Although corneal dam devices have been used, they would be impractical over a sustained period, such as weeks. Thus, a topical corneal stiffening agent over the course of several weeks could lead to a cross-linking of adjacent structure, the effects of which are uncertain. Several structures are present in the region and include the sclera, conjunctiva, lens, iris, and lacrimal duct system. In lieu of this potentially important complication of topical therapy, alternative methods of corneal drug delivery may be necessary not only to diminish the cross-linking effects in adjacent tear film structures, but also to augment corneal stromal compound levels. In this regard, the use of drug soaked collagen shields, hydrogels, or contact lenses may become useful.
Finally, parallel studies examining the cross-linking effects of nitro compounds using sclera as a collagenous tissue substrate have been reported.18 In those studies, an effort was made to determine the chemical mechanisms involved in these reactions. β-Nitro alcohols are well-known compounds that have been used extensively for synthetic processes including industrial polymer applications.38 However, to the best of our knowledge, in vivo tissue cross-linking has not been previously suggested. Although the precise mechanism is not clear at this time based on the work with scleral tissue, nitroalcohols are known to function as formaldehyde donors at neutral pH in industrial applications.38 Further studies are in progress toward understanding more fully the specific chemistry involved. Such information will be critical to efforts aimed at further enhancing the speed and efficiency of the cross-linking effect.
Acknowledgments
The authors thank James P. Dillon for his assistance with the light transmission studies and Eberhard Spoerl for his personal communications regarding UVAR cross-linking. Statistical assistance was provided by the Biostatistics Consulting Service in the Department of Biostatistics of the Mailman School of Public Health at Columbia University Medical Center.
Supported in part by Research to Prevent Blindness and by National Institutes of Health/National Center for Research Resources Grant UL1RR024156 and National Institutes of Health/National Eye Institute Grant R21EY018937 (DCP).
Footnotes
Disclosure: D.C. Paik,P; Q. Wen, None; R.E. Braunstein, None; S. Airiani, None; S.L. Trokel, P
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Meek KM, Tuft SJ, Huang Y, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 3.Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31:435–441. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- 4.Binder PS, Lindstrom RL, Stulting RD, et al. Keratoconus and corneal ectasia after LASIK. J Refract Surg. 2005;21:749–752. doi: 10.3928/1081-597X-20051101-15. [DOI] [PubMed] [Google Scholar]
- 5.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratonconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 7.Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 8.Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 9.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and UVA-light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 11.Baier J, Maisch T, Maier M, Engel E, Landthaler M, Baumler W. Singlet oxygen generation by UVA light exposure of endogenous photosensitizers. Biophys J. 2006;91:1452–1459. doi: 10.1529/biophysj.106.082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollensak G, Spoerl E, Reber F, Seiler T. Keratocyte cytotoxicity of riboflavin/UVA-treatment in vitro. Eye. 2004;18:718–722. doi: 10.1038/sj.eye.6700751. [DOI] [PubMed] [Google Scholar]
- 13.Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003;29:1786–1790. doi: 10.1016/s0886-3350(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 14.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 15.Spoerl E, Seiler T. Techniques for stiffening the cornea. J Refract Surg. 1999;15:711–713. doi: 10.3928/1081-597X-19991101-21. [DOI] [PubMed] [Google Scholar]
- 16.Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg. 2004;30:689–695. doi: 10.1016/j.jcrs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Wollensak G, Iomdina E. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J Cataract Refract Surg. 2008;34:651–656. doi: 10.1016/j.jcrs.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Paik DC, Wen Q, Airiani S, Braunstein RE, Trokel SL. Aliphatic β-nitro alcohols for non-enzymatic collagen cross-linking of scleral tissue. Exp Eye Res. 2008;87:279–285. doi: 10.1016/j.exer.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Girardin F, Orgul S, Erb C, Flammer J. Relationship between corneal temperature and finger temperature. Arch Ophthalmol. 1999;117:166–169. doi: 10.1001/archopht.117.2.166. [DOI] [PubMed] [Google Scholar]
- 20.Paik DC, Wen Q, Braunstein RE, Trokel SL. Short chain aliphatic β-nitro alcohols for corneoscleral cross-linking: feasibility and corneal endothelial toxicity studies. J Refract Surg. 2008;24:S741–S747. doi: 10.3928/1081597X-20080901-19. [DOI] [PubMed] [Google Scholar]
- 21.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 22.Ruijgrok JM, de Wijn JR, Boon ME. Glutaraldehyde crosslinking of collagen: effects of time, temperature, concentration and pre-soaking as measured by shrinkage temperature. Clin Mat. 1994;17:23–27. doi: 10.1016/0267-6605(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 23.Moore MA, Chen WM, Phillips RE, Bohachevsky IK, McIlroy BK. Shrinkage temperature versus protein extraction as a measure of stabilization of photooxidized tissue. J Biomed Mat Res. 1996;32:209–214. doi: 10.1002/(SICI)1097-4636(199610)32:2<209::AID-JBM9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Fathima NN, Madhan B, Rao JR, Nair BU, Ramasami T. Interaction of aldehydes with collagen: effect on thermal, enzymatic and conformational stability. Int J Biol Macro. 2004;34:241–247. doi: 10.1016/j.ijbiomac.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.McClain PE, Wiley ER. Differential scanning calorimeter studies of the thermal transitions of collagen. J Biol Chem. 1972;247:692–697. [PubMed] [Google Scholar]
- 26.Le Lous M, Flandin F, Herbage D, Allain J. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295–300. doi: 10.1016/0304-4165(82)90182-9. [DOI] [PubMed] [Google Scholar]
- 27.Nimni ME. Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum. 1983;13:1–86. doi: 10.1016/0049-0172(83)90024-0. [DOI] [PubMed] [Google Scholar]
- 28.Spoerl E, Wollensak G, Dittert DD, Seiler T. Thermomechanical behavior of collagen-cross-linked porcine cornea. Ophthalmologica. 2004;218:136–140. doi: 10.1159/000076150. [DOI] [PubMed] [Google Scholar]
- 29.Dillon J, Zheng L, Merriam JC, Gaillard ER. Transmission spectra of light to the mammalian retina. Photochem Photobiol. 2000;71:225–229. doi: 10.1562/0031-8655(2000)071<0225:tsoltt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 31.Paik DC, Saito LY, Sugirtharaj DD, Holmes JW. Nitrite induced cross-linking alters remodeling and mechanical properties of collagenous engineered tissues. Connect Tissue Res. 2006;47:163–176. doi: 10.1080/03008200600721569. [DOI] [PubMed] [Google Scholar]
- 32.Mannermaa E, Vellonen KS, Urtti A. Drug transport in the corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Delivery Rev. 2006;58:1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87:1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 34.Schoenwald RD, Huang HS. Corneal penetration behavior of β-blocking agent, I: physicochemical factors. J Pharm Sci. 1983;72:1266–1272. doi: 10.1002/jps.2600721108. [DOI] [PubMed] [Google Scholar]
- 35.Conaway CC, Hussain NS, Way BM, Fiala ES. Evaluation of secondary nitroalkanes, nitrocarbinols, and other aliphatic nitro compounds in the Ames Salmonella assay. Mut Res. 1991;261:197–207. doi: 10.1016/0165-1218(91)90068-w. [DOI] [PubMed] [Google Scholar]
- 36.Jung YS, Anderson RC, Edrington TS, et al. Experimental use of 2-nitro-1-propanol for reduction of Salmonella typhimurium in the ceca of broiler chicks. J Food Prot. 2004;67:1945–1947. doi: 10.4315/0362-028x-67.9.1945. [DOI] [PubMed] [Google Scholar]
- 37.Horrocks SM, Jung YS, Huwe JK, et al. Effects of short-chain nitrocompounds against Campylobacter jejuni and Campylobacter coli in vitro. J Food Sci. 2007;72:M50–M55. doi: 10.1111/j.1750-3841.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- 38.Bollmeier AF. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc.; New York: 1996. Nitro alcohols. DOI: 0.1002/0471238961.1409201802151212.a01. [Google Scholar]





